Regulation of a novel vacuolar sugar carrier is important for a range of plant functions.
Abstract
Here, we report that SUGARS WILL EVENTUALLY BE EXPORTED TRANSPORTER (SWEET16) from Arabidopsis (Arabidopsis thaliana) is a vacuole-located carrier, transporting glucose (Glc), fructose (Fru), and sucrose (Suc) after heterologous expression in Xenopus laevis oocytes. The SWEET16 gene, similar to the homologs gene SWEET17, is mainly expressed in vascular parenchyma cells. Application of Glc, Fru, or Suc, as well as cold, osmotic stress, or low nitrogen, provoke the down-regulation of SWEET16 messenger RNA accumulation. SWEET16 overexpressors (35SPro:SWEET16) showed a number of peculiarities related to differences in sugar accumulation, such as less Glc, Fru, and Suc at the end of the night. Under cold stress, 35SPro:SWEET16 plants are unable to accumulate Fru, while under nitrogen starvation, both Glc and Fru, but not Suc, were less abundant. These changes of individual sugars indicate that the consequences of an increased SWEET16 activity are dependent upon the type of external stimulus. Remarkably, 35SPro:SWEET16 lines showed improved germination and increased freezing tolerance. The latter observation, in combination with the modified sugar levels, points to a superior function of Glc and Suc for frost tolerance. 35SPro:SWEET16 plants exhibited increased growth efficiency when cultivated on soil and showed improved nitrogen use efficiency when nitrate was sufficiently available, while under conditions of limiting nitrogen, wild-type biomasses were higher than those of 35SPro:SWEET16 plants. Our results identify SWEET16 as a vacuolar sugar facilitator, demonstrate the substantial impact of SWEET16 overexpression on various critical plant traits, and imply that SWEET16 activity must be tightly regulated to allow optimal Arabidopsis development under nonfavorable conditions.
Sugars are of enormous importance for plant properties and the agronomic values of most crop species (John, 1992). In plants, they serve as energy reserves, as building blocks for carbohydrate polymers like starch or cellulose, as precursors for amino and carboxylic acids, and as osmolytes required for the molecular antifreezing program initiated after exposure to cold temperatures (Nägele et al., 2010).
Sugars in leaves are synthesized either during the day via photosynthesis or in the night as a product of starch degradation. The major sugar synthesized in most plants during the day is Suc, which, after the export of triose phosphates from the chloroplast, is synthesized in the cytosol. During nocturnal starch degradation, maltose leaves the chloroplast and serves as a substrate for the cytosolic synthesis of heteroglycans (Fettke et al., 2005). Subsequent to this, heteroglycans are degraded by phosphorylases (Fettke et al., 2005) and act as a carbon source to synthesize Suc, which can be hydrolyzed by cytosolic or vacuolar invertases to monosaccharides (Roitsch and González, 2004). These processes, in sum, enable leaf mesophyll cells to synthesize Glc and Fru, in addition to Suc, during the day and at night.
Besides these metabolic processes, sugars are transported between different intracellular compartments and between different cells in order to serve as a long-distance carbon supply for sink organs. Due to their large size and hydrate shell, the movement of neutral sugars like Suc, Glc, or Fru across membranes requires the presence of membrane-bound carriers. For example, in the plant plasma membrane, a wide number of monosaccharide- and Suc-specific carriers were identified and have been analyzed with biochemical and molecular approaches. The Arabidopsis (Arabidopsis thaliana) genome harbors more than 50 isoforms of putative monosaccharide carriers, most of which belong to the sugar transport protein subfamily (Büttner and Sauer, 2000), while about 20 putative disaccharide carriers sucrose transporters (named SUT and SUC) are present in this plant species (Lalonde et al., 2004). Most of the sugar transport protein, SUT, or SUC carriers analyzed so far reside in the plasma membrane and import, as proton-coupled transporters, apoplastic sugars against a concentration gradient (Lalonde et al., 2004). This proton-driven sugar import allows a substantial accumulation of Suc in phloem sieve elements, building the driving force for interorgan long-distance sugar transport (Turgeon and Wolf, 2009). All monosaccharide and disaccharide carriers mentioned above exhibit 12 predicted transmembrane domains and group into the large major facilitator superfamily of carriers (Marger and Saier, 1993).
In both photosynthetic active mesophyll cells as well as storage tissues, the large central vacuole represents the internal storage compartment for sugars (Martinoia et al., 2007, 2012), leading, in sugar beet (Beta vulgaris) or sugarcane (Saccharum officinarum), up to even 20% sugars per fresh biomass (John, 1992). Suc import into the vacuole occurs either via facilitated diffusion (Kaiser and Heber, 1984) or electrogenically via antiport against protons (Willenbrink and Doll, 1979). The latter process is driven by the significant proton-motive force across the vacuolar membrane (Schumacher and Krebs, 2010) and allows a substantial Suc accumulation in storage organs of high-sugar species (Getz, 1987; Getz and Klein, 1995). However, no Suc importer at the vacuolar membrane (tonoplast) has been identified on the molecular level yet, while tonoplast-located Suc exporters have been identified. This vacuolar Suc export is mediated by members of the SUT4-type clade of carriers, in cereals named SUT2 (Endler et al., 2006; Eom et al., 2011), procuring a proton-driven Suc export into the cytosol (Schulz et al., 2011). Loss of function of this type of carrier in Arabidopsis, poplar (Populus spp.), or rice (Oryza sativa) leads to an accumulation of Suc in leaves (Eom et al., 2011; Payyavula et al., 2011; Schneider et al., 2012), elegantly proving that this type of carrier fulfills an export function under in vivo conditions.
In contrast to vacuolar Suc import, the import of monosaccharides into this compartment has been deciphered on the molecular level. In the Arabidopsis tonoplast, two different monosaccharide importers have been identified, namely the vacuolar Glc transporter protein and three isoforms of the tonoplast monosaccharide transporter (TMT; Wormit et al., 2006; Aluri and Büttner, 2007). While vacuolar Glc transporter loss-of-function plants do not show significant changes in monosaccharide levels (Aluri and Büttner, 2007), decreased TMT activity correlates with impaired vacuolar sugar import and low levels of both Glc and Fru in leaves (Wormit et al., 2006). This fact and the observations that (1) TMT1 is a sugar/proton antiporter (Schulz et al., 2011), (2) increased TMT activity provokes improved seed biomass (Wingenter et al., 2010), and (3) TMT activity is highly regulated via protein phosphorylation (Wingenter et al., 2011) clearly underline the superior function of TMT for monosaccharide loading into the plant vacuole.
So far, two carriers, ESL1 and ERDL6, have been found to be responsible for Glc export from the plant vacuole (Yamada et al., 2010; Poschet et al., 2011). ESL1 (for early responsive to dehydration6-like1) represents a carrier majorly expressed in pericycle and xylem parenchyma cells and is known to be induced by drought stress (Yamada et al., 2010). Loss-of-function mutants of the ERDL6 (for early responsive to dehydration6-like6) carrier show increased leaf Glc levels and decreased seed weight, indicating that controlled Glc export via this carrier is critical for interorgan movement of sugars in Arabidopsis (Poschet et al., 2011). ESL1 seems to transport Glc in a facilitated diffusion, while in contrast to the plasma membrane-located sugar carriers and to TMT, the transport mode of ERDL6 has not been identified so far.
In marked contrast to the carriers mentioned above, the recent identification of the so-called SWEET proteins opened our understanding of how cellular sugar export is achieved. SWEET proteins occur in plants as well as in animals and humans and consist of only seven predicted transmembrane domains (Chen et al., 2010). The observation that the expression of several plant SWEET proteins is strongly induced by various pathogens indicated that they serve as sugar exporters. That hypothesis has been proven for some SWEET isoforms by heterologous expression in Xenopus laevis oocytes (Chen et al., 2010), and detailed analysis revealed that Arabidopsis SWEET11 and SWEET12 catalyze Suc export from source leaves and are critical for interorgan sugar transport (Chen et al., 2012).
In a recent quantitative trait locus analysis, we identified SWEET17 as a novel determinant of leaf Fru content, especially under cold conditions and conditions of low nitrogen supply (Chardon et al., 2013). In fact, a detailed molecular-physiological analysis revealed that SWEET17 is the first vacuole-located SWEET protein and that it serves as a Fru-specific exporter, connecting the vacuolar lumen to the cytosol. In contrast to SWEET17, the subcellular localization of its closest homolog, SWEET16, is elusive. Moreover, transport properties of SWEET16 are unknown, and the effect of increased SWEET16 activity (or any other SWEET proteins) on plant properties has not been determined. The latter aspect is of particular interest, since most genes coding for SWEET proteins are only comparably weakly expressed or are only expressed in certain cell types (Chen et al., 2010; Chardon et al., 2013).
In this report, we analyzed the intracellular localization of SWEET16 and studied its transport properties in X. laevis oocytes. Moreover, we constructed constitutive SWEET16-overexpressing Arabidopsis lines and report the impact of this overexpression of a vacuolar SWEET protein on plant development and stress tolerance. Our results support the hypothesis that the activity of a SWEET facilitator has to be controlled in planta to cope with altering environmental and developmental conditions.
RESULTS
SWEET16 Is a Vacuolar Membrane Protein
Recently, we showed that the sugar carrier SWEET17 locates to the vacuolar membrane (Chardon et al., 2013). However, the subcellular location of SWEET16, the closest homolog to SWEET17 (Chen et al., 2010), is so far unknown.
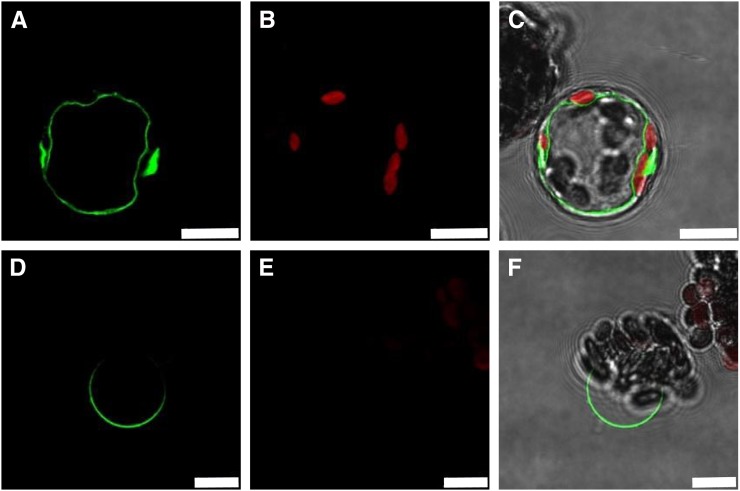
To find evidence for the membrane harboring SWEET16, we created a 35SPro:SWEET16:GFP construct and transformed isolated Arabidopsis protoplasts. The SWEET16-GFP fusion protein resides in the vacuolar membrane of isolated Arabidopsis protoplasts, since the large and space-filling GFP-labeled organelle (Fig. 1A) is indented by several red-fluorescing chloroplasts (Fig. 1B) and locates to the inner side of the plasma membrane (Fig. 1C). This defined SWEET16-GFP signal persisted also after gentle lysis of protoplasts and release of intact vacuoles (Fig. 1, D–F), confirming that the green-fluorescing organelle (Fig. 1, A and D) is the central vacuole.
