Abstract
Mitosis in the early syncytial Drosophila embryo is highly correlated in space and time, as manifested in mitotic wavefronts that propagate across the embryo. In this paper we investigate the idea that the embryo can be considered a mechanically-excitable medium, and that mitotic wavefronts can be understood as nonlinear wavefronts that propagate through this medium. We study the wavefronts via both image analysis of confocal microscopy videos and theoretical models. We find that the mitotic waves travel across the embryo at a well-defined speed that decreases with replication cycle. We find two markers of the wavefront in each cycle, corresponding to the onsets of metaphase and anaphase. Each of these onsets is followed by displacements of the nuclei that obey the same wavefront pattern. To understand the mitotic wavefronts theoretically we analyze wavefront propagation in excitable media. We study two classes of models, one with biochemical signaling and one with mechanical signaling. We find that the dependence of wavefront speed on cycle number is most naturally explained by mechanical signaling, and that the entire process suggests a scenario in which biochemical and mechanical signaling are coupled.
Introduction
The early embryos of many species, including Drosophila [1]–[4], Xenopus [5]–[7], Oryzias [8], Fundulus [9], and zebrafish [10], [11], exhibit metachronous mitosis, in which mitosis progresses as a wavefront through the embryo. Such wavefronts are reminiscent of biochemical wavefronts that are used to transmit signals across many cells in other biological systems, such as wavefronts of the molecule cAMP that propagate in a colony of Dictyostelium when it begins to aggregate to form a fruiting body [12]–[14]. Propagating wavefronts, however, need not be purely biochemical in origin. The process of mitosis is a highly mechanical one that involves significant changes in the volume occupied by chromatin [15] as well as separation of chromosomes [16]. This raises the question of whether mitotic wavefronts are purely biochemical phenomena or whether they might have a mechanical component as well.
The nuclei of the Drosophila embryo are syncytial (i.e., they share the same cytoplasm and are not separated into individual cells by plasma membranes) during their first thirteen division cycles. The nuclei migrate to the egg's surface during the ninth cycle. There they divide five more times, until the fourteenth cycle, when cell membranes form and gastrulation begins [1]. Mitotic wavefronts are observed in cycles 9 through 13 [1]. In this period, chemical diffusion is unhindered by membrane barriers. For example, it is known that calcium, a signal carrier that influences many local phenomena including mitosis [17]–[19], exhibits spikes of concentration in the syncytial embryo [20]–[24] that have been resolved into a wavefront that travels across the embryo at the same speed as the mitotic wavefront [21].
However, mitosis is also a mechanical phenomenon. In the syncytial embryo, nuclei are embedded in an elastic cytoskeleton, which contains both actin and microtubules [25]–[27]. Actin caps assemble around each of the nuclei at the end of interphase, and provide anchor points for the mitotic spindles that pull the two daughter nuclei apart [25]–[28]. Recent work shows that mechanical interactions are important for re-organization of the nuclei after mitosis [29], and optical tweezer experiments show that nuclei are mechanically coupled [30]. Moreover, mechanical deformations of the embryo are known to be able to induce morphogen expression [31]. However, little is known about how mechanical interactions affect collective phenomena such as mitotic wavefronts at the level of the entire embryo.
In this paper we report the results of both our image analysis of wavefronts in early Drosophila embryos, and our theoretical studies of models of wavefront propagation. Using novel tracking techniques, we analyzed confocal microscopy videos taken of Drosophila embryos in which the nuclear DNA/chromosomes are visualized by labeling their histones with GFP. Our analysis yields the position, shape and dynamics of the DNA/chromosomes with high temporal and spatial resolution during cycles 9–14. We observe two distinct markers of the mitotic process in each cycle, one corresponding to the onset of metaphase (at which point the chromosomes condense in the nuclear midplane, known as the metaphasic plate, see Figure S1 for an illustration of the different stages) and one corresponding to the onset of anaphase. Both onsets exhibit identical wavefront patterns, indicating that they are indeed two markers of the same process. Both onsets are also followed by displacements in the positions of the nuclei that also exhibit the same wavefront patterns. Finally, we find that the wavefront speed slows down from one cycle to the next.
We treat the embryo theoretically as an excitable medium, consisting of nuclei that can be triggered into initiating metaphase or anaphase, thereby locally exciting the medium and thus signaling their neighbors. We not only consider the well-known case of nonlinear wavefront propagation in a chemically excitable medium [32], [33], but introduce a model for the early embryo as a mechanically excitable medium [34], through which mitotic wavefronts can propagate via stress diffusion. Comparing the data with the results of these two models, we find that our observations are difficult to reconcile with a purely biochemical scenario. In such a scenario, the wavefront speed has a tendency to increase with nuclear density, and thus with cycle, contrary to our observations. The observations can, however, be explained quite naturally by a novel scenario in which nuclei not only respond to their mechanical environment, but also actively use it to signal each other. Our results suggest that mitotic wavefronts in syncytial Drosophila embryos may constitute one example of a previously unexplored form of mechanical signaling via nonlinear wavefronts that could also arise in very different biological contexts [34].
Results
Image analysis results
Nuclear cycle and shape
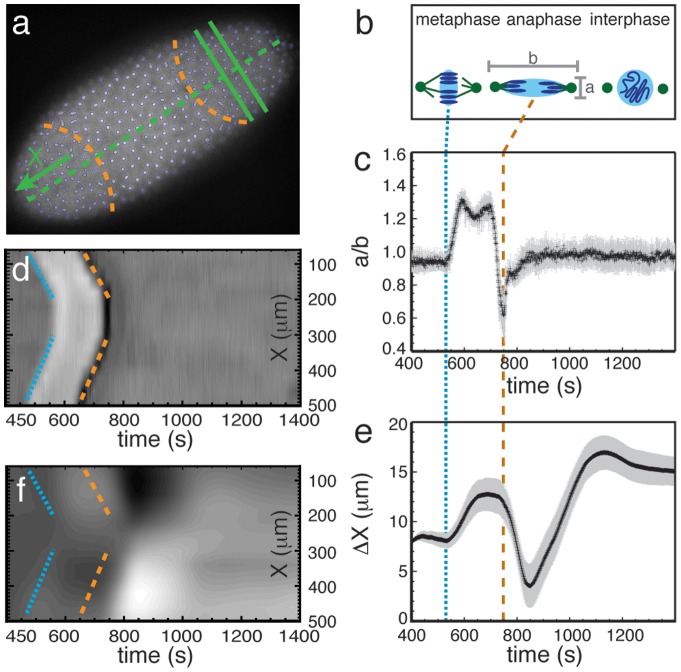
An example image of detected nuclei in a Drosophila embryo is shown in Figure 1a. In each cycle, as the nuclei progress from interphase through metaphase to anaphase, the detected shape of the DNA/chromosomes changes in a well-defined manner (Figure 1b). Newly separated nuclei are small and spherical, and thus show up in our shape tracking as small circles. During interphase, the nuclear DNA grows in size over time as it is duplicated. At the onset of metaphase, the chromosomes condense in the midplane of the nucleus, and appear to elongate into an ellipse. The final step of mitosis, the onset of anaphase, corresponds to two detectable changes in the shape: a sudden shift of the orientation axis over a  angle, and a change of aspect ratio. An example plot showing the ratio of the length of the two axes as a function of time during a cell cycle is given in Figure 1c.
angle, and a change of aspect ratio. An example plot showing the ratio of the length of the two axes as a function of time during a cell cycle is given in Figure 1c.
Figure 1. Observation of wavefronts and mechanical response.
a) Image of a Drosophila embryo during mitosis at the end of cycle 11, with the detected chromosomal contours overlaid. Anaphasic wavefronts (orange dashed curved lines), the long axis (green dashed straight line) and a typical slice perpendicular to the long axis (green parallel straight lines) are indicated. b) Sketch of the three main states in image analysis: interphase (circular contours), metaphase (compressed elliptical contours), and anaphase (highly extended elliptical contours, perpendicular to metaphase contour). See also Figure S1. c) Ratio of the two elliptical axes of the detected shape of the nuclear DNA/chromosomes vs. time in cycle 11, averaged over an  -slice (as shown in a); error bars indicate variation within the slice. The transitions between interphase and metaphase, as well as the onset of anaphase, are sharp and indicated respectively by dotted (blue) and dashed (orange) vertical lines. The slice shown was taken at
-slice (as shown in a); error bars indicate variation within the slice. The transitions between interphase and metaphase, as well as the onset of anaphase, are sharp and indicated respectively by dotted (blue) and dashed (orange) vertical lines. The slice shown was taken at 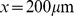 . d) Kymograph showing the elliptical axes ratio,
. d) Kymograph showing the elliptical axes ratio, 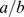 (where white indicates values larger than 1 and black indicates values smaller than 1), as a function of position
(where white indicates values larger than 1 and black indicates values smaller than 1), as a function of position  and time. The dotted and dashed lines indicate the onsets of metaphase and anaphase, as in Figure c. e) Average
and time. The dotted and dashed lines indicate the onsets of metaphase and anaphase, as in Figure c. e) Average  -displacement
-displacement 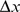 of the nuclei within one slice vs. time. After a nucleus has divided, we use the average position of its two daughters. The slice shown is identical to the one in Figure c. f) Kymograph showing the collective motion of nuclei in slices taken at different positions along the long axis of the embryo. White indicates motion in the positive
of the nuclei within one slice vs. time. After a nucleus has divided, we use the average position of its two daughters. The slice shown is identical to the one in Figure c. f) Kymograph showing the collective motion of nuclei in slices taken at different positions along the long axis of the embryo. White indicates motion in the positive  direction, black in the negative
direction, black in the negative  direction. Dotted and dashed lines again indicate the onsets of metaphase and anaphase. Note that the displacements occur sometime after these onsets, but follow the same wavefront pattern.
direction. Dotted and dashed lines again indicate the onsets of metaphase and anaphase. Note that the displacements occur sometime after these onsets, but follow the same wavefront pattern.
