Abstract
We conducted a randomized clinical trial in 45 patients with resected AJCC stage IIB-IV melanoma to characterize cellular and molecular events at sites of immunization with incomplete Freund’s adjuvant (IFA) alone, or a melanoma vaccine in IFA. At a primary vaccine site, all patients received a multi-peptide melanoma vaccine in IFA. At a replicate vaccine site, which was biopsied, group 1 received IFA only; group 2 received vaccine in IFA. Lymphocytes isolated from replicate vaccine site microenvironments (VSME) were compared to time-matched peripheral blood mononuclear cells (PBMC) in ELISpot and flow cytometry assays. Compared to PBMC, the VSME had fewer naïve and greater proportions of effector memory CD8+ T cells (TCD8). The vast majority of TCD8 within the VSME were activated (CD69+), with a concentration of antigen-specific (tetramerpos) cells in the VSME, particularly in vaccine sites with peptide (group 2). CXCR3+ lymphocytes were concentrated in the VSME of all patients, suggesting IFA-induced chemokine recruitment. TCD8 expression of retention integrins αEβ7 and α1β1 was elevated in VSME, with the highest levels observed in antigen-specific cells in VSME containing peptide (group 2). TCD8 retained in the VSME of both groups were strikingly dysfunctional, with minimal IFN-γ production in response to peptide stimulation and few tetramerpos cells producing IFN-γ. These data suggest that vaccine-induced selective retention and dysfunction of antigen-specific TCD8 within VSME may represent a significant mechanism underlying transient immune responses and low clinical response rates to peptide vaccines administered in IFA.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-013-1435-5) contains supplementary material, which is available to authorized users.
Keywords: Human, T cell, Incomplete Freund’s adjuvant, Peptide vaccine, Melanoma, CIMT 2012, Integrins, Melanoma vaccine
Introduction
Clinical activity of cancer vaccines is now supported by approval of sipuleucel-T (Provenge) for prostate cancer, and improved clinical outcome of advanced melanoma by adding a melanoma peptide vaccine to high-dose IL-2 [1]. However, melanoma vaccines alone have induced objective clinical regressions in only 3–6 % of patients [2], despite inducing circulating anti-tumor T cells in 70–100 % of patients [3, 4]. Unfortunately, the immune responses are often of low magnitude and duration [3], which likely limits their clinical activity and may explain in part the low clinical response rates.
A vaccine adjuvant is necessary to activate dendritic cells (DCs) and to induce a favorable immunologic milieu; however, little is known about the cellular and molecular events induced by such adjuvants. A widely used adjuvant is incomplete Freund’s adjuvant (IFA), which was developed in the 1930s to induce antibody responses to viral proteins [5]. It is not clear what adjuvants are best to induce durable T cell responses to defined cancer antigens. We have previously reported a histologic analysis of sites of immunization with peptides in an emulsion with Montanide ISA-51 (an IFA), using immunohistochemistry, which suggested that the vaccine site microenvironment (VSME) is not optimized for induction of strong Th1/Tc1 responses, with induction, instead, of FoxP3+ cells and of eosinophils, suggestive of a Th2 dominant microenvironment [6].
Immunization in IFA creates a depot of antigen plus adjuvant and can lead to chronic inflammation in the VSME that is clinically prominent, and may persist for years. Chronic inflammation can induce lymphoid neogenesis and development of tertiary lymphoid organs (TLO) [7–9]. This can occur at sites of cancer vaccines, where lymphoid aggregates contain separate B and T cell areas, mature dendritic cells, high endothelial venule (HEV)-like vessels and lymphoid cytokines [10]. Thus, vaccines containing IFA may induce a VSME that supports antigen presentation in the vaccine site itself [10]; however, lymphoid aggregates in the VSME may lack full function of a lymph node. Other data raise questions about whether the chronic depot of IFA-containing vaccines may have negative effects on persistent systemic immunity and tumor control: in murine models, activated, antigen specific T cells traffic selectively to such vaccine sites rather than to tumor, and may die there [11]. These findings remain to be confirmed in humans. To address whether the VSME induced by peptide vaccines in IFA may recruit and/or retain antigen-specific T cells in humans, we have evaluated the phenotype and function of antigen-specific CD8+ T cells from vaccine sites and PBMC. We present data that the VSME contains antigen-reactive effector memory T cells that are either exhausted or in a prolonged refractory state, and that are retained in the VSME, with high expression of retention integrins (α1β1 and αEβ7).
