Reduced levels of fatty alcohols in seed coat suberin increase permeability to tetrazolium salts and sensitivity to abscisic acid.
Abstract
Suberin is found in a variety of tissues, such as root endoderms and periderms, storage tuber periderms, tree cork layer, and seed coats. It acts as a hydrophobic barrier to control the movement of water, gases, and solutes as well as an antimicrobial barrier. Suberin consists of polymerized phenolics, glycerol, and a variety of fatty acid derivatives, including primary fatty alcohols. We have conducted an in-depth analysis of the distribution of the C18:0 to C22:0 fatty alcohols in Arabidopsis (Arabidopsis thaliana) roots and found that only 20% are part of the root suberin polymer, together representing about 5% of its aliphatic monomer composition, while the remaining 80% are found in the nonpolymeric (soluble) fraction. Down-regulation of Arabidopsis FATTY ACYL REDUCTASE1 (FAR1), FAR4, and FAR5, which collectively produce the fatty alcohols found in suberin, reduced their levels by 70% to 80% in (1) the polymeric and nonpolymeric fractions from roots of tissue culture-grown plants, (2) the suberin-associated root waxes from 7-week-old soil-grown plants, and (3) the seed coat suberin polymer. By contrast, the other main monomers of suberin were not altered, indicating that reduced levels of fatty alcohols did not influence the suberin polymerization process. Nevertheless, the 75% reduction in total fatty alcohol and diol loads in the seed coat resulted in increased permeability to tetrazolium salts and a higher sensitivity to abscisic acid. These results suggest that fatty alcohols and diols play an important role in determining the functional properties of the seed coat suberin barrier.
Suberin is a cell wall-linked polymeric barrier that plays a critical role in the survival of plants by protecting them against various biotic and abiotic stresses. It primarily acts as a hydrophobic barrier to control the movement of water, gases, and solutes, but also contributes to the strength of the cell wall (Ranathunge et al., 2011). Suberin is deposited at the inner face of primary cell walls next to the plasma membrane (Kolattukudy, 1980; Franke and Schreiber, 2007). It is typically found as lamellae (alternating dark and light bands when viewed by transmission electron microscopy) in the endodermis, exodermis, and peridermis of roots, as well as in the peridermis of underground storage tubers (Bernards, 2002). Suberin is also found in shoot periderms of trees (i.e. cork layer) and in seed coats (Molina et al., 2006, 2008) and is deposited in response to wounding (Kolattukudy, 2001).
Suberin is a polymer consisting of aliphatics (fatty acid derivatives), phenolics, and glycerol. The predominant aliphatic components of suberin are ω-hydroxy fatty acids, α,ω-dicarboxylic acids, very-long-chain fatty acids, and primary fatty alcohols, while the major phenolic components are p-hydroxycinnamic acids, especially ferulic acid (Kolatukudy, 1980; Bernards et al., 1995; Pollard et al., 2008; Ranathunge et al., 2011). In the periderm of underground storage organs, suberin is found in association with waxes, which are isolated either by extensive extraction in solvent (Soliday et al., 1979; Serra et al., 2009) or by brief immersion of tubers in chloroform (Espelie et al., 1980). These suberin-associated waxes consist of linear aliphatics (e.g. alkanes, fatty acids, and fatty alcohols), which are similar to cuticular wax components of aerial tissues but generally of shorter chain lengths (Espelie et al., 1980). In waxes extracted from 3-week-old wounded potato (Solanum tuberosum) periderms, alkyl ferulates (i.e. ferulic acid linked by an ester bond to a C16:0–C32:0 fatty alcohol) represent up to 60% of the total wax load (Schreiber et al., 2005). Root waxes are also found in 6- to 7-week-old mature taproots of Arabidopsis (Arabidopsis thaliana) with a fully developed periderm (Li et al., 2007; Kosma et al., 2012). They are enriched in alkyl hydroxycinnamates (AHCs) made of C18:0 to C22:0 fatty alcohols esterified with coumaric, caffeic, or ferulic acids (Kosma et al., 2012). The monomer composition (in terms of major chemical species and chain length) of both suberin and suberin-associated waxes varies considerably between plant species, tissues, and developmental stages. Aliphatic suberin and suberin-associated waxes are considered the major contributors to the diffusion resistance of suberized cell walls to radial transport of water and solutes (Soliday et al., 1979; Espelie et al., 1980; Zimmermann et al., 2000; Ranathunge and Schreiber, 2011). The organization of suberin components in the lamellated structure as well as how waxes may be associated with the polymer is a matter of debate (Graça and Santos, 2007).
Primary fatty alcohols are long-chain hydrocarbons containing a single hydroxyl group at the terminal position. They are ubiquitously detected as components of the suberin polymer, representing 1% to 10% of the total monomer mass recovered after transesterification (Holloway, 1983; Bernards, 2002; Pollard et al., 2008). Primary fatty alcohols are also typical components of suberin-associated waxes, where they can be found either in free form or linked by an ester bond with a hydroxycinnamic acid (i.e. as AHCs; Soliday et al., 1979; Espelie et al., 1980; Bernards and Lewis 1992; Li et al., 2007; Kosma et al., 2012). In mechanically isolated endodermis of soybean (Glycine max) roots, fatty alcohols represent about 1.5% and 0.2% of the total aliphatics found in suberin-associated waxes and suberin polymer, respectively (Thomas et al., 2007). In onion (Allium cepa) root exodermis, fatty alcohols (C14:0–C28:0) account for 7% to 12% of the soluble fraction, while the suberin fraction contains only C22:0 fatty alcohol, which makes up 3% of the suberin fraction across all exodermal maturation zones (Meyer et al., 2011). In suberizing potato periderms 7 d post wounding, C16:0 to C28:0 fatty alcohols represent about 10% and 18% of the total aliphatics in the insoluble poly(aliphatic) domain (suberin polymer) and in the soluble (nonpolymeric) fraction, respectively (Yang and Bernards, 2006). A similar study on native periderms from 21-d-stored potato (Serra et al., 2009) reported that fatty alcohols represent about 20% of the total aliphatic components found in the suberin polyester, while unlinked fatty alcohols and alkyl ferulates accounted for about 23% and 44% of the total aliphatics in the soluble waxes.
In Arabidopsis, C18:0, C20:0, and C22:0 fatty alcohols account for slightly less than 3% of the polymerized aliphatics in roots of soil-grown plants (Domergue et al., 2010), but as much as 36% [w/w] of the soluble wax load (Li et al., 2007). Arabidopsis fatty acyl reductases FAR1 (At5g22500), FAR4 (At3g44540), and FAR5 (At3g44550) generate, respectively, the C22:0, C20:0, and C18:0 fatty alcohol present in the suberin of root, seed coat, and wounded leaf tissues (Domergue et al., 2010). These three enzymes also generate the C18:0 to C22:0 fatty alcohol components that make up AHCs of root waxes (Kosma et al., 2012). Although one particular chain length of primary alcohol was reduced in each far single mutant line (C18:0-OH, C20:0-OH, and C22:0-OH in far5, far4, and far1, respectively), the total fatty alcohol load of the suberin polymer and its composition were only slightly affected and mutant plants had no obvious developmental or physiological defects (Domergue et al., 2010). In this study, we report on the distribution of primary fatty alcohols in the soluble (nonpolymeric) and insoluble (suberin polymer) fractions from mature roots of Arabidopsis. We report that far double and triple mutants have highly reduced fatty alcohol levels, in a chain length-specific manner, in both fractions as well as in the seed coat suberin polymer. The significant reductions in total fatty alcohol and diol levels in the seed coat of these mutants lead to increased permeability and higher sensitivity to abscisic acid (ABA), bringing to light insights on the roles of fatty alcohols and diols in determining functional properties of suberin.
RESULTS
The Majority of C18:0 to C22:0 Fatty Alcohols Are in the Soluble Nonpolymeric Fraction of Arabidopsis Roots
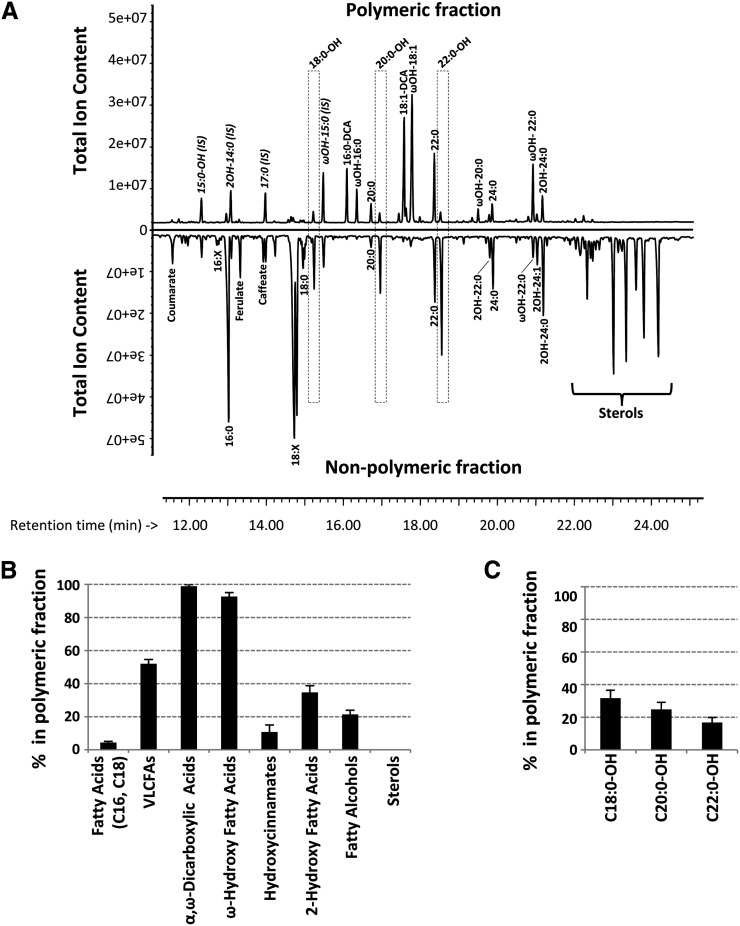
We first examined the distribution of fatty alcohols in the roots of 4-week-old tissue culture-grown wild-type (ecotype Columbia [Col-0]) seedlings. Rapid dipping of these roots in chloroform, subsequent derivatization, and analysis by gas chromatography-mass spectrometry (GC-MS) did not reveal any root waxes, in agreement with their presence only in mature Arabidopsis taproots (Kosma et al., 2012). We then analyzed (1) a nonpolymeric lipid fraction consisting of pooled sequential solvent extractions used to exhaustively delipidate roots and (2) the remaining polymeric (suberin) fraction. Both fractions were processed the same way, i.e. transmethylated, silylated, and analyzed by GC-MS (Fig. 1A). Among the various lipids, sterols were found exclusively in the nonpolymeric fraction. The nonpolymeric fraction also contained most of the C16 and C18 fatty acids, in agreement with this fraction containing soluble membrane lipids. By contrast, more than 90% of α,ω-dicarboxylic acids and ω-hydroxy fatty acids, which are typical suberin monomers, were found in the polymeric fraction (Fig. 1, A and B). Very-long-chain fatty acids were about equally distributed in both fractions because these fatty acids are found in membrane lipids (e.g. phosphatidyl-Ser or sphingolipids) as well as in suberin. The 2-hydroxy fatty acids, which are mainly found in sphingolipids, were also found in both fractions, most probably because highly glycosylated sphingolipids are not readily extracted by the chloroform/methanol extractions used to isolate the suberin polymer, ending up inappropriately in the polymeric fraction (Molina et al., 2006; Buré et al., 2011). Hydroxycinnamates were found primarily in the soluble fraction, but small amounts of coumaric and ferulic acids were also detected in the polymeric fraction. Fatty alcohols were found in both fractions, with about 20% of the total detected fatty alcohols in the polymeric fraction (i.e. suberin) and 80% in the nonpolymeric fraction. Among the different fatty alcohol chain lengths, about one-third of C18:0-OH (approximately 31%), about one-fourth of C20:0-OH (approximately 25%), and about one-sixth of C22:0-OH (approximately 16%) were found to be associated with the polymeric fraction (Fig. 1C), indicating that fatty alcohol chain length was inversely correlated with its abundance in the polymer.
