Abstract
The present study was designed to evaluate the effects of Prunella Vulgaris (P. vulgaris) on the immune function in mice. The mice were randomly divided into one control group and three treatment groups of 10 mice each. The control group received pure water and the treatment groups received P. vulgaris extract at concentrations of 0.15, 0.30 and 0.90 g/kg BW orally for 30 days, respectively. Changes in cell immune function, non-specific immunity and humoral immunity function were evaluated. Active lymphocytes and T lymphocyte subsets were determined by fluorescence-activated cell sorting (FACS). Certain Serum concentrations of cytokines were measured by enzyme-linked immunosorbent assay (ELISA). The results showed that, for cell immune function, compared with the control group, foot pad thickness in high dose group increased significantly (p<0.01), whereas no significant difference in the proliferative ability of splenic lymphocytes was observed among all groups (p>0.05). For non-specific immunity, NK cell activity increased significantly in a dose-dependent manner in P. vulgaris treated mice (p<0.01), mononuclear-macrophage function in medium and high dose P. vulgaris treated mice were significantly higher than that of the control group (p<0.05). For humoral immunity, no significant differences were observed in terms of the half value of hemolysis (HC50), number of hemolytic plaques and serum IgG level (p>0.05). The percentage of active T and Th lymphocytes of mice peripheral blood in high dose group were significantly higher than that of the control group (p<0.01). There was no significant difference in serum levels of IL-1β, IL-4, IL-10 and IFN-γ among all of the four groups (p>0.05). The data indicated that 0.90 g/kg BW P. vulgaris extract (equivalent to 7.5 g/kg BW crude drug) had some effect on cellular immune function and non-specific immune function in mice.
Introduction
P. vulgaris L. (Labiatae) belongs to Lamiaceae family Prunella genus. It is widely distributed in the temperate zone. Its Chinese name, Xia Ku Cao in pingyin, originates from a description that the herb dried and withered after the summer solstice. P. vulgaris has been used as a traditional Chinese medicine for a long time. According to the Chinese Pharmacopoeia [1], the dried fruit spike of P. vulgaris has effects of sedation, antifebrile and detumescence and is widely used for the treatment of thyroid gland malfunction, mastitis, and pulmonary tuberculosis in clinical practice [2], [3]. Modern pharmacological and clinical studies have suggested that P. vulgaris has anti-inflammatory, anti-bacterial, anti-viral and anti-tumor activities and these activities may be attributed to its immunomodulatory effect [4]–[6]. In Southeast Asia including the south of China, P. vulgaris is the main ingredient of herb tea. In vivo studies have suggested that herb tea consumption could alleviate the restraint stress-induced reduction of immune function in mice, manifested by alleviated lymphocyte impairment, increased amount and activity of lymphocytes such as natural killer (NK) cells and T lymphocytes [7]. In vitro studies revealed that both aqueous and ethanol extracts of P. vulgaris could promote lymphopoiesis and regulate the production of cytokines [8], [9]. However, most of the studies regarding the immunomodulatory effect of P. vulgaris were done in vitro, few have been reported in vivo. Therefore, in the current study, we investigated the effect of P. vulgaris on cellular immunity, humoral immunity and non-specific immunity in mice. By measuring the activities of lymphocytes and T lymphocytes subgroups as well as the serum level of humoral immunity-related cytokines such as IFN-γ, IL-1β, IL-10 and IL-4 after P. vulgaris administration, the result may provide some basis for potential mechanisms responsible for immunomodulatory effect of P. vulgaris.
Materials and Methods
Ethics Statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Ethics Committee of Guangdong Provincial Centre for Disease Control and Prevention. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Extraction
The extract of P. vulgaris was provided by Guangzhou Wanlaoji Pharmaceutical Co., Ltd. It was prepared from crude drug through water extraction followed by alcohol precipitation. One hundred g of crude drug can typically generate 11.76 g of extract; in other words, 1 g of extract is equivalent to 8.5 g of crude drug. The extract was maintained at 4°C. According to the Chinese Pharmacopoeia [1], the recommended daily consumption of P. vulgaris crude drug is 9–15 g. Assuming the body weight (BW) is 60 kg and using a recommended dosage of 15 g for calculation, the recommended daily consumption of P. vulgaris crude drug and extract are 0.25 and 0.03 g/kg BW, respectively.
Animals
Female BALB/c mice (6–8 weeks, 16–19 g, SPF grade) [10] were selected from Guangdong Medical Laboratory animal center. The mice were quarantined for three days after arrival. Mice were housed in a temperature ranging from 20 to 25°C and humidity ranging from 40 to 70%. Feed and water were supplied ad libitum.
Dose Planning and Test Substances Giving
The mice were randomly divided into one control group and three treatment groups (10 mice in each group). The control group received pure water and the treatment groups received P. vulgaris extract at 0.15, 0.30 and 0.90 g/kg BW, which were 5, 10 and 30 times of recommended dosage and equivalent to 1.25, 2.50 and 7.50 g/kg BW of crude drug respectively. To prepare the test substances for low, medium and high dose groups, 0.15,0.30 and 0.90 g P. vulgaris extract were dissolved in water to a final volume of 20.0 ml, respectively. The test substances were administrated intragastrically (0.2 ml/10 g BW) everyday for 30 days. The body weight was measured weekly. Twenty-four hours after the last administration, the mice were weighed and sacrificed.