Materials and methods
Patient selection
Specimens for this study were obtained in a clinical trial of a multi-peptide vaccine for melanoma: NCT00705640, University of Virginia (Mel48). Patients with resected AJCC stage IIB-IV arising from cutaneous, mucosal, ocular, or unknown primary site were eligible. Inclusion criteria included: expression of HLA-A1, A2, A3, or A11; age of 18 years or greater; ECOG performance status 0–1; adequate blood counts, liver, and renal function; negative serologies for HIV and Hepatitis C; and ability to give informed consent. Exclusion criteria included: multiple brain metastases; cytotoxic chemotherapy, interferon therapy, or radiation within the preceding 4 weeks; known or suspected allergies to any vaccine component; pregnancy; body weight less than 110 lbs; use of steroids; or Class III, IV heart disease. Patients were studied following informed consent, with Institutional Review Board (IRB/HSR #13498) and FDA approval (BB-IND #12191).
Patient assignment and study protocol
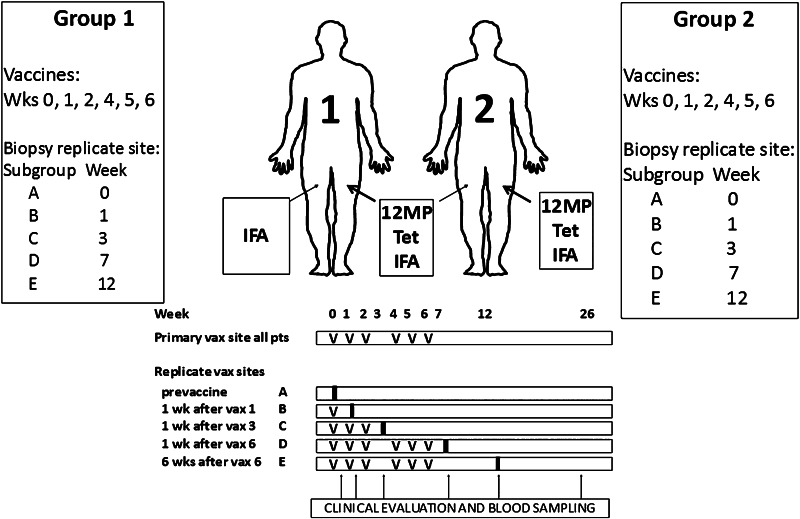
Patients were randomized to one of two groups, and one of five arms within each group (Fig. 1). All received the full vaccine (12 Class I MHC restricted melanoma peptides and a tetanus helper peptide emulsified in IFA) at the primary vaccine site (days 1, 8, 15, 29, 36, and 43), plus a 2nd vaccine at a replicate vaccine site, where the injectate differed between the two study groups: IFA only (group 1) or full vaccine (group 2). Within each study group, there were five arms: with excisional biopsy of the replicate site: pre-vaccination (week 0, Arm A), 1 week after the first vaccine (week 1, Arm B), 1 week after the third vaccination (week 3, arm C), 1 week after the sixth vaccination (week 7, arm D), or 6 weeks after the sixth vaccination (week 12, arm E).
Fig. 1.
Mel48: A multi-peptide vaccine in melanoma patients with evaluation of the injection site microenvironment. Patients with resected AJCC stage IIB-IV melanoma underwent cutaneous vaccination with 12-melanoma peptide vaccine and tetanus helper peptide in IFA. At a replicate vaccine site, Group 1 received IFA alone, Group 2 received full vaccination. Replicate vaccine sites were biopsied and peripheral blood collected as shown
Vaccine preparation and dosage
The full vaccine consisted of 12 class I MHC-restricted melanoma peptides (12-MP vaccine—Supplemental Table S1; 50 μg each peptide) and a tetanus helper peptide (Peptide-tet; 100 μg) in 0.5 mL aqueous solution, emulsified 1:1 with 0.5 mL Montanide ISA-51. The total injected volume was 1.0 mL, one half given subcutaneously (s.c.), the other half intradermally (i.d.), through a single skin puncture. The replicate vaccine containing only adjuvant was an emulsion of saline (0.5 mL) plus 0.5 mL Montanide ISA-51, also administered s.c. and i.d.