Wavefront pattern in the onset of metaphase and anaphase
The onsets of metaphase and anaphase, as determined by the axes ratio (Figure 1d) are indicated by dotted blue lines and dashed orange lines, respectively. Evidently the onset of metaphase exhibits a wavefront pattern, or rather two wavefronts, one propagating from each pole. The two wavefronts do not necessarily start at the same time. The onset of anaphase exhibits the same wavefront pattern. Mitotic waves were first observed by Foe and Alberts [1]; with better time resolution, it is evident that these wavefronts can be resolved into two distinct markers of mitosis, corresponding to the onsets of metaphase and anaphase. There may well be additional markers that cannot be resolved via histone labeling alone; for example, the work of Parry et al. [21] indicates that calcium may provide another marker for the mitotic process, and we find that the nuclear displacements also provide markers (see below).
Effect of shape changes on nuclear positions
The processes of metaphase and anaphase affect not only the shapes of the chromosomes, but also their positions. After each of the shape changes, the nuclei move collectively through the embryo, almost exclusively along the long axis (which we designate as the  -axis), resulting in a global ‘breathing mode’ of the entire embryo (see Movie S1). Remarkably, after an initial transition in which the nuclei re-organize after anaphase (studied in detail by Kanesaki et al. [29]), the nuclei hardly move with respect to their nearest neighbors during this collective movement. Figure 1e shows the average displacement
-axis), resulting in a global ‘breathing mode’ of the entire embryo (see Movie S1). Remarkably, after an initial transition in which the nuclei re-organize after anaphase (studied in detail by Kanesaki et al. [29]), the nuclei hardly move with respect to their nearest neighbors during this collective movement. Figure 1e shows the average displacement 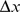 along the
along the  -axis of a small set of nuclei. Figure 1f shows the same motion for all nuclei. Note that there are subtle changes in the gray scale that parallel the metaphasic and anaphasic wavefronts but that are shifted to the right (i.e. occur later in time) with respect to each of those wavefronts. This illustrates that the nuclear displacements follow the same wavefront pattern as the axes ratio, so that the displacements also serve as markers for the mitotic wavefront. The existence of such a marker in the displacements as well as in the axes ratio and in calcium concentration underlines the important interplay of mechanics and biochemistry in the mitotic process.
-axis of a small set of nuclei. Figure 1f shows the same motion for all nuclei. Note that there are subtle changes in the gray scale that parallel the metaphasic and anaphasic wavefronts but that are shifted to the right (i.e. occur later in time) with respect to each of those wavefronts. This illustrates that the nuclear displacements follow the same wavefront pattern as the axes ratio, so that the displacements also serve as markers for the mitotic wavefront. The existence of such a marker in the displacements as well as in the axes ratio and in calcium concentration underlines the important interplay of mechanics and biochemistry in the mitotic process.
The displacement response to the onsets of metaphase and anaphase causes the nuclei to move to new equilibrium positions (Figure 1e). Note that the relaxation time of this response is fairly long, about half the length of the mitotic phase (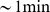 ) for the onset of metaphase and about half the length of the following interphase (up to
) for the onset of metaphase and about half the length of the following interphase (up to 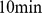 ) for the actual divisions. The displacements following the onset of metaphase therefore occur before the cytoskeletal reconstruction process, which takes place during anaphase, whereas the displacements following the onset of anaphase happen during the aftermath of the cytoskeleton reconstruction.
) for the actual divisions. The displacements following the onset of metaphase therefore occur before the cytoskeletal reconstruction process, which takes place during anaphase, whereas the displacements following the onset of anaphase happen during the aftermath of the cytoskeleton reconstruction.
Wavefront speeds
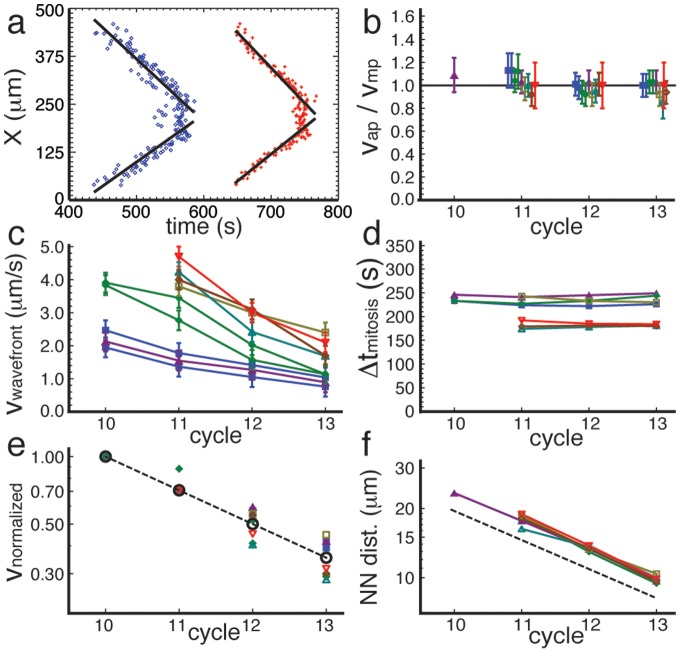
We quantify the wavefront speeds in Figure 2 for two sets of movies, where the environmental conditions (in particular the temperature) were approximately the same for all movies in a given set, but differed between the two sets (the data of the two sets were taken several months apart). Figure 2a shows an example of a position vs. time plot of all metaphase (blue diamonds) and anaphase (red pluses) onset events in a single cycle of a single embryo. The slope, corresponding to the wavefront speed, is clearly constant across the embryo. Figure 2b shows the ratio of the speeds of the wavefronts as measured by the onsets of metaphase and anaphase of all embryos, showing that for a given embryo and cycle, these are identical, confirming that they are two markers of a single process.
Figure 2. Wavefront propagation and speeds.
a)  -coordinate of nuclei at the onset of metaphase (blue diamonds) and anaphase (red pluses) vs. time for the wavefront shown in Figure 1. Both events show two clear wavefronts moving in from near the embryo poles (solid lines). b) Ratio of the speeds of the wavefronts as measured by the onset of anaphase (
-coordinate of nuclei at the onset of metaphase (blue diamonds) and anaphase (red pluses) vs. time for the wavefront shown in Figure 1. Both events show two clear wavefronts moving in from near the embryo poles (solid lines). b) Ratio of the speeds of the wavefronts as measured by the onset of anaphase (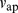 ) and metaphase (
) and metaphase (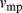 ), for different embryos and cycles. Each embryo is indicated by a different symbol and color, with the closed and open symbols representing two different measurement sets. Ratios for a given cycle and different embryos are slightly separated horizontally. c) Wavefront speed vs. cycle. Two of the embryos contribute two waves per cycle (coming in from opposite poles, as in Figure 1a; blue squares and green diamonds). Although the actual propagation speeds vary significantly from one embryo to the next, they all follow the same trend, decreasing with successive cycles. d) Time interval between the onset of metaphase and anaphase vs. cycle. e) Log-linear plot of wavefront speeds vs. cycle, normalized by the speed of its first observed wavefront (if the first observed wave front is in cycle 10) or 0.71 times its first observed wavefront (if the first observed wavefront is in cycle 11). The black open circles connected by a dashed line corresponds to a scaling of 0.71 per cycle, showing that all embryos follow the same exponentially decaying trend. f) Average distance between nearest neighbors on a logarithmic plot. The dashed line corresponds to a dependence
), for different embryos and cycles. Each embryo is indicated by a different symbol and color, with the closed and open symbols representing two different measurement sets. Ratios for a given cycle and different embryos are slightly separated horizontally. c) Wavefront speed vs. cycle. Two of the embryos contribute two waves per cycle (coming in from opposite poles, as in Figure 1a; blue squares and green diamonds). Although the actual propagation speeds vary significantly from one embryo to the next, they all follow the same trend, decreasing with successive cycles. d) Time interval between the onset of metaphase and anaphase vs. cycle. e) Log-linear plot of wavefront speeds vs. cycle, normalized by the speed of its first observed wavefront (if the first observed wave front is in cycle 10) or 0.71 times its first observed wavefront (if the first observed wavefront is in cycle 11). The black open circles connected by a dashed line corresponds to a scaling of 0.71 per cycle, showing that all embryos follow the same exponentially decaying trend. f) Average distance between nearest neighbors on a logarithmic plot. The dashed line corresponds to a dependence 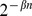 , where
, where  is the cycle number and
is the cycle number and 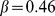 . In Figures b–f, the same symbol/color corresponds to the same embryo.
. In Figures b–f, the same symbol/color corresponds to the same embryo.
From embryo to embryo there are large variations in wavefront speed (Figure 2c), but they all show a consistent reduction in speed from one cycle to the next. This trend is illustrated in Figure 2e, where we plot the same data, normalized by the speed of the first wavefront, on a log-linear scale. Although our data only span a single decade, this figure suggests that the decrease of wavefront speed with cycle number is consistent with a decaying exponential.
Figure 2d shows that the time interval that separates the onset of metaphase from the onset of anaphase is the same for all cycles for a given embryo, but is different for the two different sets of data. By looking at the point at which the nuclear envelope breaks down and reforms, Foe and Alberts [1] also found that the duration of the mitotic phase is constant through cycles 10, 11 and 12 (3 minutes in their observations, comparable to our result), but was longer for cycle 13 (5 minutes). The re-formation of the nuclear envelope membrane may therefore take significantly longer in the last syncytial cycle, even though the actual mitotic processes continue to follow the pattern of the earlier cycles.
