Abstract
Hypofractionated radiotherapy (RT) has been employed to treat hepatocellular carcinoma (HCC). The present study aimed to report the treatment effects, the dose-response associations and the factors that are associated with radiation-induced liver disease (RILD) in a high-dose hypofractionated RT procedure. A total of 40 patients with non-metastatic HCC who underwent RT for local control of irradiated tumors were studied. The treatment technique was that of three-dimensional conformal or intensity-modulated radiation therapy, with a fraction size of 3 Gy and a total dose of 40–66 Gy in 14–23 fractions. The biologically-effective dose (BED) was 52.0–85.8 Gy10 (median, 74.1 Gy10). Tumor regression was observed in 28 patients (70.0%) with a complete response, partial response, stable disease and progressive disease status in 11 (27.5%), 17 (42.5%), five (12.5%) and seven patients (17.5%), respectively. The one-, two- and five-year overall survival (OS) and in-field control (IFC) rates were 60, 40 and 21% and 73, 62 and 56%, respectively. A positive correlation also emerged between the radiation dose and the IFC (P=0.035). Eight of the 40 patients (20%) developed non-classic RILD. A higher Cancer of the Liver Italian Program score was associated with a higher probability of non-classic RILD (P=0.02). The tumor response and IFC rate of HCC following irradiation were significantly dose-dependent. High-dose hypofractionated X-ray RT is a feasible and effective treatment for HCC in patients with good liver function and for those who meet the criteria for a curative attempt.
Keywords: hepatocellular carcinoma, hypofractionated, radiotherapy, toxicities
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers in Asia, where chronic viral hepatitis is common (1). Patients with HCC typically have impaired liver function due to virus- or alcohol-induced cirrhosis or viral hepatitis and only ~20% are appropriate candidates for surgery (2). The five-year overall survival (OS) rate for patients that are treated by surgery is 30–70% (3). For those who are not treated with surgery, liver function affected by an underlying liver disease has a strong affect on the clinical outcomes and complicates treatment strategies to a greater extent than for other tumors. Maximal preservation of the normal liver volume and function is a significant consideration in the choice of treatment.
Percutaneous ethanol injection therapy (PEIT) and radiofrequency ablation (RFA) are two major non-surgical local treatments for HCC. PEIT is often used for small HCCs. Higher local failure rates have been identified in patients with tumors of >3 cm that have been treated by PEIT, or in those with more than three tumors (4). RFA, which is able to treat tumors of ≤5 cm, has a more efficient local control rate than PEIT for small tumors (5). However, RFA is difficult to perform in patients with anatomically unfavorable tumor locations or coagulopathy, as is commonly observed in HCC patients. Transcatheter arterial chemoembolization (TACE), although not considered a curative treatment, is used in patients with poor liver function or those who are not suitable candidates for RFA or PEIT. A systemic review of randomized trials has shown that TACE improves the survival of patients with unresectable HCC (6).
Radiotherapy (RT) has not been widely adopted as a curative treatment modality for HCC due to poor liver tolerance from radiation damage. Improvements in RT techniques, including three-dimensional conformal RT (3DCRT), intensity-modulated RT (IMRT) and image-guided RT (IGRT), provide multiple treatment portals with a reduced volume of liver subjected to high-dose therapy and improved conformity and precision. These techniques increase the prescribed dose and local-control likelihood with acceptable liver toxicity (7). The parallel arrangement of liver-tissue functional subunits has facilitated the employment of hypofractionated RT. A large fraction of HCC was used in proton beam therapy as the normal liver dose may be reduced by its physical characteristic (8). For X-ray, a large fraction size for primary or metastatic liver tumors is provided through stereotactic body RT (9), and clinical trials are being conducted (10).
The present study investigated the use of X-rays with a moderate hypofractionation schema to achieve local control of the irradiated tumor in the treatment of HCC patients. The schema was 3 Gy/fraction, with a maximal total dose of up to 60–66 Gy if the liver tolerance was acceptable. The total dose of this schema was between the conventional fraction size of 2 Gy and the large fraction size provided by stereotactic body radiosurgery.
Materials and methods
Patients
The study procedure conformed to the ethical guidelines of the Declaration of Helsinki and approval for the study was obtained from the institution’s human research committee (nos. 99–1,924B). Between January 1998 and January 2008, medical records were reviewed for 40 patients with non-metastatic HCC who underwent high-dose RT, with attention to local tumor control. All the patients were treated with 3DCRT or IMRT and were administered a total radiation dose of >50 Gy10. The patients who were diagnosed with HCC and administered RT of a biologically-effective dose (BED) of >50 Gy10 using the α/β ratio of 10 Gy were selected for the present study.