Figure 1.
Distribution of lipids and fatty alcohols in the polymeric and nonpolymeric lipid fractions of 4-week-old tissue culture-grown Arabidopsis roots. A, Gas chromatography chromatograms of polymeric and nonpolymeric lipid fractions of roots from 4-week-old wild-type seedlings. Fatty alcohols are highlighted by dashed blocks. Major suberin monomers are identified in the polymeric fraction (top), while membranous fatty acids, hydroxycinnamates, and sterols are shown in the nonpolymeric fraction (bottom). X:Y indicates fatty acids with X carbon atoms and Y unsaturations, X:Y-OH indicates fatty alcohols, ωOH-X:Y indicates ω-hydroxy fatty acids, X:Y-DCA indicates α,ω-dicarboxylic fatty acids, and 2OH-X:Y indicates 2-hydroxy fatty acids. 16:X and 18:X indicate mono- plus polyunsaturated fatty acids with 16 and 18 carbons, respectively. IS, Internal standards. B, Amount (in percentage of total) of aliphatics (fatty acids and derivatives), phenolics, and sterols found in the polymeric fraction. VLCFA, Very-long-chain fatty acid (>C18). Each value is the mean of three to four biological replicates. C, Amount (in percentage of total) of fatty alcohols found in the polymeric fraction. Each value is the mean of three to four biological replicates.
Isolation of Double and Triple far1, far4, and far5 Mutant Lines
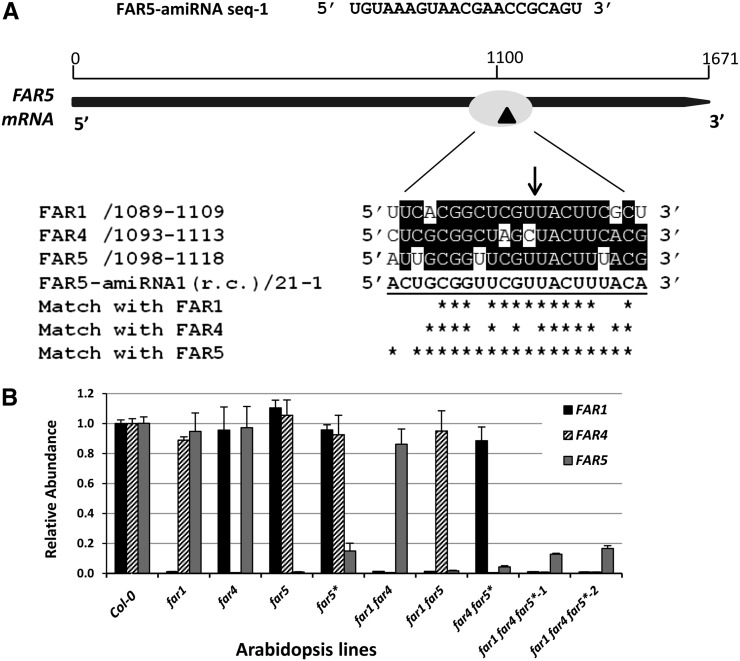
We next wanted to generate Arabidopsis mutants with highly reduced suberin-associated fatty alcohol content. Because the far1, far4, and far5 single mutant lines are only slightly affected in their total fatty alcohol content (Domergue et al., 2010), we developed double and triple mutant lines of far1, far4, and far5. FAR1 is located on chromosome 5, whereas FAR4 and FAR5 genes are positioned in tandem on chromosome 3. We generated far1 far4 and far1 far5 double mutants by conventional genetic crossing, but this approach was not feasible for the tightly linked far4 and far5 mutations. We therefore used artificial microRNA (amiRNA)-mediated gene silencing (Schwab et al., 2006) to down-regulate FAR4 and FAR5. MicroRNAs, which are typically 21 nucleotides in length, are much more transcript specific than short interfering RNAs because they do not trigger transitive silencing (Schwab et al., 2006). We used the 35S promoter for amiRNA expression because high-level expression of amiRNAs is required to achieve optimal silencing (Ossowski et al., 2008). The Web MicroRNA Designer Platform (http://wmd2.weigelworld.org) was used to design pairs of amiRNAs specific for FAR4 and FAR5. We found that the FAR4 amiRNAs were ineffective and that only one of the FAR5 amiRNAs caused significant gene silencing. This FAR5 amiRNA (sequence-1) would cause, according to the alignment, cleavage of the FAR5 transcript at position +1,110, producing a nonfunctional truncated mRNA (Fig. 2A). Ideal amiRNA sequences have no mismatches in positions 2 and 12, and only have one or two mismatches in positions 18 to 21 (Ossowski et al., 2008). As shown in Figure 2A, cross hybridization of FAR5-amiRNA-sequence1 with FAR1 or FAR4 is unlikely because of several mismatches between the amiRNA and the coding sequences, especially in the 3′ end of the amiRNA. By contrast, FAR5-amiRNA-sequence1 present only two mismatches (at positions 1 and 20) with FAR5.
Figure 2.
Generation of double and triple far mutant lines by amiRNA silencing. A, Schematic showing the FAR5-amiRNA-sequence 1 and the cleavage site on FAR5 cDNA sequence. The reverse complement (r.c.) FAR5-amiRNA-sequence 1 aligned with the coding sequence of root-expressing FARs FAR1, FAR4, and FAR5. Ideal amiRNA sequences have no mismatches in positions 2 and 12 and only have one or two mismatches in positions 18 to 21 (Ossowski et al., 2008). Cross hybridization of FAR5-amiRNA-sequence 1 with FAR1 and FAR4 is unlikely because of several mismatches in the 5′ end region. B, Transcript levels of FAR genes in wild-type, far1, far4, far5, far double, and far triple mutant lines determined by quantitative RT-PCR (*, FAR5 amiRNA lines; 1 and 2, two independent transgenic far triple mutant lines). Total RNA (0.1 µg) isolated from the roots of 2-week-old T5 seedlings grown on MS agar plates was used for quantitative RT-PCR. Results are presented as relative transcript abundances to Col-0 and normalized through geometric averaging of three constitutively expressed genes (ACT2, GAPC, and eIF4a1). The data represent means of two biological replicates, each with three technical replicates.
Constructs with FAR5-amiRNA-sequence1 were introduced into wild-type (Col-0), far4, and far1 far4 plants to generate far5*, far4 far5*, and far1 far4 far5* plants, respectively. The FAR5-amiRNA lines are denoted with an asterisk (far5*) to distinguish amiRNA-silenced FAR5 lines from far5 transfer DNA (T-DNA) insertion mutants. We found that there was significant variability in the degree of FAR5 gene silencing between individual plant lines in the T2 generation. Further screening and selection of silenced lines was carried out, and two homozygous lines were selected for far5*, far4 far5*, and far1 far4 far5*. Reverse transcriptase (RT)-PCR analysis using RNA derived from young roots of 15-d-old T4 seedlings grown in tissue culture showed that the levels of FAR5 transcripts were severely reduced in all of these 35S:FAR5-amiRNA plants (Supplemental Fig. S1), confirming the efficiency of the silencing.
We then selected one far5* single mutant line, one far4 far5* double mutant line, and two independent far1 far4 far5* triple mutant lines and grew them in tissue culture together with the other far single and far double mutants to measure FAR1, FAR4, and FAR5 transcript levels in roots. Quantitative RT-PCR analysis revealed a significant reduction (≥99%) of FAR1, FAR4, and FAR5 transcripts in far1, far4, and far5 T-DNA lines as expected (Fig. 2B). Furthermore, there was near absence of FAR1 transcript in far1 far4 and far1 far5 double mutants, near absence of FAR4 transcripts in far1 far4 and far4 far5* double mutants, and near absence of FAR5 transcripts in the far1 far5 double mutant. FAR5 transcript levels in the far5* and far4 far5* amiRNA lines were reduced greater than 85%, although none of them were reduced to the levels observed with the far5 T-DNA mutant (Fig. 2B). Both triple far1 far4 far5* mutant lines showed more than 99% reduction in FAR1 and FAR4 levels, while FAR5 transcript levels were reduced by about 85% (Fig. 2B). Altogether, these quantitative transcript analyses showed that the FAR5-amiRNA-mediated silencing functioned very efficiently in all backgrounds (i.e. in the wild type, far4, and far1 far4) and was highly specific to FAR5 because the transcript levels of FAR1 and FAR4 remained unaffected in Col-0 plants expressing FAR5-amiRNA. Consequently, these lines were selected to further study their phenotype.