Chemicals and Instruments
Chemicals
Fluorescent beads (FITC-labeled), Concanavalin A (ConA), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), agar and bovine serum albumin (BSA) were purchased from Sigma (St. Louis, MO). Antibodies against mouse CD3 FITC, CD4 PE, CD8 APC, CD19 PE, CD25 PE-Cy5 and CD25 APC were from eBioscience (San Diego, CA). Heamolysin was from Becton Dickinson (San Jose, CA). ELISA kits detecting Mouse IgG, IL-1β, IL-4, IFN-γ and IL-10 were purchased from R&D (Minneapolis, MN).
Instruments
VL-5BS biological safety cabinet was from Tsaohsin (Taibei, Taiwan). FACS Calibur flow cytometer was from Becton Dickinson (San Jose, CA, USA). Varioskan Flash Spectral Scanning Multimode Reader was from Thermo Scientific (Vantaa, FI). AutoFlow CO2 incubator was from NuAire (Plymouth, MN, USA). Digital sonofier 450 was from Branson (MX). CELL-DYN 3700 hematology analyzer was from Abbott (Abbott Park, IL, USA). CASY DT cell counter was from Schärfe System (DE).
Measurement of Spleen and Thymus Indexes
After the last measurement of body weight, mice were sacrificed by cervical dislocation. The spleens and thymus organs were removed immediately and the wet weight was measured. The spleen and thymus indexes were calculated as the wet weight of spleen and thymus divided by the body weight, respectively.
ConA-induced Mouse Splenic Lymphocyte Proliferation Assay [10]
To prepare splenocyte suspensions, spleens were removed in a sterile environment and put into a petri dish with sterile Hank’s buffered salt solution (HBSS). Each spleen was teased apart by sterile forceps and pushed through 200 BSS mesh. The resultant single splenocytes were washed with HBSS and centrifuged (1000 r/min, 10 min) three times. The cells were then re-suspended in 2 mL RPMI 1640 complete media. The live cells were counted by trypan blue exclusion assay (the percentage of which should be above 95%) and the cell number was adjusted to 3×106 cells/mL. One mL splenocyte suspension was incubated with or without 75 µL ConA solution (equivalent to 7.5 µg/mL) in a 24-well plate at 37°C with 5% CO2 for 72 hours. After 68 hours, 0.7 mL media was removed and replaced with 0.7 mL serum-free RPMI 1640 media plus 50 µL 5 mg/mL MTT. Four hours later (t = 72 h), 1 mL acidic isopropanol was added to dissolve the purple formazan product. The final solution was added into a 96-well plate in triplicate. The optical density (OD) value was measured by a microplate reader set to 570 nm.
Delayed Type Hypersensitivity (DTH) Assay [10]
Twenty-six days into the experiment, mice were immunized by 0.2 mL 2% sheep red blood cells (SRBC) (v/v) via intraperitoneal injection. Four days later (t = 30 d), the thickness of the left hind foot pad was measured by cornier caliper. Each sample was measured three times at the same place and the mean was taken. The spot of measurement then received 20 µL 20% SRBC (v/v) via subcutaneous injection. An additional 24 hours later, the thickness of left hind foot pad was again measured. The difference between two measurements was used as a measure of DTH. The result is considered positive if the value of experimental group is significantly higher than that of control group.
Measurement of Serum Hemolysin Level [10]
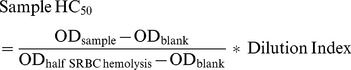
Twenty-six days into the experiment, mice were immunized by SRBC. On the last day (t = 30 d), blood was collected from the angular vein before mice were sacrificed. Serum was separated and diluted with salicylic acid (SA) buffer at a 1∶100 ratio. 100 µL diluted serum was added into a 96-well plate, followed by 50 µL 10% SRBC and 100 µL complement (diluted with SA buffer at 1∶8 ratio). For the blank control, SA buffer was used instead of diluted serum. The plate was incubated in a 37°C water bath for 30 min. After incubation, the plate was centrifuged at 2000 r/min for 10 min. Fifty µL supernatant was transferred into a new 96-well plate, followed by addition of 150 µL buffer A (1 g/L NaHCO3, 0.2 g/L KFeCN, 0.05 g/l KCN). 12.5 µL 10% SRBC was then added, again followed by buffer A to a final volume of 200 µL. The plate was incubated at room temperature for 10 min. The OD value was measured by a microplate reader set to 540 nm. The half value of hemolysis (HC50) was calculated as follows:
 |
(1) |
Measurement of Antibody Production by Splenocytes [10]
Twenty-six days into the experiment, mice were immunized by SRBC. On the last day (t = 30 d), mice were sacrificed by cervical dislocation. Splenocyte suspensions (3×106 cells/mL) were prepared as described above. Fifty µL 20% SRBC (v/v in saline) and 200 µL splenocyte suspension were mixed with 0.5 mL agar (5 g/mL in HBSS, pH 7.2–7.4), and added into a 6-well plate coated with 1 mL/well agar (5 g/L in sterile saline). Each sample was tested in duplicate. The plate was incubated at 37°C with 5% CO2 for 1 hour. 500 µL diluted complement (v/v, 1∶10 in complete media) was then added into each well, followed by further incubation for another 2 hours. After incubation, hemolytic plaques were counted by automatic image analyzer. The number of hemolytic plaques in each sample is presented as mean of duplicate/106 splenocytes.