Table I provides a summary of the characteristics of the 40 patients, consisting of 10 males and 30 females, with a median age of 63 years (range, 42–82 years). A total of 32 (80.0%) patients presented with liver cirrhosis (LC) and 15 (37.5%) had a history of esophageal variceal (EV) bleeding. Of the 40 patients, 25 (62.5%) had Child-Pugh class A (11) LC and 23 patients (57.5%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1. The previous treatments of the 28 patients who were administered RT as a salvage treatment are as follows: Surgery in one patient, TACE in 23, RFA in two, PEIT in 11 and oral chemotherapy in one. The distribution of patients with a Cancer of the Liver Italian program (CLIP) (12) score of 0, 1, 2, 3 and 4 was six, 13, 13, six and two patients, respectively. Portal vein thrombosis (PVT) was present in 13 patients (32.5%).
Table I.
Patient Characteristics.
| Characteristic | Number of patients, n (%) |
|---|---|
| Gender | |
| Female | 10 (25.0) |
| Male | 30 (75.0) |
| Age, years | |
| <63 | 20 (50.0) |
| ≥63 | 20 (50.0) |
| ECOG performance status | |
| 0–1 | 23 (57.5) |
| 2 | 17 (42.5) |
| Liver cirrhosis | |
| No | 8 (20.0) |
| Yes | 32 (80.0) |
| EV bleeding history | |
| No | 25 (62.5) |
| Yes | 15 (37.5) |
| Child-Pugh class | |
| A | 25 (62.5) |
| B | 15 (37.5) |
| Previous treatment | |
| No | 12 (30.0) |
| Yes | 28 (70.0) |
| Surgery | 1 (3.6) |
| TACE | 23 (82.1) |
| RFA | 2 (7.1) |
| PEIT | 11 (39.3) |
| C/T | 1 (3.6) |
| CLIP score | |
| 0 | 6 (15.0) |
| 1 | 13 (32.5) |
| 2 | 13 (32.5) |
| 3 | 6 (15.0) |
| 4 | 2 (5.0) |
| Hepatitis | |
| NBNC | 10 (25.0) |
| B | 6 (15.0) |
| C | 20 (50.0) |
| B+C | 4 (10.0) |
| Tumor number | |
| Single | 17 (42.5) |
| Multiple | 23 (57.5) |
| Tumor size, cm | |
| <5 | 25 (62.5) |
| 5–10 | 14 (35.0) |
| >10 | 1 (2.5) |
| PVT | |
| No | 27 (67.5) |
| Yes | 13 (32.5) |
| AJCC Stage | |
| I–II | 21 (52.5) |
| III–IV | 19 (47.5) |
CLIP, Cancer of the Liver Italian Program; C/T, chemotherapy; ECOG, Eastern Cooperative Oncology Group; EV, esophageal varices; NBNC, non-B/non-C; PEI, percutaneous ethanol injection; PVT, portal vein thrombus; RFA, radiofrequency ablation; TACE, transcatheter arterial chemoembolization; AJCC, American Joint Committee on Cancer.
Radiation therapy
The patients were immobilized in a supine position using a vacuum bag with their arms elevated overhead. Contrast-enhanced images were used for target delineation and dynamic computed tomography (CT) was performed as required for tumor identification. The CT images that were captured subsequent to 2007 were obtained by respiration-gating or 4D techniques. The delineation of the gross tumor volume (GTV) accounted for the organ motion in the 4D CT. The clinical tumor volume (CTV) was obtained by adding a 5–10-mm expansion from the GTV, and the expansion of the planning tumor volume (PTV) was typically 5 mm for the lateral directions, 0.5–1 cm for the anterior-posterior direction and 0.5–1.5 cm for the cephalic-caudal direction. The PTV extension depended on 4D or respiratory-gating CT and whether imaged-guided or respiratory gating was used in the treatment. Of the 40 patients, 15 (37.5%), 7 (17.5%) and 18 (45.0%) patients underwent 3DCRT, IMRT and 4D planning RT, respectively.
