Abstract
Skull base metastasis from differentiated thyroid carcinoma including follicular thyroid carcinoma (FTC) and papillary thyroid carcinoma (PTC) is a rare clinical entity. Eighteen FTC cases and 10 PTC cases showing skull base metastasis have been reported. The most common symptom of skull base metastasis from FTC and PTC is cranial nerve dysfunction. Bone destruction and local invasion to the surrounding soft tissues are common on radiological imaging. Skull base metastases can be the initial clinical presentation of FTC and PTC in the presence of silent primary sites. The possibility of skull base metastasis from FTC and PTC should be considered in patients with the clinical symptoms of cranial nerve dysfunction and radiological findings of bone destruction. A variety of genetic alterations in thyroid tumors have been identified to have a fundamental role in their tumorigenesis. Molecular histochemical studies are useful for elucidating the histopathological features of thyroid carcinoma. Recent molecular findings may provide novel molecular-based treatment strategies for thyroid carcinoma.
Keywords: skull base metastasis, follicular thyroid carcinoma, papillary thyroid carcinoma, iodine-131 brachytherapy, thyroid-stimulating hormone suppression
I. Introduction
In thyroid glands, there are two different types of endocrine thyroid cells, namely, follicular thyroid cells and parafollicular C cells, from both of which thyroid carcinomas are derived. There are several histological types and subtypes of thyroid carcinoma with different cellular origins, characteristics and prognoses. According to the most recent World Health Organization (WHO) classification of thyroid tumors published in 2004, follicular thyroid cell-derived tumors include papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC), poorly differentiated thyroid cancer (PDTC) and anaplastic thyroid cancer (ATC), and these follicular thyroid cell-derived carcinomas account for the majority of thyroid malignancies. This WHO classification of thyroid tumors included PDTC as a new diagnostic category [62]. Therefore, it may be noted that publications prior to this WHO classification might include misinterpretations on histological types of thyroid carcinoma. Parafollicular C cell-derived medullary thyroid cancer (MTC) accounts for a small proportion of thyroid malignancies. The primary molecular mechanism underlying MTC tumorigenesis is the aberrant activation of RET signaling [17, 75]. The aberrant activation of RET signaling is caused by RETmutations, which are not present in follicular thyroid cell-derived tumors [75].
PDTC and ATC have a very poor prognosis. In contrast to these malignant subtypes, PTC and FTC are collectively classified as differentiated thyroid carcinoma (DTC). PTC is a major differentiated subtype that has slow growing characteristics and a good prognosis. FTC is another differentiated subtype that, in contrast to PTC, has a greater tendency of distant metastasis to such organs as lung and bone. Clinical overview of follicular thyroid cell-derived carcinomas is summarized in Table 1.
Table 1.
Clinical overview of follicular thyroid cell-derived carcinomas
| Tumor type | Prevalence (% of thyroid carcinomas) | Characteristics | Subtypes |
|---|---|---|---|
| papillary thyroid carcinoma (PTC) | 80–85 | Well differentiated, with papillary architecture and characteristic nuclear features, such as enlargement, oval shape, elongation, overlapping and clearing, inclusions and grooves. Propensity for lymphatic metastasis | conventional PTC (CPTC), follicular-variant PTC (FVPTC), tall-cell PTC (TCPTC), a few rare variants |
| follicular thyroid carcinoma (FTC) | 10–15 | Well differentiated, hypercellular, microfollicular patterns, lacking nuclear features of PTC. Propensity for metastasis via the blood stream | Hurthle cell thyroid carcinoma |
| poorly differentiated thyroid carcinoma (PDTC) | 5–10 | Poorly differentiated, often overlapping with PTC and FTC. Intermediate aggressiveness between differentiated and undifferentiated thyroid carcinomas | |
| anaplastic thyroid carcinoma (ATC) | 2–3 | Undifferentiated, admixture of spindle, pleomorphic giant and epithelioid cells, extremely invasive and metastatic, highly lethal, may occur de novo or derive from PTC, FTC or PDTC |
(modified from Ref. 75.)
Despite the slowly progressive, low grade malignancy of DTC, about 10% of patients with PTC and 20–40% of patients with FTC die of the disease [79]. Most deaths result from poor control of local disease and distant metastases. The lung is the most common metastatic site of thyroid carcinoma, followed by the bone [11, 21, 45, 47]. Skull metastasis of thyroid carcinoma is rare, with a small number of reported cases [2–4, 6–8, 12, 19–24, 26–28, 30, 32–35, 38–40, 42, 44, 45, 49–51, 53, 54, 56, 57, 60, 63–65, 68–70, 73, 77, 78]. The largest series of skull metastasis from thyroid carcinoma reported a frequency of only 2.5% among 473 patients [45]. Moreover, skull base metastasis from DTC is even rarer, with only 28 reported cases, including 18 cases of skull base metastasis from FTC [6, 7, 24, 30, 39, 44, 45, 49, 50, 54, 56, 57, 60, 63, 69, 70, 78] and 10 cases from PTC [4, 12, 20, 23, 34, 38, 64, 65, 77] (Table 2). In this review article, the clinicopathological and molecular histochemical features and treatment modalities of skull base metastasis of DTC, namely, FTC and PTC, are discussed with a review of literatures on molecular pathogenesis.
Table 2.
