Abstract
Multiple myeloma (MM) is a neoplastic disorder. It results from proliferation of clonal plasma cells in bone marrow with production of monoclonal proteins, which are detectable in serum or urine. MM is clinically characterized by destructive bone lesions, anemia, hypercalcemia and renal insufficiency. Its prognosis is severe, with a median survival after diagnosis of approximately 3 years due to frequent relapses. Treatments for patients with relapsed/refractory MM include hematopoietic cell transplantation, a rechallenge using a previous chemotherapy regimen or a trial of a new regimen. The introduction of new drugs such as thalidomide, lenalidomide and bortezomib has markedly improved MM outcomes. When relapse occurs, the clinician’s challenge is to select the optimal treatment for each patient while balancing efficacy and toxicity. Patients with indolent relapse can be first treated with a 2-drug or a 3-drug combination. Patients with more aggressive relapse often require therapy with a combination of multiple active agents. Autologous stem cell transplantation should be considered as salvage therapy at first relapse for patients who have cryopreserved stem cells early in the disease course. The aim of this review is to provide an overview on the pharmacological and molecular action of treatments used for patients with relapsed/refractory multiple myeloma.
Keywords: relapsed/refractory multiple myeloma, proteasome inhibitors, immunomodulatory drugs (IMIDs), chemotherapy
Introduction
Multiple myeloma (MM) is a neoplastic disorder due to the proliferation of clonal plasma cells in bone marrow (BM) with production of monoclonal proteins. These proteins are detectable in serum and/or urine, and MM is clinically characterized by destructive bone lesions, anemia, hypercalcemia and renal insufficiency.1 Its prognosis is severe, with a median survival after diagnosis of approximately 3 years. It accounts for 1.5%–2% of all cancer deaths.1 In the last decade, the introduction of immunomodulatory drugs (IMIDs) such as thalidomide and lenalidomide, and of new front-line agents such as the proteasome inhibitor bortezomib, has significantly improved overall survival. Still, not all patients respond to these new drugs, and the development of drug resistance is common.
The term “relapsed MM” describes subjects who have previously achieved at least a minor response after salvage therapy, but then experience progressive disease, while with “refractory MM”, subjects are either unresponsive to salvage therapy or are progressing within 60 days of the last treatment.2 Although conceptually distinct, in clinical practice these 2 conditions may be grouped with the term relapsed/refractory MM and the approach to these patients still represents a challenge for specialists.1
The underlying biological mechanisms of relapsed or refractory MM have been recently clarified. For instance, the existence of minor sub-clones that can survive chemotherapy and thus become a reservoir for relapse or resistance has been demonstrated.3 The genetic instability of aggressive MM sub-clones and the selective pressures introduced by therapy during the course of the disease are currently considered the 2 primary factors conditioning relapse or resistance.4
Traditionally, relapsed/refractory MM has been treated with standard combinations of alkylating agents, anthracyclines and corticosteroids, with or without hematopoietic stem cell rescue.5,6 The new IMIDs and proteasome inhibitors have not only significantly prolonged the overall survival in relapsed/refractory patients, but also have improved the rate, the depth and the duration of responses in front-line therapy.7–9 The impact of the depth of response on survival in the relapse setting is still controversial. Based on recent evidence, complete response (CR) seems to be the only condition linked with long-term remission and prolonged survival, especially when supported by multi-parameter flow cytometry or molecular studies.10,11 Instead, near-CR, very good partial remission (VGPR) and partial remission (PR) all seem to have virtually identical outcomes.10,11
The aim of this review is to provide an overview of the pharmacological and molecular action of the drugs used to treat relapsed/refractory MM, and of the available approaches for tailoring management of such patients.
Current Treatment Options
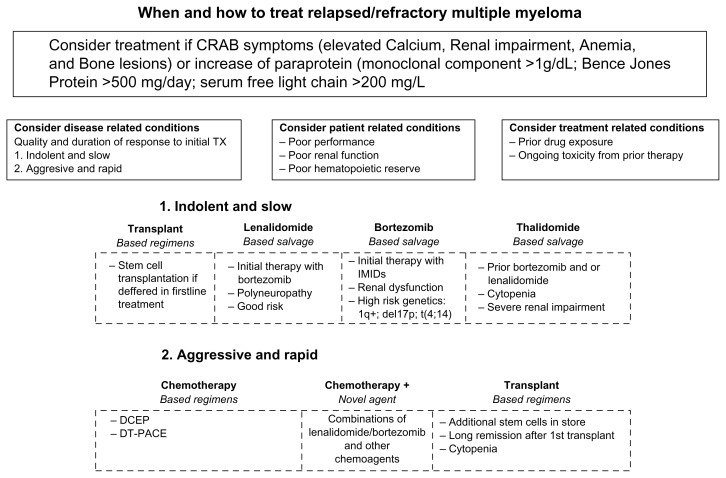
Although substantial progress has been made in recent years, MM still remains an incurable disease in most cases, due to frequent relapses. However, several new drugs active in relapsed/refractory MM are now available. When dealing with this set of patients, the challenge is to select the optimal treatment, taking into account efficacy and toxicity of the drug and both disease-and patient-related factors (Fig. 1).
Figure 1.
The clinical picture of relapsed multiple myeloma ranges from an asymptomatic form to very aggressive disease. Relapsing patients with multiple myeloma should be treated at the appearance of the typical clinical manifestations of multiple myeloma which are summarized by the CRAB symptoms (elevated Calcium, Renal impairment, Anemia, and Bone lesions), or when monoclonal protein in serum or urine has a significant growth (M spike >1 g/dL, Bence Jones protein [BJP] > 500 mg/day, or serum free light chain > 200 mg/dL). The treatment armamentarium in indolent or slow relapsing/refractory patients include, thalidomide, lenalidomide and bortezomib. Pomalidomide is considered in patients who have received at least two prior therapies, including lenalidomide and bortezomib, demonstrating disease progression.
Disease-, patient-and treatment-related conditions should be considered in the therapeutic management of relapsed/refractory multiple myeloma.