Design and sample size
This was a pilot study to obtain preliminary measures of several pre-specified endpoints of interest. For sample size determination, we characterized power in terms of effect, with planned use of regression models maximizing power for small sample size. Four patients were to be accrued to each biopsy time point for each treatment group. Maximum accrual to the study was estimated to be 44 subjects in order to accrue the target 36 eligible subjects required to meet the study objectives, plus a 5 % ineligibility rate and over-accrual of subjects scheduled to have biopsies at the later time points. Total accrual was 45.
ELISpot
ELISpot assays for interferon gamma (IFN-γ) were performed on PBMCs and on lymphocytes from the VSME (Fig. 1). Assays were performed directly ex vivo after cryopreservation (direct ELISpot), as previously reported [12]. Criteria for positive immunologic response were: magnitude of response ≥two-fold pre-vaccination value and experimental negative control, an increase of at least 0.02 % of CD8+ T cells over negative control, and no overlap between the 1SD intervals of the response and negative controls [12]. Calculated interassay coefficients of variation for normal donor PBMC for high and low responders to CEF peptides were 18 and 23 %, respectively.
Immunostaining and multi-parametric flow cytometry
Single-cell suspensions of cryopreserved PBMC and mononuclear cell specimens isolated from vaccine site biopsies using methods previously described [12] were thawed in warm RPMI-1640 (Life Technologies: Grand Island, NY) medium containing 5 % fetal bovine serum (FBS; Sigma-Aldrich: St. Louis, MO), and 100,000 U/ml deoxyribonuclease I (Cat. # LS00219; Worthington Biochemical Co.: Lakewood, NJ). Cells were washed in phosphate buffered saline (PBS) and were labeled with the LIVE/DEAD® Fixable Aqua dead cell stain (Life Technologies) for viability according to manufacturer’s instructions. Aliquots of Aqua-labeled cells were added to wells containing mixtures of Class I MHC-peptide multimers (Beckman Coulter; Indianapolis, IN) relevant to the patient’s HLA type for a total volume of 25 μL, as described [13]. Also, a pentamer (Proimmune; Oxford, UK) for SLFRAVITK (MAGE-A196–104) was incorporated in the HLA-A3 pool of tetramers. After incubation at 4 °C for 20 min, 25 μL labeled antibody mixtures for integrins, homing receptors, chemokine receptors, and activation/maturation markers were added to the cell suspensions (details in Supplemental Table S2). then incubated an additional 20 min at 4 °C, washed twice in FACS buffer (PBS + 2 % FBS), then fixed in 2 % paraformaldehyde (Sigma-Aldrich) in PBS for 10 min at room temperature, and washed once. Immunostained cells were held overnight at 4 °C then acquired on a CyAn ADP LX 9 color flow cytometer (Beckman Coulter) equipped with 3 lasers. Flow cytometry data were analyzed with FlowJo version 8.8.6 (Tree Star; Ashland, OR), gated on viable, single cell populations.
In vitro stimulation of vaccine site cell suspensions
Cells were thawed, counted, and incubated with a pool of the 12 melanoma peptides (40 μg/ml each) for 2 h, washed and cultured with IL-2 (20 IU/mL) in culture medium (RPMI1640 + 10 % human AB serum). Cultures were fed with culture media with IL-2 as needed. No media replacement was necessary. On day 14, cells were collected and viable cell recovery determined.
Intracellular cytokine stimulation and staining
Cells were stimulated with peptide-pulsed C1R-A2 cells (T:APC ratio 2:1) or with PMA + ionomycin (50 ng/mL and 1 μM, respectively). Cells were incubated 4 h in AIM V + 5 % HuAB serum in the presence of GolgiPlug (BDBiosciences; San Jose, CA), then washed in PBS and labeled with Aqua 30 min at 4 °C. Pooled tetramers, or the irrelevant tetramer were added and incubated 45 min at 4 °C. Cells were washed and fixative (BioLegend; San Diego, CA) added for 20 min at room temperature. Cells were washed twice and left overnight at 4 °C, then permeabilized, to which was added a mixture of antibodies in permeabilization buffer (BDBiosciences) consisting of anti-CD8-FITC (Beckman Coulter), CD3 Pacific Blue, CD4-PE-Texas red, CD19 PE-Texas red (Invitrogen; Grand Island, NY), and interferon-gamma-APC (eBioscience; San Diego, CA). Cells were incubated 60 min at 4 °C, then washed 3 times in permeabilization buffer and twice in PBS + 0.1 % BSA + 0.1 % sodium azide. Cells were acquired on a Beckman Coulter CyAn ADP LX 9 Color flow cytometer and analyzed using FlowJo software.