The prescribed dose was defined as a 100% and 95%, which applied to the CTV and PTV, respectively. The total dose was adjusted by considering the liver tolerance dose with the restriction that <30% of normal liver received >30 Gy (V30) and the dose restriction was reduced to 27 Gy (V27) for those with Child-Pugh class B disease. The median fraction size was 3 Gy/fraction and the radiation dose was 40–66 Gy in 14–23 fractions (BED of 52.0–85.8 Gy10 using the α/β ratio of 10 Gy; median, 74.1 Gy10). The fraction size was reduced to 2–2.5 Gy if the bowel was included in the PTV. The median of the mean liver dose for all the patients was 2,062 cGy (range, 1,008–2,415 cGy). The median of V30 for all the patients was 24% (range, 12–35%).
Follow-up
The patient cases were followed-up at least every three months by CT or ultrasonography during the first year and every six months for up to three years thereafter. The follow-up imaging studies were compared with those that were taken prior to RT and the most significant change in tumor size was regarded as the treatment response. The radiographical tumor response following RT was evaluated using the World Health Organization criteria (13). In-field failure (IFF) was defined as tumor regrowth within the current RT field. Intrahepatic recurrence outside the RT field was defined as an intrahepatic failure (IHF). Distant metastasis (DM) was defined as any recurrence outside the liver.
Classic radiation-induced liver disease (RILD) was defined by the presence of anicteric ascites and the elevation of alkaline phosphatase levels to at least a two-fold increase over the pre-treatment values in the absence of tumor progression. The end-point (occurrence of classic RILD) occurred in patients with good liver function. Non-classic RILD was defined as the elevation of alkaline phosphatase levels to more than five times the upper limit of normal or a decline in liver function (measured by a worsening of the Child-Pugh score by two or more). The end-point was described in patients with poor liver function (virus hepatitis, liver cirrhosis, portal hypertension and Child-Pugh Classes B and C) (14).
Statistics
A univariate cox regression analysis was performed to evaluate the prognostic factors and a multivariate analysis was performed with the forward stepwise procedure using a multiple Cox regression analysis. Survival and IFC were estimated from the first date of RT and the OS rates, and IFC rates were estimated using the Kaplan-Meier method. The Cox regression model was used to investigate the correlation between BED and the IFC. Fisher’s exact test and the logistic regression model were also used to evaluate the correlation between the presence of non-classic RILD and the CLIP score. P<0.05 was considered to indicate a statistically significant difference.
Results
Failure pattern and survival
The failure pattern following a minimum of a two-year follow-up period is shown in Fig. 1. IFF, IHF and DM were observed in 12, 15 and eight patients, respectively, and 10 patients experienced more than one type of recurrence. Following a median follow-up time of 7.7 years for the surviving patients, the one-, two- and five-year OS rates were 60, 40 and 21%, respectively (Fig. 2A). OS was significantly affected by the ECOG performance status (P=0.012), the Child-Pugh classification (P=0.003), the presence of LC (P=0.020), the CLIP score (P=0.001) and the tumor number (P=0.021) in the univariate analysis (Table II). The multivariate analysis showed that Child-Pugh classification (Child-Pugh class B vs. A: HR, 5.42; 95% CI, 2.27–12.95; P<0.0001) and the tumor number (multiple vs. single: HR, 4.68; 95% CI, 2.08–10.53; P<0.0001) were the most significant factors affecting OS. The one-, two- and five-year IFC were 72.7, 61.6 and 56.0%, respectively (Fig. 2B). As shown by the univariate analysis, the factors that were associated with IFC included the tumor number (P=0.026), treatment response and BED (≥60 Gy10 vs. <60 Gy10, P=0.021; ≥55 Gy10 vs. <55 Gy10; P=0.001) (Table II). The multivariate analysis revealed that the treatment response (responder vs. non-responder: HR, 0.27; 95% CI, 0.09–0.83; P=0.023) and BED (≥55 Gy10 vs. <55 Gy10: HR, 0.16; 95% CI, 0.05–0.55; P=0.023) were the most significant factors for IFC. The one-, two- and five-year intrahepatic control (IHC) and distant-metastasis free survival (DMFS) were 65.4, 56.3 and 41.7% and 79.8, 75.3 and 75.3%, respectively. No factors associated with IHC were identified. The treatment response alone affected the DMFS (P=0.032) in the univariate analysis.
Figure 1.