Fatty Alcohol Levels in the Suberin Polymer Fraction Are Significantly Reduced in far Double and Triple Mutants
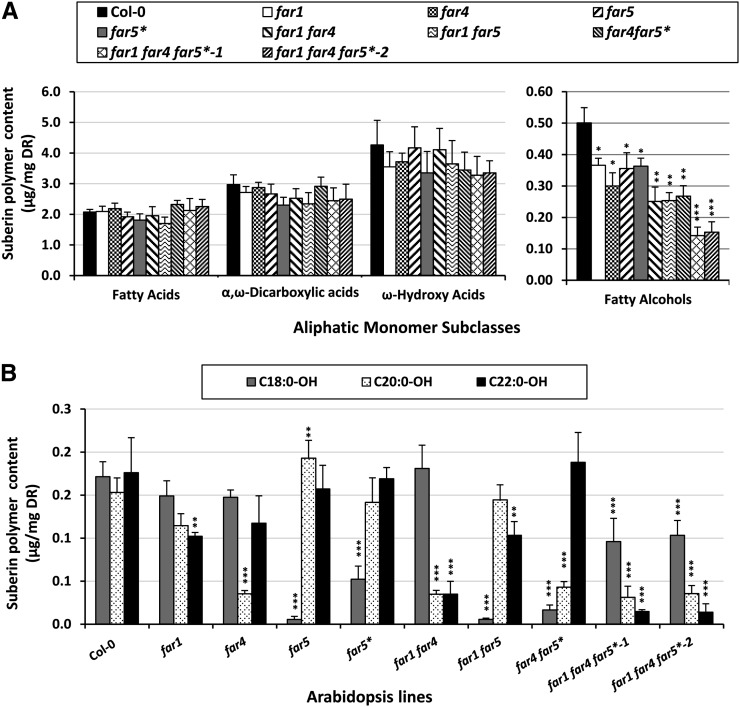
The collection of far mutant lines was grown in tissue culture for 4 weeks, their roots were isolated and solvent extracted, and the suberin (polymeric) aliphatic monomers were released by depolymerization using acid-catalyzed transmethylation, followed by silylation of hydroxy groups and analysis by gas chromatography (GC-MS and gas chromatography equipped with a flame ionization detector [GC-FID]). There were no significant differences in the quantities of most aliphatic monomer classes of suberin polymer (i.e. fatty acids, ω-hydroxy fatty acids, and α,ω-dicarboxylic fatty acids) in the far single mutants (far1, far4, far5, and far5*), far double mutants (far1 far4, far1 far5, and far4 far5*), or far triple mutants (far1 far4 far5* lines 1 and 2) compared with the wild type (Fig. 3A; Supplemental Table S1). By contrast, the total fatty alcohol content was reduced by approximately 25% in far single mutants, 50% in far double mutants, and 70% in both far triple mutant lines (Fig. 3A). There was also significant variation in the fatty alcohol chain length distributions depending on the mutant (Fig. 3B). In wild-type plants, there were nearly equal amounts of C18:0, C20:0, and C22:0 fatty alcohols in the root suberin polymer. In the single mutants, the C18:0-OH content was specifically reduced in far5 (96% reduction), the C20:0-OH content reduced in far4 (77% reduction), and the C22:0-OH content reduced in far1 (42% reduction). These data confirm our previous report (Domergue et al., 2010) and emphasize that there is partial overlap of FAR1 and FAR4 in producing C20:0-OH and C22:0-OH, but a near absence of overlap with FAR5 such that nearly all of the C18:0-OH found in suberin polymer is synthesized by FAR5. The far5* amiRNA single mutant line had about a 72% reduction in C18:0-OH, while the levels of the other two chain length alcohols (C20:0-OH and C22:0-OH) remained unaffected. Therefore, in agreement with the gene expression data (Fig. 2B), the amiRNA silencing was highly specific to FAR5 but did not cause a null phenotype. The double mutant far1 far5 had a 46% and 96% reduction in C22:0-OH and C18:0-OH contents, respectively (Fig. 3B). These values were very similar to those of the respective single far mutants, indicating that the effects were separate (little or no overlap between FAR1 and FAR5 activities) and cumulative. By contrast, the double mutant far1 far4 had a 78% reduction in C20:0-OH content (like in far4), but an 80% reduction in the C22:0-OH content (while far1 was only 42% reduced), indicating a significant contribution of FAR4 to the production of C22:0-OH fatty alcohols. The far4 far5* line had an 85% and 68% reduction in C18:0-OH and C20:0-OH levels, respectively, in agreement with the absence of overlap between FAR4 and FAR5 activities and the reductions observed in the respective single mutants (Fig. 3B). The triple mutant far1 far4 far5* line 1 had a roughly 50% reduction in C18:0-OH, while the levels of C20:0-OH and C22:0-OH were reduced by 80% and 85%, respectively. Similar levels of reduced fatty alcohol content were observed in the second independent far triple line, although the reduction of C18:0-OH was only around 45%, indicating variation in the degree of silencing of FAR5 between individual lines. It should be noted that the silencing effect of FAR5-amiRNA on C18:0-OH content was less strong in the two far triple mutant lines (45%–50%) than in the far5* single mutant and the far4 far5* double mutant (72% and 85%, respectively), but additional screening of lines did not result in identifying triple mutant lines that were more silenced in FAR5 expression. Nevertheless, the far triple mutant lines had strong reductions in all three chain lengths of primary fatty alcohols, such that the total fatty alcohol load was reduced by about 70% in the suberin polymer (Fig. 3A).
Figure 3.
Compositions of polymeric fraction of roots from wild-type and far single, double, and triple mutant lines. A, Aliphatic monomer subclasses, sorted by compound class along the x axis, of root suberin polymer. B, Fatty alcohol content, sorted into individual chain lengths, of root suberin polymer. Acyl chains were released by acid-catalyzed transmethylation, and hydroxyl groups were silylated before separation by gas chromatography and detection by flame ionization detector. Pentadecanol (C15:0-OH), ω-hydroxy-pentadecanoic acid (ωOH-C15:0), and heptadecanoic acid (C17:0) were used as internal standards for suberin monomer quantification. Mean values are shown in micrograms per milligram delipidated dry residue (DR) ± sd of three replicates. Significance was assessed by Student’s t test (*, P < 0.05; **, P < 0.01; and ***, P < 0.001).
Fatty Alcohol Levels in the Nonpolymeric Fractions and in Root Waxes Are Highly Altered in far Mutants
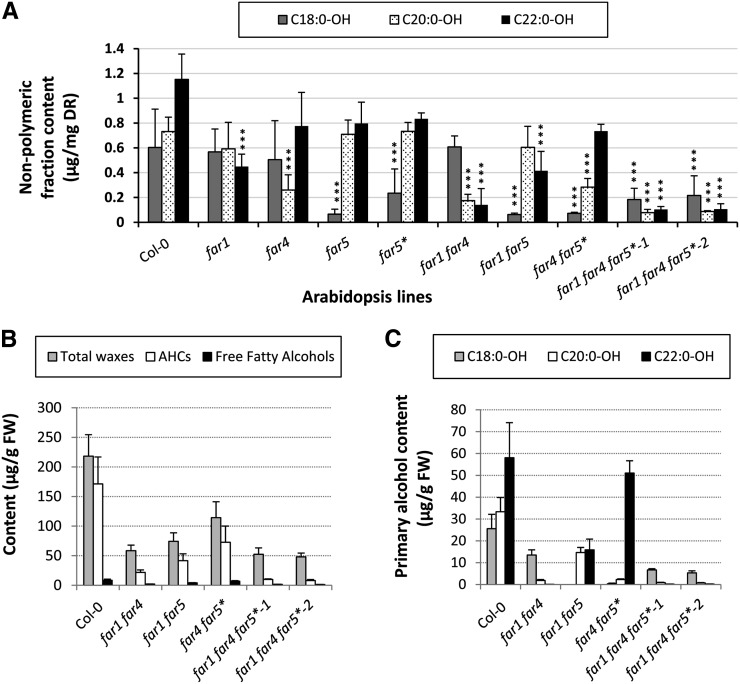
We also analyzed the nonpolymeric (soluble) lipid fractions, obtained during delipidation of the roots of the far mutant lines and observed very similar alterations of fatty alcohol levels (Fig. 4A; Supplemental Table S2). There was a 63% reduction in C22:0-OH, a 64% reduction in C20:0-OH, and an 89% reduction in C18:0-OH in far1, far4, and far5 single mutants, respectively, while the far5* amiRNA line had a 61% reduction in C18:0-OH. Among the far double mutants, far1 far4 had a reduction of 76% and 88% in C20:0-OH and C22:0-OH, respectively, far1 far5 a reduction of 89% and 66% in C18:0-OH and C22:0-OH, respectively, and far4 far5* a reduction of 88% and 61% in C18:0-OH and C20:0-OH, respectively. The far triple mutant far1 far4 far5* line 1 showed reductions in the levels of all three chain lengths of fatty alcohol, with C18:0-OH reduced by 70%, C20:0-OH reduced by 89%, and C22:0-OH reduced by 92%. Similar levels of reduction were observed in the second transgenic far triple mutant line (64%, 88%, and 91% reduction in C18:0-OH, C20:0-OH, and C22:0-OH, respectively). Altogether, these analyses showed that for each fatty alcohol chain length, the amounts in the polymeric and nonpolymeric lipid fractions were similarly reduced depending on the FAR gene(s) mutated or silenced, regardless of the method used for down-regulating gene expression (T-DNA or amiRNA silencing). The only exception concerned the C18:0-OH levels in the far triple mutant lines, which were about 40% reduced in the suberin polymer but as much as 65% decreased in the nonpolymeric fraction.
Figure 4.
Compositions of nonpolymeric and taproot wax fractions of roots from wild-type and far mutant lines. A, Fatty alcohol content, sorted into individual chain lengths, of nonpolymeric fractions from roots of far single, double, and triple mutant lines grown in tissue culture for 4 weeks. Acyl chains were released by acid-catalyzed transmethylation, and hydroxyl groups were silylated before separation by gas chromatography and detection by flame ionization detector. Pentadecanol (C15:0-OH), ω-hydroxy-pentadecanoic acid (ωOH-C15:0), and heptadecanoic acid (C17:0) were used as internal standards for suberin monomer quantification. Mean values are shown in micrograms per milligram delipidated dry residue (DR) ± sd of three replicates. Significance was assessed by Student’s t test (*, P < 0.05; **, P < 0.01; and ***, P < 0.001). B, Total amounts of taproot waxes, AHCs, and free fatty alcohols from roots of 7-week-old far double and far triple mutants grown in a soilless potting medium. C, Total fatty alcohol content (free fatty alcohols plus alcohols from AHCs) from root wax, sorted into individual chain lengths. After taproot waxes extraction, heptadecanoic acid (C17:0), tricosan-1-ol (C23:0-OH), octacosane (C28:0), tridecyl (C13:0) ferulate, and heptadecyl (C17:0) coumarate were added as internal standards, and free hydroxyl groups were silylated before separation by GC-MS. Quantification was based on peaks areas from total ion content chromatograms. Mean values are shown in micrograms per gram fresh weight (FW) of four replicates. Errors bars indicate sd.
Finally, we analyzed the content and composition of taproot waxes from 7-week-old far double and far triple mutants grown in a soilless potting medium because gene expression patterns using promoter::GUS transgenic plants revealed that FAR1, FAR4, and FAR5 genes are expressed in the peridermal cells of mature Arabidopsis taproots (Supplemental Fig. S2). In wild-type plants, about 220 µg g–1 fresh weight of root waxes were detected, with AHCs collectively representing about 80% of the total, while free fatty alcohols accounted for about 4% (Fig. 4B; Supplemental Table S3). The two triple mutant lines were most affected with the AHCs, and free fatty alcohols levels were both reduced by 82% to 95% so that about only one-fifth of the total root wax load remained (Fig. 4B). With the exception of a slight increase in C22:0 free fatty acids in both triple lines, there were no major differences in the quantities of free fatty acids, sterols, or monoacylglycerols in any of these lines (Supplemental Table S3). The chain lengths of free and combined fatty alcohols (i.e. alkyl moieties from AHCs) were also evaluated in the root waxes of the different lines. Both far1 far4 far5* triple mutant lines had dramatic reductions in all three fatty alcohol chain lengths, with C18:0-OH content reduced by about 80% and a near absence of C20:0-OH and C22:0-OH (Fig. 4C). With respect to fatty alcohol partitioning in roots from 7-week-old wild-type plants, about 20% of the total fatty alcohols were found as periderm-associated root waxes, about 24% in the suberin polymer, and more than 56% in the remaining nonpolymeric fraction (Table I). Similarly to the situation in 4-week-old tissue culture-grown roots (Fig. 1C), C18:0-OH was relatively enriched in the suberin polymer (about 35% of C18:0-OH) and relatively less abundant in the nonpolymeric fraction (47%).