Measurement of Serum Immunoglobulin G (IgG) Level by Enzyme-linked Immunosorbent Assay (ELISA)
Mice were anesthetized and blood was collected via the abdominal vein. Serum was separated by centrifugation and used for measurement of IgG concentration by ELISA according to the product manual. Briefly, 50 µL standard solution or sample was added into a 96-well plate (provided in the kit), followed by 25 µL enzyme-linked avidin. The plate was covered with adhesive strip and incubated at 37°C for 1 hour. The plate was then washed with pre-diluted washing buffer 5 times and 50 µL each of substrate I and substrate II were added into each well. The plate was incubated at room temperature and protected from light for 15 min. After incubation, 50 µL stop solution was added. The OD value was measured by a microplate reader set to 450 nm within 15 min. The IgG concentration in each sample was calculated according to the standard curve generated by standard solution. The result was considered positive if the value of the experimental group was significantly higher than that of the control group.
Measurement of NK Cell Activity by Lactate Dehydrogenase (LDH) Releasing Assay [10]
Routinely subcultured target cells were washed three times with HBSS. Sixty µL target cells were diluted with 6 mL cell counting buffer for cell number counting by automatic cell counter. The target cell number was then adjusted to 4×105 cells/mL in RPMI 1640 complete media. Splenocyte suspensions were prepared as described above. Six µL splenocyte suspension was mixed with 6 mL cell counting buffer for cell number counting by automatic cell counter. The number of splenocytes was then adjusted to 2×107 cells/mL in RPMI 1640 complete media. 100 µL target cells and 100 µL splenocytes (effector cell) (ratio of effector cells to target cell is 50∶1) were added into a 96-well plate. 100 µL target cell plus 100 µL media served as target cell spontaneous release control, whereas 100 µL target cell plus 100 µL 1% NP40 served as target cell maximum release control. The plates were incubated at 37°C in 5% CO2 for 4 h. After incubation, the plate was centrifuged at 1500 r/min for 5 min. 100 µL supernatant was transferred into a new 96-well plate, followed by addition of 100 µL LDH substrate. After 3 min, 30 µL l mol/L HCI was added to stop the reaction. The OD values were measured by a microplate reader set to 490 nm. NK cell activity was calculated and transformed as Formula (2). The result was considered positive if the transformed value of the experimental group was significantly higher than the transformed value of the control group.
 |
| (2) |
Detection of Phagocytic Activity of Peritoneal Macrophages by Flow Cytometry
Twenty-six days into the experiment, mice were immunized by SRBC. On the last day (t = 30 d), the mice were sacrificed by cervical dislocation. To collect peritoneal macrophages, 3 mL HBSS (containing 5% fetal bovine serum) was directly injected into the peritoneal cavity. After peritoneal massage (40 times), the peritoneal cavity was surgically opened and 2 mL peritoneal cavity fluid was collected. The peritoneal macrophages were then collected by filtration onto a 70 um filter. 100 µL FITC-labeled fluorescent beads was added into 10 mL PBS containing 1% BSA, incubated in a 37°C water bath for 30 min, followed by sonication for 5 min. Pretreated fluorescent beads were then mixed with peritoneal macrophages (200 µL fluorescent beads for every mL macrophages at a concentration of 4∼6×105 cells/mL) and added into a 6-well plate. The plate was incubated at 37°C for 1 hour. After incubation, the supernatant was removed and the wells were gently washed with PBS. The adherent cells were then collected and filtered onto a 70 µm filter, followed by FACS analysis. The phagocytic activity of peritoneal macrophages was presented as percentage of phagocytosis (which needs data transformation) and phagocytic index calculated as Formula (3) and (4).
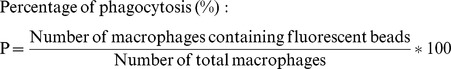 |
| (3) |
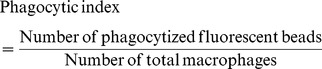 |
(4) |
Analysis of Active Lymphocytes and T Lymphocyte Subsets
Activated lymphocytes and lymphocyte subgroups in peripheral blood were analyzed by fluorescence-activated cell sorting (FACS) [11]. CD3 was used as a marker for mature T lymphocytes; CD4 and CD8 were used as markers for helper T lymphocytes (Th) and cytotoxic T lymphocytes (Tc or CTL), respectively; CD19 was used as a marker for B lymphocytes; and CD25 was used as a marker for activated lymphocytes. Before mice were sacrificed, blood was collected from the angular vein and prevented from coagulation by heparin. 100 µL anticoagulated blood was mixed with fluorescence-labeled mouse monoclonal antibodies against the above-mentioned markers and incubated at room temperature and protected from light for 20 min. After incubation, 2 mL FACS red blood cell lysis buffer was added into each tube and incubated at room temperature and protected from light for another 20 min. After washing with phosphate buffered saline, the samples were analyzed by FACS (Figure S1 and Figure S2).
Measurement of Concentrations of Serum Cytokines IL-1β, IFN-γ, IL-4 and IL-10 by ELISA
Mice were anesthetized and blood was collected via the abdominal vein. Serum was separated by centrifugation and used for the measurement of cytokine concentrations by ELISA according to the product manual. For measurement of IL-4 and IL-10, 100 µL standard solution or serum sample were added to the ELISA plate. For measurement of IFN-γ and IL-1β, 50 µL standard solution or serum sample were added. Then, 50 µL enzyme-linked avidin was added into each well. The plate was covered with adhesive strip and incubated at 37°C for 1 hour. The plate was then washed five times with pre-diluted washing buffer, followed by addition of 50 µL each of substrate I and substrate II into each well and incubation at room temperature with protection from light for 15 min. After incubation, 50 µL stop solution was added into each well. The OD values were measured by a microplate reader set to 450 nm within 15 min. The concentration of each cytokine was calculated according to the standard curve generated by the corresponding standard solution.
