Abstract
CpG island methylation in the promoter regions of the DNA mismatch repair gene mutator L homologue 1 (MLH1) and DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) genes has been shown to occur in the leukocytes of peripheral blood and colorectal tissue. However, it is unclear whether the methylation levels in the blood leukocytes and colorectal tissue are correlated. The present study analyzed and compared the levels of MGMT and MLH1 gene methylation in the leukocytes of peripheral blood and colorectal tissues obtained from patients with colorectal cancer (CRC). The methylation levels of MGMT and MLH1 were examined using methylation-sensitive high-resolution melting (MS-HRM) analysis. A total of 44 patients with CRC were selected based on the MLH1 and MGMT gene methylation levels in the leukocytes of the peripheral blood. Corresponding colorectal tumor and normal tissues were obtained from each patient and the DNA methylation levels were determined. The correlation coefficients were evaluated using Spearman’s rank test. Agreement was determined by generalized κ-statistics. Spearman’s rank correlation coefficients (r) for the methylation levels of the MGMT and MLH1 genes in the leukocytes of the peripheral blood and normal colorectal tissue were 0.475 and 0.362, respectively (P=0.001 and 0.016, respectively). The agreement of the MGMT and MLH1 gene methylation levels in the leukocytes of the peripheral blood and normal colorectal tissue were graded as fair and poor (κ=0.299 and 0.126, respectively). The methylation levels of MGMT and MLH1 were moderately and weakly correlated between the patient-matched leukocytes and the normal colorectal tissue, respectively. Blood-derived DNA methylation measurements may not always represent the levels of normal colorectal tissue methylation.
Keywords: colorectal cancers, MLH1, MGMT, methylation
Introduction
DNA methylation is a significant regulator of gene transcription, and its role in carcinogenesis has become a topic of considerable interest in the last few years. DNA cytosine methylation has been widely studied, with investigations often focusing on the methylation level of CpG dinucleotides in promoter regions that usually have higher concentrations of CpGs, known as CpG islands (1). The methylation of normally unmethylated CpG islands in the promoter regions of DNA repair genes is correlated with a loss of expression of these genes (2–5), which occurs in the early stages of colorectal cancer (CRC) development (6–8).
Methylation of DNA mismatch repair gene mutator L homologue 1 (MLH1) and DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT), is known to cause high-degree microsatellite instability (MSI-H) (4) and guanine to adenine mutations in KRAS, TP53(9) and PIK3CA(6), respectively. Methylation of the MLH1 promoter has been reported in sporadic MSI tumors and is associated with the loss of protein expression (4,7,8). MGMT encodes a DNA repair enzyme that removes the mutagenic adduct from O6-methylguanine (10). Alterations in the MGMT gene impair the ability of the MGMT protein to remove the mutagenic adduct from O6-methylguanine, thereby increasing the mutation rate (6,9) and the risk of cancer (11).
To date, studies with regard to the methylation of genes have mainly focused on the methylation level of tumor tissues (12–15). Although the majority of CpG islands are unmethylated in normal tissues, the methylation changes of a small subset of genes may be observed under physiological conditions in normal colonic mucosa (16–18). In a previous study, samples of colorectal mucosa collected from healthy individuals undergoing screening colonoscopies were analyzed for MLH1 and MGMT promoter methylation and low background methylation levels were subsequently identified (0.1–18.8%) (19). The results of another study that analyzed 13 types of normal somatic tissues, placenta, sperm and an immortalized cell line, indicated that ~18% of the genomic regions exhibited a significant difference in DNA methylation levels among the 16 tissues analyzed and were classified as tissue-specific differentially methylated regions (20). Furthermore, studies have focused on the detection of methylated DNA in peripheral blood and normal tissues (21,22), and MLH1(23–26) and MGMT(27–29) gene methylation has been reported in peripheral blood leukocytes.
Previous studies (16–18) on DNA methylation typically used paired tumor and normal surrounding tissues from cancer-bearing individuals. To the best of our knowledge, no studies with regard to the correlation between DNA methylation levels in peripheral blood leukocytes and colorectal tissue specimens, including colorectal tumor and normal colorectal tissues, from each matched patient have been published. Therefore, the present study aimed to determine whether there was a correlation between the MLH1 and MGMT methylation levels in patient-matched peripheral blood leukocytes and colorectal tissue DNA samples.