The disease-related factors include the quality and duration of response to previous therapies. If the relapse occurs after a long remission and treatment-free period, it is possible to consider the rechallenge of the same treatment but, if it occurs earlier (6–12 months) or while the patient is still undergoing treatment (which indicates aggressive, relapsed and refractory disease), the use of an alternative regimen should be considered. Cytogenetic abnormalities may indicate high-risk disease, which requires a different approach from that used in ‘slowly’ relapsing patients. Bortezomib can overcome the poor prognosis of patients with unfavorable chromosomal abnormalities, such as the del(13q14) and t(4:14) mutations. There are also initial data suggesting that high doses of bortezomib-based treatment may be effective in patients with the del(17p) mutation, which is usually associated with refractoriness to therapy.
The patient-related factors include pre-existing toxicities, comorbidities, the quality of life, age and performance status. Among the new drugs, bortezomib and thalidomide are not excreted renally, which makes them better for patients with renal impairment than lenalidomide, which is renally excreted and therefore requires dose adjustments. The use of thalidomide and bortezomib can lead to neuropathy in up to 80% of previously treated patients, whereas neuropathy is less frequent in patients treated with lenalidomide-based regimens, thus making them a reasonable choice in patients with pre-existing neuropathies. Venous thromboembolism and occasional thrombotic events have been reported in patients treated with IMIDs, especially when combined with pulsed dexamethasone instead of Bortezomib which seems. In addition lenalidomide seems to be associated with Myelosuppression.
The treatment related conditions include prior drug exposure and toxicities from prior therapy.
The challenge when treating patients with relapsed or refractory disease is to select the optimal treatment by balancing efficacy, toxicity and severity of relapse.
1. When treating indolent or slow relapse the treatment options include: lenalidomide based salvage therapy if patient have been previously exposed to bortezomib therapy, have history of polyneuropathy or have cytogenetic standard risk; bortezomib based salvage therapy if patient has been exposed to IMIDs, have renal failure or unfavorable chromosomal abnormalities; thalidomide based salvage therapies are indicated in presence of previous treatment with bortezomib or lenalidomide, cytopenia or severe renal impairment. Stem cell transplantation may be considered if deferred in first line therapy
2. Aggressive and rapid relapse requires an immediate treatment, which is likely a combination therapy including treatment with chemotherapy based regimens, chemotherapy in combination with novel agents (lenalidomide or bortezomib) or transplant based regimen.
Chemotherapy and transplantation
The use of conventional or high-dose chemotherapy is a long-standing approach to salvage therapy in patients with relapsed MM. In the past, various standard chemotherapy-based regimens have been used, among which the most widely employed are: (i) high dose melphalan, (ii) high dose prednisolone, (iii) high dose dexamethasone, (iv) vincristine, doxorubicin and pulsed high dose dexamethasone (VAD), (v) vincristine, melphalan, cyclophosphamide and prednisone (VMPC) alternating with vincristine, carmustine, doxorubicin, prednisone (VBAP), (vi) doxorubicin, vincristine, dexamethasone, etoposide and cyclophosphamide (CEVAD), (vii) cisplatin, doxorubicin, cyclophosphamide and etoposide (DTPACE), and (viii) dexamethasone, cyclophosphamide, etoposide and cisplatin (DCEP). The overall rates of response to salvage combination chemotherapy range between 30% and 60%, with morbidity and mortality rates related to the intensity of the therapy itself.12
Single or double autologous stem cell transplantation (ASCT) remains the milestone for a frontline approach in MM patients eligible for high-dose therapy, and the second ASCT represents a safe option for MM patients who were firstly treated with single ASCT.13–16 Although data support the use of a late second ASCT in patients with relapsed/progressive MM,17 researchers of the European Group for Blood and Marrow Transplantation found that outcomes were better when the second ASCT was performed before relapse, within 6–12 months from the first ASCT.18 The role of allogenic stem cell transplantation, potentially curative for myeloma, is controversial in relapsed/refractory MM.12
Immunomodulatory drugs (IMIDs)
Thalidomide
Thalidomide (α-N-phthalimido-glutarimide) represents the progenitor of IMIDs and is a synthetic derivative of glutamic acid. It was initially introduced in 1956 as a sedative hypnotic. Subsequently, it was observed that the drug can potentiate the immune response by restoring dendritic cell function and inhibiting T cell regulatory activity, leading to the activation of T lymphocytes and natural killer T (NKT) cells via interleukin 2 (IL-2) and interferon gamma (IFNγ) and activation of natural killer (NK) cells. The anti-tumoral activity of IMIDs consists in the disruption of the interactions between neoplastic clones, the BM micro-environment and anti-angiogenic activity.19 The mechanism of action of thalidomide is based on apoptosis of neoplastic cells by down-regulation of anti-apoptotic proteins via the caspase 8-mediated pathway.19
Thalidomide alone has been reported to induce partial remission in 50% of newly diagnosed patients, a rate that increases to 60%–70% when used in combination with oral dexamethasone. In 2006, the US Food and Drug Administration approved thalidomide in combination with dexamethasone for the treatment of newly diagnosed MM patients. When used in patients with relapsed/refractory MM, several studies have demonstrated that it leads to response rates of 25%–35%.20–22 It is also used as maintenance therapy in patients with progressive MM after SCT.22 In the relapsed/refractory MM setting, thalidomide used in association with dexamethasone and/or cyclophosphamide shows even higher response rates.23 Also the combination of thalidomide and conventional chemotherapy is clearly active, leading to overall response rates of 60%–75%, with CR rates of approximately 20% in a number of early phase I/II studies.24,25
Lenalidomide
Lenalidomide (Revlimid®; Celgene, NJ, USA) is an oral derivative of thalidomide with a different toxicity profile, as well as different immunomodulatory, anti-angiogenic and anti-neoplastic activity and different anti-inflammatory effects.7,26 In 2006, the US Food and Drug Administration approved lenalidomide for use in combination with dexamethasone in patients with MM who have received 1 prior therapy. In 2 parallel trials, MM-009 and MM-010 lenalidomide was used at the dose of 25 mg on days 1 to 21 of a 28-day schedule, and dexamethasone was given on days 1–4, 9–12 and 17–20 for the first 4 cycles as intravenous pulse therapy, with the dose being reduced for subsequent cycles.26 These observations provided evidence that treatment with lenalidomide plus dexamethasone leads to good responses, in the absence of disease progression and toxicity, thus obtaining a deeper remission and overall greater clinical advantages. Further trials confirmed the efficacy of lenalidomide in relapsed/refractory MM26–30 as well as in other hematological malignancies.31–33
Pomalidomide
The third available IMID, pomalidomide (CC4047), has been developed to improve the clinical efficacy and reduce the toxicity of its parent molecule thalidomide. In fact, it has a good toxicity profile. Neutropenia and thromboembolic complications are as frequent as with the other IMIDs, whereas other side effects such as neuropathy are rare.7 Pomalidomide was shown to be more effective than thalidomide in inhibiting the proliferation of malignant B cells in vitro, while in vivo increases the serum levels of IL-2 receptors and IL-12, and may promote the switch to an effector T-cell phenotype as well as the inhibition of osteoclast differentiation, reducing the destructive effects of MM in the bone microenvironment.7,34
Due to its recent introduction, pomalidomide has so far only been investigated in phase I and phase II trials involving heavily pre-treated patients. In 2013, the Food and Drug Administration approved pomalidomide for the treatment of patients with MM who have received at least 2 prior therapies, including lenalidomide and bortezomib, and have demonstrated disease progression on or within 60 days of completion of the last therapy. Pomalidomide at doses of 2 mg/day (d) has demonstrated excellent activity in patients with MM who failed to respond after treatment with lenalidomide and bortezomib.35–37 Myelosuppression was the most common toxicity. Pomalidomide overcomes resistance in myeloma refractory to both lenalidomide and bortezomib.