Figure 1.
Subcellular localization of SWEET16. The 35SPro:SWEET16:GFP fusion protein locates to the tonoplast after transformation of Arabidopsis protoplasts. A and D, GFP fluorescence. B and E, Autofluorescence of the chloroplasts. C and F, Merge including bright-field images. A to C show intact protoplast, and D to F show release of the vacuole after mild lysis of the protoplast. White bars represent 10 μm.
The SWEET16 Gene Is Mainly Expressed in Vascular Parenchyma Cells and Is Responsive to Sugars, Osmotic Stress, and Nitrogen Availability
Recently, it has been shown that the gene coding for the vacuolar Fru carrier SWEET17 is mainly expressed in vascular parenchyma cells (Chen et al., 2010, 2012; Chardon et al., 2013). To check the tissue and cellular expression pattern of the SWEET16 gene, we created SWEET16Pro:GUS transgenic lines and analyzed the corresponding gene expression.
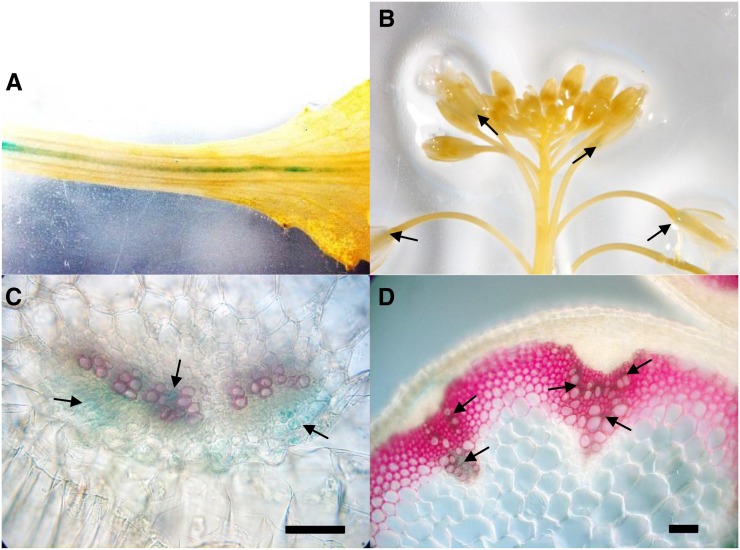
In general, the expression level of the SWEET16 gene, as revealed by GUS activity, is comparably low and only detectable in a very limited number of Arabidopsis tissues. The strongest GUS activity was found in the petiole vasculature (Fig. 2A), due to GUS expression in corresponding xylem parenchyma cells (Fig. 2C). Such a vascular location of SWEET16 expression also holds true for the xylem parenchyma in flower stalks (Fig. 2D) and is also visible at the base of individual, not fully developed flowers (Fig. 2B). On the basis of GUS activity, we have not detected substantial SWEET16 expression in other tissues.
Figure 2.
Tissue-specific localization of SWEET16. The SWEET16Pro:GUS fusion is mainly expressed in the vascular tissues. A, Petiole of a rosette leaf. B, Flower stalk. C, Transverse section of a rosette leaf petiole. D, Transverse section of a floral stem from a 7-week-old plant grown in greenhouse conditions. In B, the arrows point to the bases of developing flowers. In C and D, the arrows point to the xylem parenchyma cells. GUS staining is indicated in blue, and lignin staining with phloroglucinol is shown in pink. Bars = 50 µm.
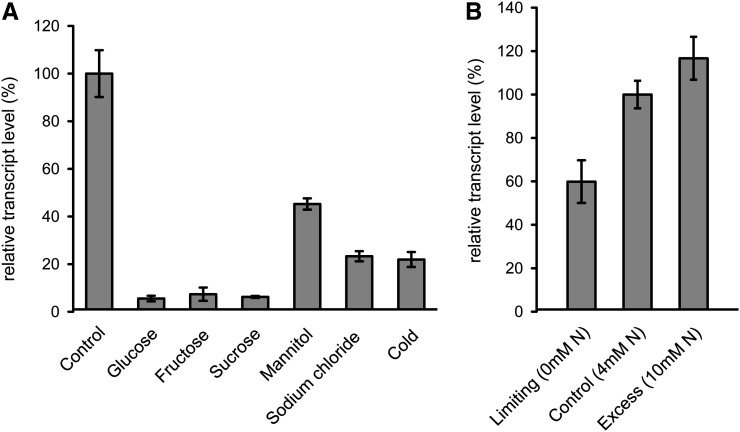
Assuming that SWEET16 is a sugar carrier, we speculated whether sugar administration, cold temperatures, or osmotic stress conditions might influence corresponding gene expression. Leaf discs incubated overnight in Glc, Fru, or Suc showed a dramatic decrease of SWEET16 mRNA, as quantified by quantitative reverse transcription-PCR analysis. All three sugars tested led to reductions of SWEET16 mRNA levels to about 5% to 7% of the corresponding control (Fig. 3A). Osmotically active solutes like mannitol and sodium chloride led to 45% and 23% reductions, respectively, of SWEET16 mRNA. Incubation of leaf discs overnight at 4°C also induced the down-regulation of SWEET16 mRNA to about 22% of the control level present in leaf discs prepared from plants incubated at a standard night-phase temperature (22°C; Fig. 3A). To check for the involvement of SWEET16 gene regulation in the response of Arabidopsis to altered nitrogen availability, we grew seedlings in liquid culture under limiting nitrogen (0 mm), standard nitrogen (4 mm), or high nitrogen (10 mm). As given, the lowest SWEET16 expression was obvious when nitrogen was absent, while increasing nitrogen concentrations led to a concomitant increase of SWEET16 mRNA (Fig. 3B).
Figure 3.
SWEET16 expression pattern. A, Expression of SWEET16 in leaf discs from 5-week-old Arabidopsis wild-type plants incubated for 16 h in different media or under cold temperature (4°C). B, Effect of nitrate availability on SWEET16 expression in liquid culture-grown wild-type seedlings. Mean values ± se of three independent biological replicates are given.
SWEET16 Catalyzes Glc, Fru, and Suc Transport
It has been shown before that the expression of several vacuolar sugar and nonsugar carriers in X. laevis oocytes allowed the decipherment of corresponding transport properties (Desbrosses-Fonrouge et al., 2005; Kovermann et al., 2007; Reinders et al., 2008). For example, after expression in X. laevis oocytes, members of the large SWEET family of plant carriers were identified as able to transport the monosaccharides Glc and Fru or the disaccharide Suc (Chen et al., 2010, 2012; Chardon et al., 2013). Thus, we exploited the recombinant oocyte system to study the substrate spectrum of SWEET16.
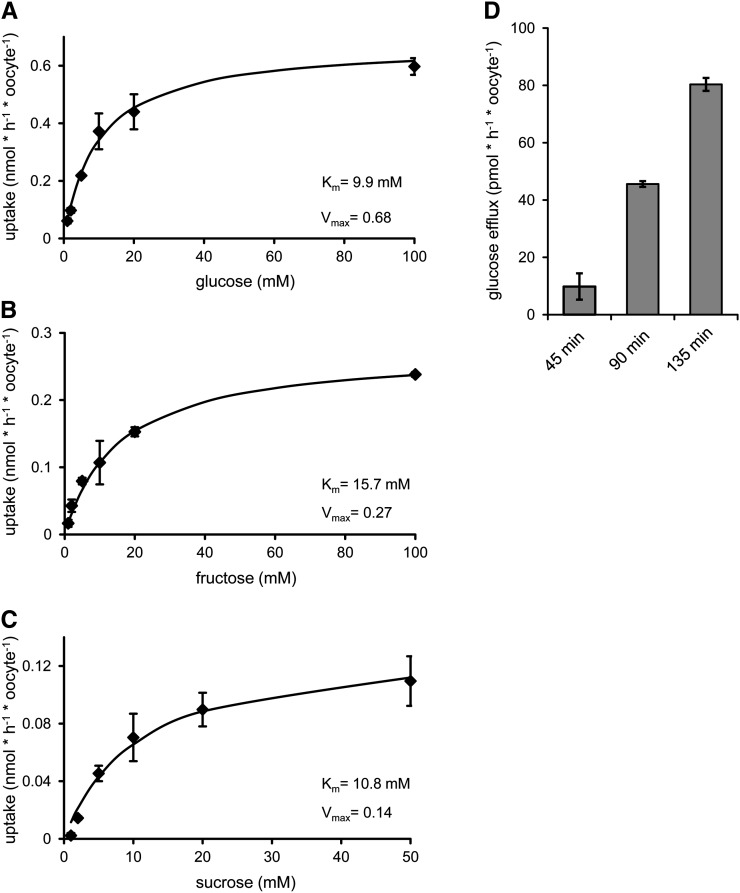
After heterologous synthesis in X. laevis oocytes, the recombinant SWEET16 protein is able to transport all three radioactively labeled sugars applied, namely [14C]Glc, [14C]Fru, and [14C]Suc (Fig. 4, A–C). The apparent Km values were 9.9 mm for Glc, 15.7 mm for Fru, and 10.8 mm for Suc (Fig. 4, A–C). The corresponding Vmax values were 0.68 nmol h−1 oocyte−1 for Glc, 0.27 nmol h−1 oocyte−1 for Fru, and 0.14 nmol h−1 oocyte−1 for Suc (Fig. 4, A–C). Exact cytosolic monosaccharide concentrations have so far not been determined, while Suc levels in the light range from 80 mm in spinach (Spinacia oleracea) to 230 mm in barley (Hordeum vulgare; Winter et al., 1993, 1994). Thus, the affinity of SWEET16 for Suc is well above the expected concentration.
Figure 4.
Sugar uptake and efflux catalyzed by SWEET16 after heterologous expression in X. laevis oocytes. A to C, Uptake of [14C]Glc (A), [14C]Fru (B), and [14C]Suc (C) into X. laevis oocytes. D, Efflux of [14C]Glc in a time-dependent manner. Each data point represents the mean value of at least nine oocytes ± se. Uptake and efflux of water-injected control oocytes were subtracted.