Materials and methods
Individuals and study samples
Samples (5 ml) of peripheral blood and colorectal tumor and normal tissues were obtained from 44 patients with CRC who underwent surgery in the Department of Surgery of the Tumor Hospital (Harbin, China). Informed consent was obtained from the surgeons and patients. No patients were administered pre-operative radiation or chemotherapy. The normal colorectal mucosa specimens were obtained from colorectal tissues at the margins of the resected specimens (≥10 cm away from the tumor). Approval for this study was obtained from the Human Subjects Committee, Harbin Medical University.
The methylation status of MLH1 and MGMT was examined in the peripheral blood leukocyte DNA of the CRC cases. Based on the MLH1 and MGMT methylation results that were detected in the peripheral blood leukocytes (0% methylation as a cut-off value), 19 individuals with methylation of either gene were selected as positive subjects and another 25 individuals without methylation for both genes were selected as negative subjects.
Sodium bisulfite conversion
The genomic DNA was extracted from the blood samples and colorectal tissue specimens, including colorectal tumor and normal colorectal tissues, using a TIAN-amp Genomic DNA kit (Tiangen, Beijing, China), according to the manufacturer’s instructions. Sodium bisulfite conversion of the genomic DNA was performed as described previously (29). DNA (1 μg) was bisulfite-modified using the EZ DNA Methylation-Gold kit (Zymo Research, Orange County, CA, USA). The eluted DNA (10 μl volume) was used for the methylation-sensitive high-resolution melting (MS-HRM) analysis.
Methylation analysis
Methylation of the MGMT and MLH1 promoter was assessed using MS-HRM (30). The primers used were those designed by Wojdacz and Dobrovic (30). For MGMT, the published primer sequences (31) and the designed sequences for MLH1 were 5′-TTTTTTTAGGAGTGAAGGAGG-3′ and 5′-AACRCCACTACRAAACTAAA-3′. The reactions were performed in 96-well LightCycler® 480 plates (Roche, Mannheim, Germany) using the LightCycler 480 High Resolution Melting Master mix, which contains a DNA intercalating dye in a final volume of 10 μl. The reaction mixture contained 1× LightCycler 480 High Resolution Melting Master mix, 200 nmol/l each primer and 1 μl bisulfite-modified DNA, with 3.0 mmol/l final MgCl2 for MLH1 and MGMT. Each reaction was performed in duplicate. The cycling conditions that were used for the two assays were as follows: SYBR Green 1 detection format; 1 cycle at 95°C for 10 min, 50 cycles at 95°C for 10 sec, a touch down from 64°C to 58°C for 10 sec (1°C/cycle) and 72°C for 20 sec, followed by an HRM step at 95°C for 1 min, 40°C for 1 min, 74°C for 5 sec and continuous acquisition to 90°C at 25 acquisitions per 1°C. Each plate included multiple water blanks for a negative control. Methylated and unmethylated genomic templates were used to calibrate the quantitative measurements of methylation. CpGenome Universal Methylated DNA (Zymo Research) was used as 100% methylated control DNA. CpGenome Universal Unmethylated DNA (Zymo Research) was used as unmethylated control DNA. Methylation standards were constructed by diluting 100% methylated bisulfite-modified control DNA in a pool of bisulfite-modified unmethylated control DNA at levels of 50, 25, 5 and 1%. These standards were included in each experimental run. Based on the standard curves, the patient data were classified into various methylation categories by two independent observers. Disagreements were settled by consensus or a third review for adjudication.
Statistical analysis
The data were analyzed using non-parametric Friedman and χ2 tests for the comparison of methylation levels in the peripheral blood leukocyte, colorectal tumor and normal colorectal tissue DNA. Spearman’s rank correlation coefficient was used for analyzing the associations of the methylation levels between the three groups. The magnitude of the correlation was specified as weak (0.00–0.39) moderate (0.40–0.79) and strong (0.80–1.00). Agreements of the levels of methylation between the peripheral blood leukocytes and normal colorectal tissues were determined using generalized weighted κ-statistics (32) Agreement was classified as excellent (κ>0.80), good (0.61≤κ≤0.80), moderate (0.41≤κ≤0.60), fair (0.21≤κ≤0.40) or poor (κ<0.20). SPSS (version 16.0; SPSS, Inc., Chicago, IL, USA) was used to analyze the data. P≤0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 44 patients, 29 males and 15 females (mean age, 55 years; range, 28–79 years), were selected for the present study. The basic characteristics of the patients are shown in Table I.