Cycle statistics
The nuclei on the surface are separated by a well-defined distance 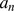 , which decreases with cycle number
, which decreases with cycle number  . Because the number of nuclei doubles from one cycle to the next, it is not surprising that
. Because the number of nuclei doubles from one cycle to the next, it is not surprising that 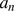 decays exponentially, scaling like
decays exponentially, scaling like 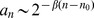 , with
, with  the cycle number and
the cycle number and 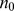 the number of the first observed cycle. We consistently found a value of
the number of the first observed cycle. We consistently found a value of 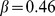 in our experiments (Figure 2f and Table 1). The value of
in our experiments (Figure 2f and Table 1). The value of 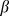 is slightly less than
is slightly less than 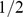 , presumably because the curved embryo is being projected onto a plane. We have also measured the duration of each cycle,
, presumably because the curved embryo is being projected onto a plane. We have also measured the duration of each cycle, 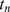 , and found that, over the observed cycles, it increases with cycle number
, and found that, over the observed cycles, it increases with cycle number  , with a weak exponential growth:
, with a weak exponential growth: 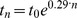 , where
, where 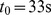 for set 1 and
for set 1 and 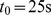 for set 2, see Table 1 and Figure S4.
for set 2, see Table 1 and Figure S4.
Table 1. Experimental data averaged over the data sets.
| Data set 1 | ||||
| cycle number | 10 | 11 | 12 | 13 |
nuclear spacing (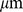 ) ) |
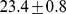
|
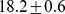
|
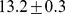
|
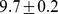
|
wavefront speed (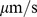 ) ) |
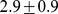
|
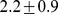
|
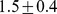
|
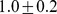
|
| cycle duration (s) |
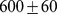
|
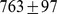
|
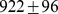
|
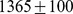
|
| mitosis duration (s) |
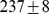
|
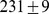
|
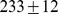
|
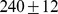
|
Theoretical analysis
Our observation that the mitotic wavefronts propagate at constant speed across the embryo suggests that the embryo can be considered as an excitable medium that supports nonlinear front propagation. Alternatively, the nuclei could all have biological clocks that determine when mitosis starts, which operate independently; in that case the wavefront would be only a result of a lucky timing of those clocks. We discuss various timing models and show that they are inconsistent with our observations in the supplementary material. Here we concentrate on two distinct classes of models for front propagation in excitable media. In the first model the nuclei communicate by releasing a small chemical species, which then diffuses to neighboring nuclei, triggering them to initiate mitosis. In the second model we explore the novel idea that mitotic wavefronts in the early embryo can be described by wavefront propagation in a medium that is mechanically rather than chemically excitable. In this model, forces exerted at the onset of the mitotic phase give rise to mechanical stresses that trigger other nuclei to proceed to mitosis as well.
Biochemical-signaling model
At the end of a cycle, when all nuclei have completed the duplication of their DNA, we assume that they are in an excitable state, meaning that they can be triggered to initiate mitosis once they receive an appropriate signal. An obvious candidate for signaling between nuclei is a small protein (e.g. a Cdk, cyclin or some other activator), which we will denote as 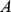 . By definition, nuclei can divide only once per cycle; therefore, in our model, we introduce a refractory period for each nucleus following anaphase, equal to the duration of the interphase.
. By definition, nuclei can divide only once per cycle; therefore, in our model, we introduce a refractory period for each nucleus following anaphase, equal to the duration of the interphase.
To introduce chemical excitability, we assume that if the local concentration of 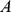 exceeds a threshold
exceeds a threshold  , the nucleus starts its program of mitosis, part of which involves releasing more
, the nucleus starts its program of mitosis, part of which involves releasing more 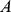 .
. 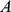 then diffuses away, raising the concentration of
then diffuses away, raising the concentration of 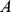 at neighboring nuclei, and so on. In our model we allow for a time delay
at neighboring nuclei, and so on. In our model we allow for a time delay 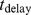 between trigger and release, meaning that a nucleus does not release more
between trigger and release, meaning that a nucleus does not release more 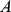 until a time
until a time 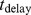 after its local concentration exceeds
after its local concentration exceeds  . We model releases of
. We model releases of 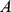 by the nuclei (or sources) as localized pulses (Dirac delta functions), and the system is initiated with a single nucleus releasing a quantity
by the nuclei (or sources) as localized pulses (Dirac delta functions), and the system is initiated with a single nucleus releasing a quantity 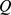 of
of 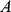 . The wavefront at any point in time corresponds to the position of all nuclei that release
. The wavefront at any point in time corresponds to the position of all nuclei that release 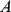 at that moment. Details on how to solve the diffusion equation and carry out the other needed calculations are given in Supplementary File S1. An example wavefront is shown in Figure 3a.
at that moment. Details on how to solve the diffusion equation and carry out the other needed calculations are given in Supplementary File S1. An example wavefront is shown in Figure 3a.
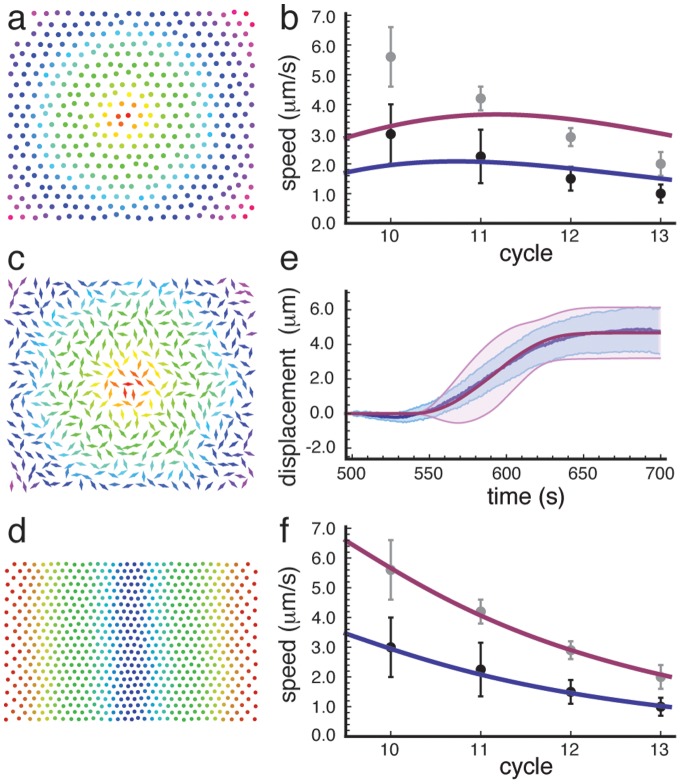
Figure 3. Propagation of wavefronts by chemical and mechanical signaling.
a) Color plot showing the chemical wavefront in two dimensions. The wave starts in the center (red dot) with a single Dirac delta peak release. The color coding indicates when a nucleus releases its chemical to the bulk, going from red through the different hues of the rainbow to violet. b) Plot showing the best fits (blue and purple lines) of the diffusion model with time delay to the to the two sets of experimental data (black and gray dots with error bars). Although the time delay manages to balance the trend that the wavefront speed increases in the region of interest (but not before), the model fails to describe the observed data. Here 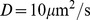 . c) Color plot showing the mechanical wavefront in two dimensions for totally anisotropic dipoles, including their orientations, which are picked at random, and free boundary conditions. The color coding is the same as in Figure a. d) Color plot showing the mechanical wavefront in two dimensions for totally isotropic dipoles and semi-periodic boundary conditions (periodic in vertical direction, free in horizontal direction). Wavefronts are initialized at both free ends simultaneously and travel to the center, as in the experimental system. e) Plot showing fit (purple) of the displacements calculated from the model to the experimentally obtained displacements (blue) following the onset of metaphase. Fit parameters same as in Figure e (set 1). Error bars obtained by averaging over a slice of
. c) Color plot showing the mechanical wavefront in two dimensions for totally anisotropic dipoles, including their orientations, which are picked at random, and free boundary conditions. The color coding is the same as in Figure a. d) Color plot showing the mechanical wavefront in two dimensions for totally isotropic dipoles and semi-periodic boundary conditions (periodic in vertical direction, free in horizontal direction). Wavefronts are initialized at both free ends simultaneously and travel to the center, as in the experimental system. e) Plot showing fit (purple) of the displacements calculated from the model to the experimentally obtained displacements (blue) following the onset of metaphase. Fit parameters same as in Figure e (set 1). Error bars obtained by averaging over a slice of 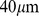 , as indicated in Figure 1a. f) Plot showing fits (blue and purple lines) of the mechanical model for isotropic force dipoles and semi-periodic boundary conditions to the two sets of experimental data (black and gray dots with error bars). Fit parameters:
, as indicated in Figure 1a. f) Plot showing fits (blue and purple lines) of the mechanical model for isotropic force dipoles and semi-periodic boundary conditions to the two sets of experimental data (black and gray dots with error bars). Fit parameters: 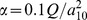 , where
, where 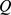 is the dipole strength and
is the dipole strength and 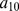 the spacing in cycle 10,
the spacing in cycle 10, 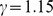 , and
, and 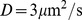 (blue line/black datapoints),
(blue line/black datapoints), 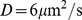 (purple line/gray datapoints).
(purple line/gray datapoints).