To verify the mode of sugar transport in more detail, we analyzed the export of radioactively labeled Glc from oocytes expressing the recombinant SWEET16 protein. For this, we preloaded SWEET16-expressing oocytes with radioactively labeled Glc and quantified the export of radioactivity in comparison with water-injected control cells (Fig. 4D). As given, the expression of SWEET16 led to a time-linear release of labeled endogenous sugar (Fig. 4D).
SWEET16 Overexpressor Lines Show Altered Sugar Metabolism
The expression of vacuole-located SWEET16 protein in the X. laevis oocyte system demonstrated that this carrier is a sugar porter (Fig. 4). Since we revealed before that overexpression of the vacuole-located carrier monosaccharide importer TMT1 modified the sugar homeostasis in Arabidopsis considerably (Wingenter et al., 2010), we were now interested to study whether an overexpression of SWEET16 also affects plant properties.
To generate SWEET16 overexpressor lines, we introduced the corresponding complementary DNA (cDNA) under the control of a constitutive 35S cauliflower mosaic virus promoter into Arabidopsis plants. The resulting SWEET16 overexpressor lines 8 and 14 were selected from 20 independent 35SPro:SWEET16 transgenic lines on the basis of substantially increased SWEET16 mRNA levels (Supplemental Fig. S1). The growth of genotypes with altered expression of vacuolar sugar transporters in liquid culture conditions allows a quick check of whether vacuolar transporter mutants affect cellular sugar homeostasis (Wingenter et al., 2010, 2011). Thus, in a first set of experiments, we searched for a putative effect of altered vacuolar SWEET16 expression during the growth of 35SPro:SWEET16 compared with wild-type plants in standard liquid culture medium, harboring 0.5% Suc (Scheible et al., 2004).
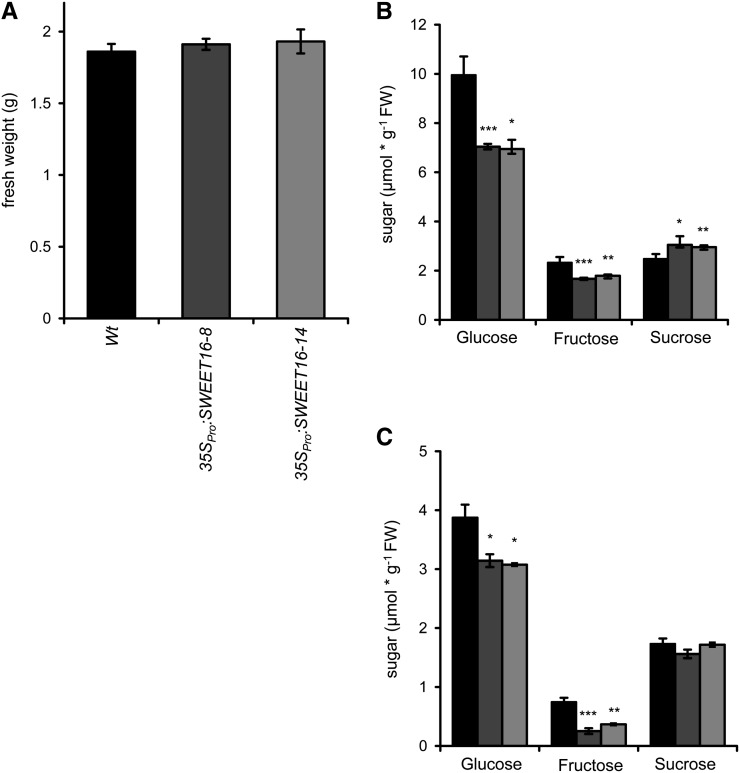
After 14 d of growth, wild-type plants and both SWEET16 overexpression lines exhibited similar fresh weight accumulation of about 1.86 g per 100 seeds (Fig. 5A). At this stage, wild-type plants contained 9.96 µmol g−1 fresh weight Glc, 2.33 µmol g−1 fresh weight Fru, and 2.48 µmol g−1 fresh weight Suc (Fig. 5B). Interestingly, the two independent 35SPro:SWEET16 lines showed significantly reduced Glc and Fru levels, while the Suc concentration was increased, when compared with wild-type plants. 35SPro:SWEET16 line 8 contained 7.04 µmol g−1 fresh weight Glc, 1.67 µmol g−1 fresh weight Fru, and 3.05 µmol g−1 fresh weight Suc, and 35SPro:SWEET16 line 14 contained similar sugar concentrations, namely 6.95 µmol g−1 fresh weight Glc, 1.79 µmol g−1 fresh weight Fru, and 2.96 µmol g−1 fresh weight Suc (Fig. 5B).
Figure 5.
Fresh weights and sugar levels of wild-type and SWEET16 overexpression lines. A, Liquid culture (supplemented with 1% Suc)-grown seedlings of 35SPro:SWEET16 lines display the same fresh weight as the wild type (Wt). B, Diversified sugar concentrations in liquid culture. C, Sugar compositions of the wild type and SWEET16 overexpression lines 8 and 14 in leaves of 6-week-old plants grown on soil. Mean values ± se of at least three independent biological replicates are given. Asterisks indicate P values (*P < 0.05, **P < 0.01, ***P < 0.005) according to Student’s t test. FW, Fresh weight.
Furthermore, we quantified sugars of wild-type plants and the two 35SPro:SWEET16 lines grown for 5 weeks on soil under controlled-climate-chamber conditions. At the end of the light period, wild-type plants contained 3.87 µmol g−1 fresh weight Glc, 0.75 µmol g−1 fresh weight Fru, and 1.68 µmol g−1 fresh weight Suc (Fig. 5C). Interestingly, 35SPro:SWEET16 lines showed specific problems in accumulating Fru. 35SPro:SWEET16 line 8 contained 3.14 µmol g−1 fresh weight Glc, only 0.25 µmol g−1 fresh weight Fru, and 1.56 µmol g−1 fresh weight Suc, and 35SPro:SWEET16 line 14 exhibited 3.08 µmol g−1 fresh weight Glc, 0.37 µmol g−1 fresh weight Fru, and 1.72 µmol g−1 fresh weight Suc (Fig. 5C).
SWEET16 Overexpression Lines Exhibit an Altered Sugar Response to Cold Conditions
It is well known that plants rapidly accumulate sugars after transfer into cold conditions (Alberdi and Corcuera, 1991; Hannah et al., 2005). To check whether increased SWEET16 expression modifies cold-induced sugar accumulation in Arabidopsis, we first grew wild-type plants and the two 35SPro:SWEET16 lines for 6 weeks under normal conditions (22°C) and transferred these plants subsequently (at the end of the light phase) into the cold (4°C). Plants were then kept under cold conditions for up to 72 h (identical light/dark regime of 10 h/14 h), and sugar and starch levels were quantified at the end of the night and day, respectively.
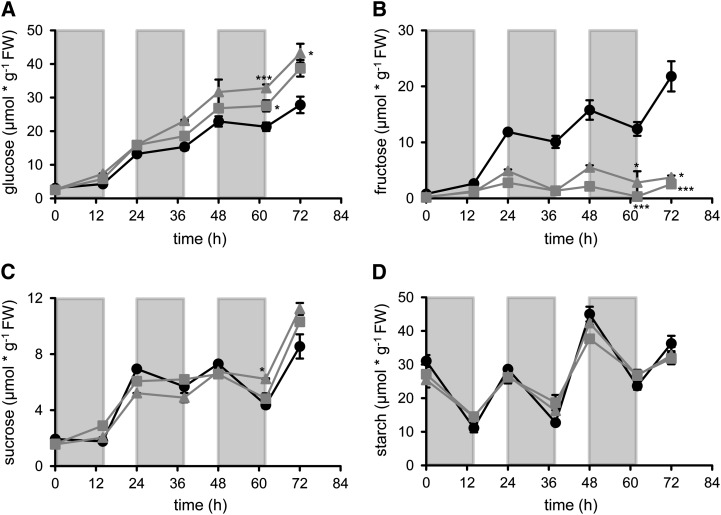
All three plant lines accumulated Glc during the 72 h of cold treatment. In wild-type plants, Glc started at a level of 3.02 µmol g−1 fresh weight, increased already in the first cold night to 4.29 µmol g−1 fresh weight, and rose further up to 27.82 µmol g−1 fresh weight within 72 h of cold treatment (Fig. 6A). In both 35SPro:SWEET16 lines, Glc levels started at comparable levels at the beginning of the experiment, namely about 2.6 µmol g−1 fresh weight (Fig. 6A). Similar to wild-type plants, the two 35SPro:SWEET16 lines increased their Glc levels within the first night phase. 35SPro:SWEET16 line 8 contained 5.8 µmol Glc g−1 fresh weight, while 35SPro:SWEET16 line 14 contained 7.32 µmol Glc g−1 fresh weight, at the end of the first cold night phase (Fig. 6A). Both 35SPro:SWEET16 lines increased their Glc levels within 72 h of cold incubation and reached higher levels than observed in wild-type plants. After 72 h of cold incubation, 35SPro:SWEET16 line 8 contained 38.71 µmol Glc g−1 fresh weight and 35SPro:SWEET16 line 14 contained 43.2 µmol Glc g−1 fresh weight (Fig. 6A).
Figure 6.
Diurnal changes in sugar accumulation in wild-type and SWEET16 overexpression lines during exposure to cold. Plants were grown for 6 weeks on soil under a 10/14-h light/dark cycle at 21°C and then transferred to 4°C at the end of the day. Black circles, The wild type; gray squares, 35SPro:SWEET16 line 8; gray triangles, 35SPro:SWEET16 line 14. The values shown represent Glc, Fru, Suc, and starch of three independent biological replicates ± se. FW, Fresh weight.
Similar to Glc, the levels of Fru increased continuously in wild-type plants during cold incubation. Wild-type plants contained 0.79 µmol Fru g−1 fresh weight at the end of the last light period under warm temperature and increased their Fru level within 72 h of cold incubation to about 21.79 µmol g−1 fresh weight. In marked contrast to this, endogenous Fru levels of both 35SPro:SWEET16 lines hardly increased when compared with wild-type plants. After 72 h in the cold, 35SPro:SWEET16 line 8 contained 2.56 µmol Fru g−1 fresh weight and 35SPro:SWEET16 line 14 contained only 3.74 µmol Fru g−1 fresh weight (Fig. 6B), representing 7- to 8-fold less Fru than present in corresponding wild-type plants.