Table I.
Demographic and clinical characteristics of 44 CRC patients.
| Patient characteristics | No. of cases (%) |
|---|---|
| Age, years | |
| ≤60 | 26 (59.1) |
| >60 | 18 (40.9) |
| Total | 44 (100.0) |
| Gender | |
| Female | 15 (34.1) |
| Male | 29 (65.9) |
| Tumor location | |
| Proximal colona | 8 (18.2) |
| Distal colonb | 6 (13.6) |
| Rectum | 30 (68.2) |
| Tumor stage | |
| I | 3 (6.8) |
| II | 22 (50.0) |
| III | 18 (40.9) |
| IV | 1 (2.3) |
Proximal colon includes the cecum through the transverse colon.
Distal colon includes the descending colon through the rectum.
CRC, colorectal cancer.
Comparing methylation status in patient-matched peripheral blood leukocyte and colorectal tissue DNA
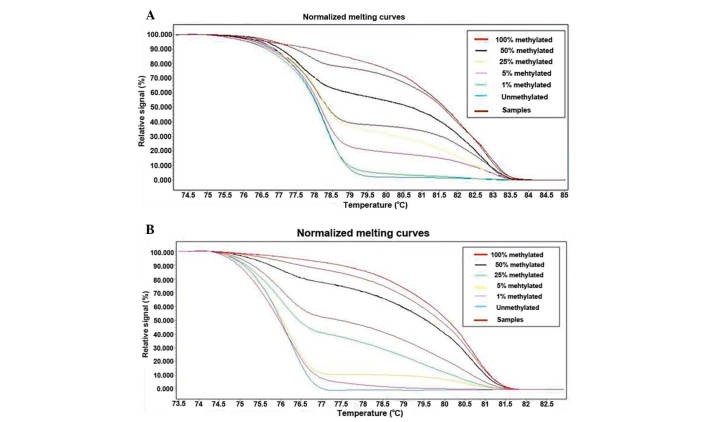
The methylation analysis results of the 44 patients are shown in Table II and illustrated in Fig. 1A and 1B. Differences in the levels of MGMT and MLH1 methylation were examined in patient-matched peripheral blood leukocyte, colorectal tumor and normal colorectal tissue DNA (Table II). There were no significant differences in the levels of MGMT and MLH1 methylation between the three groups (Friedman test, P=0.260 and P=0.464, respectively).
Table II.
MS-HRM assay of peripheral blood and colorectal tissue samples of CRC patients.
| MGMT, n | MLH1, n | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Frequencies of methylation (%) | Leukocytes | Normal tissue | Tumor tissue | Leukocytes | Normal tissue | Tumor tissue |
| 0 | 37 | 35 | 31 | 32 | 30 | 31 |
| 0–1 | 0 | 2 | 1 | 0 | 2 | 2 |
| 1–5 | 5 | 4 | 2 | 7 | 1 | 2 |
| 5–25 | 0 | 3 | 3 | 2 | 9 | 5 |
| 25–50 | 1 | 0 | 0 | 3 | 1 | 2 |
| 50–100 | 1 | 0 | 2 | 0 | 0 | 1 |
| 100 | 0 | 0 | 5 | 0 | 1 | 1 |
MS-HRM, methylation-sensitive high-resolution melting; CRC, colorectal cancer; MGMT, DNA repair gene O6-methylguanine-DNA methyltranserferase; MLH1, DNA mismatch repair gene mutator L homologue 1.
Figure 1.
Normalized methylation-sensitive high-resolution melting (MS-HRM) standard curves of MLH1 and MGMT in CRC. (A) Profile of fluorescence obtained at the melting temperature for serial dilutions of methylated DNA (100-0%) and the melting plot for the MGMT gene. (B) Profile of fluorescence obtained at the melting temperature for serial dilutions of methylated DNA (100-0%) and the melting plot for the MLH1 gene. MLH1, DNA mismatch repair gene mutator L homoloue 1; MGMT, DNA repair gene O6-methylguanine-DNA methyltransferase; CRC, colorectal cancer.