Proteasome inhibitors
Bortezomib
Bortezomib (PS-341) is the prototype proteasome inhibitor. It has potent anti-myeloma activity both when used alone and in combination with other drugs. In 2008, the US Food and Drug Administration approved bortezomib for the treatment of patients with MM.
Bortezomib was previously approved in 2003 for the treatment of refractory MM and in 2005 for the treatment of patients with MM who had received at least 1 prior therapy. The ubiquitin proteasome system is a multi-catalytic proteinase complex that, in order to maintain cell homeostasis, degrades a great variety of protein substrates both in normal and transformed cells. Consequently, proteasome inhibition affects a wide range of cell functions such as cell cycle regulation and apoptosis.5 Cancer cells, especially in MM, seem to be highly dependent on proteasome-homeostatic pathways. Furthermore, bortezomib stabilizes the nuclear factor kappa light chain-enhancer of activated B cells (NF kappa B) and up-regulates anti-apoptotic factors in tubular cells.5,12
A further large randomized trial in patients with relapsed/refractory myeloma, who had received no more than 3 previous treatment regimens, demonstrated the superiority of bortezomib given intravenously on days 1, 4, 8 and 11 of a 21-day cycle over pulse dexamethasone. The overall response rate was 38%, and the median time to progression (TTP) was 6.2 months, compared to only 18% and 3.5 months with dexamethasone.38 Bortezomib showed a minimal BM toxicity, ease of use in the case of renal failure, and absence of thrombogenicity. Bortezomib does not undergo renal clearance, and therefore needs no dose adjustments in patients with renal disease. The activity of bortezomib in patients with MM and renal insufficiency has been demonstrated in different studies.39,40 Bortezomib seems to overcome the poor prognosis of patients with unfavorable chromosomal abnormalities, such as the del(13q14) and t(4:14) mutations and there are initial data suggesting effectiveness at high doses even in patients with the del(17p) mutation, a negative prognostic factor associated with refractoriness and worse prognosis. Recent studies provide evidence that bortezomib at high doses may be of interest in patients with del(17p) mutation.41,42 In conclusion several authors have shown efficacy of bortezomib alone or in combination in relapsed refractory MM.42–49
Carfilzomib
A second-generation proteasome inhibitor PR-171 (carfilzomib) with different functional capacities has been developed. Carfilzomib is able to irreversibly inhibit the chymotryptic activity of the proteasome. Clinical studies have shown that carfilzomib has long-lasting anti-cancer activity in patients with relapsed/refractory MM, even those previously treated with bortezomib. Ongoing phase II trials have shown an overall response rate of 23.7% with a median duration of response of 7.8 months, and a median overall survival of 15.6 months. The safety profile is good, with adverse events manageable without giving rise to cumulative toxicities. The lasting responses and the drug’s acceptability demonstrate the potential of carfilzomib to offer a significant clinical benefit even in a heavily pre-treated population.50 In 2012, the US Food and Drug Administration approved carfilzomib for the treatment of patients with MM who have received at least 2 prior therapies, including bortezomib and IMIDs, and have demonstrated disease progression on or within 60 days of the completion of the last therapy. In an open-label, single-arm, multicentre pilot phase II study of carfilzomib involving 46 patients with relapsed and refractory MM after ≥2 previous therapies, the best overall response rate was 16.7%, with a median duration of response of 7.2 months.51
Other proteasome inhibitors are being developed with different spectrum of activity (eg, panproteasome inhibition with NPI-0052) and oral formulation.
IMIDs combined with bortezomib
New combinations of an IMID and bortezomib have been introduced, based on recent discoveries concerning the fundamental molecular mechanisms underlying MM cell growth and survival. Both IMIDs and bortezomib have significant activity against MM when used as single agents, so the challenge is to demonstrate whether their combination may reciprocally enhance their activity, theoretically reducing the risk of the emergence of resistant clones. The rationale for combining an IMID and bortezomib is related to their complementary mechanisms of action. However, as cumulative toxicity is a concern, various groups are evaluating the impact of other bortezomib-based combination therapies, such as the association with thalidomide and dexamethasone (BTD).
The combination of bortezomib (Velcade), thalidomide and dexamethasone (VTD) was compared with that of thalidomide and dexamethasone (TD) before ASCT in a phase III trial by the GIMEMA group.52 The 241 patients randomized to the VTD arm received standard-dose bortezomib with dexamethasone 40 mg on days 1, 2, 4, 5, 8, 9, 11 and 12, and thalidomide 200 mg/day for 63 days, whereas the 238 randomized to the TD arm received thalidomide 200 mg/day and dexamethasone 40 mg on days 1–4 and 9–12 of each 21-day cycle. As 6 patients withdrew their consent before starting treatment, the intention-to-treat analysis was based on 236 patients treated with VTD and 238 receiving TD. After induction therapy, a CR or near-CR was achieved in 31% of VTD and 11% of TD treated patients. VTD induction therapy before double ASCT significantly improved the rate of CR or near-CRs, and now represents a new standard of care for MM patients eligible for transplantation.