Similar to the Glc content, the level of Suc increased in all three plant lines during cold treatment (Fig. 6C). In wild-type plants, the Suc concentration at the end of the last light period under warm conditions was 1.93 µmol g−1 fresh weight and approached 8.55 µmol g−1 fresh weight after 72 h at 4°C (Fig. 6C). The Suc accumulation pattern in both 35SPro:SWEET16 lines resembled the Suc accumulation pattern in wild-type plants for the first 48 h (Fig. 6C). However, after 72 h in the cold, 35SPro:SWEET16 line 8 contained 10.31 µmol Suc g−1 fresh weight and 35SPro:SWEET16 line 14 contained 11.23 µmol Suc g−1 fresh weight, which is significantly more than in wild-type plants (8.55 µmol Suc g−1 fresh weight; Fig. 6C).
In contrast to the three sugars, starch decreased in all three plant lines during the first and all following night phases (Fig. 6D). Starch levels in all three plant lines approached 25.42 to 31.06 µmol C6 g−1 fresh weight at the end of the last light phase in the warm and decreased to about 11.12 to 14.47 µmol C6 g−1 fresh weight at the end of the first night phase in the cold (Fig. 6D). After 72 h in the cold, all plant lines tested exhibited similar starch levels of about 33 to 36 µmol C6 g−1 fresh weight (Fig. 6D).
SWEET16 Overexpression Mutants Show Improved Freezing Tolerance
The accumulation of soluble sugars in the cold is part of a complex metabolic reprogramming in plants, aiming to tolerate low temperatures (Alberdi and Corcuera, 1991; Hannah et al., 2005; Schulze et al., 2012). Since SWEET16 overexpression lines showed a substantially modified sugar composition in the cold, when compared with wild-type plants (Fig. 6), we were interested to analyze if those changes influence the freezing tolerance of corresponding plant lines. The quantification of the electrical conductivity of leaves provides a highly reproducible method to quantify the freezing tolerance of individual plant lines (Zuther et al., 2004; Nägele and Heyer, 2013). Therefore, we grew wild-type plants and the two 35SPro:SWEET16 lines 8 and 14 for 3 weeks and transferred them into the cold for 4 d to allow cold acclimation (Hannah et al., 2005). We then froze detached leaves gradually at −6°C and quantified the release of electrolytes, which is an indicator for the amount of destroyed cells (Ristic and Ashworth, 1993).
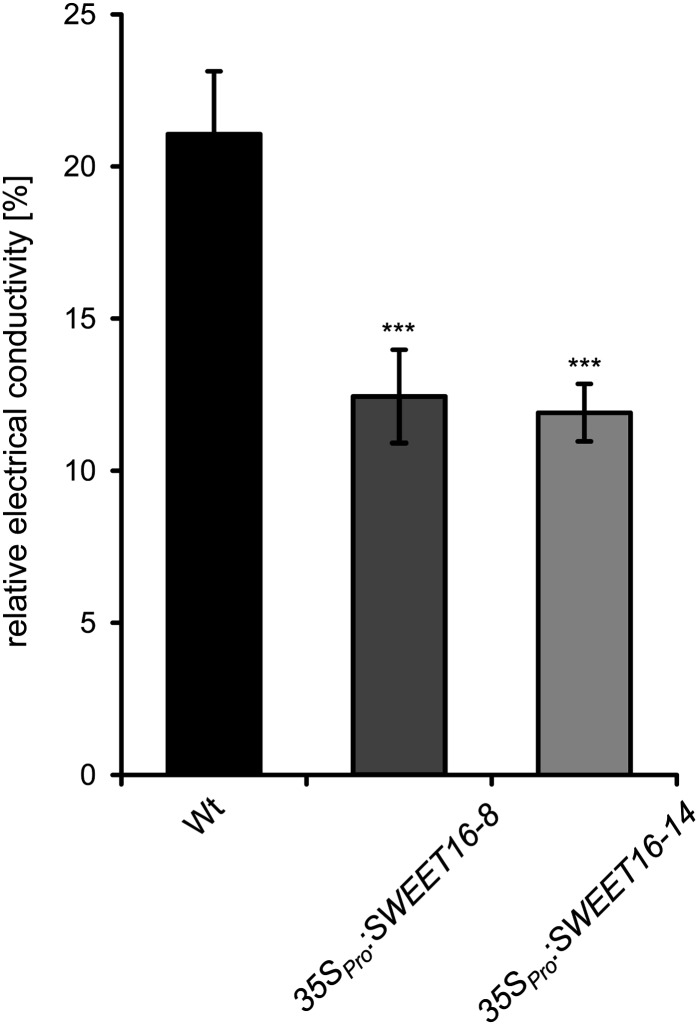
At −6°C, wild-type leaves released 21.1% of their total electrolytes, while both overexpressor lines exhibited strongly improved freezing tolerance (Fig. 7). Under identical conditions, leaves from 35SPro:SWEET16 line 8 released only 12.4% of total electrolytes and leaves from 35SPro:SWEET16 line 14 released 11.9% of their total electrolytes, highly similar to 35SPro:SWEET16 line 8 (Fig. 7).
Figure 7.
Electrolyte leakage of cold-acclimated wild-type (Wt) and SWEET16-overexpressing plants. Three-week-old plants were cold adapted at 4°C for 4 d, and electrolyte leakage was estimated by determining the electrical conductivity of the bathing solution of detached leaves frozen at −6°C (n = 10). Asterisks indicate P values (***P < 0.005) according to Student’s t test.
SWEET16 Overexpression Lines Show Altered Germination Efficiency
It is well established that increased sugar levels affect seed germination efficiency (Dekkers et al., 2004). Hence, to search for an altered early development of 35SPro:SWEET16 plants, we monitored the germination efficiency of wild-type and overexpressor lines on agar plates supplemented with Glc, Fru, or Suc (Fig. 8).
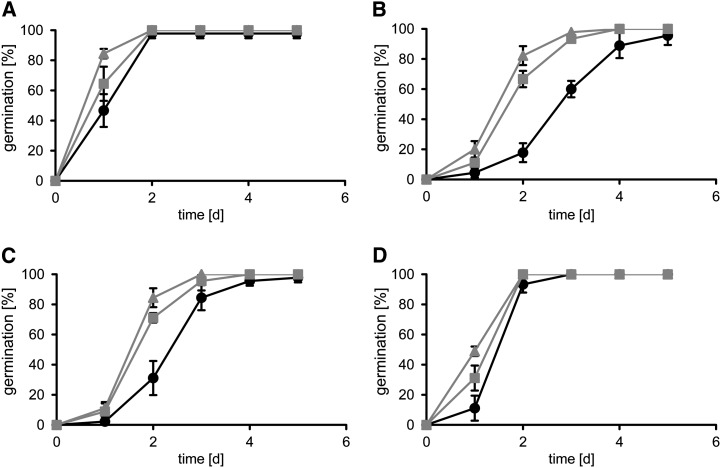
Figure 8.
Germination rates of wild-type and SWEET16 overexpression lines on different sugars. Seeds of the wild type (black circles) and 35SPro:SWEET16 lines 8 (gray squares) and 14 (gray triangles) were germinated on 1/2 MS medium containing no sugar (A), 3% Glc (B), 3% Fru (C), or 1.5% Suc (D). Data represent mean values ± se of three experimental replicates with 60 seeds per line.
After 1 d of germination under control conditions (one-half-strength Murashige and Skoog [1/2 MS] medium without sugar), 35SPro:SWEET16 line 8 developed slightly faster than the wild type and 35SPro:SWEET16 line 14, which both developed with a similar efficiency (Fig. 8A). At 2 d after the beginning of germination, seeds from all three plant lines already completed this first developmental phase and reached their maximal germination efficiency of about 95% (Fig. 8A). In contrast to this, the presence of 3% Glc during early plant development provoked a significantly different germination efficiency of wild-type and 35SPro:SWEET16 seeds (Fig. 8B). Two days after the start of germination in the presence of Glc, only 17% of wild-type seeds completed this process, while seeds from 35SPro:SWEET16 lines 8 and 14 exhibited a germination efficiency of 82% and 66%, respectively (Fig. 8B). Three days after the start of germination, seeds from the two SWEET16 overexpressor lines nearly reached their maximal germination efficiency of about 95%, while wild-type seeds required about 5 d to reach their maximal germination efficiency (Fig. 8A). Similar to this Glc-caused effect is the action of Fru on seed germination (Fig. 8C). Suc, given at a concentration of 1.5%, also decelerated early seed germination of all three plant lines (Fig. 8D). Seeds from 35SPro:SWEET16 line 8 exhibited the fastest development (48% efficiency) after 1 d, followed by 35SPro:SWEET16 line 8 seeds and wild-type seeds (Fig. 8D). However, already after 2 d of germination, seeds from all three plant lines nearly reached their maximal germination efficiency of about 95% (Fig. 8D).
SWEET16 Overexpression Mutants Show Altered Growth Phenotypes under Various Conditions
It is known that the carbon-nitrogen ratio is of importance for plant development (Coruzzi and Bush, 2001; Coruzzi and Zhou, 2001). Since SWEET16 lines exhibit an altered sugar compartmentation profile under different growth conditions (Figs. 5, A and B, and 6, A–C), we were interested to check for developmental differences between these plant lines, which depend on the nitrate availability during growth.
We observed that both 35SPro:SWEET16 lines grew faster on soil when compared with the corresponding wild-type plants (Fig. 9). After 3 weeks of growth, mutant plants exhibited about one-third increased shoot size than the corresponding wild-type plants (Fig. 9). However, growth on soil does not allow determining the root biomasses, nor is it possible to control the nutrient supply, although the latter factor is critical for biomass accumulation. Therefore, we grew wild-type and both SWEET16 overexpression lines according to an established protocol on sand (Chardon et al., 2010) and quantified root and shoot biomasses of all three plant lines in response to limiting and high nitrate availability.
Figure 9.
Growth phenotypes of wild-type (Wt) and SWEET16 overexpression lines. Plants were grown for 3 weeks on soil under standard conditions (21°C, 10/14 h of light/dark).