Summary of reported cases with skull base metastasis from follicular thyroid carcinoma (FTC) and papillary thyroid carcinoma (PTC)
| Author | Age | Sex | Histology | Location of metastasis | Period from initial diagnosis to metastasis (yrs) | symptoms | treatment |
|---|---|---|---|---|---|---|---|
| Trunnell et al. (1949) [Ref. 69] | 42 | F | FTC | sphenoid sinus | 0 | blurred vision, blindness | 131I |
| Kistler and Pribram (1975) [Ref. 30] | 69 | F | FTC | sella turcica, clivus | 9 | blurred vision, oculomotor nerve palsy | surgery |
| Song et al. (1981) [Ref. 63] | 23 | M | FTC | petrous ridge | 0 | persistent headache, one episode of consciousness loss | surgery, 131I, TSH suppression |
| Nagamine et al. (1985) [Ref. 45] | 65 | F | FTC | skull base | 0 | visual impairment, exophtalamos | surgery, 131I, external radiation |
| Ober et al. (1987) [Ref. 49] | 63 | F | FTC | clivus | 7 | six nerve palsy | 131I |
| Ruchti et al. (1987) [Ref. 57] | 71 | F | FTC | clivus, sella turcica, sphenoid sinus, petrous bone | 0 | multiple cranial nerve paralysis | none (autopsy case) |
| Ochiai et al. (1992) [Ref. 50] | 62 | F | FTC | sella turcica, clivus, cavernous sinus, sphenoid sinus | 0 | retro-orbital pain, diplopia due to abducens and oculomotor nerve paralyses | surgery, 131I |
| Casals et al. (1995) [Ref. 6] | 61 | M | FTC | clivus | 0 | palatal hypomotility, and weakness of the facial and tongue muscles | surgery, 131I, TSH suppression |
| Vargas et al. (1999) [Ref. 70] | 46 | F | FTC | clivus, cavernous sinus, skull vault | 8 | hypopituitarism | surgery, 131I, external radiation, TSH suppression |
| Rosahl et al. (2000) [Ref. 56] | 50 | F | FTC | clivus, petrous bone | 0.5 | dysphagia, dysphonia, hypoglossal paralysis | surgery, 131I, TSH suppression |
| Kachhara et al. (2001) [Ref. 24] | 50 | F | FTC | petrous apex, cavernous sinus | 0 | 5th, 6th, 7th, 8th nerve palsy | surgery, 131I, external radiation |
| Chrisoulidou et al. (2004) [Ref. 7] | 60 | M | FTC | sella turcica, cavernous sinus | 4.5 | diplopia, ptosis | surgery, external radiation |
| Simon et al. (2004) [Ref. 60] | 23 | F | FTC | sella turcica, sphenoid sinus, clivus | 0 | diplopia | surgery, 131I |
| Yilmazlar et al. (2004) [Ref. 78] | 43 | M | FTC | cavernous sinus, sphenoid sinus | 1.8 | visual impairment, galactorrhea | surgery, 131I, TSH suppression |
| Mydlarz et al. (2007) [Ref. 44] | 74 | M | FTC | clivus, sphenoid sinus, petrous apex, cavernous sinus, infratemporal fossa | 0 | blurred vision, abducens nerve palsy | surgery, TSH suppression, 131I |
| Pelaz et al. (2009) [Ref. 54] | 61 | F | FTC | infratemporal fossa | 18 | hemifacial pain, tongue and facial dysesthesia, hearing loss | surgery, 131I, TSH suppression |
| Matsuno et al. (2010) [Ref. 39] | 58 | F | FTC | temporal base, infratemporal fossa, cavernous sinus, sphenoid sinus, occipital bone, clivus, petrous bone | 7 | facial dysesthesia, hearing disturbance, paraparesis in lower extremities | external radiation, surgery, TSH suppresion, 131I |
| Matsuno et al. (2010) [Ref. 39] | 71 | F | FTC | petrous bone | 14 | 7th and 8th nerve dysfunction | surgery, external radiation, TSH suppresion, 131I |
| Johnson and Atkins (1965) [Ref. 23] | 56 | F | PTC | sella turcica, sphenoid sinus | 6 | blurred vision, 3rd and 6th nerve palsy | TSH suppression, 131I |
| Sziklas et al. (1985) [Ref. 64] | 44 | F | PTC | midline skull base, sella turcica | 18 | panhypopituitarism | surgery, 131I |
| Freeman et al. (1996) [Ref. 12] | 50 | M | PTC | skull base, sphenoid sinus | 0.25 | facial pain, exophthalmos, Horner’s syndrome | surgery, 131I, external radiation |
| Masiukiewicz et al. (1999) [Ref. 38] | 56 | M | PTC | sella turcica | 5 | panhypopituitarism | 131I |
| Masiukiewicz et al. (1999) [Ref. 38] | 55 | F | PTC | cavernous sinus, sella turcica | 14 | panhypopituitarism, blindness | 131I |
| Bell et al. (2001) [Ref. 4] | 35 | F | PTC | sella turcica | 8 | hemianopsia, diabetes insipidus, amenorrhea | surgery |
| Takami et al. (2002) [Ref. 65] | 41 | M | PTC | cavernous sinus | 10 | diplopia, subarachnoid hemorrhage | surgery, gamma knife |
| Yan et al. (2010) [Ref. 77] | 73 | M | PTC | petrous bone, sphenoid sinus, sella floor, clivus, pterygoid plate, ethmoid sinus, infratemporal fossa, cavernous sinus | 0 | visual impairment, diplopia, epistaxis | surgery, TSH suppression, 131I |
| Hugh et al. (2011) [Ref. 20] | 64 | F | PTC | petrous bone | 0 | no symptoms | surgery, external radiation |
| Kutluhan et al. (2012) [Ref. 34] | 61 | M | PTC | temporooccipital bone | NA | multiple cranial nerve paralysis | surgery, 131I, external radiation |
M: male, F: female
II. Clinical and Histopathological Features of Skull Base Metastasis from FTC and PTC
Mean age of patients with skull base metastasis from FTC was 54.6 years, ranging from 23 to 74 years, and that of those from PTC was 53.5 years, ranging from 35 to 73 years. Bone metastasis from thyroid carcinoma is often observed in the sixth and seventh decades of life [40]. Similarly, 10 out of 18 patients with skull base metastasis of FTC and 3 out of 10 those from PTC are in the sixth and seventh decades. Skull base metastasis of FTC shows female predominance (13 females and 5 males), and this female predominance is generally observed in thyroid carcinoma. Skull base metastasis of PTC shows equal gender predominance. Although PTC is more common than FTC, FTC is more prone to spread hematogenously, especially to the lungs and bone [43]. Thus, larger number of literatures concerning skull base metastasis is found in FTC compared with PTC. The metastatic lesion is usually hypervascular and osteolytic on radiological examination [26]. Bleeding is often profuse during surgical resection [45]. The most common symptom of skull base metastasis from FTC is cranial nerve dysfunction, which was observed in 16 of the 18 cases. Cranial nerve dysfunction was found in 7 of 10 cases of PTC. Bone destruction and local invasion to the surrounding soft tissues are common on radiological images, so that skull base metastasis from FTC and PTC is often mistaken as chordoma or chondrosarcoma [6, 56]. The period between initial diagnosis and skull base metastasis from FTC ranged from 0 to 18 years, with a mean of 3.9 years. The period between initial diagnosis and skull base metastasis from PTC ranged from 0 to 18 years, with a mean of 6.8 years. Skull base metastases were the initial symptom of FTC in 9 of the 19 reported cases and that of PTC in 2 of the 9 reported cases.