Various cut-off methylation levels were used for the analysis (Table III). The level of methylation was classified as positive at a cut-off value of 0–1% methylation and no statistical significant differences were observed in the levels of MGMT and MLH1 methylation among the three groups (Table III). When a level of methylation of >5% was classified as positive, there was a significant difference in the levels of MGMT methylation among the three groups (P=0.014; χ2 test), but no significant difference in the levels of MLH1 methylation (P=0.251; χ2 test). Further analysis revealed that a significant difference in MGMT methylation existed between colorectal tumor tissue DNA and leukocytes or normal colorectal tissue DNA (P=0.013 and P=0.035, respectively; χ2 test), but not between leukocyte and normal colorectal tissue DNA (P=0.645; χ2 test).
Table III.
MS-HRM assay of peripheral blood and colorectal tissue samples of CRC patients using various cut-off values.
| MGMT | MLH1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Cut-off (%) | Leukocytes, n (%) | Normal tissue, n (%) | Tumor tissue, n (%) | P-value* | P-value* | Leukocytes, n (%) | Normal tissue, n (%) | Tumor tissue, n (%) | P-value* |
| 0 | 7 (15.9) | 9 (20.5) | 13 (29.5) | 0.282 | - | 12 (27.3) | 14 (31.8) | 13 (29.5) | 0.897 |
| 1 | 7 (15.9) | 7 (15.9) | 12 (27.3) | 0.302 | - | 12 (29.5) | 12 (29.5) | 11 (25.0) | 0.962 |
| 5 | 2 (4.5) | 3 (6.8) | 10 (22.7) | 0.014 | 0.645a, 0.035b, 0.013c | 5 (11.4) | 11 (25.0) | 9 (20.5) | 0.251 |
χ2 test; leukocytes vs. normal tissue;
tumor tissue vs. normal tissue; and
tumor tissue vs. leukocytes.
MS-HRM, methylation-sensitive high-resolution melting; CRC, colorectal cancer; MGMT, DNA repair gene O6-methylguanine-DNA methyltranserferase; MLH1, DNA mismatch repair gene mutator L homologue 1.
Spearman rank correlation coefficients
Positive correlations were observed between the peripheral blood leukocyte and normal colorectal tissue DNA in the levels of MGMT and MLH1 methylation (r=0.475, P=0.001 and r=0.362, P=0.016, respectively). However, there were no positive correlations between colorectal tumor tissue and peripheral blood leukocyte or normal colorectal tissue DNA, based on the methylation levels of the assessments of the two genes (Table IV).
Table IV.
Spearman’s rank correlation coefficients (P-values) of MGMT and MLH1 methylation levels in case-matched DNA with CRC.
| DNA source | Leukocytes | Normal tissues | Tumor tissues |
|---|---|---|---|
| MGMT | |||
| Leukocytes | - | 0.475 (0.001) | −0.033 (0.833) |
| Normal tissues | 0.475 (0.001) | - | 0.025 (0.873) |
| Tumor tissues | −0.033 (0.833) | 0.025 (0.873) | - |
| MLH1 | |||
| Leukocytes | - | 0.362 (0.016) | 0.215 (0.161) |
| Normal tissues | 0.362 (0.016) | - | 0.293 (0.054) |
| Tumor tissues | 0.215 (0.161) | 0.293 (0.054) | - |
MGMT, DNA repair gene O6-methylguanine-DNA methyltranserferase; MLH1, DNA mismatch repair gene mutator L homologue 1; CRC, colorectal cancer.
Agreement
The agreement between the peripheral blood leukocyte and normal colorectal tissue DNA with CRC on the levels of MGMT and MLH1 methylation were calculated using κ coefficients (Table V). The agreement of the MGMT gene methylation levels in the leukocytes of the peripheral blood and normal colorectal tissue was graded as fair (κ=0.299). The agreement of the MLH1 gene methylation levels in the leukocytes of the peripheral blood and normal colorectal tissue was graded as poor (κ=0.126) (Table V).
Table V.