Table I. Partition of fatty alcohols in 6-week-old wild-type Arabidopsis taproots.
Root waxes from taproots of 7-week-old wild-type plants grown in soil were extracted before preparing the nonpolymeric and suberin polymer fractions. Fatty alcohol contents were determined as indicated in “Materials and Methods.” Data are expressed in micrograms per gram of delipidated roots dry weight, and each value is the mean ± sd from three replicates.
| Root Waxes | Suberin Polymer | Nonpolymeric Fraction (Excluding Waxes) | ||||
|---|---|---|---|---|---|---|
| µg/g dry wt | % | µg/g dry wt | % | µg/g dry wt | % | |
| C18:0-OH | 176.2 ± 28.0 | 18.7 | 324.6 ± 31.4 | 34.6 | 438.6 ± 31.7 | 46.8 |
| C20:0-OH | 216.0 ± 37.4 | 20.4 | 240.9 ± 10.5 | 22.9 | 599.7 ± 56.0 | 56.8 |
| C22:0-OH | 295.1 ± 58.4 | 19.4 | 269.8 ± 28.8 | 17.8 | 959.3 ± 164.9 | 62.8 |
| Fatty alcohols | 687.4 ± 122.0 | 19.5 | 835.3 ± 50.9 | 23.8 | 1997.6 ± 202.0 | 56.7 |
Altogether, these results showed that combined mutations in FAR1, FAR4, and FAR5 drastically affected the fatty alcohol content of the nonpolymeric fraction of Arabidopsis roots, including tap root waxes.
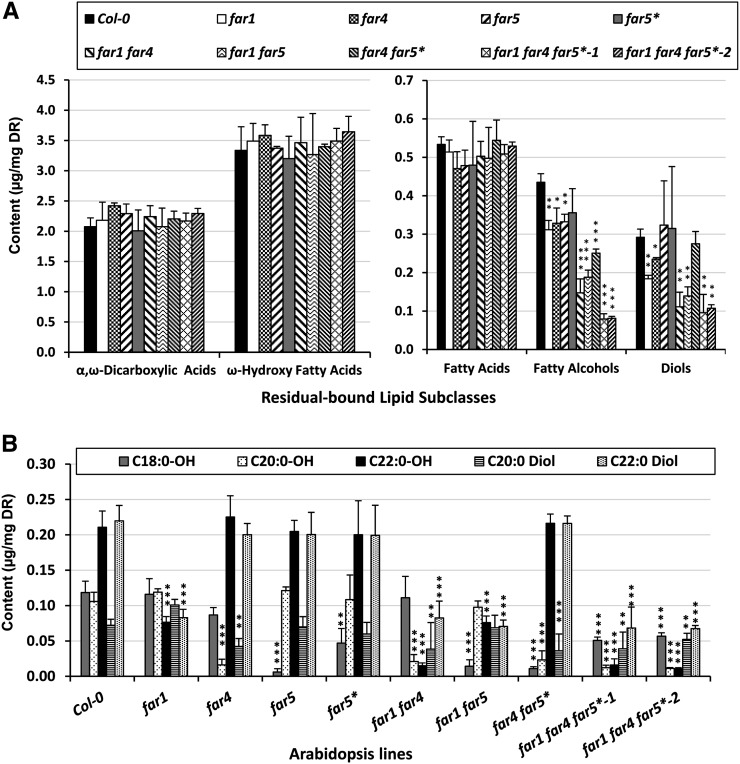
The Fatty Alcohol and Diol Composition of Seed Coat Suberin Polymer Is Highly Affected in Double and Triple far Mutants
We then analyzed the composition of seed coat suberin polymer in the double and triple far mutants compared with the wild type and the far single mutants (Fig. 5; Supplemental Table S4). No significant differences were observed in the amounts of the most abundant aliphatic monomer subclasses, α,ω-dicarboxylic acids and ω-hydroxy acids, or in the fatty acid contents of any of the far mutants compared with the wild type (Fig. 5A). By contrast, total fatty alcohol content was reduced by 18% to 28% in the single far mutants, by 42% to 66% in the double far mutants, and by more than 80% in both triple far mutant lines (Fig. 5A). In addition, the diol levels were reduced by 8% to 32% in the single far mutants, by 28% to 64% in the far double mutants, and by about 75% in both triple far mutants (Fig. 5A). Because C22:0-OH and C22:0-diol are the major components of wild-type seed coat polymer, being present at levels about twice that of C18:0-OH, C20:0-OH, and C20:0-diol, the highest reductions in total fatty alcohols were observed in lines mutated in FAR1, while the lowest ones were observed in lines mutated in FAR5 (Fig. 5A). The reduction patterns of individual chain lengths of fatty alcohols in the seed coat suberin polymer of the various far mutants were very similar to those observed with root C18:0-to-C22:0 fatty alcohols (Fig. 5B). The far1 mutant had 64% and 62% reductions in C22:0-OH and C22:0-diol, respectively, and the far4 mutant had 85% and 41% reductions in C20:0-OH and C20:0-diol, respectively. The far5 mutant had a 95% reduction in C18:0-OH, while the far5* amiRNA line had about a 60% reduction in C18:0-OH. The far1 far4 double mutant had 80% and 93% reductions in C20:0-OH and C22:0-OH amount, respectively, accompanied by reductions in C20:0-diol and C22:0-diol of 47% and 62%, respectively. The far1 far5 double mutant was affected in C18:0-OH (reduced by 88%), C22:0-OH (reduced by 64%), and C22:0-diol (reduced by 68%). The far4 far5* line had a 91% reduction in C18:0-OH and a 78% reduction in C20:0-OH along with a C20:0-diol reduction of 50%. Both triple mutant far1 far4 far5* lines were even more affected and had a significant reduction in the amounts of all three fatty alcohols (around 55%, 88%, and 93% for C18:0-OH, C20:0-OH, and C22:0-OH, respectively) and both diol amounts (around 37% and 69% for C20:0-diol and C22:0-diol, respectively; Fig. 5B). Overall, the far double and triple mutant lines had significantly altered total levels of fatty alcohols and diols, with the far1 far4 far5* triple mutant having the most drastic reduction in the fatty alcohol and diol loads (75% reduction in total compared with the wild type).
Figure 5.
Suberin polymer composition of the seed coat from wild-type and far single, double, and triple mutant seeds. A, Aliphatic suberin monomers subclasses, sorted by compound class along the x axis. B, Fatty alcohols and diols sorted into individual chain length. Acyl chains were released by acid-catalyzed transmethylation, and hydroxyl groups were silylated before separation by gas chromatography and detection by flame ionization detector. The pentadecanol (C15:0-OH), C15-hydroxypentadecanoic acid (ωOH-C15:0), and heptadecanoic acid (C17:0) were used as internal standards for suberin monomer quantification. Mean values are shown in micrograms per milligram delipidated dry residue (DR) ± sd of three replicates. Significance was assessed by Student’s t test (*, P < 0.05; **, P < 0.01; and ***, P < 0.001).
Seed Coat Permeability Is Affected in Double and Triple far Mutants
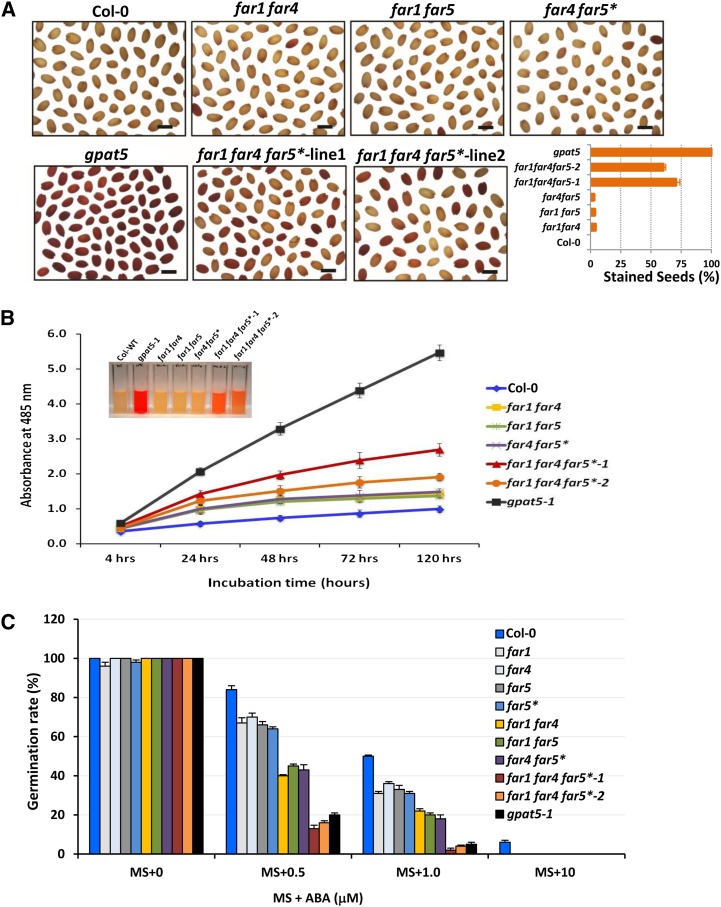
Similar to the single far1, far4, and far5 mutants (Domergue et al., 2010), the double and triple far mutants had no obvious developmental phenotype under nonstressed conditions. Examination of seedling establishment and root growth rate in tissue culture, even under salt stress conditions (0 versus 100 versus 150 mm NaCl), did not reveal any difference compared with the wild type or single far mutants, suggesting that the lower fatty alcohol levels in the suberin of these lines did not alter root development or responses to salt stress. By contrast, incubation of seed samples at 30°C for 48 h in 1% (w/v) tetrazolium red, a cationic dye widely used in seed permeability testing, showed that 60% to 70% of the seeds from the far triple mutants were stained red (tetrazolium salts penetrated through seed coat), whereas less than 5% of the far double mutant seeds and no wild-type seeds exhibited any dye penetration (Fig. 6A). The glycerol-3-phosphate acyltransferase5 (gpat5-1) seeds exhibited an intense red staining of all seeds as previously reported (Beisson et al., 2007).
Figure 6.
Effect of double and triple far mutations on the permeability of the seed coat. A, Staining pattern in seeds of Col-0 and far mutants incubated in 1% tetrazolium red at 30°C and imaged at the end of 48 h. Bar = 500 µm. B, Time course of formazan produced by reduction of tetrazolium salts by the embryos of the Arabidopsis wild-type (WT) and far double and triple mutants. The higher amount of formazan accumulation indicates increased seed coat permeability (the inset shows the extracted formazans at the 72-h time point). The data are mean value of three replicates shown as measure of A485. Error bars indicate sd. C, Seed germination rate of the wild type and far single, double, and triple mutants subjected to varying ABA concentrations. The seed germination percentage was recorded 4 d after placing seeds on MS plates supplemented with ABA at concentrations of 0.5, 1.0, and 10 μm. Values are the means of measurements from two independent experiments using different seed batches and involved three replicates of 100 seeds per plate. The data are shown in percentage of total seeds sown. Errors bars indicate sd.