No histopathological features that could predict bone metastasis, particularly skull base metastasis, of DTC were found in the literatures. Similarly, no particular histological features that could distinguish between DTC metastasizing to the skull base and the other sites were found in the literatures. Prognostic difference between DTC with skull base metastasis and those with other bone metastases was not found in the literature review. Other cancers such as lung cancer might metastasize to the skull base. Immunohistochemical studies of thyroid transcription factor-1 (TTF-1) and thyroglobulin (TGB) are useful for distinguishing between thyroid carcinoma and lung adenocarcinoma [59]. TTF-1 is an immunohistochemical marker used to confirm pulmonary and thyroid carcinoma, while TGB is expressed by thyroid carcinoma [59]. Immunohistochemical studies of high molecular weight keratin (CK 19) are also useful for discrimination between benign thyroid tumors and thyroid carcinoma. Focal CK19 staining may be found in benign disease, but diffuse and strong positivity is characteristic of PTC [5].
The possibility of skull base metastasis should be considered in the clinical course of FTC and PTC, and the patient should be meticulously followed up. Noticeably, skull base metastases can be the initial clinical presentation of more than half of the reported cases of FTC in the presence of silent primary sites, which emphasizes the unpredictable nature of FTC.
III. Treatment of Skull Base Metastasis from FTC and PTC
The treatment algorithm for primary thyroid carcinomas includes nearly total or total thyroidectomy, followed by oral administration of 131I and thyroid-stimulating hormone (TSH) suppression [68]. However, there is no clear consensus concerning the treatment of skull base metastasis from FTC and PTC because of the rarity of these lesions. Several thyroidologists have recommended, as the first-line therapy, complete excision of the thyroid gland with as many of the metastatic lesions as possible [22, 45]. Surgical debulking is hazardous in most cases of skull base metastasis because of the presence of vital structures and profuse bleeding. Therefore, the second option is internal irradiation with 131I if scintigraphy taken by the metastatic lesions [45]. External irradiation should be administered to cold lesions identified by 131I scintigraphy [45]. Chronic suppression of endogenous TSH should be induced by the administration of thyroid hormone to prevent tumor growth [56]. Measurement of circulating TGB may be useful for predicting the recurrence of the differentiated thyroid carcinoma during follow-up [71]. The calculation of TGB/(TSH × 131I uptake in 24 hr) ratio has prognostic value in the treatment including 131I ablation therapy.
Bisphosphonates have been used widely to control bone metastasis of solid tumors such as breast and prostate cancers. The use of bisphosphonates for bone metastasis of thyroid carcinoma has been reported in only 2 patients [39, 67]. Administration of zoledronic acid and alendronate sodium hydrate decreased urinary type I collagen N-telopeptide, which indicated the suppression of bone resorption. Such suppression of bone resorption by bisphosphonates may be beneficial for patients with skull base metastasis of FTC and PTC.
IV. Molecular Pathogenesis of FTC and PTC
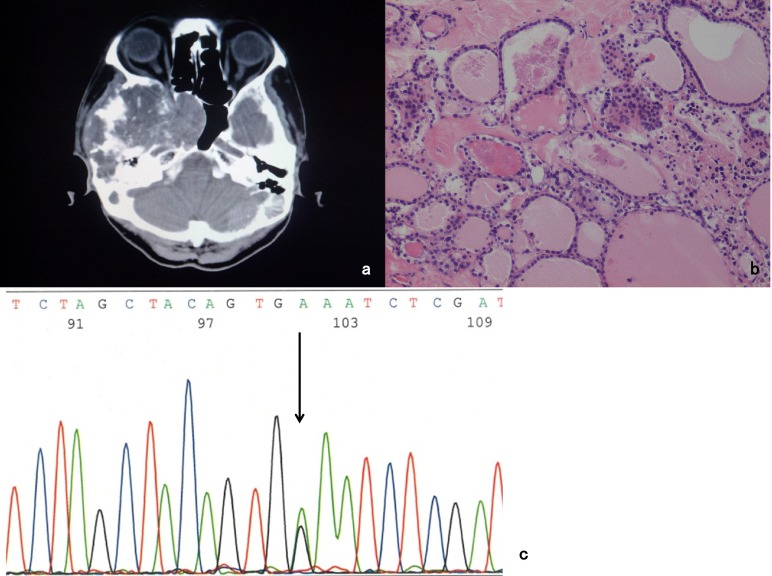
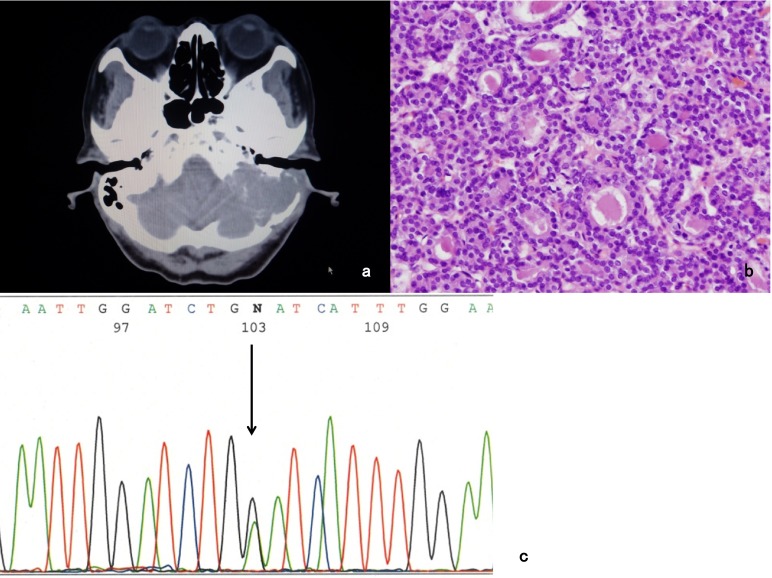
A variety of genetic alterations in thyroid tumors have been identified to have a fundamental role in their tumorigenesis. First noted is T1799A transverse point mutation of BRAF, which causes the expression of BRAF-V600E mutant protein and then evokes the constitutive activation of this serine/threonine kinase [9, 13, 29, 46, 61, 75, 76]. A multicenter study demonstrated a strong association of BRAFV600E with poor clinicopathological outcomes of PTC, namely, aggressive pathological features, increased recurrence, loss of radioiodine avidity and treatment failures [74]. The other types of BRAF mutation are also noted. BRAF-G468E and BRAF-K601E mutations were observed in our cases of FTC (Figs. 1 and 2).