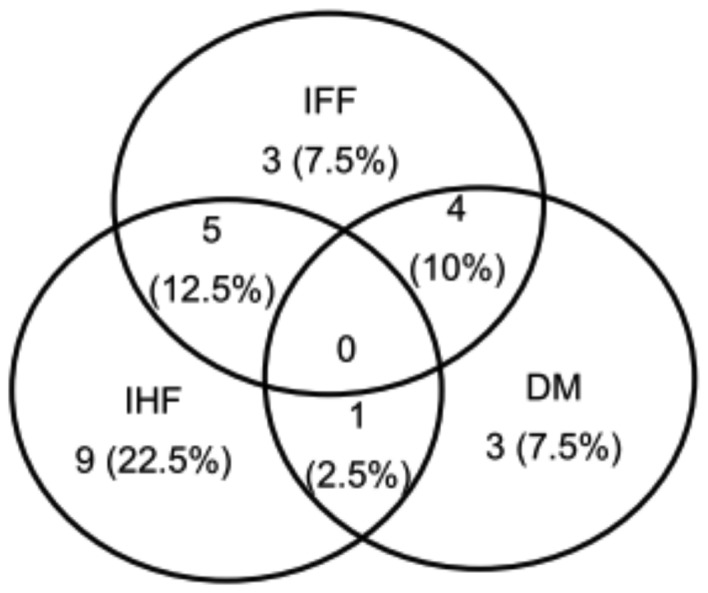
Pattern of failure. The numbers depict cumulative failure sites. IFF, in-field failure; IHF, intrahepatic failure; DM, distant metastasis.
Figure 2.
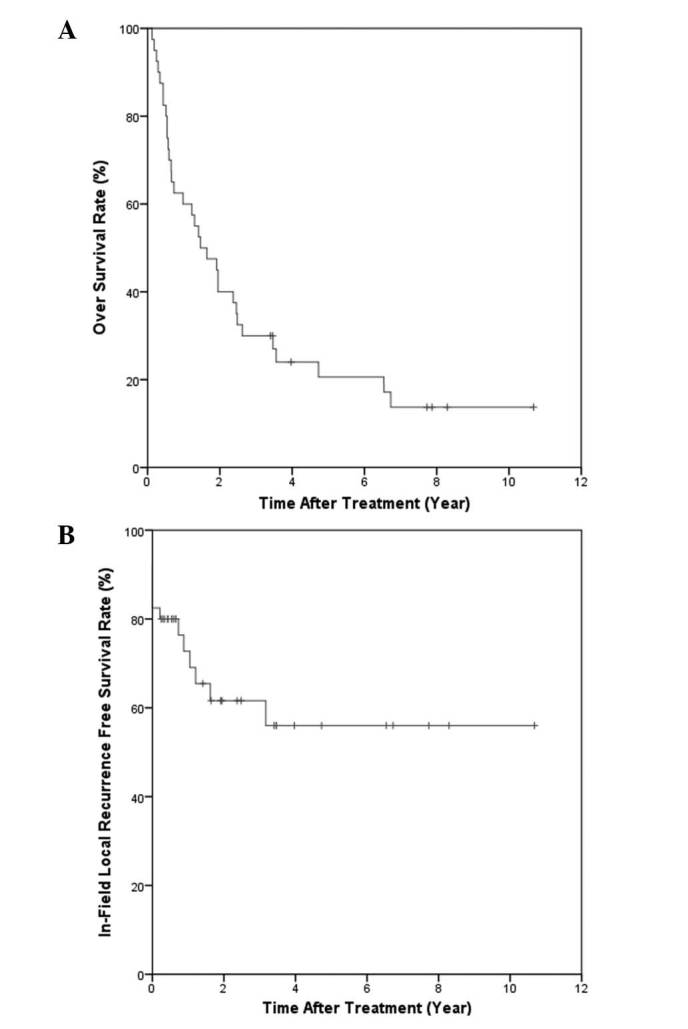
Kaplan-Meier curves of (A) survival and (B) local control. The 1-, 2- and 5-year OS rates were 60, 40 and 21%, respectively. The 1-, 2- and 5-year local control rates were 72.7, 61.6 and 56.0%, respectively.
Table II.
Univariate analysis for OS and IFC.