In the case of zero delay time, the speed  of the resulting wavefront is determined by three parameters: the diffusion constant
of the resulting wavefront is determined by three parameters: the diffusion constant 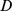 , the nuclear spacing
, the nuclear spacing  and the concentration threshold
and the concentration threshold  . We obtained the value of
. We obtained the value of  from direct measurements (Figure 2f). Gregor et al. [35] found from diffusion experiments in Drosophila that the diffusion constant of a molecule with hydrodynamic radius
from direct measurements (Figure 2f). Gregor et al. [35] found from diffusion experiments in Drosophila that the diffusion constant of a molecule with hydrodynamic radius 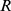 is well described by a modified Stokes-Einstein relation [36]:
is well described by a modified Stokes-Einstein relation [36]:
| (1) |
where 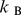 is Boltzmann's constant,
is Boltzmann's constant, 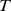 the temperature,
the temperature, 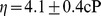 the effective viscosity of the syncytial Drosophila embryo, and
the effective viscosity of the syncytial Drosophila embryo, and 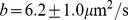 is an experimentally determined constant. Using this expression, we estimate that a reasonable value for the diffusion coefficient (from the size of the activator
is an experimentally determined constant. Using this expression, we estimate that a reasonable value for the diffusion coefficient (from the size of the activator 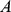 ), would correspond to a chemical with a radius of approximately
), would correspond to a chemical with a radius of approximately 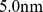 and therefore a diffusion constant of about
and therefore a diffusion constant of about 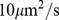 .
.
Combining the parameters of our model, we define a nondimensional threshold and speed:
| (2) |
| (3) |
In a three-dimensional model the power of  in equation (2) is 3. As shown in Supplementary File S1, for a steady-state wavefront, we then have
in equation (2) is 3. As shown in Supplementary File S1, for a steady-state wavefront, we then have 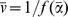 , where
, where 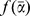 increases monotonically with
increases monotonically with 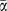 (Figure S6 in File S1). Consequently, if both
(Figure S6 in File S1). Consequently, if both 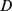 and
and  are fixed, the wavefront speed
are fixed, the wavefront speed  increases as the nuclear spacing
increases as the nuclear spacing  decreases, and thus the speed increases with cell cycle, in direct contradiction to our experimental observations. Thus, the simplest form of the biochemical signaling model cannot describe the data of Figure 2c.
decreases, and thus the speed increases with cell cycle, in direct contradiction to our experimental observations. Thus, the simplest form of the biochemical signaling model cannot describe the data of Figure 2c.
We next consider the possibility of a delay 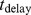 between the time when the local concentration of
between the time when the local concentration of 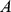 reaches the threshold value
reaches the threshold value  , and the instant when more
, and the instant when more 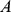 is released. In the limit where
is released. In the limit where 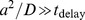 , the wavefront speed is determined by diffusion as before,
, the wavefront speed is determined by diffusion as before, 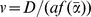 . In the opposite limit,
. In the opposite limit, 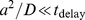 , we find
, we find 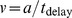 , so
, so  would decrease with cycle number for constant
would decrease with cycle number for constant 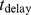 . We find that for our system, introducing a small, fixed delay time of
. We find that for our system, introducing a small, fixed delay time of 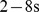 puts us in the crossover regime between these two types of behavior. Consequently, the model predicts that for the first few cycles, the wavefront speed should increase, whereas it should level off or slightly decrease in the last cycle. Changing the value of the threshold
puts us in the crossover regime between these two types of behavior. Consequently, the model predicts that for the first few cycles, the wavefront speed should increase, whereas it should level off or slightly decrease in the last cycle. Changing the value of the threshold  does not qualitatively change this result. Changing the value of the diffusion constant
does not qualitatively change this result. Changing the value of the diffusion constant 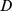 simply shifts the position of the crossover.
simply shifts the position of the crossover.
A key result of our analysis with a fixed time delay is that a physically unrealistic diffusion coefficient is required in order to reproduce our experimental observations. In order to obtain a strictly decreasing wavefront speed for the range of interest, a diffusion constant of more than 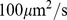 is required. This corresponds to a signaling particle that is even smaller than an ion. Thus, a biochemical-signaling model with a time delay that is independent of cell cycle cannot describe our observations either (Figure 3b).
is required. This corresponds to a signaling particle that is even smaller than an ion. Thus, a biochemical-signaling model with a time delay that is independent of cell cycle cannot describe our observations either (Figure 3b).
We also investigated the wavefront speed in the case where the delay time is allowed to vary from one cycle to the next. Naturally, given a value for the diffusion constant and the threshold, for each cycle we can find a delay time such that the speed predicted by the model matches the observed speed; these values are listed in Table S1 in File S1. The found values do not show any consistent trend, and differ quite strongly between the two data sets. There is no obvious explanation for what would set the time delay in each cycle; the time delay is not proportional to the total duration of the cycle (which increases from one cycle to the next) or any other obvious time scale. Therefore, this procedure simply shifts the problem from understanding the trend in the wavefront speed to understanding the trend in the delay time, and does not provide a satisfactory explanation of our data.
On the basis of these results, we conclude that it is very unlikely that a wavefront that propagates via diffusion of some chemical species would slow down with cycle number, as observed in our experiments. We also note that any model in which the biochemical signal is mediated by a method that is faster than diffusion (such as active transport) suffers from the same problem: the predicted wavespeed would go up with increasing cycle, because the spacing between the nuclei goes down.
Mechanical-signaling model
The early embryo cannot support ordinary elastic waves because it is heavily damped by the viscosity of the cytosol. Consequently, displacements do not propagate ballistically as in a wave, but diffusively. However, just as diffusion of 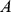 can lead to nonlinear wavefront propagation in the biochemical signaling model, diffusion of displacement could lead to wavefront propagation in a mechanical signaling model. We therefore introduce a model in which the nuclei communicate via stresses or strains that they exert on the cytoskeleton at the initiation of the mitotic phase. For example, these could be the forces that cause the chromosomes to condense into sister chromatids in prophase or to align in the nuclear midplane at the onset of metaphase.
can lead to nonlinear wavefront propagation in the biochemical signaling model, diffusion of displacement could lead to wavefront propagation in a mechanical signaling model. We therefore introduce a model in which the nuclei communicate via stresses or strains that they exert on the cytoskeleton at the initiation of the mitotic phase. For example, these could be the forces that cause the chromosomes to condense into sister chromatids in prophase or to align in the nuclear midplane at the onset of metaphase.
In our model, a nucleus starts its program when the largest eigenvalue of the local stress tensor exceeds a threshold value  . We describe the cytoskeleton as a homogeneous linear elastic medium, characterized by two elastic parameters, for example its bulk and shear moduli (
. We describe the cytoskeleton as a homogeneous linear elastic medium, characterized by two elastic parameters, for example its bulk and shear moduli (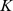 and
and 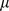 , respectively) or equivalently the Young's modulus
, respectively) or equivalently the Young's modulus 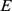 and dimensionless Poisson ratio
and dimensionless Poisson ratio  . The viscous fluid in which the elastic cytoskeleton is immersed exerts a drag force on it, characterized by a damping constant
. The viscous fluid in which the elastic cytoskeleton is immersed exerts a drag force on it, characterized by a damping constant  . Assuming that the nuclei exist in a thin layer near the surface of the embryo, we denote the deformations in the plane of the layer by
. Assuming that the nuclei exist in a thin layer near the surface of the embryo, we denote the deformations in the plane of the layer by 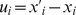 (
(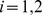 ), where the deformation maps point
), where the deformation maps point 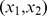 onto point
onto point 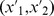 . In the overdamped limit (zero Reynolds number), the displacement
. In the overdamped limit (zero Reynolds number), the displacement 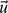 of a nucleus can be described by [37]:
of a nucleus can be described by [37]:
| (4) |
The term on the left represents the damping with damping factor  , and the two terms on the right are the elastic force per unit volume. Equation (4) is reminiscent of the diffusion equation: a time derivative on the left equals second-order space derivatives on the right. This model can therefore be thought of as describing the diffusion of the vector displacement field
, and the two terms on the right are the elastic force per unit volume. Equation (4) is reminiscent of the diffusion equation: a time derivative on the left equals second-order space derivatives on the right. This model can therefore be thought of as describing the diffusion of the vector displacement field 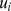 . The right hand side of equation (4) gives rise to two quantities with the dimensions of diffusion constants [34]:
. The right hand side of equation (4) gives rise to two quantities with the dimensions of diffusion constants [34]:
| (5) |
In order to introduce mechanical excitability into the model, we assume that if the largest eigenvalue of the stress tensor at a nucleus at position 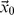 exceeds a threshold value,
exceeds a threshold value,  , at time
, at time 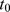 , it triggers the nucleus into action which involves adding additional stress to the system. This stress can be added in the form of a source term
, it triggers the nucleus into action which involves adding additional stress to the system. This stress can be added in the form of a source term 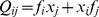 , a symmetric tensor of rank 2, corresponding to a force per unit volume
, a symmetric tensor of rank 2, corresponding to a force per unit volume 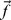 acting over a distance
acting over a distance 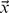 .