Fig. 1.
a: CT scan reveals skull base tumor invading the right middle cranial fossa. b: Pathological examination confirms the tumor is FTC (Hematoxylin-eosin staining). c: BRAF-K601E mutation is observed in the tumor cell (arrow).
Fig. 2.
a: CT scan reveals skull base tumor invading the left pyramidal bone. b: Pathological examination confirms the tumor is FTC (Hematoxylin-eosin staining). c: BRAF-G468E mutation is observed in the tumor cell (arrow).
Second prevalent mutations in thyroid carcinoma are RAS mutations. There are three isoforms of RAS: HRAS, KRAS and NRAS, and NRASis predominantly mutated in thyroid tumors [75]. RAS is a classical dual activator of the MAPK and PI3K-AKT pathways, and in thyroid tumorigenesis, RAS mutations seem to preferentially activate the PI3K-AKT pathway [1, 36]. The common occurrence of RAS mutations in follicular thyroid adenoma (FTA) suggests that activated RAS may have a role in early follicular thyroid cell tumorigenesis. However, additional genetic alterations other than RAS mutation are apparently required to transform FTA into thyroid carcinoma. Another study suggests that concurrent KRAS mutant expression and PTEN deletion induced a rapid occurrence of aggressive FTC [41, 75].
Mutations or deletions of the tumor suppressor gene PTENare the classical genetic alterations that activate the PI3K-AKT pathway and are the genetic basis for follicular thyroid cell tumorigenesis in Cowden’s syndrome [16]. Mutations of PIK3CA are also common in thyroid cancer, particularly FTC, PDTC and ATC [1, 14, 18, 36, 55, 58, 72, 75].
Some genes such as TRK-fused gene have important role in pathogenesis of thyroid papillary carcinoma [15, 37, 66]. TRK-fused gene is a fusion partner of the NTRK1 gene [15], which encodes a tyrosine kinase receptor for nerve growth factor [25, 31].
It has been shown that COX-2 is involved in the pathomechanisms of thyroid carcinomas and chronic thyroiditis [10, 48]. Omi et al. performed an immunohistochemical analysis for membrane-bound PGES-1 (mPGES-1) in surgically resected thyroid gland tissues including PTC [52]. They found the involvement of mPGES-1 in proliferation and differentiation of PTC as well as local invasion of PTC.
Other genetic and epigenetic alterations include mutations in TP53, β-catenin (CTNNB1), anaplastic lymphoma kinase (ALK) and isocitrate dehydrogenase 1 (IDH1), translocations (RET-PTC and paired box 8 (PAX8)-peroxisome proliferator-activated receptor-γ (PPARG)) and aberrant gene methylation [75]. Gene amplification, copy-number gain and gene translocation are also genetic mechanism in thyroid tumorigenesis [75]. Additionally, at the core of the molecular pathogenesis of thyroid carcinoma is the uncontrolled activity of various signalling pathways, including the MAPK, PI3K-AKT, nuclear factor-κB (NF-κB), RASSF1-mammalian STE20-like protein kinase 1 (MST1)-forkhead box O3 (FOXO3), WNT-β-catenin, hypoxia-inducible factor 1α (HIF1α) and TSH-TSH receptor (TSHR) pathways [75].
Molecular histochemical studies are useful for elucidating the histopathological features of thyroid carcinoma. These recent molecular findings may provide novel molecular-based treatment strategies for thyroid carcinoma.
V. Conclusion
Skull base metastasis from FTC and PTC is a rare clinical entity, and may be the initial clinical presentation of FTC and PTC in the presence of silent primary sites. Larger number of literatures concerning skull base metastasis is found in FTC compared to PTC. The possibility of skull base metastasis from FTC and PTC should be considered in patients with the clinical symptoms of cranial nerve dysfunction and radiological findings of bone destruction. Molecular histochemical studies are useful for elucidating the histopathological features of thyroid carcinoma. Recent molecular findings may provide novel molecular-based treatment strategies for thyroid carcinoma.
VI. Conflict of Interest
The authors have no conflicts of interest to disclose.
