Abstract
Subdural intracranial empyemas and brain abscesses are a rare complication of bacterial sinusitis. Pediatric parafalcine abscesses are a rare entity with different treatment compared with other brain abscesses. We present two pediatric cases with falcine abscess as a sinusitis complication and introduce our department’s treatment management. In addition a review of literature is performed. Surgical cases of our department and their management are compared with the current literature. In our cases, both of the children showed a recurrent empyema after the first surgical treatment and antibiotic therapy. A second surgical evacuation was necessary. The antibiotic therapy was given for 3 months. Short-time follow-up imaging is necessary irrespective of infection parameters in blood and patient's clinical condition. Especially in parafalcine abscesses a second look may be an option and surgical treatment with evacuation of pus is the treatment of choice if abscess remnants are visualized.
INTRODUCTION
Parafalcine and subdural empyemas generally are life-threatening diseases that require an emergent treatment [1]. They can be caused by trauma, neurosurgical procedures, hematogenious [2] and contiguous spreading of an infection [3]. The mortality depends on the time of diagnosis and is ∼10%. Around 50% of the patients show neurological deficits and seizures [4]. The symptoms are no't specific. The patients appear with headache, fever, nausea, seizures and neurological deficits [5]. For the diagnosis of subdural empyema, contrast-enhanced CT scan or better MRI are gold-standard diagnostic methods [6].
Bacterial parafalcine empyemas rarely obey as a complication of bacterial sinusitis [7]. In our study, we present two cases of children developing a parafalcine empyema as a consequence of a frontal sinusitis diagnosed by an ENT.
First we started the review with searching PubMed for articles referring to ‘brain abscesses in children’' (2056) and continued our quest with ‘interhemispheric abscesses in children' (13). Another path was looking for subdural empyemas in children (331 hits) and at last we found four articles concerning to parafalcine subdural empyemas in children.
Additionally, we describe two cases of parafalcine abscesses in children as a complication of a sinusitis frontalis treated in our own department. The patients’ charts and images were retrospectively evaluated.
The cases are presented and the literature is reviewed. Management strategies are discussed.
ILLUSTRATIVE CASE 1
The first case is a 12-year-old boy who came to our department with a diagnosed sinusitis frontalis. Irrespective of the initiated antibiotic therapy, he developed a hemiparesis on the right side and worsening headache. An MRI scan of the brain (Fig. 1A) showed a parafalcine subdural empyema frontoparietal with a left side compression of the brain. The abscess was drained by a left parasagittal approach with neuronavigation. After draining the abscess the brain did not extend sufficiently, so we decided to put in a drainage for 2days to drain potential abscess material and to avoid a relapse of the subdural empyema. We expanded the antibiotic therapy from a single therapy to a triple therapy with amoxicillin, metronidazole and cefotaxim despite the absence of bacteria in growth culture. In spite of surgery and the antibiotic therapy, the patient's condition got worse with nausea, emesis and fever. An EEG showed pathological alterations and typical epileptical activity, convulsions were observed as well and a CT scan (Fig. 1B) 4days after surgery featured a progress of the abscess. Owing to the clinical aggravation and the increase of the abscess size, we decided to perform a second surgery. Intraoperatively, we found an encapsulated conglomerate of pus. We removed the capsule mostly and evacuated the viscous pus. Afterwards, we irrigated the abscess cavity with isotonic saline solution and put in a drainage. After second surgery the patient improved significantly. The antibiotic therapy was given for 3months under regularly control of infection parameters once a week and the symptomatic epilepsy was treated with levetiracetam. Subsequently, an MRI was performed at the day of discharge (Fig. 1C), which showed a regression of the abscess and another MRI 3months after the second surgery showed no further abscess. Until then the patient was seizure free.