. 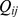 is therefore the symmetric combination of a force and a distance, with the dimensions of a stress (force per unit area), so it represents a stress source. In two dimensions,
is therefore the symmetric combination of a force and a distance, with the dimensions of a stress (force per unit area), so it represents a stress source. In two dimensions, 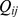 has an isotropic part of the form
has an isotropic part of the form 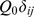 and a traceless anisotropic part of the form
and a traceless anisotropic part of the form 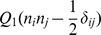 where
where 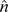 indicates the anisotropy direction. If
indicates the anisotropy direction. If 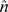 makes an angle
makes an angle  with the
with the  -axis, we find that the components of
-axis, we find that the components of 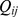 in matrix notation are given by:
in matrix notation are given by:
| (6) |
Here 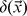 is the two-dimensional Dirac delta function. Similar active force dipoles have previously been introduced into other tissue-level models, such as those of Bischofs et al. [38] and Ranft et al. [39]. To include the force due to the added stress at
is the two-dimensional Dirac delta function. Similar active force dipoles have previously been introduced into other tissue-level models, such as those of Bischofs et al. [38] and Ranft et al. [39]. To include the force due to the added stress at 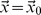 and
and 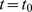 , we add the term
, we add the term 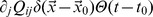 to the right hand side of equation (4):
to the right hand side of equation (4):
| (7) |
where 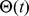 is the Heaviside step function. Equation (7) essentially describes the diffusion of the vector displacement field
is the Heaviside step function. Equation (7) essentially describes the diffusion of the vector displacement field 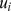 due to a tensor source term. It is similar, but not identical, to a scalar reaction-diffusion equation, which describes the evolution of a scalar concentration field
due to a tensor source term. It is similar, but not identical, to a scalar reaction-diffusion equation, which describes the evolution of a scalar concentration field  due to a scalar source term. It is therefore not surprising that the model described by equation (4) also produces wavefronts, as can be seen in Figure 3c and d.
due to a scalar source term. It is therefore not surprising that the model described by equation (4) also produces wavefronts, as can be seen in Figure 3c and d.
In order to compare the model results with the data, we need to estimate the values of the elastic constants and the damping parameter. The speed  now depends on the quantity
now depends on the quantity 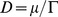 that determines the dimensional part of both diffusion constants (equation (5)), as well as the nuclear spacing
that determines the dimensional part of both diffusion constants (equation (5)), as well as the nuclear spacing  , the strengths
, the strengths 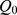 and
and 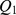 of the source term, and the threshold value
of the source term, and the threshold value  . It is well known that the values of both the elastic and the viscous modulus of a polymer network depend strongly on filament concentration [40]–[46], which can differ from one cycle to the next. Because the number of nuclei doubles in each cycle, the number of actin caps in the network doubles as well (see Figures S1 and S2). Thus, the local concentration of actin and of microtubules should effectively double with cycle number
. It is well known that the values of both the elastic and the viscous modulus of a polymer network depend strongly on filament concentration [40]–[46], which can differ from one cycle to the next. Because the number of nuclei doubles in each cycle, the number of actin caps in the network doubles as well (see Figures S1 and S2). Thus, the local concentration of actin and of microtubules should effectively double with cycle number  . We therefore write
. We therefore write 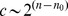 , where as before
, where as before 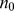 is the number of the first observed cycle. Both the storage and loss moduli of polymer networks increase with concentration approximately as power laws, but the actual powers are debated [42], [44]–[46]. Moreover, in each successive cycle the nuclei get pushed further out into the plasma membrane encompassing the entire embryo [1], increasing the friction coefficient. Because the dynamics of our system depend only on the value of the two effective diffusion constants given in equation (5), we will not be able to distinguish the dependence of the storage and loss moduli independently. Instead we assume a dependence
is the number of the first observed cycle. Both the storage and loss moduli of polymer networks increase with concentration approximately as power laws, but the actual powers are debated [42], [44]–[46]. Moreover, in each successive cycle the nuclei get pushed further out into the plasma membrane encompassing the entire embryo [1], increasing the friction coefficient. Because the dynamics of our system depend only on the value of the two effective diffusion constants given in equation (5), we will not be able to distinguish the dependence of the storage and loss moduli independently. Instead we assume a dependence 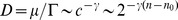 . We will use
. We will use  as a fit parameter.
as a fit parameter.
Because of the mathematical similarity between the mechanical-signaling model (equation (4)) and the diffusion model for concentration fields, we can use the same type of dimensional analysis as for the biochemical-signaling model. We again use the dimensionless threshold 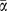 and wavefront speed
and wavefront speed 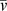 defined by equations (2) and (3), where
defined by equations (2) and (3), where 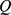 is now the typical strength of the source term, and we write
is now the typical strength of the source term, and we write 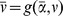 . We determine
. We determine 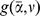 numerically, and find that it can be well described by the functional form
numerically, and find that it can be well described by the functional form 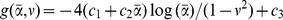 , where
, where 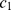 ,
, 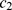 and
and 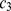 are constants that depend on the choice of source term and boundary conditions [34]. In the analysis that follows, we have adopted boundary conditions that are free along the long axis and periodic along the short axis to mimic the elongated shape of the embryo.
are constants that depend on the choice of source term and boundary conditions [34]. In the analysis that follows, we have adopted boundary conditions that are free along the long axis and periodic along the short axis to mimic the elongated shape of the embryo.
Figure 3e shows a fit to a displacement wavefront profile following the first detectable sign of the mitotic wavefront (onset of metaphase) in the initial (tenth) cycle. We find that in order to fit the profile, the source term (6) must be nearly isotropic, so that 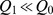 . We therefore set
. We therefore set 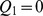 and fit to find the threshold stress, which gives
and fit to find the threshold stress, which gives 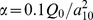 , with
, with 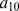 the spacing in cycle 10. Thus, the threshold stress is approximately ten percent of the force exerted per unit area.
the spacing in cycle 10. Thus, the threshold stress is approximately ten percent of the force exerted per unit area.
Figure 3f shows a fit of the wavefront speed of the two datasets, 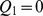 and
and 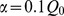 . Here, the fit parameter is the exponent
. Here, the fit parameter is the exponent  that governs the change in the displacement diffusion constant from cycle to cycle. Both datasets are well-described with a value of
that governs the change in the displacement diffusion constant from cycle to cycle. Both datasets are well-described with a value of 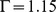 . The only difference between the two datasets is the value of the displacement diffusion constant
. The only difference between the two datasets is the value of the displacement diffusion constant 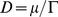 in the 10th cycle, which is about
in the 10th cycle, which is about 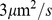 for set 1 and about
for set 1 and about 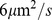 for set 2.
for set 2.
These values for the diffusion constant are comparable to those found in microrheology experiments, which have measured the frequency-dependent complex shear modulus in a variety of living cells [47]–[51]. In contrast to pure actin networks, living systems often do not exhibit a low-frequency plateau in the storage modulus 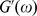 . Although this makes a precise determination of the shear modulus difficult, we can still get a decent order-of-magnitude estimate from the experimental data at
. Although this makes a precise determination of the shear modulus difficult, we can still get a decent order-of-magnitude estimate from the experimental data at 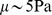 . The damping constant
. The damping constant  is given by
is given by 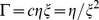 [45], [52], where
[45], [52], where  is the filament concentration,
is the filament concentration, 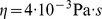 [35] is the ambient fluid viscosity, and
[35] is the ambient fluid viscosity, and 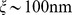 is the mesh size of the actin network. We thus estimate
is the mesh size of the actin network. We thus estimate 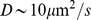 , in good agreement with our fitting results.
, in good agreement with our fitting results.
The found value for the exponent  is also reasonable. In-vitro experiments on entangled F-actin solutions indicate that the storage and loss moduli depend on the concentration in the same way [45], which leads us to expect the shear modulus
is also reasonable. In-vitro experiments on entangled F-actin solutions indicate that the storage and loss moduli depend on the concentration in the same way [45], which leads us to expect the shear modulus 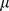 and viscosity
and viscosity 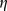 to have similar dependence on
to have similar dependence on  . On the other hand, for a semidilute solution of rigid rods, the viscosity is expected to rise as
. On the other hand, for a semidilute solution of rigid rods, the viscosity is expected to rise as 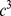 , where
, where  is the filament concentration [40]. Because the damping factor
is the filament concentration [40]. Because the damping factor  scales with the concentration and the mesh size
scales with the concentration and the mesh size  , which itself depends on the concentration as
, which itself depends on the concentration as 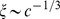 , we find that
, we find that  should be somewhere between
should be somewhere between 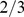 (for an entangled F-actin solution) and
(for an entangled F-actin solution) and 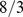 (for a semidilute solution). Our value of
(for a semidilute solution). Our value of 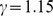 indicates that our system falls somewhere in between these two regimes, which is reasonable for the Drosophila embryo, with its hemispherical actin caps enclosing each nucleus (see Figure S2).
indicates that our system falls somewhere in between these two regimes, which is reasonable for the Drosophila embryo, with its hemispherical actin caps enclosing each nucleus (see Figure S2).
Figures 3e and f show that we can consistently fit both the wavefront velocity and the displacement profile of the nuclei as a function of time immediately after the metaphasic wavefront, with the same theory. We note that this is not possible with the chemical signaling model, which cannot provide any information about the displacement profile. The fact that we can fit both quantities with the same parameters therefore provides strong evidence in favor of the mechanical signaling model.
In addition, we note that the nuclear displacement profile provides a more discriminating test of the mechanical signaling model than the wavefront velocity. Although the velocity wavefront speed data alone can be fitted by either purely isotropic force dipoles or purely anisotropic force dipoles (and presumably anything in between), the displacement wavefront can only be fit with dipoles with a strong isotropic component. Moreover, although either the displacement or the velocity data can be fit with different combinations of the threshold and diffusion constant, the numbers given above are the only ones for which we can fit both quantities.
In summary, the mechanical signaling model agrees much better with the data than the biochemical signaling model in two important respects. First, it captures the dependence of the wavefront velocity on cell cycle number while the biochemical signaling model does not. From dimensional analysis, we have shown for both models that the wavefront velocity depends mainly on 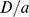 , where
, where 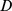 is the diffusion constant and
is the diffusion constant and  is the average distance between nuclei. Note that
is the average distance between nuclei. Note that  decreases with cycle number. In the case of biochemical signaling, the chemical diffusion coefficient
decreases with cycle number. In the case of biochemical signaling, the chemical diffusion coefficient 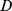 remains constant with cycle number, leading to a wavefront velocity that tends to increase with cycle number. In the case of mechanical signaling, however, the displacement diffusion coefficient,
remains constant with cycle number, leading to a wavefront velocity that tends to increase with cycle number. In the case of mechanical signaling, however, the displacement diffusion coefficient, 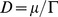 , decreases quite strongly with cycle number because the damping coefficient,
, decreases quite strongly with cycle number because the damping coefficient,  , should increase more rapidly with filament concentration than the elastic constant,
, should increase more rapidly with filament concentration than the elastic constant, 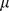 . If we make the reasonable assumption that the filament concentration increases with cycle number, then this means that the stress diffusion coefficient decreases with cycle number, leading to a wavefront velocity that decreases with cycle number, in accord with experimental observations. Second, we have shown that the mechanical signaling model describes not only the wavefront velocity but also the displacement profile following the metaphasic wavefront. In the biochemical-signaling model, a separate mechanical description would be necessary in order to describe the nuclear displacements.