Statistical Analysis
Values were expressed as mean ± SD (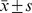 ). Statistical analysis were performed using one-way ANOVA. Then the least significant difference (LSD) test was used to compare each dose group with the control group. Differences with a p<0.05 were considered statistically significant.
). Statistical analysis were performed using one-way ANOVA. Then the least significant difference (LSD) test was used to compare each dose group with the control group. Differences with a p<0.05 were considered statistically significant.
Results
Effect of P. vulgaris on Mice Spleen and Thymus
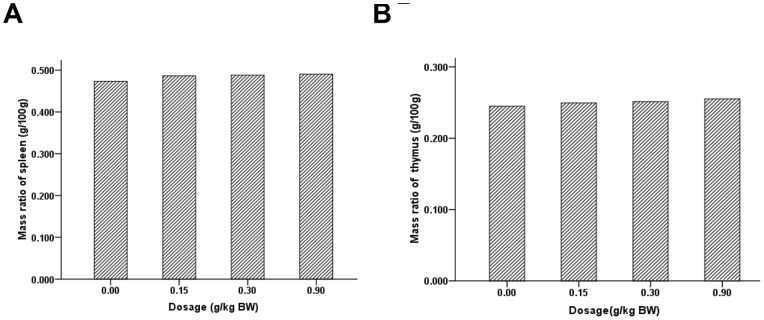
No pathological changes such as swelling, atrophy or bleeding were observed by visual observation in P. vulgaris exact (0.00, 0.15, 0.30, 0.90 g/kg BW) treated mice for 30 days. The spleen and thymus indexes were calculated as the wet weight of spleen and thymus divided by the body weight, respectively. As the result of statistical analysis, the data were expressed as mean±SD of each group (n = 10 per group). There was no significant difference in spleen index (Fig. 1A) and thymus index (Fig. 1B) among four groups (p>0.05). The results of the spleen and thymus indexes suggest that P. vulgaris has no significant effect on the major immune organs in mice.
Figure 1. The effect of P. vulgaris on mice spleen and thymus indexes.
The spleen and thymus indexes were calculated as the wet weight of spleen and thymus divided by the body weight respectively for P. vulgaris exact treated (0.00, 0.15, 0.30, 0.90 g/kg BW) mice for 30 days. The data were expressed as mean±SD of each group (n = 10 per group). There was no significant difference in spleen index (Fig. 1A) and thymus index (Fig. 1B) among four groups (p>0.05).
Effect of P. vulgaris on Mice Cellular Immune Function
To explore the the effect of P. vulgaris on mice cellular immune function, Delayed type hypersensitivity (DTH) and ConA-induced splenic lymphocyte proliferation were tested in mice treated with P. Vulgaris exact (0.00, 0.15, 0.30, 0.90 g/kg BW) for 30 days. The data were expressed as mean±SD of each group (n = 10 per group). The increase in foot pad thickness of mice immunized with SRBC was examined to represent the DTH. As the result, the thickness of the mice foot pad increased in a dose dependent manner. The increase in foot pad thickness increased significantly (p<0.01) among these four groups. Further analysis revealed that a statistically significant difference was observed between the high dose group (0.75±0.11) and the control group (0.60±0.07) (p<0.01). While there were no significant differences between low or medium dose P. vulgaris exact treated mice with the control group (p>0.05). ConA-induced splenic lymphocyte proliferation was tested by MTT assay. There was no obvious difference in the splenic lymphocyte proliferation ability among the four groups (p>0.05) (Table 1). The results suggest that high concentrations of P. vulgaris may have the potential effect on mice cellular immune function.
Table 1. The effect of P vulgaris on mice cellular immune function (mean±SD, n = 10).
| Group | Dosage (g/kg BW) | Increase in foot pad thickness (mm) | Spleniclymphocyteproliferation |
| Control group | 0.00 | 0.60±0.07 | 0.37±0.11 |
| Low-dosage group | 0.15 | 0.63±0.09 | 0.34±0.12 |
| Medium-dosage group | 0.30 | 0.68±0.14 | 0.42±0.06 |
| High-dosage group | 0.90 | 0.75±0.11** | 0.39±0.09 |
Note: Compared to the control group,
indicates P<0.01.
The tested doses are of the extract of P. vulgaris.
Effect of P. vulgaris on Mice Non-specific Immunity
Mononuclear-macrophage function
The mononuclear-macrophage function was evaluated by detecting the phagocytic activity of peritoneal macrophages by flow cytometry in P. vulgaris exact treated mice with different dosage (0.00, 0.15, 0.30, 0.90 g/kg BW) for 30 days. The percentage of phagocytosis (which needs data transformation) and phagocytic index of mononuclear-macrophage of each mice was calculated as Formula (3) and (4) according to the number of macrophages containing fluorescent beads analyzed by FACS. The data were expressed as mean±SD of each group (n = 10 per group).Both the transformed percentage of phagocytosis and the phagocytic index increased in a dose-dependent manner in mice treated with P. vulgaris. The phagocytic index of the medium (1.32±0.19) and high (1.33±0.22) dose group were significantly higher than that of the control group (1.05±0.12) (p<0.05). The transformed percentage of phagocytosis of the medium (0.89±0.06) and high (0.89±0.09) dose group remained significantly higher than that of the control group (0.80±0.05) (p<0.05). While there was no obvious difference in the transformed percentage of phagocytosis and the phagocytic index between the low dose group and the control group (p>0.05) (Table 2). The results suggest that medium and high dosage of P. vulgaris may have some effect on mice Mononuclear-macrophage function.