Another study aimed to assess efficacy, safety, and the reversal of renal impairment (RI) in previously untreated MM patients who received a combination of bortezomib, melphalan, prednisone and thalidomide followed by maintenance treatment with bortezomib and thalidomide (VMPT-VT) or bortezomib, melphalan and prednisone (VMP). There were statistically significant improvements in overall response rates and progression-free survival in the VMPT-VT arm across the renal cohorts, except in the group of patients with severe RI. In the VMPT group, severe RI reduced OS. RI was reversed in 16/63 patients receiving VMPT-VT (25.4%) and 31/77 receiving VMP (40.3%). VMPT-VT was superior to VMP in the patients with normal renal function but not in patients with severe renal insufficiency.53
Under Research Drugs
Histone deacetylase inhibitors
Panobinostat (LBH589) and vorinostat are histone deacetylase (HDAC) inhibitors, a new class of anti-myeloma agents. Inhibition of HDAC leads to histone hyperacetylation and structural alterations in chromatin causing growth arrest differentiation and/or apoptosis in tumor cells. HDAC inhibitors induce transcriptional modulations in 7%–10% of the genes in malignant cell lines as shown by microarray-based studies, by acetylating histone and non-histone proteins. HDAC inhibitor-induced cell death is one of the main mechanisms of inhibition of the survival of myeloma cells.6 In MM cells treated with an HDAC inhibitor, extrinsic and intrinsic apoptotic pathways, as well as non-apoptotic cell death such as autophagy, have been observed. The intrinsic apoptotic pathway is mediated by the mitochondria, and the pro-apoptotic signals result in the release of mitochondrial inter-membrane proteins such as cytochrome c (cyto-c), apoptosis-inducing factors (AIFs) and the second mitochondria-derived activator of caspase (Smac). HDAC inhibitors induce cell cycle arrest in the G1/S phase. The events in the G1 phase are coordinated by the three early G1 D cyclins (1, 2 and 3) and their associated cyclin-dependent kinases (CDKs) 4/6 (G1 progression) and CDK 2 (G1/S transition).6
A number of clinical trials of HDAC inhibitors alone or in combination with other anti-myeloma agents are ongoing. Phase I trials have shown that HDAC inhibitors are well tolerated by MM patients, although phase II trials have found that the activity of HDAC inhibitors as single agents is limited. However, when combined with dexamethasone and/or bortezomib, the results are more promising, even in patients with refractory and/or relapsed MM.54 Panobinostat (LBH589) and vorinostat have shown to be promising in combination with current treatment options, and panobinostat is currently being tested in a large, randomized phase III trial.6
Immune-based therapies
Monoclonal antibody therapy is a further option for MM patients. Elotuzumab is a humanized monoclonal IgG1 antibody directed against CS-1, a cell surface glycoprotein that is highly and uniformly expressed on MM cells. Elotuzumab induces significant antibody-dependent cytotoxicity against primary MM cells in the presence of peripheral lymphocytes and, in combination with lenalidomide and low-dose dexamethasone, has led to promising results.55 Another 2 agents of the same class have also showed promise: lorvotuzumab (anti-CD56) in combination with lenalidomide and dexamethasone, and mapatumumab (anti-Trail-R1) in combination with bortezomib.56
Alkylators
Among alkylating agents, bendamustine is structurally similar to purine analogues, and it has been found to be active in MM patients. The final results of a phase I/II study of bendamustine combined with lenalidomide and dexamethasone in patients with relapsed/refractory MM have been recently published, showing that this combination has anti-myeloma activity with relatively little toxicity in previously treated MM patients.57,58
Safety
The frequency and severity of IMID side-effects are dose-related and time-dependent, and should be graded based on the National Cancer Institute Common Toxicity Criteria for Adverse Events. Venous thromboembolism (VTE) and occasional thrombotic events have been reported in patients treated with thalidomide, especially when thalidomide is combined with pulsed dexamethasone (TD).59 The thrombogenic effects are likely due to many factors such as a transient reduction in soluble thrombomodulin levels during the first month of therapy, the restoration of endothelial cell PAR-1 expression after damage by cytotoxic agents such as doxorubicin and the activation of pro-coagulant tissue factor (TF) induced by phosphatylserine on the apoptotic cell membrane.59–62
Although structurally related to thalidomide, lenalidomide and pomalidomide are relatively more potent and show a different toxicity profile. Sedation, constipation and neuropathy, often associated with thalidomide, are not commonly seen with lenalidomide and pomalidomide, but the risk of developing thromboembolic events seems to be similar to that attributed to thalidomide combinations. Also, in the case of lenalidomide, the combination of dexamethasone seems to increase the risk of thromboembolic complications.59 The dose-limiting side effect of lenalidomide in phase I studies is myelosuppression (grade 3–4 neutropenia and thrombocytopenia). Although this is the most frequent adverse event, it can be effectively managed by means of dose reductions or discontinuation of the drug, although granulocyte colony-stimulating factor (G-CSF) or erythropoietin may be needed in more severe cases.63 The development of second primary malignancies (SPMs) among patients treated with lenalidomide is still controversial. On one hand, an increased incidence of SPM has been observed in MM patients after lenalidomide therapy, as compared to control patients (3.98 per 100 patient-years vs. 1.38 per 100 patient-years).64 Particularly, acute myeloid leukemia (AML) seems to be the main SPM in MM patients treated with lenalidomide, with an increase of incidence reaching 11.5-fold as compared to general population, as reported by data analysis from a Swedish register.64 On the other hand, other researchers found no differences in the incidence of SPM in myelodysplastic syndrome patients treated with lenalidomide as compared to a historic group with myelodysplastic syndrome not treated with lenalidomide.65 Indeed, the PFS benefit obtained using lenalidomide maintenance treatment is reckoned to outweigh the increased risk of SPM.66 Finally, melphalan could also be responsible for the increased risk of hematological malignancies, thus further complicating data interpretation.65 Similarly, Palumbo et al66 have recently stated that the PFS benefit obtained using lenalidomide maintenance treatment outweighs the increased risk of SPM; moreover, in patients with relapsing/refractory MM, the number and types of SPM do not seem to affect the drug’s risk/benefit profile.