. If we make the reasonable assumption that the filament concentration increases with cycle number, then this means that the stress diffusion coefficient decreases with cycle number, leading to a wavefront velocity that decreases with cycle number, in accord with experimental observations. Second, we have shown that the mechanical signaling model describes not only the wavefront velocity but also the displacement profile following the metaphasic wavefront. In the biochemical-signaling model, a separate mechanical description would be necessary in order to describe the nuclear displacements.
Finally, we note that we have assumed that the elastic constants and damping coefficients vary from cycle to cycle but do not change much during the period that we are focusing on. However, the cytoskeleton reconstructs completely during the cell cycle. Our analysis will apply as long as the elastic constants and damping coefficient do not change appreciably from the time that the original triggering wavefront is generated to the time that the anaphasic wavefront occurs. Thus, the assumption is that cytoskeletal reconstruction occurs sometime during anaphase and is finished before the process of mitosis begins in the next cycle. In particular, this also means that our model should not be able to correctly predict the much larger displacements following anaphase (see Figure 1e), which indeed it cannot.
Discussion
During the early cycles of Drosophila development, the cycles of the nuclei are strongly coupled across the entire embryo by mitotic wavefronts that travel at constant speed across the embryo. We summarize our observations as follows:
There are several markers of the mitotic process in each cycle, corresponding to the onsets of metaphase and anaphase, which are visible as wavefronts that travel across the embryo (Figure 1d).
The speed of the mitotic wavefronts slows down in each successive cycle (Figures 2c and 2e).
The onsets of metaphase and anaphase both trigger a mechanical response of the entire embryo in the form of displacements of the nuclei that also exhibit a wavefront pattern (Figure 1f).
In addition to these observations, we add those of Parry et al. [21]:
There is a visible wavefront in calcium release that coincides with the onset of anaphase.
The speed of the calcium wavefront slows down in each successive cycle, presumably matching the speed of the mitotic wavefront.
We have considered two scenarios to assess whether they are consistent with these observations. In both cases, based on observations (1), (2) and (5), we take the observed metaphase, anaphase and calcium wavefronts to be different markers of the same mitotic process, and assume that the mitotic wavefront is triggered by a single event.
Scenario A
Mitosis is triggered by a biochemical signal. Here we assume that a biochemical signal is responsible for triggering mitosis. The signal is mediated by the release and subsequent diffusion of a small ion, molecule or protein. The only chemical species that is known to exhibit a wavefront pattern during mitosis is calcium. However, because the onset of metaphase happens well before the observed calcium wavefront, which coincides with the onset of anaphase (5), calcium cannot be the signal carrier. Our theoretical analysis suggests that biochemical signaling is unlikely to be consistent with observation (3), since the natural tendency of such a model is to produce a wavefront speed that increases with cycle number. The larger the signaling molecule, the more pronounced this tendency is. Thus, we conclude that Scenario A is unlikely.
This prediction could be tested by looking for wavefronts in likely signaling species. If the wavefront propagates biochemically, then wavefronts should be observable in the appropriate signaling molecules (presumably CDKs or cyclins that are known to govern checkpoints in the cell cycle that precede the onset of metaphase [53]). If, as our model suggests, the wavefront does not propagate biochemically, then the original signaling molecule should not exhibit wavefronts.
Scenario B
Mitosis is triggered by a mechanical signal. In this scenario, there is a mechanical wavefront that triggers mitosis. The signal is transmitted via stress changes in the embryo and amplified by further release of stress as other nuclei enter the mitotic phase. Because this wavefront propagates mechanically, this speed slows down with successive cycles (2). Since we observe a metaphasic wavefront whose speed of propagation slows down with cycle, the metaphasic wavefront itself could be the triggering mechanical wavefront. It is more likely, however, that the triggering wavefront occurs earlier in the cycle and starts a clock in each nucleus, which controls the mitotic process. As a result of this clock, there are many markers of the process that exhibit the same wavefront pattern, including the onsets of metaphase and anaphase (1), the release of calcium (5), and displacements of the nuclei during metaphase and anaphase (3). This scenario is consistent with all observations.
Scenario B is consistent as well with independent observations made in Xenopus embryos. These embryos are not syncytial; instead they are divided into cells from the first cycle. It is unlikely that a biochemical signal could cross cell membranes to propagate a wavefront. Nevertheless, these embryos do exhibit metachronous mitosis [5]. They also exhibit calcium oscillations inside each cell, which precede anaphase [54]. Their behavior is therefore most consistent with Scenario B: an initial mechanical wavefront triggered by a mechanical process at the onset of metaphase or earlier, is followed by a calcium signal inside each cell and an anaphasic wavefront.
We emphasize that Scenario B does not imply that the entire process of triggering mitosis is mechanical. Indeed, the mechanism by which additional stress is generated via a force dipole in our model must be biochemical. First, there must be some sensor components that are activated when the stress exceeds its threshold value. These components must then activate other biochemical species to eventually generate additional stress by creating a force dipole. If Scenario B is correct, there should be a way of incorporating our mechanically signaling model into models of the chemical networks that control the cell cycle, such as those of Tyson and Novak [53]. One question is whether the original triggering mechanical wavefront serves as a checkpoint in the cycle. In order to understand how to include mechanical signaling into such models, it is critical to have new experiments to identify precisely the original triggering wavefront. Our model would predict that signaling molecules in stages of the cell cycle that follow this triggering wavefront should exhibit wavefronts that slow down with cycle, while those in stages that precede the triggering wavefront should not.
In principle, the estimated elastic constants and damping coefficients could be obtained directly from experiments by measuring the storage and loss moduli of the embryo surface in vivo using two-point microrheology. Optical tweezer experiments similar to the ones done by Schötz et al. [30] could also be used to extract the elastic moduli and the drag coefficient we used in our mechanical model. The actin concentration could be measured at the same time by staining the actin filaments with e.g. rhodamine, as done by Parry et al. [21] or GFP-moesin, as done by Cao et al. [55].
Even though the process of mitosis is known to require chemical activation, the key assumption in Scenario B is that the initial wavefront also propagates mechanically. This can be tested by mechanically poking the embryo at different times within the cell cycle. If the cell is poked just in advance of the original triggering wavefront, the poking itself should generate a wave that propagates from the poking site with the same speed as the mitotic wavefront. If the embryo is poked too far in advance of the original triggering wavefront, however, there should be no response. If the embryo is poked after the mitotic wavefront begins, there may be no response because the nuclei have already entered mitosis and can no longer be triggered. Thus, we would expect that poking could generate a mitotic wavefront only if it is applied in a certain time window of the cycle that could serve to identify the original triggering wavefront. Note that experiments by Farge at a slightly later stage of development in Drosophila showed that mechanical stress applied in the appropriate time window can lead dramatic changes in development [31]; Scenario B suggests that mechanical stress is important even at the syncytial stages studied here.
Finally, we note that biochemical experiments could also test the mechanical-signaling model. The most straightforward test would be to to destroy or degrade the filaments that mechanically couple the nuclei. This should prevent the mechanical wavefronts from propagating and thus the nuclei from synchronizing their mitosis. This could be done by injecting colcemid or nocodazole to disrupt the microtubules or latrunculin which affects actin filaments, for example [3]. Other means of disrupting cytoskeletal filaments, via mutation or laser ablation, should also affect the mechanical wave.
Materials and Methods
Confocal videos
The imaged flies were from a His-GFP stock with a P [w+ ubi-H2A-GFP] insertion on the third chromosome. All embryos were collected at 25°C and dechorionated in 100% bleach for 1 minute. They were picked using a  nylon strainer (BD Falcon), rinsed in distilled water and laid down on a semipermeable membrane (Biofolie). The excess water was absorbed and the embryos were immersed in Halocarbon oil 27 (Sigma Aldrich) and covered with a
nylon strainer (BD Falcon), rinsed in distilled water and laid down on a semipermeable membrane (Biofolie). The excess water was absorbed and the embryos were immersed in Halocarbon oil 27 (Sigma Aldrich) and covered with a  coverslip (Corning). Embryos were imaged with a
coverslip (Corning). Embryos were imaged with a 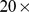 oil immersion objective plan apochromat (Leica, NA = 0.7) on a Leica SP5 laser scanning microscope with excitation wavelength of
oil immersion objective plan apochromat (Leica, NA = 0.7) on a Leica SP5 laser scanning microscope with excitation wavelength of 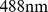 (argon laser
(argon laser 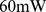 ). 8 bit images were taken every second at
). 8 bit images were taken every second at 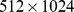
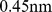 pixels and
pixels and 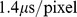 (
(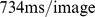 ). An example video is shown in Movie S1.
). An example video is shown in Movie S1.