Table 2. The effect of P vulgaris on mice mononuclear-macrophage function (mean±SD, n = 10).
| Group | Dosage (g/kg BW) | Phagocytic index | Transformed percentage of phagocytosis |
| Control group | 0.00 | 1.05±0.12 | 0.80±0.05 |
| Low-dosage group | 0.15 | 1.13±0.11 | 0.83±0.05 |
| Medium-dosage group | 0.30 | 1.32±0.19* | 0.89±0.06* |
| High-dosage group | 0.90 | 1.33±0.22* | 0.89±0.09* |
Note: Compared to the control group,
indicates P<0.05.
NK cell activity
The mice were treated with increasing concentrations (0.00, 0.15, 0.30, 0.90 g/kg BW) of P. vulgaris exact for 30 days. Then the NK cell activity was evaluated by the LDH releasing assay. Transformed NK cell activity of each sample was calculated and transformed as the Formula (2) according to the OD values measured by microplate reader. The data were expressed as mean±SD of each group (n = 10 per group).Transformed NK cell activity increased in a dose-dependent manner in P. vulgaris treated mice. Statistically significant differences were observed between all three experimental groups and the control group (p<0.01). As shown in Table 3,the transformed NK cell activity of the medium (0.57±0.06) and high (0.62±0.07) dose group were significantly higher than that of the control group (0.49±0.06)(p<0.01). The transformed NK cell activity of low dose group (0.54±0.03 ) remained significantly higher than that of the control group (p<0.05). The results suggest that low, medium and high dosage of P. vulgaris may have some effect on mice NK cell activity.
Table 3. The effect of P vulgaris on mice NK cell activity (mean±SD, n = 10).
| Group | Dosage (g/kg BW) | NK cell activity (%) | Transformed NK cell activity |
| Control group | 0.00 | 22.07±4.73 | 0.49±0.06 |
| Low-dosage group | 0.15 | 26.36±3.05 | 0.54±0.03* |
| Medium-dosage group | 0.30 | 29.27±4.99** | 0.57±0.06** |
| High-dosage group | 0.90 | 33.59±6.28** | 0.62±0.07** |
Note: Compared to the control group,
vindicates P<0.05,
indicates P<0.01.
Effect of P vulgaris on Mice Humoral Immunity Function
To explore the the effect of P. vulgaris on mice humoral immunity function, the mice serum hemolysin level (HC50), hemolytic plaques (duplicate/106 splenocytes) indicating antibody production of splenocytes, as well as serum IgG level were evaluated in mice treated with increasing concentrations (0.00, 0.15, 0.30, 0.90 g/kg BW) of P. Vulgaris exact for 30 days. The data were expressed as mean±SD of each group (n = 10 per group).No significant difference was detected among the three experimental groups and a control group in terms of HC50, the number of hemolytic plaques and serum IgG level (p>0.05) (Table 4).
Table 4. The effect of P vulgaris on mice humoral immunity (mean±SD, n = 10).
| Group | Dosage (g/kg BW) | HC50 | hemolytic plaque (duplicate/106 splenocytes) | Serum IgG level (µg/ml) |
| Control group | 0.00 | 12.19±2.77 | 16.03±2.65 | 37.30±3.92 |
| Low-dosage group | 0.15 | 12.89±1.77 | 16.25±3.21 | 41.35±7.47 |
| Medium-dosage group | 0.30 | 13.45±2.77 | 16.09±3.25 | 40.20±5.39 |
| High-dosage group | 0.90 | 13.08±1.77 | 18.08±3.34 | 39. 55±4.94 |
Effect of P. vulgaris on the Lymphocytes and T Lymphocyte Subsets Activity in Mice Peripheral Blood
The mice received increasing concentration (0.0, 0.15, 0.30, 0.90 g/kg BW) of P. Vulgaris exact for 30 days Then the active lymphocytes and the T lymphocyte subsets in mice peripheral blood were analyzed by FACS. According to the ratio of the active T, Th and Tc lymphocytes of each sample obtained by the flow cytometry, the detection result for each dose group can then be compared by statistical analysis. The data were expressed as mean±SD of each group (n = 10 per group).Compared with the control group, the percentages of both active T lymphocytes (CD3+CD25+) and Th cells (CD3+CD4+CD8−CD25+) increased in a dose-dependent manner in P. vulgaris treated mice. In terms of the percentages of active T lymphocytes, the high dose group (CD3+CD25+ = 8.15±0.95% ), but not the low or medium dose group, is significantly higher than the control group (CD3+CD25+ = 6.38±0.66% )(p<0.01). The active Th lymphocytes in high dose group (CD3+ CD4+CD8− CD25+ = 6.53±0.91% ) remained significantly higher than that of the control group (CD3+ CD4+CD8− CD25+ = 3.78±0.57% ) (p<0.01). While there was no significant difference in the percentage of active B lymphocytes (CD19+CD25+) and Tc lymphocytes (CD3+CD4−CD8+CD25+) among the four groups (p>0.05).(Table 5) The results suggest that the high dose P. vulgaris may have some effect on the T and Th lymphocytes activity in mice peripheral blood.