| Clinical feature | 2-year OS, % | P-value | 2-year IFC, % | P-value |
|---|---|---|---|---|
| Gender | ||||
| Female | 10.0 | 75.0 | ||
| Male | 50.0 | 0.151 | 57.5 | 0.369 |
| Age, years | ||||
| <63 | 30.0 | 65.4 | ||
| ≥63 | 50.0 | 0.217 | 57.1 | 0.763 |
| ECOG | ||||
| 0–1 | 52.2 | 61.5 | ||
| 2 | 23.5 | 0.012 | 61.8 | 0.874 |
| Child-Pugh class | ||||
| A | 56.0 | 60.8 | ||
| B | 13.3 | 0.003 | 61.9 | 0.746 |
| Liver Cirrhosis | ||||
| No | 62.5 | 72.9 | ||
| Yes | 34.4 | 0.02 | 58.2 | 0.763 |
| EV Bleeding | ||||
| No | 48.0 | 46.3 | ||
| Yes | 26.7 | 0.288 | 93.3 | 0.046 |
| CLIP Score | ||||
| ≥3 | 12.5 | 25.0 | ||
| <3 | 46.9 | 0.019 | 76.3 | 0.034 |
| HBV | ||||
| No | 36.7 | 57.1 | ||
| Yes | 50.0 | 0.227 | 75.0 | 0.312 |
| HCV | ||||
| No | 31.3 | 39.3 | ||
| Yes | 45.8 | 0.522 | 75.4 | 0.08 |
| Tumor no. | ||||
| Single | 58.8 | 86.9 | ||
| Multiple | 26.1 | 0.003 | 38.6 | 0.026 |
| Tumor size, cm | ||||
| <5 | 48.0 | 0.447 | 74.3 | 0.402 |
| 5–10 | 28.6 | 0.479 | 39.3 | 0.177 |
| >10 | 0.0 | 0.251 | - | - |
| PVT | ||||
| No | 44.4 | 66.2 | ||
| Yes | 30.8 | 0.286 | 52.9 | 0.704 |
| AJCC stage | ||||
| I–II | 47.6 | 74.7 | ||
| III–IV | 31.6 | 0.062 | 43.9 | 0.227 |
| Response | ||||
| Non-responder | 41.7 | 31.3 | ||
| Responder | 39.3 | 0.624 | 74.6 | 0.009 |
CLIP, Cancer of the Liver Italian Program; ECOG, Eastern Cooperative Oncology Group; EV, esophageal varices; HBV, hepatitis B virus; HCV, hepatitis C virus; IFC, in-field control; OS, overall survival; PVT, portal vein thrombus; AJCC; American Joint Committee on Cancer.
Tumor response
Of the 40 patients, 11 (27.5%) achieved a complete response (CR) following RT and a partial response (PR) was noted in 17 (42.5%) patients. The overall response rate was 70.0%. Stable disease (SD) was observed in five patients (12.5%) and progressive disease (PD) in seven patients (17.5%) (Table III). A positive correlation trend existed between the radiation dose and the tumor response. A higher BED indicated a higher probability of IFC. Using the Cox regression model, the estimated two-year IFC rates for a BED of <60 Gy10, 60–70 Gy10 and >70 Gy10 were 43, 55 and 70%, respectively (P=0.035).
Table III.
Tumor response to radiation.
| Response | Number of patients, (%) |
|---|---|
| CR | 11 (27.5) |
| PR | 17 (42.5) |
| SD | 5 (12.5) |
| PD | 7 (17.5) |
CR, complete response; PR, partial response; SD, stable disease; PD, progresive disease.
Toxicity
Eight of the 40 patients (20%) were noted to experience a deterioration of the Child-Pugh score by two or more. The median time of non-classic RILD occurrence from RT completion was 39.5 days (range, 15–85 days). Among the patients who developed non-classic RILD, one (12.5%), five (62.5%) and two (25%) demonstrated CR, PR and PD, respectively. Six of the eight patients with non-classic RILD exhibited ascites and an increased serum total bilirubin level. Using the logistic regression model, the estimated probability of non-classic RILD for CLIP scores 0, 1, 2, 3 and 4 were 3, 8.2, 21, 43.9 and 69.8%, respectively (P=0.02). A higher CLIP score was associated with a higher probability of non-classic RILD. A positive association between BED to the tumor and non-classic RILD was not identified by the dose constraints. The probability of non-classic RILD did not increase when the BED to the tumor increased. However, the mean liver dose for the patients who developed RILD was significantly higher than that for the non-RILD patients (2,322 cGy vs. 1764 cGy; P=0.048). Classic RILD was not noted in any patients. One patient had a duodenal ulcer confirmed by panendoscopy. The patient who developed the duodenal ulcer underwent 3DCRT with a dose of 54 Gy in 18 fractions to PTV and the ulcer location was in the 90–95% isodose region.