To better quantify the seed coat permeability alterations of the different mutants, we measured tetrazolium salt uptake over 5 d using the triphenyl tetrazolium chloride reduction method (Molina et al., 2008). Tetrazolium salts are amphipathic cations, which, after penetrating the dead cells of the seed coat, are reduced in the embryo to a red-colored insoluble precipitate made up of formazans by active dehydrogenases (Berridge et al., 1996). As shown in Figure 6B, both triple far mutants and the three double far mutants produced more red formazans than the wild type, but less than gpat5 seeds, over the time course. Between the two triple mutant lines, the production of formazans was slightly higher in far1 far4 far5* line 1 seeds than in far1 far4 far5* line 2 seeds. Between 4 and 120 h of incubation, the increase in formazans was about 2.9-fold in the control (wild-type) seeds, about 3.2-fold in the far double mutant seeds, about 4.2-fold in the far1 far4 far5* line 2 seeds, about 5.5-fold in the far1 far4 far5* line 1 seeds, and about 9-fold in the gpat5 seeds. Because analysis of the seed waxes of the different lines did not show any difference in content or composition (Supplemental Table S5), these analyses strongly suggest that the reduced levels of fatty alcohols and diols in the suberin polymer of the seed coat affected its permeability.
To further establish that seed coat permeability was affected, we examined the effects of the hormone ABA on germination percentage of the far single, double, and triple mutant seeds. ABA has been reported to inhibit Arabidopsis seed germination by restricting the availability of energy and metabolites (Garciarrubio et al., 1997). Matured seeds of the wild type and far mutants were placed on Murashige and Skoog medium lacking ABA or supplemented with ABA at concentrations of 0.5 µm, 1.0 µm, and 10 µm, and after 4 d, the germination percentage was scored (Fig. 6C). In the absence of ABA, all the lines showed germination percentages close to 100%. At 0.5 µm ABA, the germination percentage of wild-type seeds dropped to 84%, while that of the far single mutants was between 60% and 70%, that of the double far mutants was around 40%, and that of both triple far mutants was below 20% (Fig. 6C). At 1.0 µm ABA concentration, wild-type seeds showed about 50% germination rate, while far single mutants and far double mutants showed about 35% and 20% germination, respectively. At this concentration, the far1 far4 far5* triple mutant line as well as the gpat5 line had greater than 95% reductions in germination. Finally, at a concentration of 10 µm ABA, wild-type seeds showed about 6% germination, while none of the far mutant seeds germinated.
DISCUSSION
Suberin is a protective hydrophobic barrier consisting of phenolics, glycerol, and a variety of fatty acid derivatives, including C18:0-to-C22:0 primary fatty alcohols. Our present results suggest that C18:0-to-C22:0 primary fatty alcohols represent about 5% of the total aliphatic monomers found in the root suberin polymer of Arabidopsis and that about 80% of the C18:0-to-C22:0 fatty alcohols present in Arabidopsis roots are not covalently linked to the polymer. Down-regulation of Arabidopsis FAR1, FAR4, and FAR5 strongly reduced fatty alcohols levels in the polymeric and nonpolymeric fractions from roots and seed coat suberin polymer. In this study, we also show that a 75% reduction in total fatty alcohol and diol loads in the seed coat resulted in increased permeability to tetrazolium salts and a higher sensitivity to ABA.
C18:0-to-C22:0 Fatty Alcohols in Arabidopsis Roots Are Mostly Not Part of Suberin Polymer
In this work, we analyzed the distribution of fatty alcohols in the nonpolymeric (soluble) and polymeric (suberin) fractions of roots from wild-type (Col-0) Arabidopsis plants grown 4 weeks in tissue culture or 7 weeks in soilless potting medium. The polymeric fraction corresponds to the monomers released from the suberin polymer by depolymerization, whereas the nonpolymeric fraction, obtained by exhaustive organic solvent extraction of roots, includes all the membranous lipids as well as the aliphatic or phenolic components of the suberin barrier that are not covalently linked (and root waxes when present). We found that most of the fatty alcohols (75%–80%) were not directly part of the suberin polymer. By contrast, most of the ω-hydroxy fatty acids and α,ω-dicarboxylic acids (>90%), which are the two most abundant suberin monomer classes in Arabidopsis roots, were almost exclusively found in the polymeric fraction. This distribution of suberin-type monomers between the polymer and the soluble fraction is consistent with previous reports describing such distribution in onion, soybean, and potato (Dean and Kolattukudy, 1977; Yang and Bernards, 2006; Thomas et al., 2007; Meyer et al., 2011). In all of these studies, most of the ω-hydroxy fatty acids and α,ω-dioic acids were reported in the polymeric fraction, while most of the fatty alcohols were detected in the soluble fraction. In a model where suberin is an extended polyaliphatic network, ω-hydroxy fatty acids and α,ω-dicarboxylic acids, which contain two functional groups, would promote the extension of a cross-linked polymer, whereas monofunctional aliphatics, such as fatty alcohols and fatty acids, would terminate the polymerization process when incorporated into the suberin. Nevertheless, fatty alcohols are systematically reported as representing 1% to 10% of the total monomers of the suberin polymer (Holloway, 1983; Bernards, 2002; Pollard et al., 2008). When the dried root residue obtained after the delipidation process was reduced to a fine powder using a polytron and reextracted a second time, as described in “Materials and Methods,” 85% to 90% of the fatty alcohols remained associated with the residue (Supplemental Fig. S3). In addition, in the Arabidopsis enhanced suberin1 (esb1) mutant, which is characterized by a doubling of total suberin aliphatics, the levels of ω-hydroxy fatty acids, α,ω-dioic acids, fatty acids, and fatty alcohols are all about doubled (Baxter et al., 2009; Ranathunge and Schreiber, 2011). Together, these data support the idea that about 20% of the fatty alcohols present in Arabidopsis wild-type roots are covalently linked to the suberin polymer and account for about 5% of the total aliphatic monomers of the suberin polymer. In addition, if the fatty alcohols present in the nonpolymeric fraction are “suberin (soluble) components,” fatty alcohols should not be considered anymore as minor components of Arabidopsis root suberin, because, in total, they may be as abundant as α,ω-dicarboxylic fatty acids.
Among the different chain lengths, C18:0-OH was found preferentially incorporated into the polymer, even if, in the combined nonpolymeric and polymeric fractions, it was the least abundant fatty alcohol. Whether this is a result of subsequent enzyme chain length specificity will be answered with the discovery of the enzymes responsible for suberin assembly. Because most of the fatty alcohols present in Arabidopsis wild-type roots are found in the nonpolymeric fraction, this raises questions about their exact location in planta. As shown in Figure 1A, these fatty alcohols are relatively abundant molecules in the nonpolymeric fraction, especially when considering that the C16:0 and C18:X acyl chains represent the membranous lipids from all root cells. Because the enzymes generating these fatty alcohols, FAR1, FAR4, and FAR5, are specifically expressed in the root endoderm (Domergue et al., 2010) and periderm (Supplemental Fig. S2) of Arabidopsis roots, their products are expected to only accumulate in these cellular layers. In soybean roots, suberin-associated waxes containing fatty alcohols have been detected in both root endodermis and epidermis (Thomas et al., 2007). Although direct evidence is still missing, it is highly probable that the fatty alcohols found in the nonpolymeric fraction are noncovalently associated with suberin. Because the cell layers producing suberin export the fatty alcohols found in the polymeric fraction, the same most probably also occurs for the nonpolymerized fatty alcohols. Considering the relatively low amounts of fatty alcohols found in the suberin, it might be that other aliphatic monomers (especially those containing two functional groups for cross linking) are preferentially used for generating the suberin polymer, resulting in most of the fatty alcohols remaining noncovalently associated with suberin. Also, in other plants where the aliphatic composition of the soluble lipid fraction and suberin polymer were compared, the levels of fatty alcohols were higher in the former than in the later (Schreiber et al., 2005; Yang and Bernards, 2006; Thomas et al., 2007; Serra et al., 2009; Meyer et al., 2011). It remains to be determined in which form, free or esterified, these fatty alcohols are found in the nonpolymeric fraction of Arabidopsis roots. Because it was shown that the FATTY ALCOHOL:CAFFEOYL-CoA TRANSFERASE (FACT), the enzyme generating alkyl caffeates in Arabidopsis, is expressed in the periderm of mature taproots as well as in the endoderm of young roots (Kosma et al., 2012) like FAR1, FAR4, and FAR5 (Domergue et al., 2010), it might be that AHCs are also present as suberin-associated waxes in the endodermis. Another distinct possibility is that the fatty alcohols present in the nonpolymeric extracts represent residual periderm-associated AHCs that were not removed by the rapid solvent dipping used for root wax extraction. However, the fatty alcohol-to-hydroxycinnamate molar ratio in the nonpolymeric fraction was close to 2, suggesting that the fatty alcohols of this fraction are not only in the form of AHCs.
Fatty Alcohol Levels in the Suberin Polymer and the Soluble Lipid Fraction (Nonpolymeric) Are Significantly Reduced in Double and Triple far Mutants
To efficiently decrease suberin-associated fatty alcohol content, we down-regulated FAR1, FAR4, and FAR5 by conventional genetic crossing and amiRNA-mediated gene silencing. Quantitative RT-PCR analysis revealed a near absence of FAR1, FAR4, and FAR5 transcripts (reduction ≥99%) in T-DNA lines and as much as 85% reduction in FAR5 transcript in the best FAR5-amiRNA-mediated silencing lines, with the transcript levels of FAR1 or FAR4 remaining unaffected, confirming the efficiency and specificity of our strategy. In the best triple far1 far4 far5* mutant lines, FAR1 and FAR4 levels were reduced by more than 99%, while FAR5 transcript levels were reduced by at least 80%. Nevertheless, the fatty alcohols in root suberin could not be completely eliminated due to the partial silencing of the FAR5 gene. There is also a possibility that the complete elimination of fatty alcohols is lethal.
The various lipid analyses that we conducted in this study showed that strong reductions in FAR1, FAR4, and FAR5 transcript levels similarly affected the fatty alcohol compositions in root and seed coat suberin polymers and in the root soluble (nonpolymeric) fraction. In agreement with the results obtained with far1, far4, and far5 single mutant lines (Domergue et al., 2010), C18:0-OH, C20:0-OH, and C22:0-OH levels were mostly affected by down-regulation of FAR5, FAR4, and FAR1, respectively. Analyses of the double and triple far mutants yielded additional information about the distinct but overlapping specificities of these three FARs. Because C18:0-OH was barely affected in the far1 far4 double mutant, FAR5 appears to be responsible for most of the C18:0-OH produced in planta. The fact that the reduction in C22:0-OH never exceeded 60% in the far1 far5 double mutant indicates that FAR4 contributes significantly to C22:0-OH production. Similarly, analyses of the far4 far5* double mutant suggests that FAR1 contributes to C20:0-OH production, but to a lesser extent, confirming the overlapping specificities of FAR1 and FAR4. The systematic presence of C20:0 and C22:0-OH in the analyses of far1 far4 double mutant and far1 far4 far5* triple mutant lines, where FAR1 and FAR4 transcript levels are barely detected, suggests that FAR5 is also able to produce small amounts of these longer fatty alcohols in planta, at least in the absence of FAR1 and FAR4.