VII. References
- 1.Abubaker J., Jehan Z., Bavi P., Sultana M., Al-Harbi S., Ibrahim M., Al-Nuaim A., Ahmed M., Amin T., Al-Fehaily M., Al-Sanea O., Al-Dayel F., Uddin S., Al-Kuraya K. S. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J. Clin. Endocrinol. Metab. . 2008;93:611–618. doi: 10.1210/jc.2007-1717. [DOI] [PubMed] [Google Scholar]
- 2.Akdemir I., Erol F. S., Akpolat N., Ozveren M. F., Akfirat M., Yahsi S. Skull metastasis from thyroid follicular carcinoma with difficult diagnosis of the primary lesion. Neurol. Med. Chir. (Tokyo) 2005;45:205–208. doi: 10.2176/nmc.45.205. [DOI] [PubMed] [Google Scholar]
- 3.Aoki A., Kwak R. [A case of skull metastasis of malignant struma] No To Shinkei. 1972;24:1657–1660. (in Japanese with English abstract) [Google Scholar]
- 4.Bell C. D., Kovacs K., Horvath E., Smythe H., Asa S. Papillary carcinoma of thyroid metastatic to the pituitary gland. Arch. Pathol. Lab. Med. 2001;125:935–938. doi: 10.5858/2001-125-0935-PCOTMT. [DOI] [PubMed] [Google Scholar]
- 5.Bose D., Das R. N., Chatterjee U., Banerjee U. Cytokeratin 19 immunoreactivity in the diagnosis of papillary thyroid carcinoma. Indian J. Med. Paediatr. Oncol. 2012;33:107–111. doi: 10.4103/0971-5851.99746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casals M. M., Hunter S. B., Olson J. J., Gussack G., Blevins L. S., Jr. Metastatic follicular thyroid carcinoma masquerading as a chordoma. Thyroid. 1995;5:217–221. doi: 10.1089/thy.1995.5.217. [DOI] [PubMed] [Google Scholar]
- 7.Chrisoulidou A., Pazaitou-Panayiotou K., Flaris N., Drimonitis A., Giavroglou I., Ginikopoulou E., Vainas I. Pituitary metastasis of follicular thyroid carcinoma. Horm. Res. . 2004;61:190–192. doi: 10.1159/000076387. [DOI] [PubMed] [Google Scholar]
- 8.Coconu M., Berdan G., Rosculescu I., Herlea V. An unusual metastasis of a papillary thyroidian carcinoma with follicular pattern. Rom. J. Morphol. Embryol. 1998;44:183–185. [PubMed] [Google Scholar]
- 9.Cohen Y., Xing M., Mambo E., Guo Z., Wu G., Trink B., Beller U., Westra W. H., Ladenson P. W., Sidransky D. BRAF mutation in papillary thyroid carcinoma. J. Natl. Cancer Inst. . 2003;95:625–627. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 10.Cornetta A. J., Russell J. P., Cunnane M., Keane W. M., Rothstein J. L. Cyclooxygenase-2 expression in human thyroid carcinoma and Hashimoto’s thyroiditis. Laryngoscope. 2002;112:238–242. doi: 10.1097/00005537-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Dinsmore R. S., Hicken N. F. Metastases from malignant tumors of the thyroid. A study of 124 cases. Am. J. Surg. 1934;24:202–224. [Google Scholar]
- 12.Freeman J. L., Gershon A., Liavaag P. G., Walfish P. G. Papillary thyroid carcinoma metastasizing to the sphenoid-ethmoid sinuses and skull base. Thyroid. 1996;6:59–61. doi: 10.1089/thy.1996.6.59. [DOI] [PubMed] [Google Scholar]
- 13.Fukushima T., Suzuki S., Mashiko M., Ohtake T., Endo Y., Takebayashi Y., Sekikawa K., Hagiwara K., Takenoshita S. BRAF mutations in papillary carcinomas of the thyroid. Oncogene . 2003;22:6455–6457. doi: 10.1038/sj.onc.1206739. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Rostan G., Costa A. M., Pereira-Castro I., Salvatore G., Hernandez R., Hermsem M. J., Herrero A., Fusco A., Cameselle-Teijeiro J., Santoro M. Mutation of the PIK3CA gene in anaplastic thyroid cancer. Cancer Res. . 2005;65:10199–10207. doi: 10.1158/0008-5472.CAN-04-4259. [DOI] [PubMed] [Google Scholar]
- 15.Greco A., Fusetti L., Miranda C., Villa R., Zanotti S., Pagliardini S., Pierotti M. A. Role of the TFG N-terminus and coiled-coil domain in the transforming activity of the thyroid TRK-T3 oncogene. Oncogene. 1998;16:809–816. doi: 10.1038/sj.onc.1201596. [DOI] [PubMed] [Google Scholar]
- 16.Gustafson S., Zbuk K. M., Scacheri C., Eng C. Cowden syndrome. Semin. Oncol. . 2007;34:428–434. doi: 10.1053/j.seminoncol.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Hofstra R. M., Landsvater R. M., Ceccherini I., Stulp R. P., Stelwagen T., Luo Y., Pasini B., Höppener J. W., van Amstel H. K., Romeo G., Lips C. J. M., Buys C. H. C. M. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature . 1994;367:375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- 18.Hou P., Liu D., Shan Y., Hu S., Studeman K., Condouris S., Wang Y., Trink A., El-Naggar A. K., Tallini G., Vasko V., Xing M. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin. Cancer Res. . 2007;13:1161–1170. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- 19.Hubeny M. J., Mass M. Roentgenologic aspects of metastases. Radiology. 1940;35:315–321. [Google Scholar]
- 20.Hugh S. C., Enepekides D., Wong J., Yeung R., Lin V. Y. Metastasis of follicular variant of papillary thyroid carcinoma masquerading as primary temporal bone tumour. J. Laryngol. Otol. . 2011;125:528–532. doi: 10.1017/S0022215110002926. [DOI] [PubMed] [Google Scholar]