Image analysis
We visualized nuclear DNA/chromosomes by tagging their histones with GFP. To determine the positions, sizes, aspect ratios and orientations of the DNA/chromosomes from each video frame, we developed a new image analysis technique, explained in detail in [56]. In brief, we first applied a bandpass filter to eliminate high-frequency noise. We then made a contour plot of the resulting image, found the locally highest-level contour (i.e., the contours with no other contour inside them), and identified each of them as a single nucleus. For each nucleus, we fit the contour at half-height with an ellipse to get its position, shape and orientation. An example of an experimental image with the chromosomal tracking overlaid is given in Figure 1a.
Because the images were taken at high frequency (typically 1 Hz), the nuclei move less than their own radius from one frame to the next, simplifying tracking. The obvious exception is when nuclei divide during anaphase, and the observed shape splits in two. Because we detect shapes as well as positions of the chromosomes in each nucleus, tracking divisions is easy as well: when a nucleus divides, the chromosomes become highly elongated just before they split, and produce two almost circular daughters close to the endpoints of the long axis of the mother immediately after it splits, which are easily identified.
Experimental data sets
Our image analysis results are for two different sets of experiments, which were carried out at ambient room temperature several months apart. The ambient temperature was higher for the second set, resulting in faster embryo development. We only used the data from those embryos which we could track from cycle 10–14 in the first set (Dataset 1, 3 embryos) and cycle 11–14 in the second set (Dataset 2, 4 embryos). Movie S1 is the raw data of one of the embryos from set 1. This confocal microscopy imaging movie shows a developing Drosophila embryo. The chromosomal histones are visualized by labeling with GFP. The version of the movie shown here shows 1 image per 15 s, displayed at 5fps, so sped up 75×. Movies for data analysis were recorded at 1fps. The dimensions of each frame are 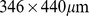 .
.
Additional image analysis results
The average data from the two sets are given in Table 1, and their average speeds are plotted on a log-linear scale in Figure S3. The data from set 1 are given as closed symbols (blue, purple and green) in Figure S3, the data from set 2 as open symbols (cyan, orange, gold and red). In Figures 3c and S6, the black dots correspond to the mean wavefront speeds of set 1, and the gray ones to the mean speeds of set 2.
In addition to the data shown in Figure 2, we also measured the duration of each of the cycles (Figure S4a). The numbers we found are consistent with those reported by Foe and Alberts [1] and Parry et al. [21]. Averaging over the embryos in each set, we find that the cycle duration can be reasonably approximated by a quadratic dependence on the cycle number (Figure S4b).
Supporting Information
Illustration showing the four stages of the Drosophila embryo replication cycle that can be detected from our movies: interphase (DNA replication), metaphase (condensation of chromosomes in the nuclear midplane), anaphase (division of the nucleus in two daughter nuclei) and telophase (separation of daughter nuclei). The plasma membrane is shown in gray, the actin cap (made of actin filaments) in red, the microtubules in green, the centrosomes in yellow, and the DNA/chromosomes in blue.
(TIFF)
Sketch of a cross-section through a Drosophila embryo valid for stages 9–13. Most nuclei are located at the surface of the embryo. The nuclei are pushed outwards into the plasma membrane (gray), resulting in the formation of somatic buds. Each nucleus is enclosed in a microtubule basket (green) and contained in an individual actin cap (red), which gets disassembled after mitosis and re-assembled during interphase. DNA/chromosomes are shown in blue and centrosomes in yellow. The yolk (light blue) is a viscoelastic fluid containing water, cytoskeletal elements and necessary building blocks for the nuclei. The yolk is bounded by an actin cortex over which the nuclei can move. Also shown in this sketch are the small number of nuclei that reside inside the yolk, and the also small number of somatic cells that already form in cycle 10 at the posterior end (the pole cells that divide out of sync with the rest of the embryo). See Foe and Alberts [1] for sketches for each of the first 14 cycles and Schejter and Wieschaus [4] for a review on the cytoskeletal elements in the early embryo.
(TIFF)
Average speed of each of the two sets of data, on a log-linear plot. The data are fitted by an exponential 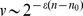 ,
, 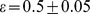 . The black dots correspond to the mean wavefront speeds of set 1, and the gray ones to the mean speeds of set 2.
. The black dots correspond to the mean wavefront speeds of set 1, and the gray ones to the mean speeds of set 2.
(TIFF)
Duration of the measured cycles. a) Experimental data. The different symbols and colors correspond to the ones in Figure 2. b) Cycle duration averaged over all experimentally observed embryos (black and gray dots for sets 1 and 2 respectively). The cycle durations can be fitted reasonably well by a weak exponential 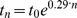 , where
, where 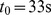 (set 1) and
(set 1) and 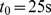 (set 2).
(set 2).
(TIFF)
Supplementary information, in which we discuss the various models in more detail: (1) Timing models for wavefront propagation; (2) Diffusion model for wavefront propagation, including models with time delay; and (3) Mechanical model for wavefront propagation. We also analyze the steady-state of a propagating wavefront in both the diffusion and mechanical model.
(PDF)
Confocal microscopy imaging movie of a developing Drosophila embryo. The chromosomal histones are visualized by labeling with GFP. The version of the movie shown here shows one image per 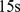 , displayed at
, displayed at 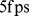 , so sped up
, so sped up 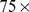 . Movies for data analysis were recorded at
. Movies for data analysis were recorded at  . The dimensions of each frame are
. The dimensions of each frame are 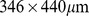 .
.
(AVI)
Acknowledgments
We thank Thomas Gregor for providing resources for the experiments and for his careful reading of the manuscript, and Xiaoyang Long for assistance with acquiring the experimental data. We also thank Gareth Alexander and Michael Lampson for helpful discussions.
Funding Statement
The study was supported by a Rubicon grant from the Netherlands Organization for Scientific Research (NWO) to T.I., http://www.nwo.nl/rubicon; Grant NSF-DMR-1104637 from the National Science Foundation (NSF) to A.J.L., and grant NSF-DMR-1104707 from the NSF to L.K. and T.C.L., http://www.nsf.gov/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Foe VE, Alberts BM (1983) Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci 61: 31–70. [DOI] [PubMed] [Google Scholar]
- 2. Foe VE (1989) Mitotic domains reveal early commitment of cells in Drosophila embryos. Development 107: 1–22. [PubMed] [Google Scholar]
- 3.Foe VE, Odell G, Edgar BA (1993) Mitosis and morphogenesis in the Drosophila embryo: Point and Counterpoint. In: Bate M, Martinez-Arias A, editors, Development of Drosophila melanogaster. Cold Spring Harbor: Cold Spring Harbor Press, pp. 149–300.
- 4. Schejter ED, Wieschaus E (1993) Functional elements of the cytoskeleton in the early Drosophila embryo. Annu Rev Cell Biol 9: 67–99. [DOI] [PubMed] [Google Scholar]
- 5. Satoh N (1977) Metachronous cleavage and initiation of gastrulation in amphibian embryos. Develop, Growth and Differ 19: 111–117. [DOI] [PubMed] [Google Scholar]
- 6. Newport J, Kirschner M (1982) A major developmental transition in early Xenopus embryos: I. Characterization and timing of cellular changes at the midblastula stage. Cell 30: 675–686. [DOI] [PubMed] [Google Scholar]
- 7. Saka Y, Smith JC (2001) Spatial and temporal patterns of cell division during early Xenopus embryogenesis. Dev Biol 229: 307–318. [DOI] [PubMed] [Google Scholar]
- 8. Kageyama T (1986) Mitotic wave in the yolk syncytial layer of embryos of Oryzias latipes originates in the amplification of mitotic desynchrony in early blastomeres. Zool Sci 3: 1046. [Google Scholar]
- 9. Trinkaus JP (1992) The midblastula transition, the YSL transition and the onset of gastrulation in Fundulus . Development 116: 75–80. [PubMed] [Google Scholar]
- 10. Kane DA, Warga RM, Kimmel CB (1991) Mitotic domains in the early embryo of the zebrafish. Nature 360: 735–737. [DOI] [PubMed] [Google Scholar]
- 11. Kane DA, Kimmel CB (1993) The zebrafish midblastula transition. Development 119: 447–456. [DOI] [PubMed] [Google Scholar]
- 12. Devreotes P (1989) Dictyostelium discoideum: a model system for cell-cell interactions in development. Science 245: 1054–1058. [DOI] [PubMed] [Google Scholar]
- 13. Levine H, Reynolds W (1991) Streaming instability of aggregating slime mold amoebae. Phys Rev Lett 66: 2400–2403. [DOI] [PubMed] [Google Scholar]
- 14. Lee KJ, Cox EC, Goldstein RE (1996) Competing patterns of signaling activity in Dictyostelium Discoideum . Phys Rev Lett 76: 1174–1177. [DOI] [PubMed] [Google Scholar]
- 15. Kleckner N, Zickler D, Jones GH, Dekker J, Padmore R, et al. (2004) A mechanical basis for chromosome function. Proc Natl Acad Sci USA 101: 12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, et al.. (2008) Molecular biology of the cell. New York, NY, U.S.A.: Garland Science, 5th edition.