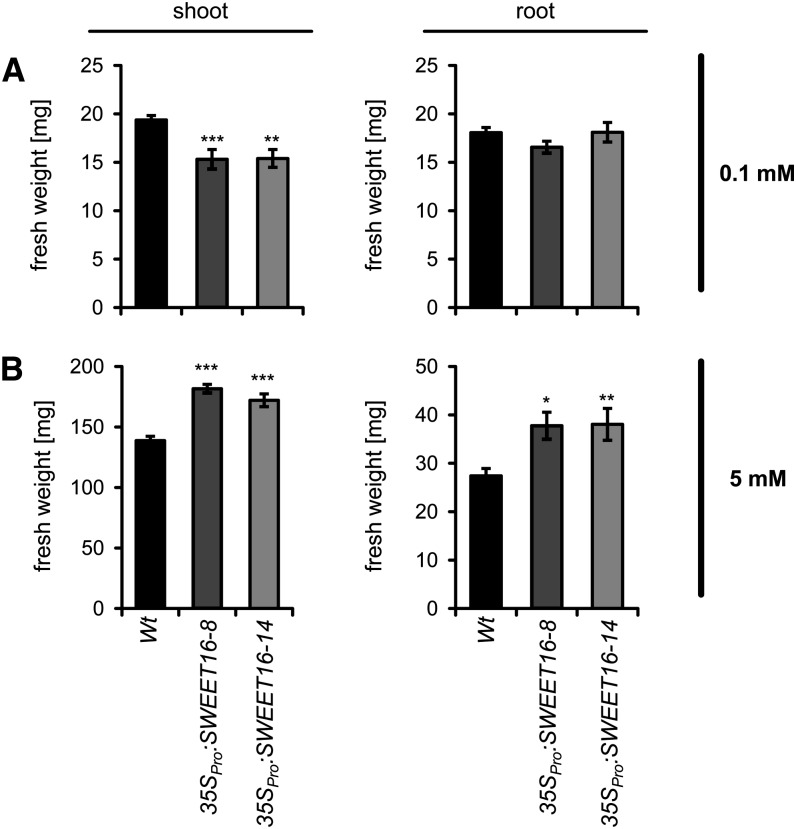
At limiting nitrate supply (0.1 mm), shoot and root growth of all three plant lines was reduced compared with ample nitrogen (5 mm nitrate). Wild-type shoots accumulated a biomass of 19.4 mg plant−1, and both 35SPro:SWEET16 lines exhibited even smaller shoot biomasses of only about 15.3 mg plant−1 (Fig. 10A). Root biomasses of all three plant lines accumulated in the range of 16.56 to 18 mg plant−1, without any significant differences (Fig. 8A). At high nitrate (5 mm), the shoot biomass of wild-type plants was further increased and reached 138.7 mg plant−1 (Fig. 10B). However, similar to the situation on soil, under these conditions both 35SPro:SWEET16 lines exhibited higher shoot biomasses than the corresponding wild-type plants, namely 181.64 mg plant−1 for 35SPro:SWEET16 line 8 and 172.05 mg plant−1 for 35SPro:SWEET16 line 14 (Fig. 10B). Similar to shoot biomasses, roots from both 35SPro:SWEET16 lines were again markedly larger than wild-type roots and reached about 37 mg plant−1 (Fig. 10B).
Figure 10.
Shoot and root biomasses of wild-type and SWEET16 overexpression lines grown either on limiting or high nitrate. Fresh weights of shoots and roots of 35-d-old plants grown on sand supplied either with 0.1 mm (A) or 5 mm (B) nitrate were determined. Mean values ± se of four to five biological replicates are given. Asterisks indicate P values (*P < 0.05, **P < 0.01, ***P < 0.005) according to Student’s t test.
Since the growth patterns of wild-type plants and the two SWEET16 overexpressors differed considerably (Figs. 9 and 10) and because SWEET16 has been identified as a vacuole-located sugar carrier (Figs. 1 and 4), we were interested to check for putatively altered sugar levels in all three plant lines grown under either low or high nitrogen supply (Fig. 11). For this approach, we grew the wild type and the two SWEET16 overexpressor lines under either limiting (0.1 mm) or high (5 mm) nitrate, prepared roots and shoots, and quantified, after extraction, the corresponding levels of Glc, Fru, and Suc.
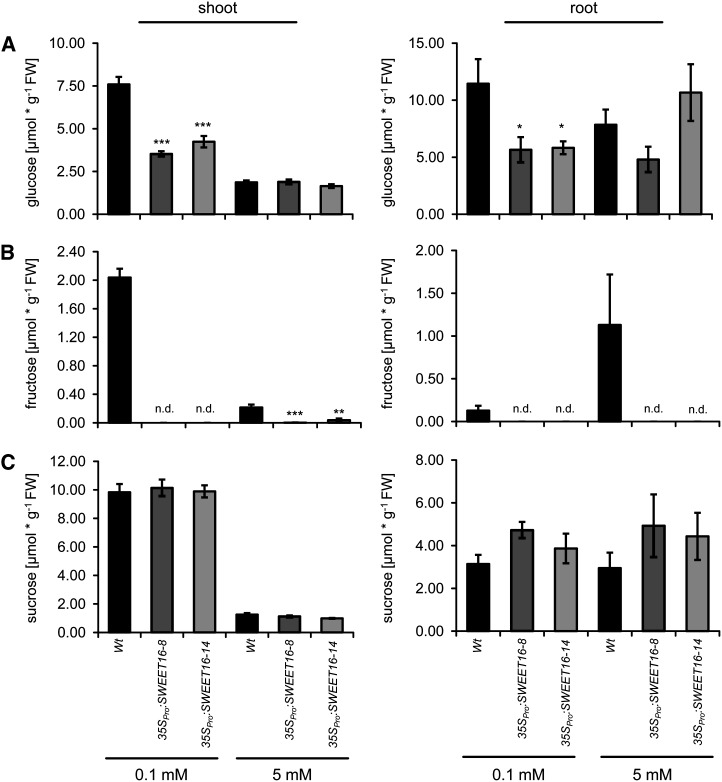
Figure 11.
Altered sugar concentrations of wild-type and SWEET16 overexpression lines in shoot and root under different nitrogen concentrations. Contents of Glc (A), Fru (B), and Suc (C) were determined in shoots and roots of the wild type (Wt) and 35SPro:SWEET16 overexpression lines 8 and 14 grown for 35 d on sand supplemented with 0.1 or 5 mm nitrate. Data represent mean values ± se of four to five biological replicates. n.d., Not detectable. Asterisks indicate P values (*P < 0.05, **P < 0.01, ***P < 0.005) according to Student’s t test. FW, Fresh weight.
Under limiting nitrate, wild-type shoots contained about 7.6 µmol Glc g−1 fresh weight, while both SWEET16 overexpressor plants showed markedly decreased Glc levels, namely only 3.5 and 4.2 µmol g−1 fresh weight for 35SPro:SWEET16 lines 8 and 14, respectively (Fig. 11A). At high nitrate, all three plant lines exhibited similar Glc levels of about 1.9 µmol g−1 fresh weight (Fig. 11A). Also in roots from SWEET16 overexpressor lines grown under limiting nitrate, Glc levels were markedly decreased when compared with wild-type tissues, while under high nitrate, no statistically significant differences between the three plant lines were detectable (Fig. 11A). Similar to Glc, the levels of Fru in shoots from SWEET16 overexpressor plants grown under limiting nitrate were strongly decreased when compared with wild-type tissues. Under the latter conditions, Fru in SWEET16 overexpressor shoots was below the detection level, while wild-type plants contained 2.4 µmol Fru g−1 (Fig. 11B). Under high nitrate, wild-type shoots contained only 0.22 µmol Fru g−1 fresh weight, SWEET16 overexpressor line 8 contained Fru below the detection level, and SWEET16 overexpressor line 14 contained only 0.04 µmol Fru g−1 fresh weight (Fig. 11B). In all three plant lines grown under either low or high nitrate, the Suc levels were similar, namely about 10 µmol g−1 fresh weight at low nitrate and about 1.2 µmol g−1 fresh weight at high nitrate (Fig. 11C). Similar to the situation in shoots, also in roots no significant changes in the Suc levels were detectable in the three plant lines depending on the nitrogen supply (Fig. 11C).
DISCUSSION
Dynamic storage of sugars in the plant vacuole is critical to adjust carbon homeostasis in phases of excess sugar availability or sugar depletion. Several proton-driven importers or exporters able to shuttle monosaccharides and disaccharides across the vacuolar membrane have been identified at the molecular level in the recent past (Wormit et al., 2006; Reinders et al., 2008; Wingenter et al., 2010; Eom et al., 2011; Schulz et al., 2011; Schneider et al., 2012). With the discovery of ERDL6 and SWEET17 in Arabidopsis, the first vacuolar carriers available for Glc and Fru export, respectively, have been identified (Poschet et al., 2011; Chardon et al., 2013). The closest homolog to SWEET17 in Arabidopsis is the carrier SWEET16 (Chen et al., 2010), but the subcellular localization of latter carrier is unknown, nor has its transport characteristics or impact on plant properties after overexpression been studied.
The SWEET16-GFP fusion protein locates exclusively to the vacuolar membrane from Arabidopsis mesophyll protoplasts (Fig. 1). This observation appears in line with the localization of the closest homolog, SWEET17, which has been described as a vacuolar Fru porter (Chardon et al., 2013). Hence, it seems that the two members of this SWEET family subclass locate differently from the 15 other SWEET members, which are proposed to reside in the plasma membrane (Chen et al., 2010). It has been shown in various plant species that the majority of leaf monosaccharides reside in the vacuolar lumen (Gerhardt et al., 1987; Winter et al., 1993, 1994; Szecowka et al., 2013). Thus, the nearly complete inability of both 35SPro:SWEET16 lines to accumulate Fru under various conditions (Figs. 6B and 11B) speaks further for a vacuolar localization of the authentic protein.
It is interesting that the sweet16 gene is mainly expressed, at a generally low level, in xylem parenchyma cells representing part of the vascular structures in leaves and flower stalks (Fig. 2). A similar limited gene expression has also been documented for the homologous gene sweet17 from Arabidopsis (Chardon et al., 2013) as well as for the vacuolar sugar exporter ESL1 (Yamada et al., 2010). In summary, these observations point to a novel, so far unresolved, function of xylem parenchyma cells in interorgan sugar transport, which is not easy to analyze because of the very small size and limited accessibility of these plant structures. However, it has also been demonstrated that sugar homeostasis in the xylem parenchyma is critical for the refilling of xylem vessels to repair gas embolism (Zwieniecki and Holbrook, 2009).
The recombinant SWEET16 synthesized in X. laevis oocytes transports Glc, Fru, and Suc with similar apparent affinities and comparable velocities (Fig. 4, A–C). This finding fully concurs with the observation that SWEET11 and SWEET14 from rice, and SWEET11 and SWEET12 from Arabidopsis, also transport disaccharides and monosaccharides (Chen et al., 2012). Obviously, some SWEET proteins accept substrates of significantly different molecular dimensions, representing a phenomenon that is unknown for the quite substrate-specific plasma membrane-located sugar transporters of the MST and SUT/SUC clades (Büttner and Sauer, 2000; Lalonde et al., 2004). Moreover, SWEET16 is able to catalyze a facilitated transport into and out of oocytes synthesizing the recombinant transporter protein (Fig. 4D), which concurs with our findings on the vacuolar Fru carrier SWEET17 (Chardon et al., 2013).