Discussion
RT by X-ray is not routinely used in the curative treatment of HCC. However, HCC has been observed to be more radiosensitive than was previously believed (15). The major limitation has been the poor radiation tolerance of the adjacent normal liver. The first study of the correlation between the dose and complication rate for whole-liver RT was reported by Ingold et al, who demonstrated that the RILD incidence was 12.5 and 44% for patients who were treated with 30–35 Gy and >35 Gy whole liver RT, respectively (16). In a Radiation Therapy Oncology Group study for liver metastasis, no RILD was observed in patients who were administered 30 Gy whole liver irradiation provided by 1.5 Gy/per fraction in two factions per day (17). Lawrence et al(18) revealed that a higher radiation dose (30 Gy whole liver irradiation with a 15 or 30 Gy boost) resulted in a higher tumor response than whole liver RT alone (64% for the boost group and 39% for whole liver RT alone). In addition, it has been shown that the tolerance for liver irradiation may be 35 Gy for the whole liver, 42 Gy for 70% of the liver, 52 Gy for 50% of the liver and 70 Gy for 30% of the liver (19).
Dawson et al(20) showed that liver doses associated with a 5% risk of RILD for uniform irradiation of one-third, two-thirds and the whole liver were 90, 47 and 31 Gy, respectively. Advancements in RT technology have created the possibility of delivering a higher local radiation dose to the liver tumor. A positive correlation between radiation dose and tumor response was observed by Park et al(21). The response rates for doses of <40, 40–50 and >50 Gy were 29, 69 and 77%, respectively. The dose response was established in <50 Gy radiation. A PR was observed in 90% of patients with tumors of <5 cm, but only in 60% of those with tumors >5 cm. Park et al(7) showed that the IFF rate was 46.7 vs. 16.9% for patients treated with doses of ≤50 Gy10 and >50 Gy10, and concluded that a BED of 50 Gy10 was a criterion for an effective radiation dose. Liu et al(22) showed that an improved OS rate was correlated with the dose delivered to the tumor, particularly for doses of >50.4 Gy (1.8 Gy per fraction). Although previous studies have shown that the radiation dose and tumor size affect the treatment results, only a specific dose obtains an enhanced tumor response.
All 40 patients in the present study were administered a greater radiation dose of 52.0–85.8 Gy10 with a median BED of 74.1 Gy10, showing a positive correlation between the radiation dose and tumor response. A higher BED indicated a higher probability of a tumor response. In addition, a BED of ≥55 Gy10 was significantly associated with an improved IFC rate. The results show this schema is feasible for HCC curative treatment and that a dose-response correlation exists for tumor control.
The corresponding BED for the hypofractionated RT schema was 52.0–85.8 Gy10 using the α/β ratio of 10 Gy (median, 74.1 Gy10). The doses published from 3DCRT with conventional fractionation for HCC were between 33 and 66 Gy (23). A wide range of response (55–92%), one-year local control (61–78%) and one-year OS (43–61%) rates were reported for these doses. Studies have reported similar results to the present treatment strategy with a fraction size of 3–6 Gy/fx, a total dose of 38–68 Gy (24,25), a 55–70% response rate, a 73–85% one-year local control rate and a 60–100% one-year OS rate. Stereotactic body radiotherapy (SBRT) with a diversified fractionation schema showed a 65–100% one-year local control rate and a 48–93% one-year OS rate (9,26). However, stringent patient-selection criteria for liver function, tumor location, tumor size and the high-technique demand limit the routine use of SBRT. The present data and data from other published studies have shown that high-dose hypofractionated conformal RT is feasible and yields an improved local control compared with 3DCRT conventional fractionation.
Although higher-dose RT for HCC is achievable with careful patient selection and an improved radiation technique, RILD remains a significant complication. Data from Western countries indicate that a mean liver dose of 28 Gy in 2-Gy/fx is associated with a 5% risk of classic RILD, which is characterized by fatigue, weight gain, increased abdominal girth, hepatomegaly, anicteric ascites and an isolated elevation in alkaline phosphatase that is out of proportion with the other liver enzymes (27). For the HCC patients in regions with endemic viral infection, the tolerance dose for RILD was shown to be lower and HBV infection predisposed patients to RILD, particularly for non-classic RILD presenting with jaundice or markedly elevated serum transaminases of more than five times the upper limit of the reference range (28,29). Radiation was shown to induce HBV reactivation, possibly through the bystander effect, and non-classic RILD further complicated the RILD for viral hepatitis-related HCC (30,31).