Frequencies of MGMT and MLH1 methylation in the peripheral blood and the normal colorectal tissues.
| Frequencies of normal colorectal tissues methylation (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Frequencies of blood methylation (%) | 0 | 0–1 | 1–5 | 5–25 | 25–50 | 50–100 | 100 | n |
| MGMT | ||||||||
| 0 | 32 | 2 | 3 | 0 | 0 | 0 | 0 | 37 |
| 0–1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1–5 | 3 | 0 | 1 | 1 | 0 | 0 | 0 | 5 |
| 5–25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25–50 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 50–100 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| n | 35 | 2 | 4 | 3 | 0 | 0 | 0 | 44 |
| κ | 0.299 (P=0.002) | |||||||
| MLH1 | ||||||||
| 0 | 24 | 2 | 1 | 5 | 0 | 0 | 0 | 32 |
| 0–1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1–5 | 5 | 0 | 0 | 2 | 0 | 0 | 0 | 7 |
| 5–25 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| 25–50 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 3 |
| 50–100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| n | 30 | 2 | 1 | 9 | 1 | 0 | 1 | 44 |
| κ | 0.126 (P=0.098) | |||||||
κ: The κ statistics are parameters of agreement that take chance agreement into account.
MGMT, DNA repair gene O6-methylguanine-DNA methyltranserferase; MLH1, DNA mismatch repair gene mutator L homologue 1.
Discussion
DNA promoter methylation has previously been shown to be a well-characterized event in tumor biology and has been extensively documented in CRC (33,34). However, few studies have compared DNA promoter methylation in DNA from various patient-matched sources. The present results revealed that the levels of MLH1 and MGMT methylation were not significantly different in patient-matched peripheral blood leukocyte, colorectal tumor tissue and normal colorectal tissue DNA as original semi-quantitatively rank data. Since low background methylation levels have previously been reported for various genes in normal samples (35,36), several studies have used 0.1–10% as a cut-off for the scoring criteria of gene methylation using the MS-HRM assay (31,37–40) and there has not been a unified standard to define methylation. Therefore, in the present study, in order to identify the various methylation levels between cancer and normal samples, a range of cut-off methylation levels were used. When samples with >5% methylation were considered as methylated, distinctive MGMT gene promoter methylation levels were identified between colorectal tumor tissue and leukocyte or normal colorectal tissue DNA, but no significant differences were observed between leukocyte and normal colorectal tissue DNA. Thus, in samples containing >5% methylation, the analysis of the MGMT gene appeared to increase the sensitivity for discriminating cancer from normal colorectal tissues or leukocytes.
Previous studies have focused on DNA methylation measured in the leukocytes (41,42) or the normal mucosa tissues (17,43,44), rarely reporting the correlation between the two. Ally et al reported significant positive correlations between the estrogen receptor-α methylation index in leukocytes and normal colonic tissue in CRC patients (r=0.570; P=0.003) (45). However, the samples were not case matched. Spearman rank correlations were performed to investigate the correlation of the MLH1 and MGMT methylation levels in patient-matched peripheral blood leukocyte, colorectal tumor and normal colorectal tissue DNA samples. The most significant positive correlations were observed between the leukocyte and normal colorectal tissue DNA for methylation detection of MGMT and MLH1 (r=0.475 and r=0.362, respectively).
In human studies, ethical and practical barriers may make it difficult or impossible to collect specimens from the target tissue. Hence, the use of surrogate samples, including DNA derived from easily accessible peripheral blood, is widely accepted when the target tissue is unobtainable. Several studies with regard to DNA methylation biomarkers tested in leukocytes suggest the suitability of epigenetic biomarkers for the detection of several cancers relative to controls (46–48). Widschwendter et al identified that particular methylation patterns in peripheral blood DNA may serve as surrogate markers for the risk of breast cancer (49). To investigate the use of blood as a surrogate for DNA methylation in tissues, the present study measured the MGMT and MLH1 gene methylation levels in the leukocytes of the peripheral blood and normal colorectal tissue, which were graded as fair and poor (κ=0.299 and 0.126, respectively); therefore, blood-derived DNA methylation level measurements may not always represent the levels of target colorectal tissue methylation (50). While all somatic cells in a given individual are genetically identical, differing cell types form highly distinct anatomical structures and perform a wide range of disparate physiological functions (51). It has been conjectured that during tissue differentiation and development, transcription-relevant control regions in the genome become selectively de- or upmethylated to enable the transcription of a restricted set of genes within a given tissue (52). It is also plausible that methylation patterns in DNA obtained from blood may be more ‘plastic’ compared with that of other tissues, due to the close proximity of the blood to environmental influences, such as nutrition and smoking (50).