There is ample evidence that vacuolar sugar transport is tightly regulated on several levels. For example, the ERDL6 gene, coding for the first identified vacuolar Glc exporter, is repressed under conditions requiring Glc accumulation in the vacuole or increasingly expressed under conditions of Glc demand in the cytosol, such as wounding (Poschet et al., 2011), while the ESL1 gene, coding for a vacuole-located Glc facilitator, is mainly stress induced (Yamada et al., 2010). The activity of the main vacuolar monosaccharide importer TMT is even more complex regulated, since the TMT1 and TMT2 genes are under the control of sugars, salt, and temperature and both genes are cell type-specifically expressed (Wormit et al., 2006). Moreover, TMT activity is tuned by a recently identified protein kinase, VIK1, belonging to a subclade of the large MAP3K family of protein kinases (Wingenter et al., 2011). We show here that SWEET16 transports Glc, Fru, and Suc in the recombinant oocyte system (Fig. 4). However, the complex regulation of cellular sugar homeostasis on the levels of (1) transporter activity, (2) sugar synthesis, and (3) sugar degradation (see above) might explain why the overexpression of SWEET16 in the intact plant mainly affects Glc/Fru levels while the corresponding Suc levels are less affected (Figs. 5, B and C, and 6, A and B).
In line with these findings is the inhibitory function of exogenously applied sugars on SWEET16 expression (Fig. 3). Under the latter conditions, cytosolic sugar levels are already high, and a decrease of the SWEET16 mRNA concentration prevents further export of sugars into the cytosol. Such a comprehensive regulation of import and export of sugars across the vacuolar membrane makes perfect sense, since it has been demonstrated that altered activity of either sugar importers or sugar exporters in various species affects plant properties like intracellular sugar compartmentation, sugar sensing, germination efficiency, plant growth, and fruit yield (Wingenter et al., 2010; Eom et al., 2011; Poschet et al., 2011; Schneider et al., 2012).
Thus, given that a tight control of vacuolar sugar transport is obviously critical for plants to cope with different developmental or environmental constraints, it was tempting to analyze the consequences caused by a constitutively expressed SWEET16 gene. As generally expected for facilitators, like all members of the SWEET family analyzed so far (Chen et al., 2010, 2012; Chardon et al., 2013), the transport properties under the cellular in vivo condition depend inter alia upon the relative concentrations of all substrates on both sites of the corresponding membrane. That the complex metabolic scenario on both sites of the vacuolar membrane governs net fluxes mediated by SWEET16 is further supported by a closer analysis of metabolite changes in SWEET16 overexpression lines. For example, when grown in liquid culture or on soil (at the end of the day), both SWEET16 overexpression mutants contain less of both Glc and Fru than the corresponding wild-type plants, while Suc levels are similar in the three plant lines (Fig. 5, B and C). In contrast, at the end of the night, SWEET16 overexpression lines exhibited decreased levels of all three types of sugars when compared with wild-type plants (Fig. 5), whereas during cold adaptation, or when grown under limiting nitrogen supply, SWEET16 overexpression lines harbored less Glc and (especially) Fru, while Suc levels were barely affected when compared with wild-type plants (Figs. 6, A–C, and 11). As a consequence of this, the exact physiological impact of a modified activity of carriers like SWEET16 can vary from condition to condition tested, because the individual external stimuli influence the relative rates of sugar synthesis and degradation in the cytosol and in the vacuolar lumen. Moreover, we cannot exclude that SWEET16 is dependent upon the external stimulus posttranslationally modified, as shown for the vacuolar TMT1 protein (Wingenter et al., 2011), which might affect transport properties considerably when compared with the recombinant situation in the oocyte.
It was astonishing to see that the 35SPro:SWEET16 lines exhibit markedly improved tolerance against freezing temperatures, as revealed by quantification of the electrical conductivity caused by subzero temperatures (Fig. 7). Upon transfer into the cold, both 35SPro:SWEET16 lines accumulated nearly no Fru but showed increased levels of Glc and Suc when compared with the corresponding wild-type plants (Fig. 6). The additional presence of Glc and Suc in SWEET16-overexpressing lines under cold conditions roughly compensates for the osmotically missing Fru (Fig. 6) and obviously contributes strongly to the antifreezing ability of Arabidopsis. Detailed analysis by other groups made plain that the previously cold-protecting-classified sugar raffinose (Flinn and Ashworth, 1995; Cox and Stushnoff, 2001) acts, in fact, as a long-term cold protection metabolite and that Suc and hexoses represent the early-arising cold-protecting sugars (Zuther et al., 2004; Nägele and Heyer, 2013). Our data here give rise to the assumption that Glc and Suc exhibit superior freezing protection when compared with Fru (Figs. 6 and 7). Thus, it will be interesting to check for altered freezing tolerance in other Arabidopsis mutants exhibiting modified vacuolar Suc (e.g. SUT4 overexpression lines) and/or Glc (e.g. ERDL6 overexpression lines) levels. Given that SWEET16 gene expression is cold repressed (Fig. 3A), we propose that a reduction of SWEET16 activity contributes to an accumulation of sugars in the leaf during this stress condition.
Both 35SPro:SWEET16 lines showed improved germination efficiency (Fig. 8). It is well known that high sugar concentrations arrest plant development, especially during early seed germination (Dekkers et al., 2004, 2008), and that this process is due to sugar repression initiated by cytosolically located hexokinase isoforms (Jang et al., 1997; Rolland et al., 2002; Granot et al., 2013). Thus, we explain the improved germination efficiency and growth of 35SPro:SWEET16 plants in response to high sugar supply by the subcellular location of SWEET16 in the vacuolar membrane (Fig. 1) and its ability for Glc, Fru, and Suc transport (Fig. 4). During feeding of external sugars, the corresponding cytosolic levels will consequently increase, since plasma membrane-located sugar porters actively pump the external sugars into the cell (Büttner and Sauer, 2000). Obviously, in the case of wild-type cells, the subsequent transport of sugars from the cytosol into the vacuole is limited, while in SWEET16 overexpression lines, the presence of a facilitator, able to transport all three types of sugars, increases the fluxes from the cytosolic compartment into the large vacuole, occupying up to 85% of the cell volume (Neuhaus, 2007). We previously documented that the removal of cytosolic sugars into the vacuole is limited by sugar transport activity at the tonoplast (Wingenter et al., 2010). We assume that increased SWEET16 activity facilitates the transfer of cytosolic sugars into the vacuole, which prevents the sugar-induced synthesis of abscisic acid (Arenas-Huertero et al., 2000), known to be an inhibitor of seed germination (Shan et al., 2012).
We observed, similar to the increased germination efficiency, that both 35SPro:SWEET16 lines grew more efficiently on both soil and sand (Figs. 9 and 10). However, on sand, an improved growth of 35SPro:SWEET16 mutants is only obvious in the presence of high nitrogen supply (Fig. 10). This observation points to a tight control of the intracellular sugar compartmentation in response to altered nitrogen availability and an improved nitrogen use efficiency of SWEET16 overexpressor lines under sufficient (Fig. 9) or high (Fig. 10) nitrate. Plants grown at sufficient or high nitrogen availability use most of the photosynthetically synthesized sugars for anabolic processes, leading to fast growth, while under conditions of limiting nitrogen, growth stops and, as a consequence, sugars accumulate (Coruzzi and Bush, 2001; Coruzzi and Zhou, 2001; Krapp and Truong, 2005; Krapp et al., 2011). We propose that under sufficient or high nitrogen availability (e.g. on soil or at 5 mm nitrate), increased SWEET16 activity drains parts of the vacuolar sugars permanently into the cytosol, where, after phosphorylation, they serve as carbon acceptors for reduced nitrogen. This assumption is based on the observations that SWEET16 overexpressors show fewer monosaccharides and disaccharides on soil when compared with the wild type (Fig. 5C) and less Fru in shoots and in roots when grown under high nitrogen (Fig. 11B). A general aim of plant breeders, among others, is the improvement of nitrogen use efficiency (Hirel et al., 2007; McAllister et al., 2012). Since both 35SPro:SWEET16 lines exhibit increased shoot and root biomasses under conditions of soil growth or high nitrogen supply (Figs. 9 and 10), corresponding modifications in crop plants seem attractive.
Under limiting nitrogen, wild-type Arabidopsis accumulates Glc, Fru, and Suc in shoots when compared with growth at high nitrogen availability (Fig. 11), while in contrast, SWEET16 overexpressor lines exhibit significantly fewer monosaccharides than the corresponding wild-type plants (Fig. 11, A and B). The fact that high cytosolic monosaccharide levels are sensed and induce the down-regulation of genes required for anabolism (Koch, 1996; Smeekens and Rook, 1997; Rolland et al., 2002) explains why increased SWEET16 activity under these conditions is inhibitory on developmental processes. Such an accumulation of sugars in the cytosol under low nitrogen in SWEET16 overexpressor lines is likely, because the additional carbon cannot be used for growth, as amino acid synthesis is inefficient under these conditions. The fact that SWEET16 expression is low under limiting nitrate but high under high nitrate (Fig. 3B) is thus fully in line with these observations, because sugars themselves have been identified as inhibitors of SWEET16 expression (Fig. 3A).
We discussed above that vacuolar sugar import and export are highly regulated at the levels of gene expression, stress stimuli, and posttranslational modifications. Having seen that the overexpression of SWEET16 improves traits like freezing tolerance (Fig. 7), germination efficiency (Fig. 8), and growth on soil and sand (Figs. 9 and 10) led us to question why a plant like Arabidopsis does not permanently exhibit higher activities of this vacuolar carrier. However, under limiting nitrogen availability, the additional activity of SWEET16 exerts strong negative effects on plant growth (Fig. 10). This observation per se makes clear that a constitutively increased activity of SWEET16 is not of general benefit for plant growth.
We cannot exclude that overexpression of the SWEET16 gene in wild-type plants causes cosuppression in cells specifically expressing the endogenous SWEET16 gene. However, given the significant alterations of sugar levels in SWEET16 overexpression lines (Figs. 5 and 6) and the influence of SWEET16 overexpression on processes like seed germination, freezing tolerance, and nitrogen use efficiency (Figs. 7–9), it seems justified to assume that the increased carrier activity in various types of cells is responsible for the multiple effects on plant performance.