Eight of the 40 patients in the present study developed non-classic RILD, six of whom had viral hepatitis under the liver-dose constraints of V30 <30%. The most significant prognostic factor for non-classic RILD was a high CLIP score. Previous studies have shown that in addition to a normal liver dose and HBV carrier status, the underlying liver function is also a significant predictive factor for RILD (32,33). The Child-Pugh classification has often been used to evaluate liver reserve in cirrhosis patients. The present study revealed that the CLIP score, assigning points for the Child-Pugh score, the tumor morphology (solitary, ≤50% of the liver, massive), the serum α-fetoprotein level and the presence or absence of PVT (12), not only serve as prognostic factors for the survival of HCC patients (34,35), but that they also strongly correlate with RILD incidence.
In the spectrum of local-regional therapy for HCC, the percentage of good surgical candidates for resection is ~20% and the five-year survival rate following surgery is ~50%. RFA results in five-year survival rates of 50%, with up to 20% recurrence rates for larger tumors. However, the result is often limited by the size and location of the tumor. For patients with large or multifocal tumors, TACE offers survival benefits compared with the best supportive treatment alone (36–38). However, the five-year survival rate is <2% and the recurrence rate is nearly 100%. In the present study, ~40% of patients had Child-Pugh class B liver cirrhosis and >75% had viral hepatitis. The tumors in the majority of the patients (70%) failed to respond to previous local-regional therapies and were recurrent multiple HCCs of a moderate size. Within this patient population, the strategy resulted in a five-year IFC rate of 56% and a five-year OS rate of 20.6%. The present study shows that this strategy achieves long-term survival and good local control in certain patients.
In summary, the present study showed that high-dose hypofractionated RT is a feasible and effective treatment for HCC. A positive correlation was identified between the radiation dose and IFC. It was shown that a higher BED indicates a higher probability of IFC. The baseline liver function and the CLIP score should also be evaluated carefully to avoid RILD.
Acknowledgements
This study was presented as poster in American Society for Radiation Oncology 51th Annual Meeting, Chicago. The authors would like to thank the Statistical Center For Clinical Research, Chang Gung Memorial Hospital, Taoyuan, Taiwan for their assistance with this study.
References
- 1.Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist. 2010;15(Suppl 4):5–13. doi: 10.1634/theoncologist.2010-S4-05. [DOI] [PubMed] [Google Scholar]
- 2.Liu CL, Fan ST. Nonresectional therapies for hepatocellular carcinoma. Am J Surg. 1997;173:358–365. doi: 10.1016/S0002-9610(96)00384-4. [DOI] [PubMed] [Google Scholar]
- 3.Ye SL, Takayama T, Geschwind J, Marrero JA, Bronowicki JP. Current approaches to the treatment of early hepatocellular carcinoma. Oncologist. 2010;15(Suppl 4):34–41. doi: 10.1634/theoncologist.2010-S4-34. [DOI] [PubMed] [Google Scholar]
- 4.Khan KN, Yatsuhashi H, Yamasaki K, et al. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol. 2000;32:269–278. doi: 10.1016/s0168-8278(00)80072-0. [DOI] [PubMed] [Google Scholar]
- 5.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomised controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54:1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 7.Park W, Lim DH, Paik SW, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Kawashima M, Furuse J, Nishio T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;23:1839–1846. doi: 10.1200/JCO.2005.00.620. [DOI] [PubMed] [Google Scholar]
- 9.Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 10.Toronto UHN. Stereotactic Body Radiation Therapy (SBRT) Hepatocellular Carcinoma. [Accessed April 25, 2012];Journal. Available from: http://www.clinicaltrials.gov/ct2/show/NCT00914355?term=HCC+SBRT&rank=00914355. NLM Identifier: NCT00914355.