- 21.Inci S., Akbay A., Bertan V., Gedikoglu G., Onol B. Solitary skull metastasis from occult thyroid carcinoma. J. Neurosurg. Sci. 1994;38:63–66. [PubMed] [Google Scholar]
- 22.Inoue T., Tomita T., Kawasaki A., Hoshi M., Koide N. [A case of follicular carcinoma of the thyroid gland with giant skull metastasis] Jibiinnkoka Tenbou. 1995;2:216–221. (in Japanese with English abstract) [Google Scholar]
- 23.Johnson P. M., Atkins H. L. Functioning metastasis of thyroid carcinoma in the sella turcica. J. Clin. Endocrinol. Metab. . 1965;25:1126–1130. doi: 10.1210/jcem-25-8-1126. [DOI] [PubMed] [Google Scholar]
- 24.Kachhara R., Nair S., Radhakrishnan V. V., Pandey M., Ahmed M. I., Kumar A., Bhattacharya R. N. Solitary metastasis from occult follicular carcinoma of the thyroid mimicking trigeminal neurinoma. Case Report. Neurol. Med. Chir. (Tokyo) 2001;41:360–363. doi: 10.2176/nmc.41.360. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan D. R., Hampstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science. 1991;252:554–558. doi: 10.1126/science.1850549. [DOI] [PubMed] [Google Scholar]
- 26.Kearns D. B., Robinson L. D., Wright G. L., Wickersham J. K., Parke R. B., Jr. Skull metastases from follicular thyroid carcinoma. Arch. Otolaryngol. Head Neck Surg. . 1988;114:454–456. doi: 10.1001/archotol.1988.01860160098030. [DOI] [PubMed] [Google Scholar]
- 27.Kelessis N. G., Prassas E. P., Dascalopoulou D. V., Apostolikas N. A., Tavernaraki A. P., Vassilopoulos P. P. P. Unusual metastatic spread of follicular thyroid carcinoma: report of a case. Surg. Today. 2005;35:300–303. doi: 10.1007/s00595-004-2922-2. [DOI] [PubMed] [Google Scholar]
- 28.Kim S. H., Kosnik E., Madden C., Morran S., Rusin J., Gordon T., Boue D. Lytic skull metastasis from a follicular thyroid carcinoma in a child. Pediatr. Neurosurg. 1998;28:84–88. doi: 10.1159/000028626. [DOI] [PubMed] [Google Scholar]
- 29.Kimura E. T., Nikiforova M. N., Zhu Z., Knauf J. A., Nikiforov Y. E., Fagin J. A. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. . 2003;63:1454–1457. [PubMed] [Google Scholar]
- 30.Kistler M., Pribram H. W. Metastatic disease of the sella turcica. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1975;123:13–21. doi: 10.2214/ajr.123.1.13. [DOI] [PubMed] [Google Scholar]
- 31.Klein R., Jing S. Q., Nanduri V., O’Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- 32.Kung V. W., Wong G. K., Zhu X. L., Ying S. Y., Ahuja A. T., Poon W. S., Ng H. K. Solitary skull metastasis of thyroid papillary carcinoma. A.N.Z. J. Surg. 2007;77:1030–1031. doi: 10.1111/j.1445-2197.2007.04311.x. [DOI] [PubMed] [Google Scholar]
- 33.Kusunoki T., Urano K., Saito K., Murata K. A case of skull metastasis from thyroid papillary carcinoma. Thyroid. 2003;13:889–890. doi: 10.1089/105072503322401113. [DOI] [PubMed] [Google Scholar]
- 34.Kutluhan A., Yalçıner G., Bozdemir K., Ozdemir E., Tarlak B., Bilgen A. S. Papillary thyroid carcinoma with metastasis to the temporooccipital skull: a case report. Kulak. Burun. Bogaz. Ihtis. Derg. . 2012;22:160–163. doi: 10.5606/kbbihtisas.2012.030. [DOI] [PubMed] [Google Scholar]
- 35.Lin K. D., Lin J. D., Huang H. S., Jeng L. B., Ho Y. S. Skull metastasis with brain invasion from thyroid papillary microcarcinoma. J. Formos. Med. Assoc. 1997;96:280–282. [PubMed] [Google Scholar]
- 36.Liu Z., Hou P., Ji M., Guan H., Studeman K., Jensen K., Vasko V., El-Naggar A. K., Xing M. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J. Clin. Endocrinol. Metab. . 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 37.Maebayashi H., Takeuchi S., Masuda C., Makino S., Fukui K., Kimura H., Tooyama I. Expression and localization of TRK-fused gene products in the rat brain and retina. Acta Histochem. Cytochem. 2012;45:15–23. doi: 10.1267/ahc.11015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masiukiewicz U. S., Nakchbandi I. A., Stewart A. F., Inzucchi S. E. Papillary thyroid carcinoma metastatic to the pituitary gland. Thyroid. 1999;9:1023–1027. doi: 10.1089/thy.1999.9.1023. [DOI] [PubMed] [Google Scholar]
- 39.Matsuno A., Katakami H., Okazaki R., Yamada S., Sasaki M., Nakaguchi H., Yamada S. M., Hoya K., Murakami M., Yamazaki K., Ishida Y., Iwasaki H., Kuyama J., Kakudo K. Skull base metastasis from follicular thyroid carcinoma-Two case reports. Neurol. Med. Chir. (Tokyo) . 2010;50:421–425. doi: 10.2176/nmc.50.421. [DOI] [PubMed] [Google Scholar]