The total fatty alcohol load of far1 far4 far5* triple mutant lines was reduced by at least 70% in all the lipid fractions we analyzed. By contrast, we did not observe significant variations in the levels of the other lipid constituents of the root soluble (nonpolymeric) and the taproot waxes fraction (besides AHCs). Similarly, in the root and seed coat suberin polymer, the other main lipid polyester monomers (unsubstituted fatty acids, ω-hydroxyacids, and α,ω-dicarboxylic acids) were not significantly altered, indicating that reduced levels of fatty alcohols did not influence the incorporation of the other monomer classes into the suberin polymer.
Fatty Alcohols and Diols Are Important Determinants of Seed Coat Permeability
Despite a 70% reduction in their total fatty alcohol level, triple far mutant plants had no obvious developmental phenotype under nonstressed conditions. Seedling establishment and root growth rate in tissue culture were also not altered, even under osmotic stress. By contrast, tetrazolium salt assays showed increased seed coat permeability in far double and triple mutants compared with wild-type seeds. Similarly, decreased levels of fatty alcohols and diols in the various far single, double, and triple lines correlated with increased seed germination sensitivity to ABA, suggesting that fatty alcohols and derivatives play a major role in the diffusion resistance of seed coat suberin.
Because FAR1 is mainly responsible for C22:0-OH production, and C22:0 fatty alcohols and diols together constitute about 60% of the total fatty alcohol load in the seed suberin polyester, this load was reduced by only 30% in far4 far5 and by at least 60% in far1 far4 and far1 far5. In the far triple mutant analyzed, the reduction in total fatty alcohol and diol load reached 75%. Tetrazolium salt staining of seeds showed that seed coat permeability was most affected in triple lines and similarly affected in all the double lines, whereas no tetrazolium salt penetration into the seeds of the wild type was observed. In our germination assays, in the presence of 0.5 or 1 µm ABA, the triple far mutant seeds were more sensitive to ABA than the double far mutants, the double mutants were more sensitive than the single far mutants, and the single mutants were more sensitive than wild-type seeds. Because ABA is known to prevent postgermination vegetative growth (Lopez-Molina et al., 2001), these results are most probably due to increased exposure of the far mutants seed embryo to ABA as a result of altered seed coat permeability. The effect of ABA on germination of triple far mutant seeds was similar to that observed with gpat5 seeds, which had total polyester monomer loads decreased by 40% (Beisson et al., 2007).
In Arabidopsis, the seed coat constitutes about 20% of mature dry seed weight (Li et al., 2006) and is associated with complex physiological processes such as seed dormancy and germination (Haughn and Chaudhury, 2005). Suberization is present over the entire coat of matured seeds (Molina et al., 2008). The inner seed coat polyester has been reported to be rich in cutin-type monomers, while typical suberin monomers are abundant in the outer integument (oi1 layer) of the seed coat in Arabidopsis and Brassica napus (Espelie et al., 1980; Molina et al., 2008). In Arabidopsis, the seed coat suberin is enriched in longer aliphatics, especially C22 and C24 ω-hydroxy fatty acids and α,ω-dicarboxylic acids, when compared with the root polymer (Beisson et al., 2007; Domergue et al., 2010). C22:0-OH and C22:0-diol are also more abundant, and together they constitute about 60% of the total fatty alcohol load in the seed coat suberin polyester. In the far triple mutants, this load was reduced by about 80% and resulted in increased permeability to tetrazolium dye and higher sensitivity to ABA, suggesting that fatty alcohols play an important role in the barrier properties of the seed coat.
The altered diffusion resistance of the suberin polymer may be more evident in seeds than in roots because, in root suberin, primary alcohols only constitute 5% of the total suberin monomer load, whereas, in the seed coat suberin, primary alcohols, together with diols, represent about 10% of the total suberin monomer load. In most plant extracellular lipid barriers, waxes play a critical role as a barrier for water passage (Bernard and Joubès, 2013). In seed coats, however, waxes are minor components (Beisson et al., 2007; Shao et al., 2007), and it was found by comparing the penetration of the tetrazolium dye into waxed versus dewaxed seeds that permeability was not dependent on seed waxes (Molina et al., 2008). In this study, seed wax composition and content was not affected in single, double, or triple far mutant lines. By contrast, Arabidopsis mutants apetala2, aberrant induction of type three genes 1, fatty acyl-ACP thioesterase B, and gpat5, which all have altered seed suberin polyester, showed increased permeabilities in dye penetration assays (Beisson et al., 2007; Molina et al., 2008), suggesting that the fatty alcohol and diol content and composition of the suberin polymer play important roles in controlling seed coat permeability.
Putative Functions of Free and Combined Fatty Alcohols in Planta
Suberin is deposited during secondary cell wall differentiation in specialized internal cell layers (e.g. root endodermis and bundle sheath of monocots) and external cell layers (e.g. periderms of roots and stems that have undergone secondary growth), as well as in response to environmental cues (e.g. wounding and salt stress; Domergue et al., 2010; Ranathunge et al., 2011). Suberin prevents the uncontrolled flow of water, gases, and ions and provides resistance to pathogens (Enstone et al., 2003; Franke and Schreiber, 2007; Ranathunge and Schreiber, 2011; Franke et al., 2012). Soliday et al. (1979) proposed that waxes associated with the suberin polymer, rather than the polymerized lipids, constitute the major diffusion barrier formed during wound healing in potato tubers. This conclusion was made from experiments using a chemical inhibitor of fatty acid chain elongation that severely affected the formation of suberin-associated waxes, but with very little effect on the deposition of the major suberin polymer aliphatic components. Recently, the periderm from potato tubers of CYP86A33-silenced plants, which contain less suberin polymer and more soluble waxes, were shown to have higher water permeabilities than the wild type (Serra et al., 2009), suggesting, by contrast, that the polymerized lipids, rather than the waxes associated with the suberin polymer, constitute the major diffusion barrier. By comparing the water and solute permeabilities of mutants containing more (esb1) or less (horst) suberin amount, it was concluded that both the aliphatic monomer arrangement (in the suberin polymer, but most probably also its association with waxes) and the macromolecular structure of the suberin biopolymer play roles in apoplastic barrier formation (Ranathunge and Schreiber, 2011).
Fatty alcohols found in the nonpolymeric fraction may also play an important role in biotic interactions. Long-chain fatty alcohols are reported to have antibacterial action (Masao et al., 1987) and C12:0-OH and C13:0-OH have high-growth inhibitory activity against Staphylococcus aureus (Togashi et al., 2007). Similarly, AHCs, especially alkyl coumarates and alkyl caffeates, have antibacterial activity toward the gram-negative bacterium Pseudomonas fluorescens (Baranowski and Nagel, 1982). Accumulation of alkyl ferulates in the wax fraction of wound periderm also suggests their role in pathogen interactions (Schreiber et al., 2005). Studies with roots extracts from Kalanchoe daigremontiana suggested that AHCs are not toxic to the plant, but that upon cleavage, the released ferulic acid acts as an allelochemical in the soil (Nair et al., 1988). Low-Mr phenols such as cinnamic acid and derivatives are often produced by plants in the early step of pathogen infection (Nicholson and Hammerschmidt, 1992). It is also known that the soil pathogen Rhysoctonia solani induces suberization of potato tubers during infection (Buskila et al., 2011), suggesting that suberin is not only produced upon mechanical stress, but also in response to pathogens. Finally, it was shown in wound-healing potato tubers that suberin phenolics provide resistance to bacterial soft rot (Erwinia carotovora subsp. carotovora), while deposition of suberin aliphatics is necessary for resistance to fungal infection (Lulai and Corsini, 1998). Altogether, these results indicate that free and combined forms of fatty alcohols likely have a role in protecting plant roots from soil-borne plant pathogens.
In conclusion, the double and triple far mutant lines reported here provide a means to further study the exact localizations and functions of free and combined fatty alcohols in Arabidopsis roots. It remains to be determined in which form (free or esterified) the fatty alcohols are found in the nonpolymeric fraction and whether the nonpolymerized fatty alcohols are physically associated with the suberin polymer as proposed here. Pathogen susceptibility tests may also provide a better understanding of their putative biological roles in plant-pathogen interactions. Finally, these double and triple far mutant lines could be used to test for alterations of water potential and ion mobility to further investigate the suberin barrier properties.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) plants were in the Col-0 background. The T-DNA insertion lines far1-2 (SALK_149469), far4-1 (SALK_000229), and far5-1 (SALK_152963), described in Domergue et al. (2010), were used in this study. Surface-sterilized seeds were sown on Murashige and Skoog (MS) medium with the pH adjusted to 5.7 and supplemented with 0.7% (w/v) agar. Seeds were stratified in the dark for 3 to 4 d at 4°C and then transferred to long-day conditions (16-h/8-h light/dark cycle) at 22°C or to continuous light conditions at 20°C. When required, approximately 2-week-old seedlings were transferred either to a soil-vermiculite mixture and further grown at 20°C to 22°C in a controlled-environment growth chamber with ambient humidity and under long-day conditions or, for root wax analysis, were transferred to a 1:1:1 mixture of Promix PGX soilless medium (Premier Horticulture), vermiculite, and perlite and grown in a growth chamber at 21°C to 22°C , 40% to 60% humidity, and a 16-h/8-h light/dark cycle.