Table 5. The effect of P vulgaris on mice active lymphocytes and T lymphocyte subsets in mice peripheral blood (mean±SD, n = 10).
| Group | Dosage (g/kg BW) | CD3+ CD25+ (%) | CD3+CD4+CD8− CD25+(%) | CD3+CD4−CD8+ CD25(%) | CD19+ CD25+ (%) |
| Control group | 0.00 | 6.38±0.66 | 3.78±0.57 | 0.15±0.02 | 0.28±0.05 |
| Low-dosage group | 0.15 | 6.70±0.87 | 4.27±0.86 | 0.17±0.04 | 0.28±0.13 |
| Medium-dosage group | 0.30 | 6.73±0.82 | 4.95±1.53 | 0.16±0.07 | 0.32±0.12 |
| High-dosage group | 0.90 | 8.15±0.95 ** | 6.53±0.91** | 0.20±0.07 | 0.26±0.10 |
Note: Compared to the control group,
indicates P<0.01.
Effect of P vulgaris on Serum Concentrations of Cytokines
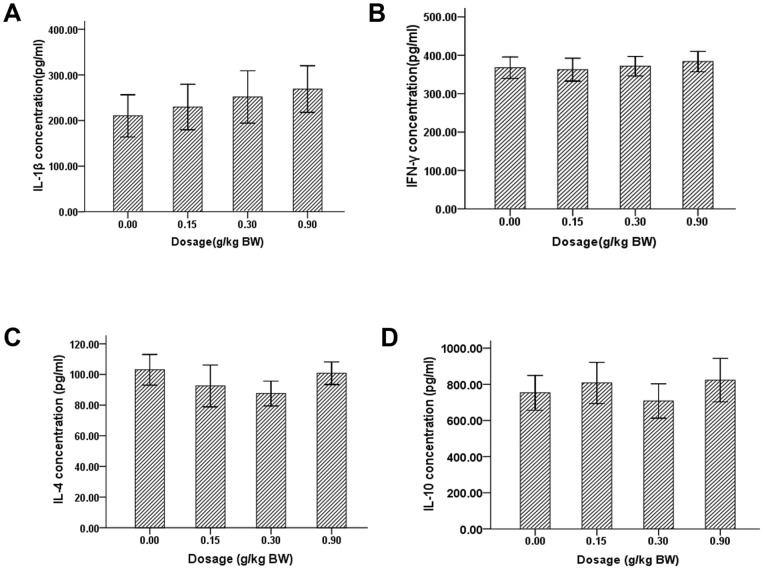
Serum concentrations of different cytokines including IL-1β, IFN-γ, IL-4 and IL-10 in P. vulgaris exact (0.00, 0.15, 0.30, 0.90 g/kg BW) treated mice were tested by ELISA according to the manual of ELISA kits. The data were expressed as mean±SD of each group (n = 10 per group).The serum concentration of IL-1β tended to increase in a dose-dependent manner in P. vulgaris treated mice. However, no significant difference in serum levels of IL-1β (Fig. 2A), IFN-γ (Fig. 2B), IL-4 (Fig. 2C) and IL-10 (Fig. 2D) was observed among four groups (p>0.05).
Figure 2. The effect of P vulgaris on mice serum concentrations of cytokines.
The serum levels of IL-1β, IFN-γ, IL-4 and IL-10 in P. vulgaris exact treated (0.00, 0.15, 0.30, 0.90 g/kg BW) mice for 30 days were detected by ELISA according to the manual of ELISA kits. The data were expressed as mean±SD of each group (n = 10 per group). No significant difference in serum levels of IL-1β (Fig. 2.A), IFN-γ (Fig. 2B), IL-4(Fig. 2C) and IL-10 (Fig. 2D) was observed among these four groups of mice (p>0.05).
Discussion
Studies have suggested that the anti-inflammatory and antitumor activities of P. vulgaris are closely related to its immunomodulatory effect [4], [12], [13]. Our current study showed that 0.90 g/kg BW P. vulgaris extract had some effect on mice cellular immune function and non-specific immune function. While 0.30 and 0.15 g/kg BW Prunella extract may have some potential effect on mice cellular immune function and non-specific immune function. For non-specific immunity, both NK cell activity and macrophage function increased significantly in a dose-dependent manner in P. vulgaris treated mice. Compared with the control group, significant increases in NK cell activity were observed in all three experimental groups and significant increases in macrophage function were observed in medium and high dose groups. These results suggest that P. vulgaris is able to effect mice non-specific immunity in a dose-dependent manner, which is consistent with other studies. For example, aqueous extract of P. vulgaris enhanced macrophage function by regulating the NF-κB pathway in RAW 264.7 mouse macrophage cell line [14], [15]. Compound Ajuga decumbens Thunb, another species in the same family as P. vulgaris, showed similar ability to enhance phagocytic activity of mouse macrophages in a dose-dependent manner [16]. Moreover, studies have indicated that the bioactive components of P. vulgaris such as polysaccharides, oleanolic acid and ursolic acid may be responsible for boosting non-specific immunity in mice, presented as a dose-dependent increase of phagocytic activity of mononuclear-macrophages and IL-1β secretion from macrophages [17]–[19]. Our current study also suggests that P. vulgaris may affect mice cell immune function, manifested by a dose-dependent increase in foot pad thickness in mice treated with P. vulgaris. There was a significant difference between high dose group and the control group in terms of foot pad thickness. It has been reported that the aqueous extract of P. vulgaris can stimulate mouse T lymphocyte proliferation [8]. Polysaccharides from P. vulgaris were shown to affect cell immune function in mice and induce the secretion of interferon (IFN) in a dose-dependent manner [20]. Furthermore, no influence of P. vulgaris on mouse humoral immunity was observed in the current study, evident by no significant difference between the experimental groups and the control group in terms of serum hemolysin levels, antibody producing splenic lymphocytes and serum IgG levels.