Statistical analysis
For each assay, data from all patients with available samples from the vaccine site and time-matched PBMC were evaluated using SPSS 20 (IBM: Armonk, NY). Linear regression models were used to assess the difference between vaccine site values and time-matched PBMC, accounting for two variables: Patient group and Study Arm (biopsy time point). To minimize the impact of outliers in this small patient population and to satisfy model assumptions, square root transformation of endpoints was employed for all analyses. In analyses of tetramerpos and tetramerneg subsets of cells, samples containing fewer than 5 tetramerpos cells were excluded, which resulted in the elimination of 1 group 2C patient from CCR7/CD45RA, CXCR3, CLA and CD62L analyses. In this pilot study, no adjustments were made for multiple comparisons. All tests were two-sided and considered significant for p ≤ 0.05. All results are reported after adjusting for patient group and study arm (time of biopsy).
Results
Patients studied
Forty-five patients with resected AJCC stage IIB-IV melanoma were enrolled in a clinical trial of a multi-peptide vaccine (Mel 48, Fig. 1), from which histologic findings have been reported [6]. All were vaccinated with 12MP plus tetanus helper peptide in a primary vaccine site. Each had “replicate vaccine” injection at a second site, which was biopsied. The replicate vaccine consisted of either IFA alone (group 1), or IFA with peptides (group 2). Patients enrolled in the two study arms were similar (Table 1). Replicate site biopsies were obtained in 38 patients (84 %). Vaccine site biopsies from study arms A (week 0), B (week 1), and E (week 12) contained insufficient lymphocytic infiltrates for both ELIspot and flow cytometric evaluation. Data from both VSME and time matched PBMC were obtained from 13 patients from vaccine site biopsies at weeks 3 and 7: 2, 3, 5, and 3 in groups 1C, 1D, 2C, and 2D, respectively.
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristics | Group 1 (n = 23) | Group 2 (n = 22) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Gender | ||||
| Male | 16 | 70 | 17 | 77 |
| Stage at enrollment | ||||
| IIB | 3 | 13 | 0 | 0 |
| IIC | 1 | 4 | 0 | 0 |
| IIIA | 2 | 9 | 3 | 14 |
| IIIB | 8 | 35 | 8 | 36 |
| IIIC | 4 | 17 | 9 | 41 |
| IV | 5 | 22 | 2 | 9 |
| ECOG PS | ||||
| 0 | 22 | 96 | 20 | 91 |
| 1 | 1 | 4 | 2 | 9 |
| Median Age, years | 56 | 53 | ||
| HLA-expressiona | ||||
| HLA-A1 | 12 | 52 | 10 | 45 |
| HLA-A2 | 8 | 35 | 9 | 41 |
| HLA-A3 | 13 | 57 | 9 | 41 |
| HLA-A11 | 2 | 9 | 3 | 14 |
ECOG PS eastern cooperative oncology group performance status, HLA human leukocyte antigen
aMany patients express more than 1 eligible HLA type
Composition of cells in the VSME
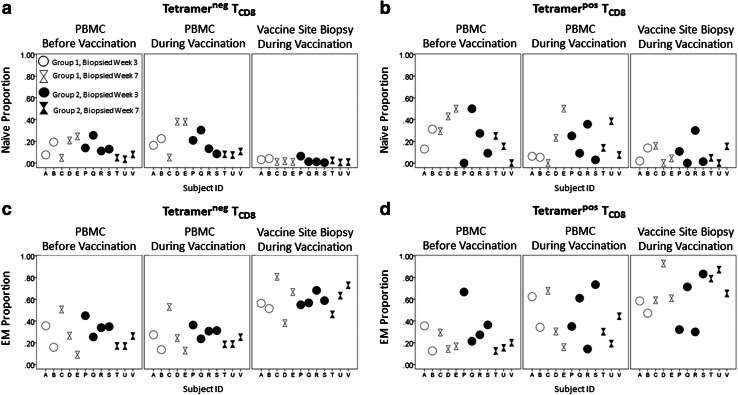
CD8+ T cells were characterized based on CCR7 and CD45RA expression [14]. Compared to PBMC, the VSME had fewer naïve cells (Tnaïve, CD45RA+/CCR7+) and greater proportions of effector memory cells (TEM, CD45RAneg/CCR7neg, Fig. 2). Among tetramerneg cells, Tnaïve were lower in VSME than PBMC (p = 0.003) and TEM were higher (p < 0.001, Fig. 2a, c). Among tetramerpos cells, TEM were more prevalent in the VSME than in PBMC (p = 0.033, Fig. 2d). Proportions of central memory T cells (TCM, CD45RAneg/CCR7+) were very low for all samples, were slightly higher in the VSME than PBMC overall (p = 0.004), but not among tetramerpos cells (Supplemental Fig. SF1). There were no significant differences in CD45RApos effector memory (TEMRA, CD45RApos/CCR7neg) proportions between sites (Supplemental Fig. SF1).