Generation of Double and Triple far Mutant Lines
The far1 far4 and far1 far5 double mutants were made using conventional genetic crossing. Homozygous insertion lines for both the genes were identified among the segregating plants in the F2 generation by PCR screening for the presence of a T-DNA insertion at both loci. Genotyping PCR was carried out using genomic DNA isolated from the leaves of segregating plants.
amiRNA silencing (Schwab et al., 2006; Ossowski et al., 2008) was used to generate far4 far5* double and far1 far4 far5* triple mutant lines. For the amiRNAs, the key 21 nucleotides of the naturally occurring Arabidopsis miR319a were replaced with sequences specific for FAR4 and FAR5. Two amiRNAs each for FAR4 and FAR5 that hybridized to different parts of the respective transcripts were designed using WMD2-Web MicroRNA Designer (Supplemental Table S6; Schwab et al., 2006), although only one of the amiRNAs targeting FAR5 was found to be effective. Primers FAR5-amiX-I and FAR5-amiX-II were used to generate this amiRNA. The plasmid pRS300 contains the endogenous Arabidopsis miR319a microRNA precursor in a pBluescript SK+ backbone (Schwab et al., 2006), and this was used as template to amplify by overlap PCR the 701-bp full-length precursor amiRNA for FAR5. The purified PCR product was digested with EcoRI and XbaI and inserted between the corresponding sites of pBluescript II SK+ plasmid, and the PCR-amplified region was verified by DNA sequencing. The EcoRI-to-XbaI FAR5 amiRNA-containing DNA fragment from this plasmid was then cloned between the corresponding sites of the plant binary vector pBAR1, followed by insertion of a 35S promoter derived from the pCAMBIA1301 vector between HindIII at the upstream end and EcoRI at the downstream end of the 35S promoter. The pBAR1-35S-FAR5-amiRNA plasmid was transformed into GV3101::pMP90 Agrobacterium tumefaciens cells by electroporation. Col-0, far4, and far1 far4 plants were transformed using the floral dip method (Zhang et al., 2006). Successful Arabidopsis transformants containing the T-DNA harboring 35S-FAR5-amiRNA were selected on minimal media containing 10 μg mL–1 BASTA (DL-phosphinothricin, Duchefa Biochemie/Gold Biotechnology) herbicide.
cDNA Synthesis and Transcript Analysis by Quantitative RT-PCR
Total RNA was extracted from roots (approximately 50–60 mg) of 15-d-old seedlings grown vertically on MS medium using the RNAqueous Kit (Applied Biosystems-Ambion AM1912). The column-purified samples were subjected to DNase treatment using TURBO DNase (Applied Biosystems) and complementary DNA (cDNA) generated using SuperScript III RT (Invitrogen). Quantitative PCR was performed on a StepOnePlus real-time PCR system (Applied Biosystem) using Power SYBR Green qPCR Master Mix (Applied Biosystems). The cDNA from the RT reactions was diluted ten times with nuclease-free water. Each quantitative PCR reaction contained 5 μL of 5× Power SYBR mix, 2 μL of primer mix (5 μm each of forward and reverse primer), 2 μL of the diluted cDNA, and 1 μL of sterile water (for primer sequences, see Supplemental Table S6). Transcripts were quantified as relative abundance using the comparative cycle threshold (Ct) method (Δ-Δ Ct: 2–ΔΔCt; Pfaffl, 2001, 2006). The geometric mean of the relative transcript abundance of the endogenous controls ACTIN2 (ACT2; At1g49240), EUKARYOTIC TRANSLATION INITIATION FACTOR4A1 (eIF4a1; At3g13920), and GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE (GAPC; At3g04120) in each sample was used to normalize for differences of total RNA amounts between the samples. Two biological replications were carried out for each sample, with each reaction having three technical replicates.
Lipid Extractions
For analysis of polymeric and nonpolymeric lipid fractions, roots were collected from 4-week-old plants grown under long-day conditions and on plates containing MS medium. Each mutant line was analyzed in three replicates, with each replicate representing a pool of approximately 20 plants. Freshly collected roots were immediately immersed in 4 mL hot isopropanol and incubated for 30 min at 85°C in an oven incubator. After cooling, samples were extensively delipidated by extracting the nonpolymerized (soluble) lipids successively for 24 h each with 4 mL chloroform:methanol (2:1, v/v), 4 mL chloroform:methanol (1:1, v/v), 4 mL chloroform:methanol (1:2, v/v), and 4 mL 100% methanol, all performed at room temperature on a tube rotator. These successive delipidation steps were collected in the same 9-mL glass tube (by evaporating, each day, the newly collected solvent under a gentle stream of nitrogen gas) and corresponded to the nonpolymeric fraction. The resulting solvent-extracted tissues were dried in a fume hood at room temperature for 2 to 3 d and then in a dessicator for another 2 d and corresponded to the polymeric fraction.
For seed coat analysis, 50 to 60 mg of dry seeds were ground in isopropanol using a mortar and pestle, and then the mixture was transferred to glass gas chromatography tubes and heated for 30 min at 85°C in an oven incubator. After cooling, samples were extensively delipidated by extracting the soluble lipids successively for 24 h with chloroform:methanol (2:1, v/v), chloroform:methanol (1:2, v/v), and 100% methanol, all performed at room temperature on a tube rotator. This was followed by a series of washes with water (1 h), 2 m NaCl (1 h), water (1 h), and 100% methanol (1 h). The delipidation was continued with chloroform:methanol (1:2, v/v), chloroform:methanol (2:1, v/v), and 100% methanol for 24 h each. Samples were dried in a fume hood at room temperature for 2 to 3 d and then in a desiccator for another 2 d.
For root wax analyses, Arabidopsis plants were grown for 7 weeks in soilless potting medium for compositional analysis or in soil-perlite-vermiculite mixture for fatty alcohol content measurements. Each replicate consisted of taproots (up to 5 cm from the base of the rosette) from 12 to 20 plants. Arabidopsis root waxes were extracted by dipping of roots in chloroform for 1.5 min. After extraction, internal standards were added to chloroform extracts, which were heptadecanoic acid (C17:0), tricosan-1-ol (C23:0-OH), octacosane (C28:0), tridecyl (C13:0) ferulate, and heptadecyl (C17:0) coumarate, for quantification of the respective chemical compounds. The chloroform extracts were filtered through glass wool and then evaporated under a gentle stream of nitrogen gas to dryness.
For seed wax analysis, about 100 mg of seeds were extracted for 2 min in 4 mL of chloroform containing 1µg of tetracosane. The chloroform extracts were filtered through glass wool and then evaporated under a gentle stream of nitrogen gas to dryness.
Lipid Analyses
The solvent-extracted dried residues were weighed, and 10 to 30 mg of each sample was depolymerized by transmethylation at 85°C for 3 h using 2 mL of 5% (v/v) sulfuric acid in methanol. The soluble fractions were processed in the same way. Ten micrograms each of heptadecanoic acid (C17:0), pentadecanol (C15:0-OH), ω-hydroxy-pentadecanoic acid (ω-OH-C15:0), and 2-hydroxy tetradecanoic acid (2OH-C14:0) were added as internal standards. After cooling, 2 mL of NaCl (2.5%, w/v) and 2.5 ml of dichloromethane (DCM) were added and mixed vigorously for 5 min followed by centrifugation at 800g for 5 min at room temperature to promote phase separation. The lower DCM phase was transferred to a fresh glass tube. An additional 2.5 mL of DCM was added to the original mixture for a second extraction. The pooled DCM extracts were then washed with 2 mL of Tris-NaCl solution (100 mm Tris base pH 8.0 in 0.09% [w/v] NaCl), and the lower DCM phase was collected in a fresh gas chromatography tube, avoiding any aqueous phase. The lower organic phase was evaporated under a gentle stream of nitrogen.
These lipid extracts, as well as seed and root waxes, were then incubated at 110°C for 15 min with either 99% (v/v) N,O-bis(trimethylsilyl)-trifluoroacetamide plus 1% chlorotrimethylsilane or a 1:1 mix of N,O-bis(trimethylsilyl)-trifluoroacetamide and pyridine to trimethylsilylate free hydroxyl groups. Total fatty alcohol content of root waxes was determined by transmethylation and subsequent silylation of root wax extracts.
Gas Chromatography
Silylated samples were evaporated to dryness (without heating) and dissolved in heptane:toluene (1:1, v/v) for analysis by gas chromatography. Polymeric fractions (root and seed coat suberin), nonpolymeric (soluble) fractions, and seed waxes were quantified with a Varian-3900 GC-FID and a HP-5MS capillary column (30-m length, 0.25-mm i.d., and 0.25-mm film thickness). A 1-μL aliquot of the sample was injected in splitless mode, and high-purity helium was used as the carrier gas at a flow rate of 1.5 mL min–1. The temperature of the injector was at 250°C, and the column oven temperature was held at 50°C for 1 min and then increased from 50°C to 200°C at a rate of 25°C per minute, followed by a 1-min hold, and then it was ramped up again at the rate of 10°C per minute to a final temperature of 320°C, which was held for 8 min. The detector was at 350°C. Quantification of monomers was based on peak areas in the GC-FID chromatograms and identified using their retention time and the peak area of the respective internal standards (C17:0, C15:0-OH, ω-OH-C15:0, or 2OH-C14:0). The identities of the peaks were verified by GC-MS. Root waxes were analyzed on an Agilent 6850 gas chromatograph equipped with an Agilent 5975 mass spectrometer. Splitless injection was used with an HP5-MS column (30-m length, 0.25-mm i.d., and 0.25-µm film thickness). Temperature settings were as follows: inlet, 350°C; detector, 320°C; and oven temperature program, 130°C for 2 min and increased to 325°C at a rate of 5°C per minute. The helium flow rate was set at 1.5 mL per min–1.
Seed Coat Permeability Test
Tetrazolium red assays were used for seed coat permeability tests (Debeaujon et al., 2000; Beisson et al., 2007). Dried Arabidopsis seeds were incubated in the dark in an aqueous solution of 1% (w/v) tetrazolium red (2,3,5-triphenyltetrazolium chloride, Sigma-Aldrich) at 30°C for 4 to 48 h. The seeds were observed for change in color and imaged using a stereomicroscope. The quantitative formazan assay was carried out as described by Molina et al. (2008). If the tetrazolium salts penetrate into the seed, they are reduced by the embryo to a red precipitate called formazans. Seed samples (50 ± 1 mg each) of the Arabidopsis wild type and mutants were incubated in 500 μL of 1% (w/v) aqueous solution of tetrazolium red at 30°C for 4, 24, 48, 72, and 120 h in darkness. After incubation, the samples were washed twice with water, resuspended in 1 mL 95% (v/v) ethanol, and finely ground with a mortar and pestle to extract formazans. The final volume was adjusted to 2 mL with 95% (v/v) ethanol and immediately centrifuged for 3 min at 15,000g, and the supernatant was recovered. This procedure was performed quickly to avoid reaction of tetrazolium red with the embryo cells after seed disruption. Formazan concentration was determined by measuring the absorbance at 485 nm (Candler et al., 1997). Each sample was assayed in triplicates.
Germination Percentage Assay
Germination rates of the Arabidopsis wild-type and far mutant seeds were observed on MS medium free or supplemented with ABA (Phyto Technology Laboratories) at concentrations 0.5, 1, and 10 μm. Wild-type and far mutant seeds were harvested immediately after maturity from plants of the same age and grown under the same conditions. Mature seeds were sown in triplicate on petri plates (100 seeds per plate) and were transferred to a controlled environment growth chamber after cold treatment in the dark for 3 d at 4°C. Germination was scored at 4 d after transfer to the growth chamber. Seeds that showed penetration of the radicle through the seed coat were counted as germinated seeds. The test was done with two separate batches of seeds harvested from sets of plants grown at different times.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. FAR5 expression in far5 single, far4 far5 double, and far1 far4 far5 triple amiRNA mutant lines.
Supplemental Figure S2. FAR1, FAR4, and FAR5 expression in suberized periderm of Arabidopsis taproots detected in transgenic promoter:GUS lines.
Supplemental Figure S3. Effect of a second delipidation procedure on fatty alcohol partitioning.http://www.plantphysiol.org/cgi/content/full/pp.113.224410/DC1
Supplemental Table S1. Composition of suberin polymer from roots of wild-type and far single, double, and triple mutant lines.