- 11.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 12.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. No authors listed. [DOI] [PubMed] [Google Scholar]
- 13.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94–S100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng SH, Lin YM, Chuang VP, et al. A pilot study of three-dimensional conformal radiotherapy in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14:1025–1033. doi: 10.1046/j.1440-1746.1999.01994.x. [DOI] [PubMed] [Google Scholar]
- 16.Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200–208. [PubMed] [Google Scholar]
- 17.Russell AH, Clyde C, Wasserman TH, Turner SS, Rotman M. Accelerated hyperfractionated hepatic irradiation in the management of patients with liver metastases: results of the RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys. 1993;27:117–123. doi: 10.1016/0360-3016(93)90428-x. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence TS, Dworzanin LM, Walker-Andrews SC, et al. Treatment of cancers involving the liver and porta hepatis with external beam irradiation and intraarterial hepatic fluorodeoxyuridine. Int J Radiat Oncol Biol Phys. 1991;20:555–561. doi: 10.1016/0360-3016(91)90069-g. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence TS, Ten Haken RK, Kessler ML, et al. The use of 3-D dose volume analysis to predict radiation hepatitis. Int J Radiat Oncol Biol Phys. 1992;23:781–788. doi: 10.1016/0360-3016(92)90651-w. [DOI] [PubMed] [Google Scholar]
- 20.Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210–2218. doi: 10.1200/JCO.2000.18.11.2210. [DOI] [PubMed] [Google Scholar]
- 21.Park HC, Seong J, Han KH, Chon CY, Moon YM, Suh CO. Dose-response relationship in local radiotherapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2002;54:150–155. doi: 10.1016/s0360-3016(02)02864-x. [DOI] [PubMed] [Google Scholar]
- 22.Liu MT, Li SH, Chu TC, et al. Three-dimensional conformal radiation therapy for unresectable hepatocellular carcinoma patients who had failed with or were unsuited for transcatheter arterial chemoembolization. Jpn J Clin Oncol. 2004;34:532–539. doi: 10.1093/jjco/hyh089. [DOI] [PubMed] [Google Scholar]
- 23.Seong J, Park HC, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55:329–336. doi: 10.1016/s0360-3016(02)03929-9. [DOI] [PubMed] [Google Scholar]
- 24.Liang SX, Zhu XD, Lu HJ, et al. Hypofractionated three-dimensional conformal radiation therapy for primary liver carcinoma. Cancer. 2005;103:2181–2188. doi: 10.1002/cncr.21012. [DOI] [PubMed] [Google Scholar]
- 25.Bae SH, Park HC, Lim do H, et al. Salvage treatment with hypofractionated radiotherapy in patients with recurrent small hepatocellularcarcinoma. Int J Radiat Oncol Biol Phys. 2012;82:e603–e607. doi: 10.1016/j.ijrobp.2011.09.053. [DOI] [PubMed] [Google Scholar]
- 26.Kwon JH, Bae SH, Kim JY, et al. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson LA, Ten Haken RK. Partial volume tolerance of the liver to radiation. Semin Radiat Oncol. 2005;15:279–283. doi: 10.1016/j.semradonc.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Cheng JC, Wu JK, Lee PC, et al. Biologic susceptibility of hepatocellular carcinoma patients treated with radiotherapy to radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2004;60:1502–1509. doi: 10.1016/j.ijrobp.2004.05.048. [DOI] [PubMed] [Google Scholar]
- 29.Cheng JC, Liu HS, Wu JK, Chung HW, Jan GJ. Inclusion of biological factors in parallel-architecture normal-tissue complication probability model for radiation-induced liver disease. Int J Radiat Oncol Biol Phys. 2005;62:1150–1156. doi: 10.1016/j.ijrobp.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Park JW, Kim TH, Koh DW, Lee WJ, Kim CM. Hepatitis B virus reactivation after three-dimensional conformal radiotherapy in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2007;69:813–819. doi: 10.1016/j.ijrobp.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Chou CH, Chen PJ, Lee PH, Cheng AL, Hsu HC, Cheng JC. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin Cancer Res. 2007;13:851–857. doi: 10.1158/1078-0432.CCR-06-2459. [DOI] [PubMed] [Google Scholar]
- 32.Liang SX, Zhu XD, Xu ZY, et al. Radiation-induced liver disease in three-dimensional conformal radiation therapy for primary liver carcinoma: the risk factors and hepatic radiation tolerance. Int J Radiat Oncol Biol Phys. 2006;65:426–434. doi: 10.1016/j.ijrobp.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 33.Xu ZY, Liang SX, Zhu J, et al. Prediction of radiation-induced liver disease by Lyman normal-tissue complication probability model in three-dimensional conformal radiation therapy for primary liver carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:189–195. doi: 10.1016/j.ijrobp.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 34.Levy I, Sherman M Liver Cancer Study Group of the University of Toronto. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:881–885. doi: 10.1136/gut.50.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueno S, Tanabe G, Sako K, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program Hepatology. 2001;34:529–534. doi: 10.1053/jhep.2001.27219. [DOI] [PubMed] [Google Scholar]
- 36.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 37.Llovet JM, Real MI, Montana X, et al. Barcelona Liver Cancer Group: Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 38.Lin DY, Liaw YF, Lee TY, Lai CM. Hepatic arterial embolization in patients with unresectable hepatocellular carcinoma - a randomized controlled trial. Gastroenterology. 1988;94:453–456. doi: 10.1016/0016-5085(88)90436-2. [DOI] [PubMed] [Google Scholar]