In conclusion, our results identify SWEET16 as a vacuolar sugar facilitator, demonstrate the substantial impact of SWEET16 overexpression on various critical plant traits, and imply that SWEET16 activity must be tightly regulated to allow the optimal development of Arabidopsis under favorable and nonfavorable conditions. Thus, a thorough analysis of the properties of SWEET16 mutants and overexpressor lines under even more growth and cultivation conditions than used here is required to further understand why vacuolar sugar transport is so tightly regulated.
MATERIALS AND METHODS
Plant Material
Wild-type and mutant Arabidopsis (Arabidopsis thaliana) plants were grown in a growth chamber on standard soil (ED-73; Patzer; www.einheitserde.de) at 21°C (day and night), and light was present at 125 µmol quanta m−2 s−1 under a 10/14-h day/night regime (standard growth conditions). Growth on sand was done according to (Chardon et al., 2010) under the same light and temperature regime.
Alternatively, plants were cultivated under short-day conditions in liquid culture according to a protocol suitable for fresh weight determination and feeding of effector molecules (Scheible et al., 2004). The seedlings were grown for 14 d in standard liquid medium, harvested, washed, and then used for metabolite quantification. For germination experiments as well as for liquid culture, seeds were surface sterilized in 5% sodium hypochloride and incubated for 24 h in the dark at 4°C for stratification. Germination efficiency was determined on 1/2 MS (pH 5.7, KOH) plates supplemented with or without sugars from triplicate assays with 20 seeds per line. Generally, for RNA isolation and metabolite quantification, plant tissues were collected and immediately frozen in liquid nitrogen until use. Sugar extraction from Arabidopsis tissue and spectroscopic quantification were performed as described earlier (Quick et al., 1989).
Generation of SWEET16 Overexpression Lines
For the generation of SWEET16 overexpression lines, the SWEET16-encoding sequence (inserted into pBSK) was excised using XbaI and XhoI and inserted into the correspondingly prepared original pHannibal vector. Then, the SWEET16 construct was cut by NotI and inserted into the correspondingly prepared original pART27 vector. The correctness of all constructs was proven by complete sequencing.
Transformations of Arabidopsis wild-type plants (Columbia) were performed by the floral dip method (Clough and Bent, 1998) using the Agrobacterium tumefaciens strain GV3101 harboring the overexpression construct of SWEET16. This resulted in 20 strong SWEET16 overexpression lines, of which lines 8 and 14 were identified as the strongest via northern-blot analysis (Supplemental Fig. S1 and Supplemental Materials and Methods S1).
Xenopus laevis Oocytes, Injection, Uptake, and Efflux Studies
The preparation and injection of X. laevis oocytes were performed as described (Bröer et al., 1999). Copy RNA (cRNA) synthesis of SWEET16 was carried out using the mMESSAGE mMACHINE T7 Ultra Kit (Ambion Life Technologies) according to the manufacturer’s advice. cRNA was injected at 25 ng per 27.6 µL of SWEET16 or RNase-free water (as a control) per oocyte. For uptake studies, eight to 10 injected oocytes were incubated for 1 h in 200 µL of oocyte Ringer buffer medium (OR2+; pH 7.0) supplemented with the given concentrations of sugar including 5 µCi mL−1 14C-labeled sugar. After 1 h, uptake was stopped, oocytes were washed four times with 4 mL of ice-cold OR2+, and single oocytes were transferred in scintillation vials. Each oocyte was lysed with 100 µL of 10% (w/v) SDS for 1 h, and radioactivity was quantified in a scintillation counter. For efflux studies, 3 d after injection of SWEET16 cRNA or water (control), oocytes were preloaded with 50 nL of 100 mm Glc, including 0.2 µCi µL [14C]Glc, and each batch, consisting of three oocytes, was placed in 2 mL of OR2+ (pH 7.0). After 45, 90, and 135 min, 100 µL of buffer medium was sampled and radioactivity was quantified in a scintillation counter.
Subcellular Localization of SWEET16
To analyze the subcellular localization of SWEET16 in Arabidopsis, we generated a SWEET16-GFP fusion construct using the Gateway-specific destination vector pK7FWG2.0 (Karimi et al., 2002). Therefore, the cDNA of SWEET16 was amplified and the stop codon was removed via PCR using SWEET16-specific primers SWEET16_gw_fwd (5′-ggggacaagtttgtacaaaaaagcaggcttaATGGCAGACTTGAGTTTT-3′) and SWEET16_gw_rev-stop (5′-ggggaccactttgtacaagaaagctgggtaTGCAGCGAGGAGAGGTTG-3′), harboring the attB1 and attB2 sites (lower case portions of the sequence), then cloned via BP reaction into pDONRZEO (Invitrogen) followed by an LR reaction into pK7FWG2.0. Transformation of Arabidopsis mesophyll protoplast was performed as described (Yoo et al., 2007). A Leica TCS SP5 II confocal laser-scanning microscope (http://www.leica-microsystems.com) was used for imaging. All images were taken using a Leica HCX PL APO 63·/1.20w motCORR CS objective. The laser used was VIS-Argon for GFP constructs (488 nm/500–535 nm).
Cloning of SWEET16Pro:GUS and Histochemical GUS Staining
The cloning of SWEET16Pro:GUS and histochemical GUS staining were performed as described earlier (Chardon et al., 2013). The 1,295-bp fragment of the SWEET16 promoter was amplified from genomic DNA with the following primers: SWEET16_pro_fwd (5′-caccTGATTACAACATTACAACATTCAGTG-3′) and SWEET16_pro_rev (5′-CTCTGAGGATGGGTTTCTGAG-3′).
Quantitative Real-Time PCR
RNA of the wild type and the two overexpression lines was extracted from frozen plant leaves or leaf discs using the NucleoSpin RNA Plant Kit (Macherey-Nagel), and the iScript cDNA Synthesis Kit (Bio-Rad) was used for the synthesis of cDNA according to the manufacturer’s instructions. Quantitative real-time PCR was accomplished according to Wormit et al. (2006). Primers for the quantification of SWEET16 were SWEET16_RT_fwd (5′-GAGATGCAAACTCGCGTTCTAGT-3′) and SWEET16_RT_rev (5′-GCACACTTCTCGTCGTCACA-3′; Chen et al., 2010). For transcript normalization, protein phosphatase 2A (At1g13320) was used as a reference gene (Czechowski et al., 2005).
Electrical Conductivity
The electrical conductivity of the wild type and SWEET16-overexpressing lines was assessed as described (Ristic and Ashworth, 1993) with minor modifications as follows. Three-week-old plants were acclimated to cold (4°C) for 4 d, and fully expanded leaves were taken from 10 plants and placed in glass tubes (one leaf per tube) containing 2 mL of deionized water. The tubes were transferred to a freezing bath set at 2°C for 1 h followed by a temperature decrease at a rate of 2°C h−1 to −6°C. Freezing of the water around the leaf tissue was initiated at −2°C by the addition of ice chips to each tube. After the last step, tubes were taken from the bath and thawed overnight in a cold room (4°C) with gentle shaking. After thawing, 2 mL of deionized water was added to the tubes and gently shaken for 1 h at room temperature. The electrical conductivity was measured at room temperature using a conductivity meter (WTW LF521; Wissenschaftlich-Technische Werkstätten). Samples were then boiled for 2 h and shaken overnight at 4°C, and the electrical conductivity was measured again.
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under accession number At3g16690 (AtSWEET16).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Northern-blot analysis of SWEET16 overexpression lines.
Supplemental Materials and Methods S1. Northern-blot analysis clarification.
Acknowledgments
We thank Nelly Wolf for excellent technical assistance.