A limitation of the present study is the fact that the subjects that were selected. The methylation results may not reflect the natural frequencies of methylation in leukocytes or colorectal tissues. Another limitation of the study is a lack of inclusion of healthy controls. Studies using cancer subjects may not exclude the possibility of disseminated tumor cells or the effect the disease itself may have on the systemic methylation status in leukocytes and normal colorectal tissues. Further studies with disease-free individuals and an investigation of tumor suppressor gene methylation are required to clarify this issue.
In summary, the correlation of MGMT and MLH1 methylation levels between patient-matched leukocytes and normal colorectal tissues was classified as moderate and weak, respectively. Blood-derived DNA methylation measurements may not always represent the levels of normal colorectal tissue methylation.
Acknowledgements
This study was supported by grants from the Education Bureau of Heilongjiang Province (11531100).
References
- 1.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 2.Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9826. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–167. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 4.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 5.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 6.Derks S, Postma C, Moerkerk PT, et al. Promoter methylation precedes chromosomal alterations in colorectal cancer development. Cell Oncol. 2006;28:247–257. doi: 10.1155/2006/846251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esteller M. Epigenetic lesions causing genetic lesions in human cancer: promoter hypermethylation of DNA repair genes. Eur J Cancer. 2000;36:2294–2300. doi: 10.1016/s0959-8049(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 8.Frigola J, Solé X, Paz MF, et al. Differential DNA hypermethylation and hypomethylation signatures in colorectal cancer. Hum Mol Genet. 2005;14:319–326. doi: 10.1093/hmg/ddi028. [DOI] [PubMed] [Google Scholar]
- 9.Esteller M, Herman JG. Generating mutations but providing chemosensitivity: the role of O6-methylguanine DNA methyltransferase in human cancer. Oncogene. 2004;23:1–8. doi: 10.1038/sj.onc.1207316. [DOI] [PubMed] [Google Scholar]
- 10.Pegg AE. Mammalian O6-alkylguanine-DNA alkyltransferase: regulation and importance in response to alkylating carcinogenic and therapeutic agents. Cancer Res. 1990;50:6119–6129. [PubMed] [Google Scholar]
- 11.Povey AC, Badawi AF, Cooper DP, et al. DNA alkylation and repair in the large bowel: animal and human studies. J Nutr. 2002;132:3518S–3521S. doi: 10.1093/jn/132.11.3518S. [DOI] [PubMed] [Google Scholar]
- 12.Anacleto C, Leopoldino AM, Rossi B, et al. Colorectal cancer ‘methylator phenotype’: fact or artifact? Neoplasia. 2005;7:331–335. doi: 10.1593/neo.04502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anacleto C, Rossi B, Lopes A, et al. Development and application of a multiplex PCR procedure for the detection of DNA methylation in colorectal cancer. Oncol Rep. 2005;13:325–328. [PubMed] [Google Scholar]
- 14.Fox EJ, Leahy DT, Geraghty R, et al. Mutually exclusive promoter hypermethylation patterns of hMLH1 and O6-methylguanine DNA methyltransferase in colorectal cancer. J Mol Diagn. 2006;8:68–75. doi: 10.2353/jmoldx.2006.050084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YH, Petko Z, Dzieciatkowski S, et al. CpG island methylation of genes accumulates during the adenoma progression step of the multistep pathogenesis of colorectal cancer. Genes Chromosomes Cancer. 2006;45:781–789. doi: 10.1002/gcc.20341. [DOI] [PubMed] [Google Scholar]
- 16.Shen L, Kondo Y, Rosner GL, et al. MGMT promoter methylation and field defect in sporadic colorectal cancer. J Natl Cancer Inst. 2005;97:1330–1338. doi: 10.1093/jnci/dji275. [DOI] [PubMed] [Google Scholar]
- 17.Nagasaka T, Goel A, Notohara K, et al. Methylation pattern of the O6-methylguanine-DNA methyltransferase gene in colon during progressive colorectal tumorigenesis. Int J Cancer. 2008;122:2429–2436. doi: 10.1002/ijc.23398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KH, Lee JS, Nam JH, et al. Promoter methylation status of hMLH1, hMSH2, and MGMT genes in colorectal cancer associated with adenoma-carcinoma sequence. Langenbecks Arch Surg. 2011;396:1017–1026. doi: 10.1007/s00423-011-0812-9. [DOI] [PubMed] [Google Scholar]