The activity of T lymphocyte subsets is an important indicator of immune homeostasis [21]. It was reported that intragastric administration of P. vulgaris capsules at a concentration of 0.21–0.86 g/kg·d for 30 days induced up-regulation of certain T lymphocyte subgroups in rats [3]. The current study showed that P. vulgaris treatment enhanced the activity of T lymphocytes in general and its subgroup Th cells; in contrast, it had minimal effects on B lymphocytes and Tc cells, another subset of T lymphocytes. The thickness of the mice foot pad and the percentages of active T and Th lymphocytes increased in a dose-dependent manner in P. vulgaris treated mice. Compared with the control group, the high dose group showed significantly higher percentage of active T lymphocytes and its subgroup Th cells, further proving that P. vulgaris is able to effect the cellular immune function in mice. No significant changes in B lymphocytes and Tc cells were observed among the four groups, which is consistent with the finding that P. vulgaris had minimal effect on mouse humoral immunity.
Conclusion
In conclusion, 0.90 g/kg BW P. vulgaris extract (equivalent to 7.50 g/kg BW crude drug) had some effect on cellular immune function and non-specific immune function in mice.
Supporting Information
Flow cytometry detection of the active T lymphocyte subsets. These pictures are the detection results of a sample. I gate in Fig. S1A indicates the lymphocyte region. The labeled cells were acquired in the two-dimensional Dot-Plot graph by Forward scatter (FSC) and Side scatter (SSC). In Fig. S1 B–D, the right upper quadrant and lower right quadrant were lymphocyte region. Right upper quadrant represents active lymphocyte region, while lower right quadrant represents non active lymphocyte region. Active T, Th, TC lymphocytes were detected through three fluorescent antibodies of FITC-CD3/PE-CD19/APC-CD25. The right upper quadrant in Fig. S1B, Fig. S1C and Fig. S1D was respectively the region of active T(CD3+ CD25+), Th(CD3+ CD4+CD8− CD25+) and Tc(CD3+ CD4−CD8+ CD25+) lymphocytes. According to the ratio of the active T, Th and Tc lymphocytes of each sample obtained by flow cytometry, the detection result for each dose group can then be analyzed.
(TIF)
Flow cytometry detection of the active B lymphocyte. These pictures are the detection results of a sample. I gate in Fig. S2A indicates the lymphocyte region. The labeled cells were acquired in the two-dimensional Dot-Plot graph, the right upper quadrant and lower right quadrant in Fig. S2 B was lymphocyte region. Active B lymphocytes were detected through two fluorescent antibody simultaneously of PE-CD19/APC-CD25. The right upper quadrant in Fig. S2B was the region of active B lymphocytes (CD19+ CD25+). According to the ratio of the active B lymphocytes of each sample obtained by flow cytometry, the detection result for each dose group can then be analyzed.
(TIF)
Funding Statement
The authors thank the National High Technology Research and Development Program 863 (2010AA023001), Guangdong Natural Science Foundation project (S2013010013289), Guangdong science and technology plan project (201300000161), and the Guangdong provincial bureau of Chinese medicine project (20132108) for their financial contributions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.The Pharmacopoeia Commission of the People’s Republic of China (2010) Pharmacopoeia of the People,s Republic of China Part I. Beijing:China Medical Science Press. 263 p.
- 2. Yang K, Guo KQ, Wu HY (2007) Clinical Effect of Prunellae Oral Liquid on Goiter with Different Thyroid Function. Chinese Journal of Integrated Traditional and Western Medicine 27(1): 37–39. [PubMed] [Google Scholar]
- 3. Zheng Y, Qiao CD, Yuan W, Zhao L (2004) Influence of Self heal Capsule on the Expression of Subsets of T Lymphocyte in Peripheral Blood of Rats with Ulcerative Colitis. Chin J Integr Trad West Med Dig 12(1): 10–12. [Google Scholar]
- 4. Liu GM, Jia XB, Wang HB, Feng L, Chen Y (2009) Review about current status of cancer prevention for the chemical composition or composition and function mechanism of Prunella vulgaris.Journal of Chinese Medicine Materials. 3(12): 1920–1926. [Google Scholar]
- 5. Chen CY, Wu G, Zhang MZ (2009) The effects and mechanism of action of Prunella vulgaris Lextract on Jurkat human T lymphoma cell proliferation. Chinese-German Journal of Clinical Oncology 8(7): 426–429. [Google Scholar]
- 6. Chlopcíková S, Psotová J, Miketová P, Sousek J, Lichnovský V, et al. (2005) Miketová, et al. Cytoprotectivity of Prunella vulgaris on doxorubicin-treated rat cardiomyocytes. Fitoterapia 76: 556–561. [DOI] [PubMed] [Google Scholar]
- 7. He R, Yao X (2009) The “Xiehuo” Effect of Guangdong Herbal Tea and Its Composition. World Science and Technology/Modernization of Traditional Chinese Medicine and Materia Medica 11(6): 834–839. [Google Scholar]
- 8. Harput US, Saracoglu I, Ogihara Y (2006) Effects of two Prunella species on lymphocyte proliferation and nitric oxide production. Phytother Res 20(2): 157–159. [DOI] [PubMed] [Google Scholar]
- 9. Lu J, Qing R, Ye S, Deng Y, Hu DZ (2012) Effects of Prunella vulgaris extract on cellular immune function in MDR-MTB infection mice. Chin J Clin Lab Sci’Jan 30(1): 49–51. [Google Scholar]
- 10.Ministry of Health of the people’s Republic of China (2003) Inspection and evaluation of health food. Health law enforcement and supervision of the Ministry of Health 22–35 p.