Fig. 2.
Vaccine sites are depleted of naïve and enriched for effector memory CD8+ T cells compared to peripheral blood. Phenotypes of CD8+ T cells (TCD8) were determined by multi-parameter flow cytometry staining with CCR7 and CD45RA. Cells were sorted by their ability to bind to tetramers relevant to the HLA type of the patient. Gating was performed on live, singlet, CD8+/CD4neg/CD14neg/CD19neg, then tetramerpos or tetramerneg lymphocyte populations. The figure depicts percentages of CD8+ T cells staining with naïve (Naïve, CCR7+/CD45RA+) and effector memory (EM, CCR7neg/CD45RAneg) phenotypes. a Tnaive of tetramerneg cells. Values from vaccine site and/or peripheral blood at each time point are shown for each individual patient with a unique Subject ID. b Tnaive of tetramerpos cells. c TEM of tetramerneg cells. d TEM of tetramerpos cells
Activation and antigen specificity in the vaccine site
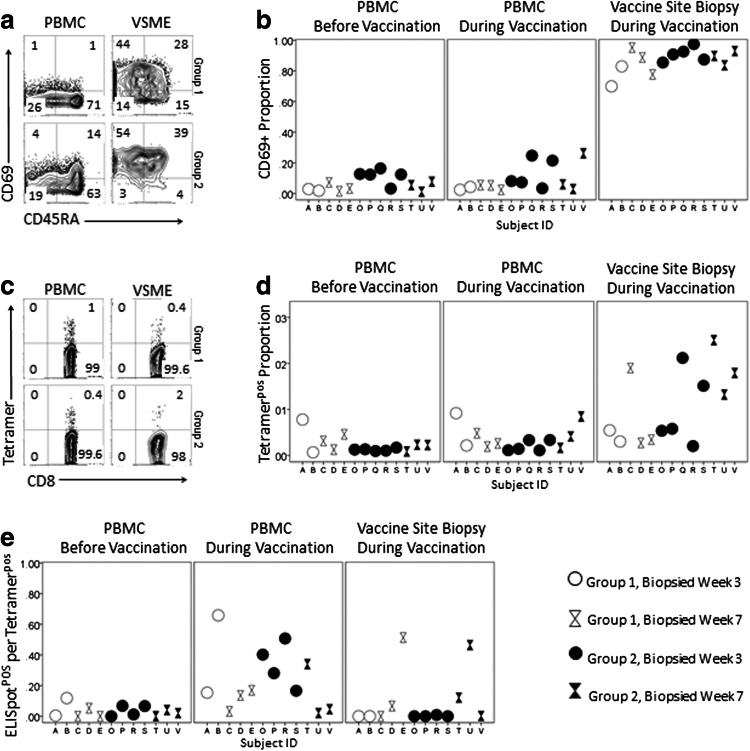
Among all subjects, CD69+ CD8+ T cells were enriched in the VSME compared to PBMC (p < 0.001, Fig. 3b). The proportion of tetramerpos cells in VSME trended higher than in PBMC overall but did not reach statistical significance (Fig. 3d); review of graphical data prompted examination of group 2 alone: among those patients with antigen in the biopsied VSME, there were more tetramerpos CD8+ T cells than in PBMC (p = 0.003 Fig. 3d). CD4+ T cell responses to the tetanus helper peptide were not a focus of this report and were not evaluable with multimers. Results of direct IFN-γ ELISpot assay for response to tetanus helper peptide are provided in Supplemental Table S4.