- 19.Menigatti M, Truninger K, Gebbers JO, Marbet U, Marra G, Schär P. Normal colorectal mucosa exhibits sex- and segment-specific susceptibility to DNA methylation at the hMLH1 and MGMT promoters. Oncogene. 2009;28:899–909. doi: 10.1038/onc.2008.444. [DOI] [PubMed] [Google Scholar]
- 20.Rakyan VK, Down TA, Thorne NP, et al. An integrated resource for genome-wide identification and analysis of human tissue-specific differentially methylated regions (tDMRs) Genome Res. 2008;18:1518–1529. doi: 10.1101/gr.077479.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lofton-Day C, Model F, Devos T, et al. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54:414–423. doi: 10.1373/clinchem.2007.095992. [DOI] [PubMed] [Google Scholar]
- 22.Grützmann R, Molnar B, Pilarsky C, et al. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One. 2008;3:e3759. doi: 10.1371/journal.pone.0003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gazzoli I, Loda M, Garber J, Syngal S, Kolodner RD. A hereditary nonpolyposis colorectal carcinoma case associated with hypermethylation of the MLH1 gene in normal tissue and loss of heterozygosity of the unmethylated allele in the resulting microsatellite instability-high tumor. Cancer Res. 2002;62:3925–3928. [PubMed] [Google Scholar]
- 24.Miyakura Y, Sugano K, Akasu T, et al. Extensive but hemiallelic methylation of the hMLH1 promoter region in early-onset sporadic colon cancers with microsatellite instability. Clin Gastroenterol Hepatol. 2004;2:147–156. doi: 10.1016/s1542-3565(03)00314-8. [DOI] [PubMed] [Google Scholar]
- 25.Sheng JQ, Zhang H, Ji M, et al. Genetic diagnosis strategy of hereditary non-polyposis colorectal cancer. World J Gastroenterol. 2009;15:983–989. doi: 10.3748/wjg.15.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou HH, Yan SY, Zhou XY, et al. MLH1 promoter germline-methylation in selected probands of Chinese hereditary non-polyposis colorectal cancer families. World J Gastroenterol. 2008;14:7329–7334. doi: 10.3748/wjg.14.7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Psofaki V, Kalogera C, Tzambouras N, et al. Promoter methylation status of hMLH1, MGMT, and CDKN2A/p16 in colorectal adenomas. World J Gastroenterol. 2010;16:3553–3560. doi: 10.3748/wjg.v16.i28.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Candiloro IL, Dobrovic A. Detection of MGMT promoter methylation in normal individuals is strongly associated with the T allele of the rs16906252 MGMT promoter single nucleotide polymorphism. Cancer Prev Res (Phila) 2009;2:862–867. doi: 10.1158/1940-6207.CAPR-09-0056. [DOI] [PubMed] [Google Scholar]
- 29.Vineis P, Chuang SC, Vaissière T, et al. DNA methylation changes associated with cancer risk factors and blood levels of vitamin metabolites in a prospective study. Epigenetics. 2011;6:195–201. doi: 10.4161/epi.6.2.13573. [DOI] [PubMed] [Google Scholar]
- 30.Wojdacz TK, Dobrovic A. Methylation-sensitive high resolution melting (MS-HRM): a new approach for sensitive and high-throughput assessment of methylation. Nucleic Acids Res. 2007;35:e41. doi: 10.1093/nar/gkm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balic M, Pichler M, Strutz J, et al. High quality assessment of DNA methylation in archival tissues from colorectal cancer patients using quantitative high-resolution melting analysis. J Mol Diagn. 2009;11:102–108. doi: 10.2353/jmoldx.2009.080109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 33.Esteller M, Sparks A, Toyota M, et al. Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res. 2000;60:4366–4371. [PubMed] [Google Scholar]
- 34.Chan AO, Broaddus RR, Houlihan PS, Issa JP, Hamilton SR, Rashid A. CpG island methylation in aberrant crypt foci of the colorectum. Am J Pathol. 2002;160:1823–1830. doi: 10.1016/S0002-9440(10)61128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahlquist T, Lind GE, Costa VL, et al. Gene methylation profiles of normal mucosa, and benign and malignant colorectal tumors identify early onset markers. Mol Cancer. 2008;7:94. doi: 10.1186/1476-4598-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakagawa H, Nuovo GJ, Zervos EE, et al. Age-related hypermethylation of the 5′ region of MLH1 in normal colonic mucosa is associated with microsatellite-unstable colorectal cancer development. Cancer Res. 2001;61:6991–6995. [PubMed] [Google Scholar]