Bortezomib toxicity is characterized by nausea, diarrhea, cyclic reversible thrombocytopenia, fatigue, and peripheral neuropathy. Peripheral neuropathy occurs in about one-third of patients and may have a painful component. It improves or resolves in a high proportion of patients, although recovery often takes several months after dose modification or discontinuation. An increased incidence of herpes zoster reactivation has been reported, requiring acyclovir prophylaxis in all patients receiving bortezomib.67
Based on clinical trial data, HDAC inhibitors are generally well tolerated, but there have been reports of various different toxicities, such as reversible QT prolongation, pericardial effusion, hypokalemia and thrombocytopenia.68,69
Supportive Care
Considerable progress has been made for patients with MM in terms of supportive and palliative care. These measures can improve their quality of life (QoL) when integrated with conventional medical treatment.
Approximately 85% of patients develop bone disease manifesting as osteopenia, osteolytic lesions and related complications, all of which reduce their performance status and QoL.70 Patients should be treated with analgesics such as acetaminophen, whereas non steroidal anti-inflammatory drugs should be avoided because they can affect renal function. Opioids should be considered when patients fail to respond to first-step therapy. Local radiotherapy can also relieve the pain of skeletal disease and may palliate soft tissue disease.71
Also, bisphosphonates can help in the management of bone pain in MM patients and can help in preventing occurrence of new bone lesions and pathological fractures.72,73 Moreover, there is evidence that zoledronic acid may affect OS and PFS, probably interfering with the MM microenvironment in BM.73
Blood and platelet transfusions can help maintain the QoL by relieving exertional dyspnoea and preventing bleeding, even when a patient is approaching the terminal stage of the disease.
Treatment Strategies and Patients Preference
The clinical picture of relapsed/refractory MM ranges from an asymptomatic form to a very aggressive disease. Relapsed/refractory patients with MM should be treated at the appearance of the typical clinical manifestations of MM, which are summarized by the CRAB symptoms (elevated Calcium, Renal impairment, Anemia, and Bone lesions), or when monoclonal protein in serum or urine has a significant growth. (Monoclonal spike >1 g/dL, Bence Jones protein [BJP] > 500 mg/day, or serum free light chain >200 mg/dL).
There are still no standard therapies for relapsed/refractory MM, and treatment remains a challenge, especially in the case of patients who already received several lines of therapies.70 Nevertheless, the use of IMIDs and proteasome inhibitors has led to deepest and longest remissions in front line therapies, allowing patients to obtain longer event-free survivals. The lesson from clinical trials shows that the new agents work also in relapsed/refractory MM patients, thus rendering crucial the sequence and the timing of drug choosing. In addition, various disease-and patient-related factors, as well as pharmacological characteristics of antimyeloma agents, should be considered for therapeutic management. The quality and duration of response to previous therapies and the aggressiveness of the relapse are important disease-related factors and represent key points in treatment strategies. Deep and prolonged responses are more likely in patients with a late relapse (after a remission of >12 months) than in those relapsing early (a remission of <6 months). If the relapse occurs after a long remission and treatment-free period, it is possible to consider repeating the same treatment,70–74 but if it occurs earlier (6–12 months) or while the patient is still undergoing treatment (which indicates aggressive, relapsed and refractory disease), the use of an alternative regimen should be considered. In addition, the presence of clinical risk factors such as cytogenetic abnormalities may indicate high-risk disease, which requires a different approach from that used in ‘slowly’ relapsing patients. Bortezomib can overcome the poor prognosis of patients with unfavorable chromosomal abnormalities, such as the del(13q14) and t(4:14) mutations. There are also initial data suggesting that high doses of bortezomib-based treatment may be effective in patients with the del (17p) mutation, which is usually associated with refractoriness to therapy.36
Patient-related factors include pre-existing toxicities, comorbidities, the quality of life, age and performance status. Some degree of renal impairment frequently occurs in MM patients and, as many therapeutic agents are renally excreted, this may affect drug pharmacokinetics and limit the choice. Among the new drugs, bortezomib and thalidomide are not renally excreted, which makes them better for patients with renal impairment than lenalidomide, which instead is renally excreted and therefore requires dose adjustments. Moreover, bortezomib has shown beneficial effects in patients with MM and renal insufficiency32,33 by rapidly reducing light chain production. On the contrary, as neither lenalidomide nor thalidomide are metabolized by the liver, they are more suitable for patients with impaired liver function than bortezomib.
The use of thalidomide and bortezomib can lead to neuropathy in up to 80% of previously treated patients, whereas neuropathy is less frequent in patients treated with lenalidomide-based regimens, thus making them a reasonable choice in patients with pre-existing neuTreatment of relapsed myeloma ropathies. Subcutaneous administration of bortezomib results in less polyneuropathy related symptoms, than intravenous infusion.75
Bortezomib alone has not been associated with any increase in VTE, and is therefore a good choice for patients with a history of thromboembolic events. Thalidomide or lenalidomide treatment generally requires appropriate anti-thrombotic prophylaxis. It is generally accepted that acetylsalicylic acid (ASA) is a good thromboprophylaxis in patients without a previous history of thrombotic events and with no thrombotic risk factors, whereas anti-coagulant prophylaxis is mandatory for those patients who have previously experienced a thromboembolic event or who are at high thrombotic risk.61,62 The thromboprophylaxis, with low molecular weight heparin, should be performed almost for 3 months before switching to aspirin, since thromboembolic events are more likely in the first three months of treatment with IMIDs.76 When an aggressive relapse occurs, patients whose performance status allows them to tolerate an aggressive treatment should be treated to obtain the deepest response in order to improve survival. Aggressive and rapid relapse requires an immediate treatment, which is likely a combination therapy including treatment with chemotherapy based regimens, chemotherapy in combination with novel agents (lenalidomide or bortezomib) or transplant based regimen. In contrast, when the patient’s global health status does not allow them to tolerate an aggressive therapy, best supportive care should be considered in order to improve the quality of life.
The fact that one-third of MM patients are 75 years old or older at diagnosis raises some concerns about the tolerability and the toxicity of the treatment. Although age should not be considered an exclusion criterion for treatment, only a few clinical trials have investigated the safety and efficacy of drugs in elderly and/or frail patients. Modified treatment regimens and dose reductions should be used to improve tolerability.