Fig. 3.
Marked activation, antigen specificity and cellular dysfunction in vaccine sites. CD69 expression and staining with MHC-peptide tetramers in live, singlet, CD8+/CD4neg/CD14neg/CD19neg lymphocyte populations were evaluated by multi-parameter flow cytometry. Functionality was assessed by IFN-γ response to vaccine peptides in direct ELISpot assay. CD69 Expression. a Upper (group 1C) and lower (group 2C) panels display representative flow cytometry from the VSME and time-matched PBMC for individual patients. b Percentages of CD8+ T cell populations staining positive for CD69 are shown for individual patient samples. Tetramer staining. Cells were sorted by their ability to bind MHC-peptide tetramers relevant to the HLA type of the patient. c Upper (group 1C) and lower (group 2C) panels display representative flow cytometry from the VSME and time-matched PBMC for representative patients. Total numbers of cells evaluated per assay from the VSME were lower than in PBMC. d Tetramerpos proportion of CD8+ T cell populations for individual patients. e Ratio of ELISpot reactivity to tetramer. The number of cells producing IFN-γ in response to vaccine peptides was compared to the number of tetramer positive cells per 105 CD8+ T cells at each time point. Ratios of IFN- γ+ to tetramerpos cells are depicted for individual patients
Dysfunction of antigen-specific cells
Antigen-specific CD8+ T cells in the VSME appeared to be dysfunctional for all patients, regardless of the presence or absence of peptides at the VSME. Despite increased tetramerpos cells in the VSME, there were fewer ELISpot+ cells per 105 CD8+ T cells in the VSME than in PBMC (p = 0.033, Supplemental Fig. SF2). Vaccine sites contained fewer functional cells per tetramerpos T cells than PBMC (p = 0.053, Fig. 3e).
To evaluate preliminarily whether the apparent dysfunction of tetramerpos cells in the VSME was reversible, a stimulated ELISpot assay was performed. Cryopreserved cell suspensions from 5 vaccine site biopsies were stimulated with the 12MP in vitro and placed in a 14 day culture with IL-2, using methods previously reported [4]. Lymphocytes expanded very poorly in all samples; only 2 yielded adequate cell numbers for flow cytometry (viable cells only 3 and 15 % of the input cell number), which revealed no expansion of tetramerpos cells after stimulation (data not shown).
Expression of homing molecules
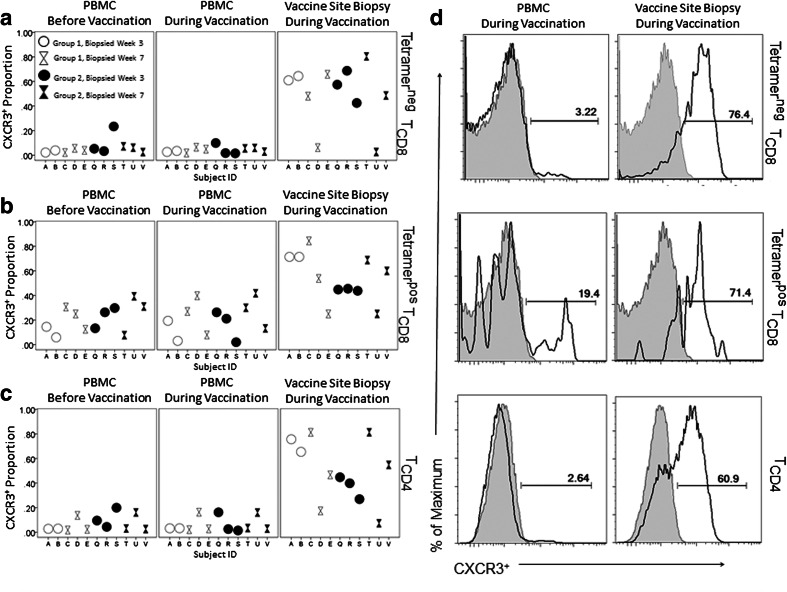
The VSME overall contained greater proportions of T cells expressing the chemokine receptor CXCR3 than PBMC (Fig. 4a, b). This was true among CD4+ T cells (p < 0.001, Fig. 4c), tetramerneg and tetramerpos CD8+ T cells (p = 0.001, p < 0.001, respectively, Fig. 4a, b).