- 40.McCormack K. R. Bone metastases from thyroid carcinoma. Cancer. 1966;19:181–184. doi: 10.1002/1097-0142(196602)19:2<181::aid-cncr2820190207>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 41.Miller K. A., Yeager N., Baker K., Liao X. H., Refetoff S., Di Cristofano A. Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res. 2009;69:3689–3694. doi: 10.1158/0008-5472.CAN-09-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyawaki S., Yamazaki R., Harada T., Takanashi S., Nagashima T., Nakaguchi H., Okazaki R., Yamazaki K., Ishida Y., Matsuno A. Skull metastasis of thyroid papillary carcinoma. J. Clin. Neurosci. 2007;14:481–484. doi: 10.1016/j.jocn.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 43.Mizukami Y., Michigishi T., Nonomura A., Hashimoto T., Terahata S., Noguchi M., Hisada K., Matsubara F. Distant metastases in differentiated thyroid carcinomas: a clinical and pathologic study. Hum. Pathol. . 1990;21:283–290. doi: 10.1016/0046-8177(90)90228-w. [DOI] [PubMed] [Google Scholar]
- 44.Mydlarz W. K., Wu J., Aygun N., Olivi A., Carey J. P., Westra W. H., Tufano R. P. Management considerations for differentiated thyroid carcinoma presenting as a metastasis to the skull base. Laryngoscope. 2007;117:1146–1152. doi: 10.1097/MLG.0b013e318058192e. [DOI] [PubMed] [Google Scholar]
- 45.Nagamine Y., Suzuki J., Katakura R., Yoshimoto T., Matoba N., Takaya K. Skull metastasis of thyroid carcinoma. Study of 12 cases. J. Neurosurg. 1985;63:526–531. doi: 10.3171/jns.1985.63.4.0526. [DOI] [PubMed] [Google Scholar]
- 46.Namba H., Nakashima M., Hayashi T., Hayashida N., Maeda S., Rogounovitch T. I., Ohtsuru A., Saenko V. A., Kanematsu T., Yamashita S. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J. Clin. Endocrinol. Metab. . 2003;88:4393–4397. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 47.Niederle B., Roka R., Schemper M., Fritsch A., Weissel M., Ramach W. Surgical treatment of distant metastases in differentiated thyroid cancer: indication and results. Surgery. 1986;100:1088–1097. [PubMed] [Google Scholar]
- 48.Nose F., Ichikawa T., Fujisawa M., Okayasu I. Up-regulation of cyclooxygenase-2 expression in lymphocytic thyroiditis and thyroid tumors. Am. J. Clin. Pathol. 2002;117:546–551. doi: 10.1309/9CCJ-XQ8P-PMFM-M65K. [DOI] [PubMed] [Google Scholar]
- 49.Ober K. P., Cowen R. J., Sevier R. E., Poole G. J. Thyrotoxicosis caused by functioning metastatic thyroid carcinoma. A rare and elusive cause of hyperthyroidism with low radioactive iodine uptake. Clin. Nucl. Med. 1987;12:345–348. [PubMed] [Google Scholar]
- 50.Ochiai H., Nakano S., Goya T., Wakisaka S., Kinoshita K. Pituitary metastasis of thyroid follicular adenocarcinoma —case report. Neurol. Med. Chir. (Tokyo) 1992;32:851–853. doi: 10.2176/nmc.32.851. [DOI] [PubMed] [Google Scholar]
- 51.Ogawa Y., Sugawara T., Seki H., Sakuma T. Thyroid follicular carcinoma metastasized to the lung, skull, and brain 12 years after initial treatment for thyroid gland—case report. Neurol. Med. Chir. (Tokyo) . 2006;46:302–305. doi: 10.2176/nmc.46.302. [DOI] [PubMed] [Google Scholar]
- 52.Omi Y., Shibata N., Okamoto T., Obara T., Kobayashi M. Immunohistochemical demonstration of membrane-bound prostaglandin E2 synthase-1 in papillary thyroid carcinoma. Acta Histochem. Cytochem. 2009;42:105–109. doi: 10.1267/ahc.09014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozdemir N., Senoglu M., Acar U. D., Canda M. S. Skull metastasis of follicular thyroid carcinoma. Acta Neurochir. (Wien) 2004;146:1155–1158. doi: 10.1007/s00701-004-0290-8. [DOI] [PubMed] [Google Scholar]
- 54.Pelaz A. C., Llorente J. L., Suarez C. Infratemporal metastasis from occult follicular thyroid carcinoma. J. Craniofacial. Surg. 2009;20:165–167. doi: 10.1097/SCS.0b013e318191cec0. [DOI] [PubMed] [Google Scholar]
- 55.Ricarte-Filho J. C., Ryder M., Chitale D. A., Rivera M., Heguy A., Ladanyi M., Janakiraman M., Solit D., Knauf J. A., Tuttle R. M., Ghossein R. A., Fagin J. A. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. doi: 10.1158/0008-5472.CAN-09-0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosahl S. K., Erpenbeck V., Vorkapic P., Samii M. Solitary follicular thyroid carcinoma of the skull base and its differentiation from ectopic adenoma—review, use of galectin-3 and report of a new case. Clin. Neurol. Neurosurg. 2000;102:149–155. doi: 10.1016/s0303-8467(00)00088-3. [DOI] [PubMed] [Google Scholar]
- 57.Ruchti C., Balli-Antunes M., Gerber H. A. Follicular tumor in the sellar region without primary cancer of the thyroid. Heterotopic carcinoma? Am. J. Clin. Pathol. . 1987;87:776–780. doi: 10.1093/ajcp/87.6.776. [DOI] [PubMed] [Google Scholar]
- 58.Santarpia L., El Naggar A. K., Cote G. J., Myers J. N., Sherman S. I. Phosphatidylinositol 3-kinase/akt and ras/raf-mitogen-activated protein kinase pathway mutations in anaplastic thyroid cancer. J. Clin. Endocrinol. Metab. . 2008;93:278–284. doi: 10.1210/jc.2007-1076. [DOI] [PubMed] [Google Scholar]
- 59.Sathiyamoorthy S., Maleki Z. Cytomorphologic overlap of differentiated thyroid carcinoma and lung adenocarcinoma and diagnostic value of TTF-1 and TGB on cytologic material. Diagn. Cytopathol. 2013 doi: 10.1002/dc.22997. doi: 10.1002/dc.22997. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 60.Simon N., Quyyumi S. A., Rothman J. G. Follicular thyroid cancer presenting as a sellar mass: case report and review of the literature. Endocr. Pract. 2004;10:62–66. doi: 10.4158/EP.10.1.62. [DOI] [PubMed] [Google Scholar]