Conclusions
Novel agents targeting the MM and its microenvironment such as thalidomide, lenalidomide and bortezomib have improved outcomes and extended survival in patients with relapsed and/or refractory MM. Several evidences have demonstrated that their usage at the time of a first relapse is linked with better outcomes rather than as salvage treatment after 2 or more previous therapies. Combination therapy using agents with different mechanisms of action is becoming an attractive means of increasing efficacy and/or overcoming resistance to standard treatment regimens. Other new agents are in clinical development. Pomalidomide has led to encouraging results in heavily pre-treated patients. The oral histone deacetylase inhibitors panobinostat and vorinostat can synergistically enhance the cytotoxic activity of lenalidomide and bortezomib, and overcome possible resistance. The second-generation proteasome inhibitor carfilzomib can be used as monotherapy and in combination with lenalidomide plus low-dose dexamethasone. Combined therapy can be used to prevent or overcome treatment resistance, and increase the efficacy of standard treatment regimens.
Footnotes
Author Contributions
Conceived the concept: RC, MC. Analyzed the data: RC, MC, NO, AL. Wrote the first draft of the manuscript: RC. Contributed to the writing of the manuscript: RC, MC, NO. Agree with manuscript results and conclusions: RC, RG, NO, AL, SG, MC. Jointly developed the structure and arguments for the paper: RC, MC, RG, NO. Made critical revisions and approved final version: RC, RG, NO, AL, SG, MC. All authors reviewed and approved of the final manuscript.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests. Provenance: the authors were invited to submit this paper.
Funding
Author(s) disclose no funding sources.
References
- Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351(18):1860–73. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P ASH/FDA Panel on Clinical Endpoints in Multiple Myeloma. Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22(2):231–9. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- Magrangeas F, Avet-Loiseau H, Gouraud W, et al. Minor clone provides a reservoir for relapse in multiple myeloma. Leukemia. 2013;27(2):473–81. doi: 10.1038/leu.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P, Minvielle S. Multiple myeloma: so much progress, but so many unsolved questions. Haematologica. 2013;98(4):487–9. doi: 10.3324/haematol.2013.083592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, San-Miguel JF, Anderson KC. Emerging therapies for the treatment of relapsed or refractory multiple myeloma. Eur J Haematol. 2011;86(1):1–15. doi: 10.1111/j.1600-0609.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- Moreau P. The future of therapy for relapsed/refractory multiple myeloma: emerging agents and novel treatment strategies. Semin Hematol. 2012;49( Suppl 1):S33–46. doi: 10.1053/j.seminhematol.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Castelli R, Cannavò A, Conforti F, Grava G, Cortelezzi A. Immunomodulatory drugs in multiple myeloma: from molecular mechanisms of action to clinical practice. Immunopharmacol Immunotoxicol. 2012;34(5):740–53. doi: 10.3109/08923973.2012.658921. [DOI] [PubMed] [Google Scholar]
- Moehler T, Goldschmidt H. Therapy of relapsed and refractory multiple myeloma. Recent Results Cancer Res. 2011;183:239–71. doi: 10.1007/978-3-540-85772-3_11. [DOI] [PubMed] [Google Scholar]
- Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Français du Myélome. N Engl J Med. 1996;335(2):91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- Harousseau JL, Dimopoulos MA, Wang M, et al. Better quality of response to lenalidomide plus dexamethasone is associated with improved clinical outcomes in patients with relapsed or refractory multiple myeloma. Haematologica. 2010;95(10):1738–44. doi: 10.3324/haematol.2009.015917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, Kastritis E, Christoulas D, et al. Treatment of patients with relapsed/refractory multiple myeloma with lenalidomide and dexamethasone with or without bortezomib: prospective evaluation of the impact of cytogenetic abnormalities and of previous therapies. Leukemia. 2010;24(10):1769–78. doi: 10.1038/leu.2010.175. [DOI] [PubMed] [Google Scholar]
- Lonial S. Relapsed multiple myeloma. Hematology Am Soc Hematol Educ Program. 2010;2010:303–9. doi: 10.1182/asheducation-2010.1.303. [DOI] [PubMed] [Google Scholar]
- Lokhorst H, Einsele H, Vesole D, et al. International Myeloma Working Group. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010;28(29):4521–30. doi: 10.1200/JCO.2010.29.7929. [DOI] [PubMed] [Google Scholar]
- Bladé J, Rosiñol L, Cibeira MT, Rovira M, Carreras E. Hematopoietic stem cell transplantation for multiple myeloma beyond 2010. Blood. 2010;115(18):3655–63. doi: 10.1182/blood-2009-08-238196. [DOI] [PubMed] [Google Scholar]
- Jimenez-Zepeda VH, Mikhael J, Winter A, et al. Second autologous stem cell transplantation as salvage therapy for multiple myeloma: impact on progression-free and overall survival. Biol Blood Marrow Transplant. 2012;18(5):773–9. doi: 10.1016/j.bbmt.2011.10.044. [DOI] [PubMed] [Google Scholar]
- Olin RL, Vogl DT, Porter DL, et al. Second auto-SCT is safe and effective salvage therapy for relapsed multiple myeloma. Bone Marrow Transplant. 2009;43(5):417–22. doi: 10.1038/bmt.2008.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis LC, Saad A, Zhong X, et al. Plasma Cell Disorders Working Committee of the Center for International Blood and Marrow Transplant Research. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant. 2013;19(5):760–6. doi: 10.1016/j.bbmt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C, Iacobelli S, Brand R, et al. Chronic Leukaemia Working Party Myeloma Subcommittee, European Group for Blood and Marrow Transplantation. Benefit and timing of second transplantations in multiple myeloma: clinical findings and methodological limitations in a European Group for Blood and Marrow Transplantation registry study. J Clin Oncol. 2004;22(9):1674–81. doi: 10.1200/JCO.2004.06.144. [DOI] [PubMed] [Google Scholar]
- Quach H, Ritchie D, Steward AK, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24(1):22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneller A, Raanani P, Hardan I, et al. Therapy with thalidomide in refractory multiple myeloma patients—the revival of an old drug. Br J Haematol. 2000;108(2):391–3. doi: 10.1046/j.1365-2141.2000.01835.x. [DOI] [PubMed] [Google Scholar]
- Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 2000;342(5):364. doi: 10.1056/NEJM200002033420523. [No authors listed] [DOI] [PubMed] [Google Scholar]
- Mohty M, Attal M, Marit G, et al. Thalidomide salvage therapy following allogeneic stem cell transplantation for multiple myeloma: a retrospective study from the Intergroupe Francophone du Myélome (IFM) and the Société Française de Greffe de Moelle et Thérapie Cellulaire (SFGM-TC) Bone Marrow Transplant. 2005;35(2):165–9. doi: 10.1038/sj.bmt.1704756. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Hamilos G, Zomas A, et al. Pulsed cyclophosphamide, thalidomide and dexamethasone: an oral regimen for previously treated patients with multiple myeloma. Hematol J. 2004;5(2):112–7. doi: 10.1038/sj.thj.6200326. [DOI] [PubMed] [Google Scholar]
- García-Sanz R, González-Porras JR, Hernández JM, et al. The oral combination of thalidomide, cyclophosphamide and dexamethasone (ThaCyDex) is effective in relapsed/refractory multiple myeloma. Leukemia. 2004;18(4):856–63. doi: 10.1038/sj.leu.2403322. [DOI] [PubMed] [Google Scholar]
- Kropff M, Baylon HG, Hillengass J, et al. Thalidomide versus dexamethasone for the treatment of relapsed and/or refractory multiple myeloma: results from OPTIMUM, a randomized trial. Haematologica. 2012;97(5):784–91. doi: 10.3324/haematol.2011.044271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–32. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- Knop S, Gerecke C, Liebisch P, et al. Lenalidomide, adriamycin, and dexamethasone (RAD) in patients with relapsed and refractory multiple myeloma: a report from the German Myeloma Study Group DSMM (Deutsche Studiengruppe Multiples Myelom) Blood. 2009;113(18):4137–43. doi: 10.1182/blood-2008-10-184135. [DOI] [PubMed] [Google Scholar]
- Schey SA, Morgan GJ, Ramasamy K, et al. The addition of cyclophosphamide to lenalidomide and dexamethasone in multiply relapsed/refractory myeloma patients; a phase I/II study. Br J Haematol. 2010;150(3):326–33. doi: 10.1111/j.1365-2141.2010.08250.x. [DOI] [PubMed] [Google Scholar]
- Reece D, Masih-Khan Phase I–II Trial of Oral Cyclophosphamide, Prednisone and Lenalidomide (Revlimid®) (CPR) for the Treatment of Patients with Relapsed and Refractory Multiple Myeloma. 50th ASH Annual Meeting and Exposition. 2010;116:3055. doi: 10.1111/bjh.13100. [DOI] [PubMed] [Google Scholar]
- Kastritis E, Dimopoulos MA. The evolving role of lenalidomide in the treatment of hematologic malignancies. Expert Opin Pharmacother. 2007;8(4):497–509. doi: 10.1517/14656566.8.4.497. [DOI] [PubMed] [Google Scholar]
- Castelli R, Cassin R, Cannavò A, Cugno M. Immunomodulatory drugs: new options for the treatment of myelodysplastic syndromes. Clin Lymphoma Myeloma Leuk. 2013;13(1):1–7. doi: 10.1016/j.clml.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Thieblemont C, Delfau-Larue MH, Coiffier B. Lenalidomide in diffuse large B-cell lymphoma. Adv Hematol. 2012;2012:861060. doi: 10.1155/2012/861060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach H, Ritchie D, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24(1):22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schey S, Ramasamy K. Pomalidomide therapy for myeloma. Expert Opin Investig Drugs. 2011;20(5):691–700. doi: 10.1517/13543784.2011.567265. [DOI] [PubMed] [Google Scholar]
- Lacy MQ, Allred JB, Gertz MA, et al. Pomalidomide plus low-dose dexamethasone in myeloma refractory to both bortezomib and lenalidomide: comparison of 2 dosing strategies in dual-refractory disease. Blood. 2011;118(11):2970–5. doi: 10.1182/blood-2011-04-348896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Siegel D, Baz R, et al. Phase 1study of pomalidomide MTD, safety, and efficacy in patients with refractory multiple myeloma who have received lenalidomide and bortezomib. Blood. 2013;121(11):1961–7. doi: 10.1182/blood-2012-08-450742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg PA, Mark TM. Pomalidomide in the treatment of relapsed multiple myeloma. Future Oncol. 2013;9(7):939–48. doi: 10.2217/fon.13.105. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110(10):3557–60. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- Haynes R, Leung N, Kyle R, Winearls CG. Myeloma kidney: improving clinical outcomes? Adv Chronic Kidney Dis. 2012;19(5):342–51. doi: 10.1053/j.ackd.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Roussou M, Gkotzamanidou M, et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia. 2013;27(2):423–9. doi: 10.1038/leu.2012.182. [DOI] [PubMed] [Google Scholar]
- Bergsagel PL, Mateos MV, Gutierrez NC, Rajkumar SV, San Miguel JF. Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high-risk multiple myeloma. Blood. 2013;121(6):884–92. doi: 10.1182/blood-2012-05-432203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath S, Richardson PG, Sonneveld P, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. Leukemia. 2007;21(1):151–7. doi: 10.1038/sj.leu.2404442. [DOI] [PubMed] [Google Scholar]
- Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119(4):940–8. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- Jagannath S, Barlogie B, Berenson JR, et al. Updated survival analyses after prolonged follow-up of the phase 2, multicenter CREST study of bortezomib in relapsed or refractory multiple myeloma. Br J Haematol. 2008;143(4):537–40. doi: 10.1111/j.1365-2141.2008.07359.x. [DOI] [PubMed] [Google Scholar]
- Berenson JR, Yang HH, Sadler K, et al. Phase I/II trial assessing bortezomib and melphalan combination therapy for the treatment of patients with relapsed or refractory multiple myeloma. J Clin Oncol. 2006;24(6):937–44. doi: 10.1200/JCO.2005.03.2383. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25(25):3892–901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- Kropff M, Bisping G, Schuck E, et al. Deutsche Studiengruppe Multiples Myelom. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol. 2007;138(3):330–7. doi: 10.1111/j.1365-2141.2007.06656.x. [DOI] [PubMed] [Google Scholar]
- Palumbo A, Gay F, Bringhen S, et al. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. Ann Oncol. 2008;19(6):1160–5. doi: 10.1093/annonc/mdn018. [DOI] [PubMed] [Google Scholar]