Figure 1:
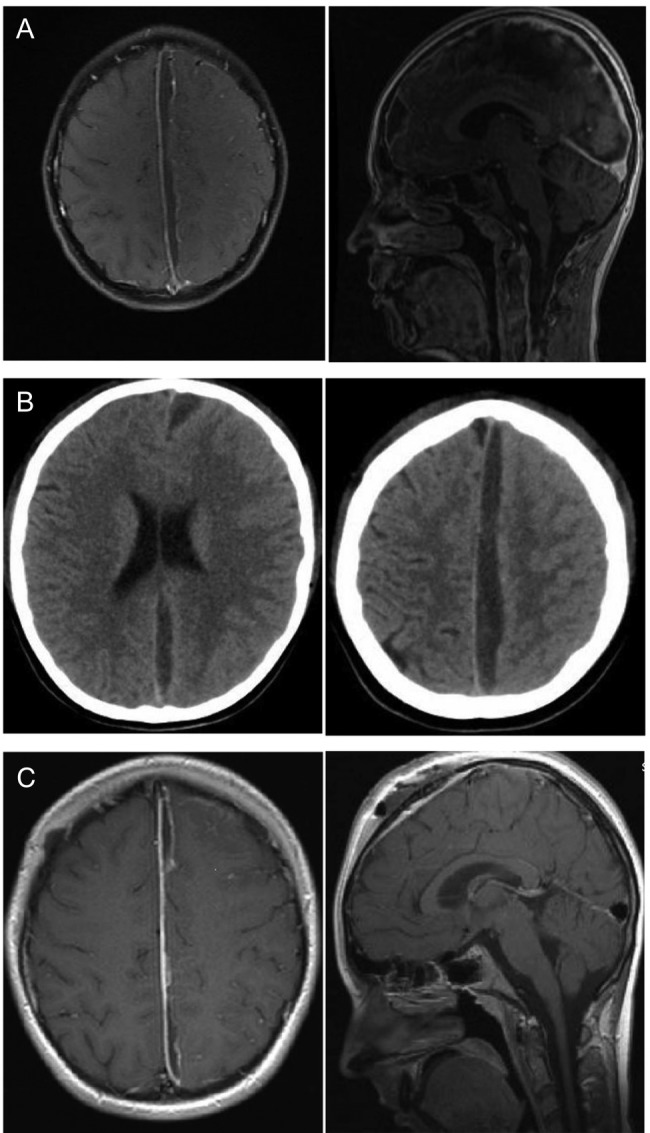
Twelve-year-old boy with parafalcine subdural empyema. (A) Axial and saggital MRI section with administration of gadolinium. A parafalcine subdural empyema frontoparietal with left-side compression of the brain. It is seen a diffuse edema of the left hemisphere. (B) Axial native CT section. An enlargement of the empyema is visible 4days after first surgery with a diffuse edema of the left hemisphere without a significant midline shift. (C) Axial and sagittal MRI section with administration of gadolinium. Regression of the empyema is visible after the second surgery. A regressive edema of the left hemisphere is visible, especially the frontal abscess conglomerate is reduced.
ILLUSTRATIVE CASE 2
An 8-year-old girl with a diagnosed sinusitis frontalis, already under antibiotic therapy, developed progressive headaches and increasing infection parameters. The brain MRI showed a subdural frontal parafalcine empyema (Fig. 2A). The girl's symptoms were nausea, emesis, headache and fever but no neurological disturbance. We drained the empyema by a right frontal craniotomy and clear the abscess cavity with isotonic saline solution. The brain extended rapidly after removing the space-occupying pus. Microbiologically, streptococcus intermedius infection was verified. The antibiotic therapy was optimized after resistogram. After surgery, the girl's condition got worse with seizures and fever. In a repeated MRI (Fig. 2B), the subdural empyema showed an enlargement. We decided to revise and extend the approach to remove the capsule of the empyema which could be seen perioperatively. The empyema was released by an osteoplastic right parasagittal trepanation.