- 61.Soares P., Trovisco V., Rocha A. S., Lima J., Castro P., Preto A., Máximo V., Botelho T., Seruca R., Sobrinho-Simões M. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 62.Sobrinho-Simões M., Albores-Saavedra J., Tallini G., Santoro M., Volante M., Pilotti S., Carcangiu M. L., Papotti M., Matias-Guiu X., Guiter G. E., Zakowski M., Sakamoto A. In “World Health Organization Classification of Tumors: Pathology and Genetics of Tumours of Endocrine Organs”, eds. R. A. DeLellis, R. V. Lloyd, P. U. Heitz and C. Eng. IARC Press; Lyon: 2004. Poorly differentiated carcinoma; pp. 73–76. [Google Scholar]
- 63.Song I. S., Chan K. F., Tey P. H., Choi H. S. An unusual case of thyroid carcinoma metastasis to the posterior fossa. Mt. Sinai J. Med. 1981;48:281–285. [PubMed] [Google Scholar]
- 64.Sziklas J. J., Mathews J., Spencer R. P., Rosenberg R. J., Ergin M. T., Bower B. F. Thyroid carcinoma metastatic to pituitary. J. Nucl. Med. 1985;26:1097. [PubMed] [Google Scholar]
- 65.Takami T., Ohata K., Tsuyuguchi N., Mao Y., Inoue Y., Wakasa K., Hara M. Cavernous sinus metastasis from thyroid papillary adenocarcinoma. J. Clin. Neurosci. 2002;9:598–600. doi: 10.1054/jocn.2002.1101. [DOI] [PubMed] [Google Scholar]
- 66.Takeuchi S., Masuda C., Maebayashi H., Tooyama I. Immunohistochemical mapping of TRK-fused gene products in the rat brainstem. Acta Histochem. Cytochem. 2012;45:57–64. doi: 10.1267/ahc.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tassinari D., Poggi B., Nicoletti S., Fantini M., Tamburini E., Possenti C., Sartori S. Zoledronic acid treatment at home: safety data from an observational prospective trial. J. Palliat. Med. 2007;10:352–358. doi: 10.1089/jpm.2006.0122. [DOI] [PubMed] [Google Scholar]
- 68.Thyroid Carcinoma Task Force., authors AACE/AAES medical/surgical guidelines for clinical practice: management of thyroid carcinoma. American Association of Clinical Endocrinologists. American College of Endocrinology. Endocr. Pract. 2001;7:202–220. [PubMed] [Google Scholar]
- 69.Trunnell J. B., Marinelli L. D., Duffy B. J., Jr., Hill R., Peacock W., Rawson R. W. The treatment of metastatic thyroid cancer with radioactive iodine; credits and debits. J. Clin. Endocrinol. Metab. . 1949;9:1138–1152. doi: 10.1210/jcem-9-11-1138. [DOI] [PubMed] [Google Scholar]
- 70.Vargas G. E., UY H., Bazan C., Guise T. A., Bruder J. M. Hemiplegia after thyrotropin alfa in a hypothyroid patient with thyroid carcinoma metastatic to the brain. J. Clin. Endocrinol. Metab. 1999;84:3867–3871. doi: 10.1210/jcem.84.11.6161. [DOI] [PubMed] [Google Scholar]
- 71.Verkooijen R. B., Rietbergen D., Smit J. W., Romijn J. A., Stokkel M. P. A new functional parameter measured at the time of ablation that can be used to predict differentiated thyroid cancer recurrence during follow-up. Eur. J. Endocrinol. 2007;156:41–47. doi: 10.1530/eje.1.02322. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Hou P., Yu H., Wang W., Ji M., Zhao S., Yan S., Sun X., Liu D., Shi B., Zhu G., Condouris S., Xing M. High prevalence and mutual exclusivity of genetic alterations in the phosphatidylinositol-3-kinase/akt pathway in thyroid tumors. J. Clin. Endocrinol. Metab. . 2007;92:2387–2390. doi: 10.1210/jc.2006-2019. [DOI] [PubMed] [Google Scholar]
- 73.Wong G. K., Boet R., Poon W. S., Ng H. K. Lytic skull metastasis secondary to thyroid carcinoma in an adolescent. Hong Kong Med. J. 2002;8:149–151. [PubMed] [Google Scholar]
- 74.Xing M., Westra W. H., Tufano R. P., Cohen Y., Rosenbaum E., Rhoden K. J., Carson K. A., Vasko V., Larin A., Tallini G., Tolaney S., Holt E. H., Hui P., Umbricht C. B., Basaria S., Ewertz M., Tufaro A. P., Califano J. A., Ringel M. D., Zeiger M. A., Sidransky D., Ladenson P. W. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J. Clin. Endocrinol. Metab. . 2005;90:6373–6379. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 75.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu X., Quiros R. M., Gattuso P., Ain K. B., Prinz R. A. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res. . 2003;63:4561–4567. [PubMed] [Google Scholar]
- 77.Yan B., Liu D. G., Lü H. L., Zhang Q. H. Images for diagnosis. Papillary thyroid microcarcinoma presenting as skull base metastasis. Chin. Med. J. (Engl) 2010;123:2750–2752. [PubMed] [Google Scholar]
- 78.Yilmazlar S., Kocaeli H., Cordan T. Sella turcica metastasis from follicular carcinoma of thyroid. Neurol. Res. 2004;26:74–78. doi: 10.1179/016164104773026561. [DOI] [PubMed] [Google Scholar]
- 79.Zohar Y., Strauss M. Occult distant metastases of well-differentiated thyroid carcinoma. Head Neck . 1994;16:438–442. doi: 10.1002/hed.2880160508. [DOI] [PubMed] [Google Scholar]