- 37.Meng W, Huebner A, Shabsigh A, Chakravarti A, Lautenschlaeger T. Combined RASSF1A and RASSF2A promoter methylation analysis as diagnostic biomarker for bladder cancer. Mol Biol Int. 2012;2012:701814. doi: 10.1155/2012/701814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morandi L, Franceschi E, de Biase D, et al. Promoter methylation analysis of O6-methylguanine-DNA methyltransferase in glioblastoma: detection by locked nucleic acid based quantitative PCR using an imprinted gene (SNURF) as a reference. BMC Cancer. 2010;10:48. doi: 10.1186/1471-2407-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Avraham A, Uhlmann R, Shperber A, et al. Serum DNA methylation for monitoring response to neoadjuvant chemotherapy in breast cancer patients. Int J Cancer. 2012;131:E1166–E1172. doi: 10.1002/ijc.27526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kristensen LS, Mikeska T, Krypuy M, Dobrovic A. Sensitive Melting Analysis after Real Time- Methylation Specific PCR (SMART-MSP): high-throughput and probe-free quantitative DNA methylation detection. Nucleic Acids Res. 2008;36:e42. doi: 10.1093/nar/gkn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lim U, Flood A, Choi SW, et al. Genomic methylation of leukocyte DNA in relation to colorectal adenoma among asymptomatic women. Gastroenterology. 2008;134:47–55. doi: 10.1053/j.gastro.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pufulete M, Al-Ghnaniem R, Leather AJ, et al. Folate status, genomic DNA hypomethylation, and risk of colorectal adenoma and cancer: a case control study. Gastroenterology. 2003;124:1240–1248. doi: 10.1016/s0016-5085(03)00279-8. [DOI] [PubMed] [Google Scholar]
- 43.Lind GE, Thorstensen L, Løvig T, et al. A CpG island hypermethylation profile of primary colorectal carcinomas and colon cancer cell lines. Mol Cancer. 2004;3:28. doi: 10.1186/1476-4598-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee S, Hwang KS, Lee HJ, Kim JS, Kang GH. Aberrant CpG island hypermethylation of multiple genes in colorectal neoplasia. Lab Invest. 2004;84:884–893. doi: 10.1038/labinvest.3700108. [DOI] [PubMed] [Google Scholar]
- 45.Ally MS, Al-Ghnaniem R, Pufulete M. The relationship between gene-specific DNA methylation in leukocytes and normal colorectal mucosa in subjects with and without colorectal tumors. Cancer Epidemiol Biomarkers Prev. 2009;18:922–928. doi: 10.1158/1055-9965.EPI-08-0703. [DOI] [PubMed] [Google Scholar]
- 46.Moore LE, Pfeiffer RM, Poscablo C, et al. Genomic DNA hypomethylation as a biomarker for bladder cancer susceptibility in the Spanish Bladder Cancer Study: a case-control study. Lancet Oncol. 2008;9:359–366. doi: 10.1016/S1470-2045(08)70038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Aakre JA, Jiang R, et al. Methylation markers for small cell lung cancer in peripheral blood leukocyte DNA. J Thorac Oncol. 2010;5:778–785. doi: 10.1097/JTO.0b013e3181d6e0b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsiung DT, Marsit CJ, Houseman EA, et al. Global DNA methylation level in whole blood as a biomarker in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16:108–114. doi: 10.1158/1055-9965.EPI-06-0636. [DOI] [PubMed] [Google Scholar]
- 49.Widschwendter M, Apostolidou S, Raum E, et al. Epigenotyping in peripheral blood cell DNA and breast cancer risk: a proof of principle study. PLoS One. 2008;3:e2656. doi: 10.1371/journal.pone.0002656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKay JA, Xie L, Harris S, Wong YK, Ford D, Mathers JC. Blood as a surrogate marker for tissue-specific DNA methylation and changes due to folate depletion in post-partum female mice. Mol Nutr Food Res. 2011;55:1026–1035. doi: 10.1002/mnfr.201100008. [DOI] [PubMed] [Google Scholar]
- 51.Christensen BC, Houseman EA, Marsit CJ, et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009;5:e1000602. doi: 10.1371/journal.pgen.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walsh CP, Bestor TH. Cytosine methylation and mammalian development. Genes Dev. 1999;13:26–34. doi: 10.1101/gad.13.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]