Figure 2:
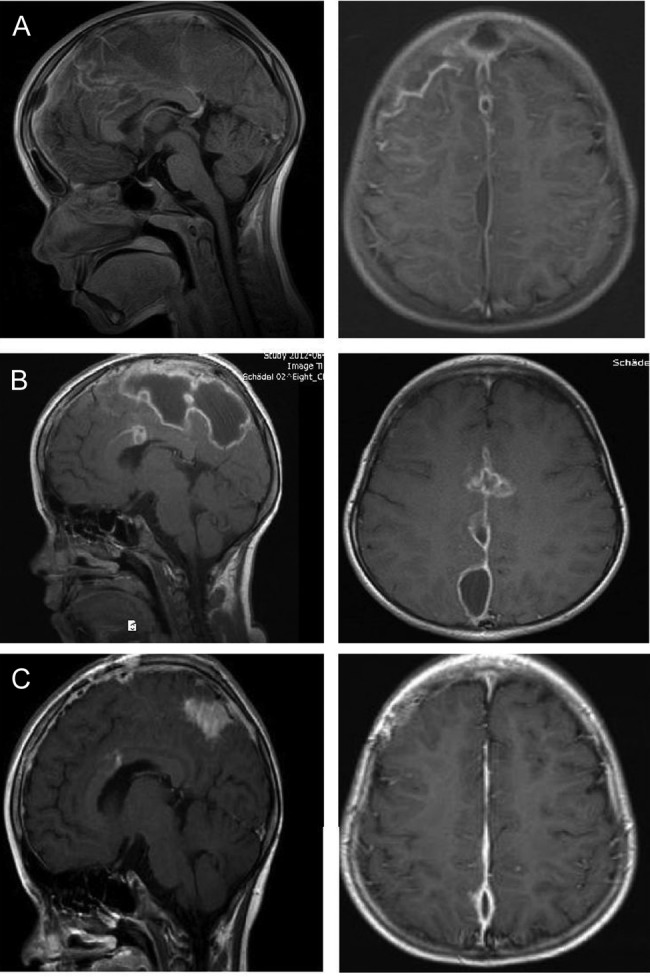
Eight-year-old girl with parafalcine subdural empyema. (A) Axial and sagittal MRI section with administration of gadolinium. Space occupying empyema with the largest expansion right frontal and contact to the falx. Perifocal edema without a significant midline shift is seen. (B) Axial and sagittal MRI section with administration of gadolinium. An enlargement of the empyema is visible after first surgery. The empyema is localized in central parasagittal region with a significant space-occupying component with a compression of the right central region. (C) Axial and sagittal MRI section with administration of gadolinium. A regression is visible after second surgery. In comparison to preoperative MRI, a notable decrease in the space-occupying part of the empyema is seen.
The symptomatic epilepsy was treated with levetiracetam. Afterwards, the girl stayed for another week in our department. At the day of discharge, an MRI (Fig. 2C) was repeated and showed a regressive parafalcine abscess. The infection parameters decrease steadily under antibiotics and no seizures were noticed anymore. An MRI was performed 3 months after second surgery. After the last MRI, the antibiotic and the antiepileptic therapy were stopped.
DISCUSSION
The review showed that especially parafalcine subdural empyemas are infrequently described in PubMed. We found only four articles concerning parafalcine subdural empyemas in children. For example, Banerjee et al. described 65 pediatric patients with supratentorial subdural empyemas and recommend an early surgical drainage, preferably craniotomy, eradication of pus and sensitive broad-spectrum antibiotics [1]. Pathak et al. described 41 cases with subdural empyemas from 1977 until 1988, only four of patients had parafalcine collection [8].
Our both children had parafalcine empyemas as a complication of a sinusitis frontalis. In spite of antibiotic therapy and surgery, the empyemas in both patients showed an enlargement after a few days after the first craniotomy.
The symptoms of parafalcine empyemas can vary from unspecific symptoms like fever and headache to neurological symptoms like hemiparesis, impairment of consciousness until seizures. The most pediatric brain abscesses generally occur with headache (48%), fever (48%), nausea–vomiting (36%) and seizures (29.3%) [9]. The neurological symptoms like cranial nerve palsy and hemiparesis of course depends on the localization of the lesion.