- 11. Warnatz K, Schlesier M (2008) Flowcytometric phenotyping of common variable immunodeficiency. Cytometry B Clin Cytom 74(5): 261–27. [DOI] [PubMed] [Google Scholar]
- 12. Ryu SY, Oak MH, Yoon SK, Cho DI, Yoo GS, et al. (2000) Anti-allergic and antiinflammatory triterpenes from the herb of Prunela vulgaris. Planta Med 66(4): 358–360. [DOI] [PubMed] [Google Scholar]
- 13. Zdarilova A, Svobodova A, Simanek V, Ulrichova J (2009) Prunella vulgaris extract and rosmarinicacid suppress lipopolysaccharide-induced alteration in human gingival fibroblasts. Toxicol In Vitro 23(3): 386–392. [DOI] [PubMed] [Google Scholar]
- 14. Han EH, Choi JH, Hwang YP, Park HJ, Choi CY, et al. (2009) Immunostimulatory activity of aqueous extract isolated from Prunella vulgaris. Food Chem Toxicol 47(1): 62–9. [DOI] [PubMed] [Google Scholar]
- 15. Huang N, Hauck C, Man-Yu Y (2009) Rosmarinic Acid in Prunella vulgaris Ethanol Extract Inhibits LPS induced Prostaglandin E2 and Nitric Oxide in RAW264.7 Mouse Macrophages. Agric Food Chem 57(22): 10579–10589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang WJ, Li HF, Ou JQ, Tian WY (2010) An experimental study on immunomodulating activity of the Ajuga decumbens Thunb. lishizhen medicine and Materia Medica Research 21(1): 107–108. [Google Scholar]
- 17.Ikeda Y, Murakami A, Fujimura Y, Tachibana H, Yamada K, et al. (2007) Aggregated Ursolic Acid, a Natural Triterpenoid, Induces IL-1β Release from Murine Peritoneal Macrophages: Role of CD36. The Journal of Immunology 178; 4854–4864. [DOI] [PubMed]
- 18. Wang GX, Wang SR, Lin WM, Zhao RF, Lin N (2009) Effects of polysacchar ide2comb inations on immune activity of m ice in vivo. Ch in J Public Health 25 (12): 1468–1470. [Google Scholar]
- 19. Fan YL, Cui FD, Wang XY, Yan PG (2001) The Comparative studies on the influence of Oleanolic acid (OLA) and Oleanolic acid 3β-phthalic monoester disodinm salt (OLA2Pena) on the mononuclear macrophage phagocytosis in mice. Pharmacology and Clinics of Chinese Materia Medica 17 (5): 10–11. [Google Scholar]
- 20. Jiang LH, Feng Y, Xu DS (2007) The Anti-Herpesvirus and Related Immunocompetence Effect of Polysaccharides from Prunella vulgaris L. Lishizhen medicine and Materia Medica Research. 18(11): 2622–2623. [Google Scholar]
- 21. Fuentes-Arderiu X, Mestre M (2009) Description of flow cytometry examinations related to human cell differentiation molecules in clinical immunology. Cytometry B Clin Cytom 76(4): 291–293. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flow cytometry detection of the active T lymphocyte subsets. These pictures are the detection results of a sample. I gate in Fig. S1A indicates the lymphocyte region. The labeled cells were acquired in the two-dimensional Dot-Plot graph by Forward scatter (FSC) and Side scatter (SSC). In Fig. S1 B–D, the right upper quadrant and lower right quadrant were lymphocyte region. Right upper quadrant represents active lymphocyte region, while lower right quadrant represents non active lymphocyte region. Active T, Th, TC lymphocytes were detected through three fluorescent antibodies of FITC-CD3/PE-CD19/APC-CD25. The right upper quadrant in Fig. S1B, Fig. S1C and Fig. S1D was respectively the region of active T(CD3+ CD25+), Th(CD3+ CD4+CD8− CD25+) and Tc(CD3+ CD4−CD8+ CD25+) lymphocytes. According to the ratio of the active T, Th and Tc lymphocytes of each sample obtained by flow cytometry, the detection result for each dose group can then be analyzed.
(TIF)
Flow cytometry detection of the active B lymphocyte. These pictures are the detection results of a sample. I gate in Fig. S2A indicates the lymphocyte region. The labeled cells were acquired in the two-dimensional Dot-Plot graph, the right upper quadrant and lower right quadrant in Fig. S2 B was lymphocyte region. Active B lymphocytes were detected through two fluorescent antibody simultaneously of PE-CD19/APC-CD25. The right upper quadrant in Fig. S2B was the region of active B lymphocytes (CD19+ CD25+). According to the ratio of the active B lymphocytes of each sample obtained by flow cytometry, the detection result for each dose group can then be analyzed.
(TIF)