Glossary
- TMT
tonoplast monosaccharide transporter
- cDNA
complementary DNA
- 1/2 MS
one-half-strength Murashige and Skoog
- cRNA
copy RNA
- OR2+
oocyte Ringer buffer medium
References
- Alberdi M, Corcuera LJ. (1991) Cold-acclimation in plants. Phytochemistry 30: 3177–3184 [Google Scholar]
- Aluri S, Büttner M. (2007) Identification and functional expression of the Arabidopsis thaliana vacuolar glucose transporter 1 and its role in seed germination and flowering. Proc Natl Acad Sci USA 104: 2537–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, León P. (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14: 2085–2096 [PMC free article] [PubMed] [Google Scholar]
- Bröer S, Bröer A, Schneider HP, Stegen C, Halestrap AP, Deitmer JW. (1999) Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J 341: 529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büttner M, Sauer N. (2000) Monosaccharide transporters in plants: structure, function and physiology. Biochim Biophys Acta 1465: 263–274 [DOI] [PubMed] [Google Scholar]
- Chardon F, Barthélémy J, Daniel-Vedele F, Masclaux-Daubresse C. (2010) Natural variation of nitrate uptake and nitrogen use efficiency in Arabidopsis thaliana cultivated with limiting and ample nitrogen supply. J Exp Bot 61: 2293–2302 [DOI] [PubMed] [Google Scholar]
- Chardon F, Bedu M, Calenge F, Klemens PA, Spinner L, Clement G, Chietera G, Léran S, Ferrand M, Lacombe B, et al. (2013) Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Curr Biol 23: 697–702 [DOI] [PubMed] [Google Scholar]
- Chen LQ, Hou BH, Lalonde S, Takanaga H, Hartung ML, Qu XQ, Guo WJ, Kim JG, Underwood W, Chaudhuri B, et al. (2010) Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 468: 527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LQ, Qu XQ, Hou BH, Sosso D, Osorio S, Fernie AR, Frommer WB. (2012) Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 335: 207–211 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Coruzzi G, Bush DR. (2001) Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol 125: 61–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi GM, Zhou L. (2001) Carbon and nitrogen sensing and signaling in plants: emerging ‘matrix effects.’ Curr Opin Plant Biol 4: 247–253 [DOI] [PubMed] [Google Scholar]
- Cox SE, Stushnoff C. (2001) Temperature-related shifts in soluble carbohydrate content during dormancy and cold acclimation in Populus tremuloides. Can J Res 31: 730–737 [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers BJ, Schuurmans JA, Smeekens SC. (2004) Glucose delays seed germination in Arabidopsis thaliana. Planta 218: 579–588 [DOI] [PubMed] [Google Scholar]
- Dekkers BJ, Schuurmans JA, Smeekens SC. (2008) Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Mol Biol 67: 151–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbrosses-Fonrouge AG, Voigt K, Schröder A, Arrivault S, Thomine S, Krämer U. (2005) Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS Lett 579: 4165–4174 [DOI] [PubMed] [Google Scholar]
- Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, Keller F, Baginsky S, Martinoia E, Schmidt UG. (2006) Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol 141: 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom JS, Cho JI, Reinders A, Lee SW, Yoo Y, Tuan PQ, Choi SB, Bang G, Park YI, Cho MH, et al. (2011) Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol 157: 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettke J, Eckermann N, Tiessen A, Geigenberger P, Steup M. (2005) Identification, subcellular localization and biochemical characterization of water-soluble heteroglycans (SHG) in leaves of Arabidopsis thaliana L.: distinct SHG reside in the cytosol and in the apoplast. Plant J 43: 568–585 [DOI] [PubMed] [Google Scholar]
- Flinn CL, Ashworth EN. (1995) The relationship between carbohydrates and flower bud hardiness among three forsythia taxa. J Am Soc Hortic Sci 120: 607–613 [Google Scholar]
- Gerhardt R, Stitt MN, Heldt HW. (1987) Subcellular metabolite levels in spinach leaves: regulation of sucrose synthesis during diurnal alterations in photosynthesis. Plant Physiol 83: 339–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz HP. (1987) Accumulation of sucrose into vacuoles released from isolated sugar beet root protoplasts by both, direct sucrose uptake and UDP-glucose-dependent group translocation. Plant Physiol Biochem 25: 573–580 [Google Scholar]
- Getz HP, Klein M. (1995) Characteristics of sucrose transport and sucrose-induced H+ transport on the tonoplast of red beet (Beta vulgaris L.) storage tissue. Plant Physiol 107: 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot D, David-Schwartz R, Kelly G. (2013) Hexose kinases and their role in sugar-sensing and plant development. Front Plant Sci 4: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah MA, Heyer AG, Hincha DK. (2005) A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genet 1: e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirel B, Le Gouis J, Ney B, Gallais A. (2007) The challenge of improving nitrogen use efficiency in crop plants: towards a more central role for genetic variability and quantitative genetics within integrated approaches. J Exp Bot 58: 2369–2387 [DOI] [PubMed] [Google Scholar]
- Jang JC, León P, Zhou L, Sheen J. (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John P (1992) Biosynthesis of the Major Crop Products. John Wiley & Sons, Chichester, UK [Google Scholar]
- Kaiser G, Heber U. (1984) Sucrose transport into vacuoles isolated from barley mesophyll protoplasts. Planta 161: 562–568 [DOI] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. (2002) Gateway vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Koch KE. (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 509–540 [DOI] [PubMed] [Google Scholar]
- Kovermann P, Meyer S, Hörtensteiner S, Picco C, Scholz-Starke J, Ravera S, Lee Y, Martinoia E. (2007) The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J 52: 1169–1180 [DOI] [PubMed] [Google Scholar]
- Krapp A, Berthomé R, Orsel M, Mercey-Boutet S, Yu A, Castaings L, Elftieh S, Major H, Renou JP, Daniel-Vedele F. (2011) Arabidopsis roots and shoots show distinct temporal adaptation patterns toward nitrogen starvation. Plant Physiol 157: 1255–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Truong HN (2005) Regulation of C/N interaction in model plant species. In S Goyal, R Tischner, A Basra, eds, Enhancing the Efficiency of Nitrogen Utilization in Plants. Haworth, New York, pp 127–173 [Google Scholar]
- Lalonde S, Wipf D, Frommer WB. (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55: 341–372 [DOI] [PubMed] [Google Scholar]
- Marger MD, Saier MH., Jr (1993) A major superfamily of transmembrane facilitators that catalyse uniport, symport and antiport. Trends Biochem Sci 18: 13–20 [DOI] [PubMed] [Google Scholar]
- Martinoia E, Maeshima M, Neuhaus HE. (2007) Vacuolar transporters and their essential role in plant metabolism. J Exp Bot 58: 83–102 [DOI] [PubMed] [Google Scholar]
- Martinoia E, Meyer S, De Angeli A, Nagy R. (2012) Vacuolar transporters in their physiological context. Annu Rev Plant Biol 63: 183–213 [DOI] [PubMed] [Google Scholar]
- McAllister CH, Beatty PH, Good AG. (2012) Engineering nitrogen use efficient crop plants: the current status. Plant Biotechnol J 10: 1011–1025 [DOI] [PubMed] [Google Scholar]
- Nägele T, Henkel S, Hörmiller I, Sauter T, Sawodny O, Ederer M, Heyer AG. (2010) Mathematical modeling of the central carbohydrate metabolism in Arabidopsis reveals a substantial regulatory influence of vacuolar invertase on whole plant carbon metabolism. Plant Physiol 153: 260–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nägele T, Heyer AG. (2013) Approximating subcellular organisation of carbohydrate metabolism during cold acclimation in different natural accessions of Arabidopsis thaliana. New Phytol 198: 777–787 [DOI] [PubMed] [Google Scholar]
- Neuhaus HE. (2007) Transport of primary metabolites across the plant vacuolar membrane. FEBS Lett 581: 2223–2226 [DOI] [PubMed] [Google Scholar]
- Payyavula RS, Tay KH, Tsai CJ, Harding SA. (2011) The sucrose transporter family in Populus: the importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. Plant J 65: 757–770 [DOI] [PubMed] [Google Scholar]
- Poschet G, Hannich B, Raab S, Jungkunz I, Klemens PA, Krueger S, Wic S, Neuhaus HE, Büttner M. (2011) A novel Arabidopsis vacuolar glucose exporter is involved in cellular sugar homeostasis and affects the composition of seed storage compounds. Plant Physiol 157: 1664–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick WP, Siegl G, Neuhaus HE, Feil R, Stitt M. (1989) Short-term water stress leads to a stimulation of sucrose synthesis by activating sucrose-phosphate synthase. Planta 177: 535–546 [DOI] [PubMed] [Google Scholar]
- Reinders A, Sivitz AB, Starker CG, Gantt JS, Ward JM. (2008) Functional analysis of LjSUT4, a vacuolar sucrose transporter from Lotus japonicus. Plant Mol Biol 68: 289–299 [DOI] [PubMed] [Google Scholar]
- Ristic Z, Ashworth EN. (1993) Ultrastructural evidence that intracellular ice formation and possibly cavitation are the sources of freezing injury in supercooling wood tissue of Cornus florida L. Plant Physiol 103: 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch T, González MC. (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9: 606–613 [DOI] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J. (2002) Sugar sensing and signaling in plants. Plant Cell (Suppl) 14: S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M. (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136: 2483–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Hulpke S, Schulz A, Yaron I, Höll J, Imlau A, Schmitt B, Batz S, Wolf S, Hedrich R, et al. (2012) Vacuoles release sucrose via tonoplast-localised SUC4-type transporters. Plant Biol (Stuttg) 14: 325–336 [DOI] [PubMed] [Google Scholar]
- Schulz A, Beyhl D, Marten I, Wormit A, Neuhaus E, Poschet G, Büttner M, Schneider S, Sauer N, Hedrich R. (2011) Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J 68: 129–136 [DOI] [PubMed] [Google Scholar]
- Schulze W, Schneider T, Starck S, Martinoia E, Trentmann O. (2012) Cold acclimation induces changes in Arabidopsis tonoplast protein abundance and activity and alters phosphorylation of tonoplast monosaccharide transporters. Plant J 69: 529–541 [DOI] [PubMed] [Google Scholar]
- Schumacher K, Krebs M. (2010) The V-ATPase: small cargo, large effects. Curr Opin Plant Biol 13: 724–730 [DOI] [PubMed] [Google Scholar]
- Shan X, Yan J, Xie D. (2012) Comparison of phytohormone signaling mechanisms. Curr Opin Plant Biol 15: 84–91 [DOI] [PubMed] [Google Scholar]
- Smeekens S, Rook F. (1997) Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiol 115: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szecowka M, Heise R, Tohge T, Nunes-Nesi A, Vosloh D, Huege J, Feil R, Lunn J, Nikoloski Z, Stitt M, et al. (2013) Metabolic fluxes in an illuminated Arabidopsis rosette. Plant Cell 25: 694–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R, Wolf S. (2009) Phloem transport: cellular pathways and molecular trafficking. Annu Rev Plant Biol 60: 207–221 [DOI] [PubMed] [Google Scholar]
- Willenbrink J, Doll S. (1979) Characteristics of the sucrose uptake system of vacuoles isolated from red beet tissue: kinetics and specificity of the sucrose uptake system. Planta 157: 159–162 [DOI] [PubMed] [Google Scholar]
- Wingenter K, Schulz A, Wormit A, Wic S, Trentmann O, Hoermiller II, Heyer AG, Marten I, Hedrich R, Neuhaus HE. (2010) Increased activity of the vacuolar monosaccharide transporter TMT1 alters cellular sugar partitioning, sugar signaling, and seed yield in Arabidopsis. Plant Physiol 154: 665–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenter K, Trentmann O, Winschuh I, Hörmiller II, Heyer AG, Reinders J, Schulz A, Geiger D, Hedrich R, Neuhaus HE. (2011) A member of the mitogen-activated protein 3-kinase family is involved in the regulation of plant vacuolar glucose uptake. Plant J 68: 890–900 [DOI] [PubMed] [Google Scholar]
- Winter H, Robinson DG, Heldt HW. (1993) Subcellular volumes and metabolite concentrations in barley leaves. Planta 191: 180–190 [Google Scholar]
- Winter H, Robinson DG, Heldt HW. (1994) Subcellular volumes and metabolite concentrations in spinach leaves. Planta 193: 530–535 [Google Scholar]
- Wormit A, Trentmann O, Feifer I, Lohr C, Tjaden J, Meyer S, Schmidt U, Martinoia E, Neuhaus HE. (2006) Molecular identification and physiological characterization of a novel monosaccharide transporter from Arabidopsis involved in vacuolar sugar transport. Plant Cell 18: 3476–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Osakabe Y, Mizoi J, Nakashima K, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K. (2010) Functional analysis of an Arabidopsis thaliana abiotic stress-inducible facilitated diffusion transporter for monosaccharides. J Biol Chem 285: 1138–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SD, Cho YH, Sheen J. (2007) Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zuther E, Büchel K, Hundertmark M, Stitt M, Hincha DK, Heyer AG. (2004) The role of raffinose in the cold acclimation response of Arabidopsis thaliana. FEBS Lett 576: 169–173 [DOI] [PubMed] [Google Scholar]
- Zwieniecki MA, Holbrook NM. (2009) Confronting Maxwell’s demon: biophysics of xylem embolism repair. Trends Plant Sci 14: 530–534 [DOI] [PubMed] [Google Scholar]