The results of our case series showed that parafalcine empyemas seem to be a rare complication with high mortality if not treated quickly. In both cases, sinusitis frontalis seemed to be the infection focus. After the first surgery with drainage of the abscess, the symptoms reappeared and the abscess increased as seen in the follow-up CT and MRI. A second surgery, despite sufficient antibiotic treatment was indicated. It is important in pediatric cases with parafalcine empyemas to perform a follow-up CT or MRI 4–7 days after the surgical evacuation. Since both children showed pathological activity in EEG, we suggest an EEG 4–7 days post-surgery.
The treatment of choice should be a combination of surgery and antibiotic therapy and if it is necessary antiepileptic therapy too. Optimal and fast microbiological probe acquisition and communication with the laboratory is extremely important. For early diagnosis, a contrast-enhanced CT scan and a non-enhanced scan are essential, but the procedure of choice is an MRI with contrast agent.
REFERENCES
- 1.Banerjee AD, Pandey P, Devi BI, Sampath S, Chandramouli BA, et al. Pediatric supratentorial subdural empyemas: a retrospective analysis of 65 cases. Pediatr Neurosurg. 2009;45:11–8. doi: 10.1159/000202619. doi:10.1159/000202619. [DOI] [PubMed] [Google Scholar]
- 2.Salunke PS, Malik V, Kovai P, Mukherjee KK. Falcotentorial subdural empyema: analysis of 10 cases. Acta Neurochir (Wien) 2011;153:164–9. doi: 10.1007/s00701-010-0695-5. discussion 170 doi:10.1007/s00701-010-0695-5. [DOI] [PubMed] [Google Scholar]
- 3.Ak HE, Ozkan U, Devecioglu C, Kemaloglu MS. Treatment of subdural empyema by burr hole. Isr J Med Sci. 1996;32:542–4. [PubMed] [Google Scholar]
- 4.Dill SR, Cobbs CG, McDonald CK. Subdural empyema: analysis of 32 cases and review. Clin Infect Dis. 1995;20:372–86. doi: 10.1093/clinids/20.2.372. doi:10.1093/clinids/20.2.372. [DOI] [PubMed] [Google Scholar]
- 5.Helweg-Larsen J, Astradsson A, Richhall H, Erdal J, Laursen A, Brennum J, et al. Pyogenic brain abscess, a 15 year survey. BMC Infect Dis. 2012;12:332. doi: 10.1186/1471-2334-12-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weingarten K, Zimmerman RD, Becker RD, Heier LA, Haimes AB, Deck MD, et al. Subdural and epidural empyemas: MR imaging. AJR Am J Roentgenol. 1989;152:615–21. doi: 10.2214/ajr.152.3.615. doi:10.2214/ajr.152.3.615. [DOI] [PubMed] [Google Scholar]
- 7.Bustos BR, Pavez MPA, Bancalari MBJ, Miranda ARM, Escobar S, Hr, et al. Subdural empyema secondary to sinusitis. Rev Chilena Infectol. 2006;23:73–6. doi: 10.4067/s0716-10182006000100011. [DOI] [PubMed] [Google Scholar]
- 8.Pathak A, Sharma BS, Mathuriya SN, Khosla VK, Khandelwal N, Kak VK, et al. Controversies in the management of subdural empyema. A study of 41 cases with review of literature. Acta Neurochir (Wien) 1990;102:25–32. doi: 10.1007/BF01402182. doi:10.1007/BF01402182. [DOI] [PubMed] [Google Scholar]
- 9.Ozsurekci Y, Kara A, Cengiz AB, Celik M, Ozkaya-Parlakay A, Karadag-Oncel E, et al. Brain abscess in childhood: a 28-year experience. Turk J Pediatr. 2000;54:144–9. [PubMed] [Google Scholar]