Supplemental Table S2. Composition of nonpolymeric fractions from roots of wild-type and far single, double, and triple mutant lines.
Supplemental Table S3. Composition of waxes from taproots of wild-type and far double and triple mutant lines.
Supplemental Table S4. Composition of suberin polymer from seeds of wild-type and far single, double, and triple mutant lines.
Supplemental Table S5. Composition of seed waxes from wild-type and far single, double, and triple mutant seeds.
Supplemental Table S6. Sequences of DNA primers used in this study.
Acknowledgments
We thank Tracey Tenreira (Unité Mixte de Recherche 1332, Institut National de la Recherche Agronomique/Université de Bordeaux 1) for participating in this work, the Metabolome facility in Bordeaux, France (http://www.cgfb.u-bordeaux2.fr/en/metabolome) for lipid analyses, and John Ohlrogge and Mike Pollard (Department of Plant Biology, Michigan State University) for providing space and facilities and for critical assessment of the root wax analysis.
Glossary
- AHC
alkyl hydroxycinnamate
- ABA
abscisic acid
- Col-0
ecotype Columbia
- GC-MS
gas chromatography-mass spectrometry
- amiRNA
artificial microRNA
- T-DNA
transfer DNA
- RT
reverse transcriptase
- GC-FID
gas chromatography equipped with a flame ionization detector
- cDNA
complementary DNA
- Ct
cycle threshold
- DCM
dichloromethane
- MS
Murashige and Skoog
References
- Baranowski JD, Nagel CW. (1982) Inhibition of Pseudomonas fluorescens by hydroxycinnamic acids and their alkyl esters on. J Food Sci 47: 1587–1589 [Google Scholar]
- Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE. (2009) Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genet 5: e1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge JB. (2007) The acyltransferase GPAT5 is required for the synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19: 351–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A, Joubès J. (2013) Arabidopsis cuticular waxes: advances in synthesis, export and regulation. Prog Lipid Res 52: 110–129 [DOI] [PubMed] [Google Scholar]
- Bernards MA. (2002) Demystifying suberin. Can J Bot 80: 227–240 [Google Scholar]
- Bernards MA, Lewis NG. (1992) Alkyl ferulates in wound healing potato tubers. Phytochemistry 31: 3409–3412 [DOI] [PubMed] [Google Scholar]
- Bernards MA, Lopez ML, Zajicek J, Lewis NG. (1995) Hydroxycinnamic acid-derived polymers constitute the polyaromatic domain of suberin. J Biol Chem 270: 7382–7386 [DOI] [PubMed] [Google Scholar]
- Berridge MV, Tan AS, McCoy KD, Wang R. (1996) The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica (Indianap, Ind) 4: 15–20 [Google Scholar]
- Buré C, Cacas JL, Wang F, Gaudin K, Domergue F, Mongrand S, Schmitter JM. (2011) Fast screening of highly glycosylated plant sphingolipids bytandem mass spectrometry. Rapid Commun Mass Spectrom 25: 3131–3145 [DOI] [PubMed] [Google Scholar]
- Buskila Y, Tsror Lahkim L, Sharon M, Teper-Bamnolker P, Holczer-Erlich O, Warshavsky S, Ginzberg I, Burdman S, Eshel D. (2011) Postharvest dark skin spots in potato tubers are an oversuberization response to Rhizoctonia solani infection. Phytopathology 101: 436–444 [DOI] [PubMed] [Google Scholar]
- Candler JW, Abrams SR, Bartels D. (1997) The effect of ABA analogs on callus viability and gene expression in Craterostigma plantagineum. Physiol Plant 99: 465–469 [Google Scholar]
- Dean BB, Kolattukudy PE. (1977) Biochemistry of suberization: incorporation of [1-14C]oleic acid and [1-14C]acetate into aliphatic components of suberin in potato-tuber disks (Solanum tuberosum). Plant Physiol 59: 48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Léon-Kloosterziel KM, Koornneef M. (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122: 403–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domergue F, Vishwanath SJ, Joubès J, Ono J, Lee JA, Bourdon M, Alhattab R, Lowe C, Pascal S, Lessire R, et al. (2010) Three Arabidopsis fatty acyl-coenzyme A reductases, FAR1, FAR4, and FAR5, generate primary fatty alcohols associated with suberin deposition. Plant Physiol 153: 1539–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA, Ma F. (2003) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21: 335–351 [Google Scholar]
- Espelie KE, Sadek NZ, Kolattukudy PE. (1980) Composition of suberin-associated waxes from the subterranean storage organs of seven plants, parsnip, carrot, rutabaga, turnip, red beet, sweet potato and potato. Planta 148: 468–476 [DOI] [PubMed] [Google Scholar]
- Franke R, Schreiber L. (2007) Suberin—a biopolyester forming apoplastic plant interfaces. Curr Opin Plant Biol 10: 252–259 [DOI] [PubMed] [Google Scholar]
- Franke RB, Dombrink I, Schreiber L. (2012) Suberin goes genomics: use of a short living plant to investigate a long lasting polymer. Front Plant Sci 3: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciarrubio A, Legaria JP, Covarrubias AA. (1997) Abscisic acid inhibits germination of mature Arabidopsis seeds by limiting the availability of energy and nutrients. Planta 203: 182–187 [DOI] [PubMed] [Google Scholar]
- Graça J, Santos S. (2007) Suberin: a biopolyester of plants’ skin. Macromol Biosci 7: 128–135 [DOI] [PubMed] [Google Scholar]
- Haughn G, Chaudhury A. (2005) Genetic analysis of seed coat development in Arabidopsis. Trends Plant Sci 10: 472–477 [DOI] [PubMed] [Google Scholar]
- Holloway P. (1983) Some variations in the composition of suberin from the cork layers of higher plants. Phytochemistry 22: 495–502 [Google Scholar]
- Kolattukudy PE. (1980) Biopolyester membranes of plants: cutin and suberin. Science 208: 990–1000 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE (2001). Plant Cuticle and Suberin. John Wiley & Sons, Hoboken, NJ [Google Scholar]
- Kosma DK, Molina I, Ohlrogge JB, Pollard M. (2012) Identification of an Arabidopsis fatty alcohol:caffeoyl-Coenzyme A acyltransferase required for the synthesis of alkyl hydroxycinnamates in root waxes. Plant Physiol 160: 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Beisson F, Ohlrogge J, Pollard M. (2007) Monoacylglycerols are components of root waxes and can be produced in the aerial cuticle by ectopic expression of a suberin-associated acyltransferase. Plant Physiol 144: 1267–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Beisson F, Pollard M, Ohlrogge J. (2006) Oil content of Arabidopsis seeds: the influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 67: 904–915 [DOI] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH. (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lulai EC, Corsini DL. (1998) Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiol Mol Plant Pathol 53: 209–222 [Google Scholar]
- Masao H, Kumi M, Sumitra H. (1987) Effect of long chain fatty acids and fatty alcohols on streptococcus mutans. Chem Pharm Bull 35: 3507–3510 [DOI] [PubMed] [Google Scholar]
- Meyer CJ, Peterson CA, Bernards MA. (2011) Spatial and temporal deposition of suberin during maturation of the onion root exodermis. Botany 89: 119–131 [Google Scholar]
- Molina I, Bonaventure G, Ohlrogge J, Pollard M. (2006) The lipid polyester composition of Arabidopsis thaliana and Brassica napus seeds. Phytochemistry 67: 2597–2610 [DOI] [PubMed] [Google Scholar]
- Molina I, Ohlrogge JB, Pollard M. (2008) Deposition and localization of lipid polyester in developing seeds of Brassica napus and Arabidopsis thaliana. Plant J 53: 437–449 [DOI] [PubMed] [Google Scholar]
- Nair MG, Epp MD, Burke BA. (1988) Ferulate esters of higher fatty alcohols and allelopathy in Kalanchöe daigremontiana. J Chem Ecol 14: 589–603 [DOI] [PubMed] [Google Scholar]
- Nicholson RL, Hammerschmidt R. (1992) Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol 30: 369–389 [Google Scholar]
- Ossowski S, Schwab R, Weigel D. (2008) Gene silencing in plants using artificial microRNAs and other small RNAs. Plant J 53: 674–690 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW (2006). Relative quantification. In T Dorak, ed, Real-Time PCR. International University Line, La Jolla, CA, pp 63–82 [Google Scholar]
- Pollard M, Beisson F, Li Y, Ohlrogge JB. (2008). Building lipid barriers: biosynthesis of cutin and suberin. Trends Plant Sci 13: 236–246 [DOI] [PubMed] [Google Scholar]
- Ranathunge K, Schreiber L. (2011) Water and solute permeabilities of Arabidopsis roots in relation to the amount and composition of aliphatic suberin. J Exp Bot 62: 1961–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranathunge K, Schreiber L, Franke R. (2011) Suberin research in the genomics era—new interest for an old polymer. Plant Sci 180: 399–413 [DOI] [PubMed] [Google Scholar]
- Schreiber L, Franke R, Hartmann K. (2005) Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Planta 220: 520–530 [DOI] [PubMed] [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D. (2006) Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra O, Soler M, Hohn C, Sauveplane V, Pinot F, Franke R, Schreiber L, Prat S, Molinas M, Figueras M. (2009) CYP86A33-targeted gene silencing in potato tuber alters suberin composition, distorts suberin lamellae, and impairs the periderm’s water barrier function. Plant Physiol 149: 1050–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Meyer CJ, Ma F, Peterson CA, Bernards MA. (2007) The outermost cuticle of soybean seeds: chemical composition and function during imbibition. J Exp Bot 58: 1071–1082 [DOI] [PubMed] [Google Scholar]
- Soliday CL, Kolattukudy PE, Davis RW. (1979) Chemical and ultrastructural evidence that waxes associated with the suberin polymer constitute the major diffusion barrier to water vapor in potato tuber (Solanum tuberosum L.). Planta 146: 607–614 [DOI] [PubMed] [Google Scholar]
- Thomas R, Fang X, Ranathunge K, Anderson TR, Peterson CA, Bernards MA. (2007) Soybean root suberin: anatomical distribution, chemical composition, and relationship to partial resistance to Phytophthora sojae. Plant Physiol 144: 299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi N, Shiraishi A, Nishizaka M, Matsuoka K, Endo K, Hamashima H, Inoue Y. (2007) Antibacterial activity of long-chain fatty alcohols against Staphylococcus aureus. Molecules 12: 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WL, Bernards MA. (2006) Wound-induced metabolism in potato (Solanum tuberosum) tubers. Plant Signal Behav 1: 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Henriques R, Lin SS, Niu QW, Chua NH. (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1: 641–646 [DOI] [PubMed] [Google Scholar]
- Zimmermann HM, Hartmann K, Schreiber L, Steudle E. (2000) Chemical composition of apoplastic transport barriers in relation to radial hydraulic conductivity of corn roots (Zea mays L.). Planta 210: 302–311 [DOI] [PubMed] [Google Scholar]