- 17. Silver RB (1989) Nuclear envelope breakdown and mitosis in sand dollar embryos is inhibited by microinjection of calcium buffers in a calcium-reversible fashion, and by antagonists of intracellular Ca2+ channels. Dev Biol 131: 11–26. [DOI] [PubMed] [Google Scholar]
- 18. Silver RB (1990) Calcium and cellular clocks orchestrate cell division. Ann N Y Acad Sci 582: 207–221. [DOI] [PubMed] [Google Scholar]
- 19. Thomas AP, Bird GSJ, Hajnóczky G, Robb-Gaspers LD, Putney JW (1996) Spatial and temporal aspects of cellular calcium signaling. FASEB J 10: 1505–1517. [PubMed] [Google Scholar]
- 20. Allbritton NL, Meyer T (1993) Localized calcium spikes and propagating calcium waves. Cell Calcium 14: 691–697. [DOI] [PubMed] [Google Scholar]
- 21. Parry H, McDougall A, Whitaker M (2005) Microdomains bounded by endoplasmic reticulum segregate cell cycle calcium transients in syncytial Drosophila embryos. J Cell Biol 171: 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parry H, McDougall A, Whitaker M (2006) Endoplasmic reticulum generates calcium signalling microdomains around the nucleus and spindle in syncytial Drosophila embryos. Biochem Soc Trans 34: 385–388. [DOI] [PubMed] [Google Scholar]
- 23. Jaffe LF (2008) Calcium waves. Phil Trans R Soc B 363: 1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whitaker M (2008) Calcium signalling in early embryos. Phil Trans R Soc B 363: 1401–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warn RM, Magrath R, Webb S (1984) Distribution of f-actin during cleavage of the Drosophila syncytial blastoderm. J Cell Biol 98: 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karr TL, Alberts BM (1986) Organization of the cytoskeleton in early Drosophila embryos. J Cell Biol 102: 1494–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan W, Theurkauf WE (1995) The cytoskeleton and morphogenesis of the early Drosophila embryo. Curr Opin Cell Biol 7: 18–22. [DOI] [PubMed] [Google Scholar]
- 28. Stevenson VA, Kramer J, Kuhn J, Theurkauf WE (2001) Centrosomes and the scrambled protein coordinate microtubule-independent actin reorganization. Nat Cell Biol 3: 68–75. [DOI] [PubMed] [Google Scholar]
- 29. Kanesaki T, Edwards CM, Schwarz US, Grosshans J (2011) Dynamic ordering of nuclei in syncytial embryos: a quantitative analysis of the role of cytoskeletal networks. Integr Biol 3: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 30.Schötz EM (2004) In vivo manipulation of Drosophila syncytial blastoderm embryos using optical tweezers. Ph.D. thesis, Universität Konstanz, Konstanz, Germany.
- 31. Farge E (2003) Mechanical induction of twist in the drosophila foregut/stomodeal primordium. Curr Biol 13: 1365–1377. [DOI] [PubMed] [Google Scholar]
- 32. Winfree AT (1972) Spiral waves of chemical activity. Science 175: 634–636. [DOI] [PubMed] [Google Scholar]
- 33. Agladze KI, Krinsky VI (1982) Multi-armed vortices in an active chemical medium. Nature 296: 424–426. [Google Scholar]
- 34.Idema T, Liu AJ (2013) Mechanical signaling via nonlinear wavefront propagation in a mechanically-excitable medium. arXiv: 1304.3657. [DOI] [PubMed]
- 35. Gregor T, Bialek W, de Ruyter van Steveninck RR, Tank DW, Wieschaus EF (2005) Diffusion and scaling during early embryonic pattern formation. Proc Natl Acad Sci USA 102: 18403–18407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lang I, Scholz M, Peters R (1986) Molecular mobility and nucleocytoplasmic flux in hepatoma cells. J Cell Biol 102: 1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaikin PM, Lubensky TC (1995) Principles of condensed matter physics. Cambridge, U.K.: Cambridge University Press.
- 38. Bischofs IB, Safran SA, Schwarz US (2004) Elastic interactions of active cells with soft materials. Phys Rev E 69: 021911. [DOI] [PubMed] [Google Scholar]
- 39. Ranft J, Basan M, Elgeti J, Joanny JF, Prost J, et al. (2010) Fluidization of tissues by cell division and apoptosis. Proc Natl Acad Sci USA 107: 20863–20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doi M, Edwards SF (1986) The theory of polymer dynamics. Oxford, U.K.: Oxford University Press.
- 41. Müller O, Gaub HE, Bärmann M, Sackmann E (1991) Viscoelastic moduli of sterically and chem ically cross-linked actin networks in the dilute to semidilute regime: measurements by oscillating disk rheometer. Macromolecules 24: 3111–3120. [Google Scholar]
- 42. Janmey PA, Hvidt S, Käs J, Lerche D, Maggs A, et al. (1994) The mechanical properties of actin gels. J Biol Chem 269: 32503–32513. [PubMed] [Google Scholar]
- 43. MacKintosh FC, Käs J, Janmey PA (1995) Elasticity of semiflexible biopolymer networks. Phys Rev Lett 75: 4425–4428. [DOI] [PubMed] [Google Scholar]
- 44. Palmer A, Mason TG, Xu J, Kuo SC, Wirtz D (1999) Diffusing wave spectroscopy microrheology of actin filament networks. Biophys J 76: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gardel ML, Valentine MT, Crocker JC, Bausch AR, Weitz DA (2003) Microrheology of entangled F-actin solutions. Phys Rev Lett 91: 158302. [DOI] [PubMed] [Google Scholar]
- 46. Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira PA, et al. (2004) Scaling of F-actin network rheology to probe single filament elasticity and dynamics. Phys Rev Lett 93: 188102. [DOI] [PubMed] [Google Scholar]
- 47. Yamada S, Wirtz D, Kuo SC (2000) Mechanics of living cells measured by laser tracking microrheology. Biophys J 78: 17361747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, et al. (2001) Scaling the microrheology of living cells. Phys Rev Lett 87: 148102. [DOI] [PubMed] [Google Scholar]
- 49. Guigas G, Kalla C, Weiss M (2007) Probing the nanoscale viscoelasticity of intracellular fluids in living cells. Biophys J 93: 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilhelm C (2008) Out-of-equilibrium microrheology inside living cells. Phys Rev Lett 101: 028101. [DOI] [PubMed] [Google Scholar]
- 51. Wirtz D (2009) Particle-tracking microrheology of living cells: principles and applications. Annu Rev Biophys 38: 301–326. [DOI] [PubMed] [Google Scholar]
- 52. Schmidt CF, Baermann M, Isenberg G, Sackmann E (1989) Chain dynamics, mesh size, and diffusive transport in networks of polymerized actin: a quasielastic light scattering and microuo-rescence study. Macromolecules 22: 3638–3649. [Google Scholar]
- 53. Tyson JJ, Novak B (2011) Cell cycle: who turns the crank? Curr Biol 21: R185–R187. [DOI] [PubMed] [Google Scholar]
- 54. Keating TJ, Cork RJ, Robinson KR (1994) Intracellular free calcium oscillations in normal and cleavage-blocked embryos and artificially activated eggs of Xenopus laevis . J Cell Sci 107: 2229–2237. [DOI] [PubMed] [Google Scholar]
- 55. Cao J, Crest J, Fasulo B, Sullivan W (2010) Cortical actin dynamics facilitate early-stage centro- some separation. Curr Biol 20: 770–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Idema T (2013) A new way of tracking motion, shape and divisions. Eur Biophys J 42: 647–654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Illustration showing the four stages of the Drosophila embryo replication cycle that can be detected from our movies: interphase (DNA replication), metaphase (condensation of chromosomes in the nuclear midplane), anaphase (division of the nucleus in two daughter nuclei) and telophase (separation of daughter nuclei). The plasma membrane is shown in gray, the actin cap (made of actin filaments) in red, the microtubules in green, the centrosomes in yellow, and the DNA/chromosomes in blue.
(TIFF)
Sketch of a cross-section through a Drosophila embryo valid for stages 9–13. Most nuclei are located at the surface of the embryo. The nuclei are pushed outwards into the plasma membrane (gray), resulting in the formation of somatic buds. Each nucleus is enclosed in a microtubule basket (green) and contained in an individual actin cap (red), which gets disassembled after mitosis and re-assembled during interphase. DNA/chromosomes are shown in blue and centrosomes in yellow. The yolk (light blue) is a viscoelastic fluid containing water, cytoskeletal elements and necessary building blocks for the nuclei. The yolk is bounded by an actin cortex over which the nuclei can move. Also shown in this sketch are the small number of nuclei that reside inside the yolk, and the also small number of somatic cells that already form in cycle 10 at the posterior end (the pole cells that divide out of sync with the rest of the embryo). See Foe and Alberts [1] for sketches for each of the first 14 cycles and Schejter and Wieschaus [4] for a review on the cytoskeletal elements in the early embryo.
(TIFF)
Average speed of each of the two sets of data, on a log-linear plot. The data are fitted by an exponential 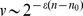 ,
, 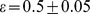 . The black dots correspond to the mean wavefront speeds of set 1, and the gray ones to the mean speeds of set 2.
. The black dots correspond to the mean wavefront speeds of set 1, and the gray ones to the mean speeds of set 2.
(TIFF)
Duration of the measured cycles. a) Experimental data. The different symbols and colors correspond to the ones in Figure 2. b) Cycle duration averaged over all experimentally observed embryos (black and gray dots for sets 1 and 2 respectively). The cycle durations can be fitted reasonably well by a weak exponential 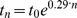 , where
, where 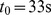 (set 1) and
(set 1) and 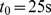 (set 2).
(set 2).
(TIFF)
Supplementary information, in which we discuss the various models in more detail: (1) Timing models for wavefront propagation; (2) Diffusion model for wavefront propagation, including models with time delay; and (3) Mechanical model for wavefront propagation. We also analyze the steady-state of a propagating wavefront in both the diffusion and mechanical model.
(PDF)
Confocal microscopy imaging movie of a developing Drosophila embryo. The chromosomal histones are visualized by labeling with GFP. The version of the movie shown here shows one image per 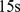 , displayed at
, displayed at 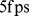 , so sped up
, so sped up 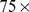 . Movies for data analysis were recorded at
. Movies for data analysis were recorded at 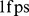 . The dimensions of each frame are
. The dimensions of each frame are 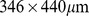 .
.
(AVI)