- Popat R, Oakervee H, Williams C, et al. Bortezomib, low-dose intravenous melphalan, and dexamethasone for patients with relapsed multiple myeloma. Br J Haematol. 2009;144(6):887–94. doi: 10.1111/j.1365-2141.2008.07572.x. [DOI] [PubMed] [Google Scholar]
- Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–25. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannath S, Vij R, Stewart AK, et al. An open-label single-arm pilot phase II study (PX-171-003-A0) of low-dose, single-agent carfilzomib in patients with relapsed and refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2012;12(5):310–8. doi: 10.1016/j.clml.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Cavo M, Tacchetti P, Patriarca F, et al. GIMEMA Italian Myeloma Network. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376(9758):2075–85. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- Morabito F, Gentile M, Mazzone C, et al. Safety and efficacy of bortezomib-melphalan-prednisone-thalidomide followed by bortezomib-thalidomide maintenance (VMPT-VT) versus bortezomib-melphalan-prednisone (VMP) in untreated multiple myeloma patients with renal impairment. Blood. 2011;118(22):5759–66. doi: 10.1182/blood-2011-05-353995. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Mitsiades CS, Laubach JP, et al. Preclinical data and early clinical experience supporting the use of histone deacetylase inhibitors in multiple myeloma. Leuk Res. 2013;37(7):829–37. doi: 10.1016/j.leukres.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Lonial S, Vij R, Harousseau JL, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. J Clin Oncol. 2012;30(16):1953–9. doi: 10.1200/JCO.2011.37.2649. [DOI] [PubMed] [Google Scholar]
- Allegra A, Penna G, Alonci A, et al. Monoclonal antibodies: potential new therapeutic treatment against multiple myeloma. Eur J Haematol. 2013;90(6):441–68. doi: 10.1111/ejh.12107. [DOI] [PubMed] [Google Scholar]
- Lentzsch S, O’Sullivan A, Kennedy RC, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. Blood. 2012;119(20):4608–13. doi: 10.1182/blood-2011-12-395715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pönisch W, Heyn S, Beck J, et al. Lenalidomide, bendamustine and prednisolone exhibits a favourable safety and efficacy profile in relapsed or refractory multiple myeloma: final results of a phase 1 clinical trial OSHO – #077. Br J Haematol. 2013;162(2):202–9. doi: 10.1111/bjh.12361. [DOI] [PubMed] [Google Scholar]
- Cavo M, Zamagni E, Cellini C, et al. Deep-vein thrombosis in patients with multiple myeloma receiving first-line thalidomide-dexamethasone therapy. Blood. 2002;100(6):2272–3. doi: 10.1182/blood-2002-06-1674. [DOI] [PubMed] [Google Scholar]
- Barbui T, Falanga A. Thalidomide and thrombosis in multiple myeloma. J Thromb Haemost. 2003;1(3):421–2. doi: 10.1046/j.1538-7836.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- Castelli R, Ferrari B, Cortelezzi A, Guariglia A. Thromboembolic complications in malignant haematological disorders. Curr Vasc Pharmacol. 2010;8(4):482–94. doi: 10.2174/157016110791330799. [DOI] [PubMed] [Google Scholar]
- Elice F, Rodeghiero F. Hematologic malignancies and thrombosis. Thromb Res. 2012;129(3):360–6. doi: 10.1016/j.thromres.2011.11.034. [DOI] [PubMed] [Google Scholar]
- Niesvizky R, Naib T, Christos PJ, et al. Lenalidomide-induced myelosuppression is associated with renal dysfunction: adverse events evaluation of treatment-naïve patients undergoing front-line lenalidomide and dexamethasone therapy. Br J Haematol. 2007;138(5):640–3. doi: 10.1111/j.1365-2141.2007.06698.x. [DOI] [PubMed] [Google Scholar]
- Thomas A, Mailankody S, Korde N, Kristinsson SY, Turesson I, Landgren O. Second malignancies after multiple myeloma: from 1960s to 2010s. Blood. 2012;119(12):2731–7. doi: 10.1182/blood-2011-12-381426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adès L, Le Bras F, Sebert M, et al. Treatment with lenalidomide does not appear to increase the risk of progression in lower risk myelodysplastic syndromes with 5q deletion. A comparative analysis by the Groupe Francophone des Myelodysplasies. Haematologica. 2012;97(2):213–8. doi: 10.3324/haematol.2011.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366(19):1759–69. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol. 2008;26(29):4784–90. doi: 10.1200/JCO.2007.14.9641. [DOI] [PubMed] [Google Scholar]
- Giles F, Fischer T, Cortes J, et al. A phase I study of intravenous LBH589, a novel cinnamic hydroxamic acid analogue histone deacetylase inhibitor, in patients with refractory hematologic malignancies. Clin Cancer Res. 2006;12(15):4628–35. doi: 10.1158/1078-0432.CCR-06-0511. [DOI] [PubMed] [Google Scholar]
- Iancu-Rubin C, Gajzer D, Mosoyan G, Feller F, Mascarenhas J, Hoffman R. Panobinostat (LBH589)-induced acetylation of tubulin impairs megakaryocyte maturation and platelet formation. Exp Hematol. 2012;40(7):564–74. doi: 10.1016/j.exphem.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowiak A. Management strategies for relapsed/refractory multiple myeloma: current clinical perspectives. Semin Hematol. 2012;49( Suppl 1):S16–32. doi: 10.1053/j.seminhematol.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Mill WB, Griffith R. The role of radiation therapy in the management of plasma cell tumors. Cancer. 1980;45(4):647–52. doi: 10.1002/1097-0142(19800215)45:4<647::aid-cncr2820450405>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Berenson JR, Lichtenstein A, Porter L, et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J Clin Oncol. 1998;16(2):593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- Morgan GJ, Davies FE, Gregory WM, et al. National Cancer Research Institute Haematological Oncology Clinical Study Group. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. 2010;376(9757):1989–99. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty B, El-Cheikh J, Yakoub-Agha I, Avet-Loiseau H, Moreau P, Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: a focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia. 2012;26(1):73–85. doi: 10.1038/leu.2011.310. [DOI] [PubMed] [Google Scholar]
- Chaudhry V, Cornblath DR, Polydefkis M, Ferguson A, Borrello I. Characteristics of bortezomib-and thalidomide-induced peripheral neuropathy. J Peripher Nerv Syst. 2008;13(4):275–82. doi: 10.1111/j.1529-8027.2008.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119(4):933–9. doi: 10.1182/blood-2011-03-344333. ; quiz 1093. [DOI] [PubMed] [Google Scholar]