Fig. 4.
In vaccine sites, chemokine receptor CXCR3 expression is elevated. Live, singlet, CD14neg/CD19neg cells were identified and gated on CD4+, CD8+/Tetramerpos or CD8+/Tetramerneg populations. TCD8 = CD8+ T cells. TCD8 = CD8+ T cells. TCD4 = CD4+ T cells.CXCR3 expression among a tetramerneg and b tetramerpos TCD8 and c TCD4 are shown for individual patients. d CXCR3 populations in a representative group 2C patient. Each grey histogram is a negative control (FMO-CXCR3)
Cutaneous leukocyte antigen (CLA) was inconsistently elevated in the VSME. Among tetramerneg CD8+ T cells, CLA+ cells were increased in the VSME (p = 0.004, Supplemental Fig. SF3a), but no evident enhancement of CLA was observed among tetramerpos CD8+ T cells in the VSME (Supplemental Fig. SF3b).
CD62L (L-selectin), a HEV homing molecule, was present on PBMC, but CD62L+ CD8+ T cells were notably decreased or absent in all VSME, for both tetramerneg and tetramerpos subsets of VSME CD8+ T cells (p = 0.004; p < 0.001, respectively; Supplemental Fig. SF3c, d).
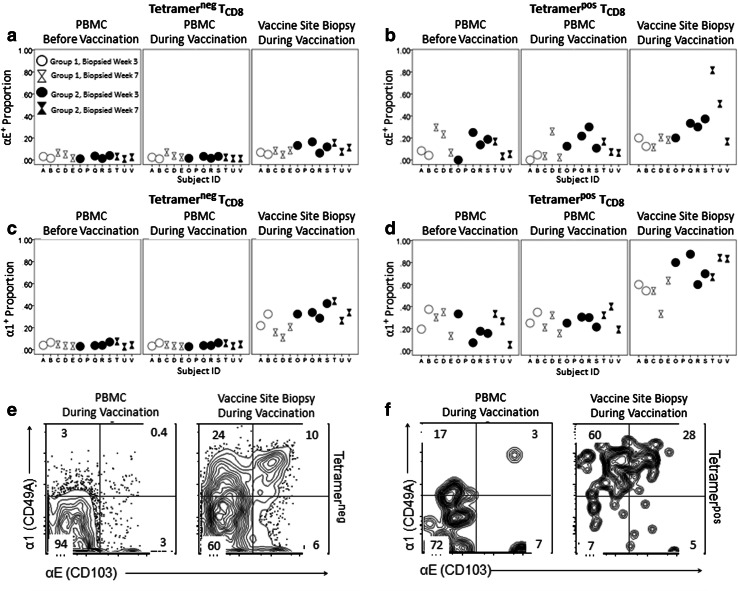
Increased expression of retention integrins
Expression of retention integrins αEβ7 and α1β1 was elevated in the VSME, particularly in group 2 (Fig. 5). Among tetramerneg CD8+ T cells αEβ7 expression was higher in the VSME than in PBMC (p < 0.001, Fig. 5a). Among tetramerpos CD8+ T cells in the vaccine site, αEβ7 expression was not significantly increased in this small sample of patients; however, graphical depiction of the data showed that the greatest αEβ7 expression was among tetramer positive cells in group 2 (Fig. 5b): average percentages of cells expressing αEβ7 in VSME and PBMC were 30 and 16 %, respectively, at week 3 and 50 and 10 %, respectively, at week 7 (Fig. 5b). In all patients, αEβ7 was nearly absent from tetramerneg cells in PBMC at every time point.
Fig. 5.
Retention integrin expression is elevated in vaccine sites. Gating was performed on live, singlet, CD8+/CD4neg/CD14neg/CD19neg, then tetramerpos or tetramerneg lymphocyte populations. TCD8 = CD8+ T cells. a Epithelial retention integrin αEβ7 among tetramerneg cells. Percentages of tetramerneg TCD8 populations staining positive for αEβ7 are shown for individual patients. b αEβ7 among tetramerneg cells. c Collagen retention integrin α1β1 among tetramerneg cells. Percentages of tetramerneg TCD8 populations staining positive for α1β1 are shown for individual patients. d Collagen retention integrin α1β1 among tetramerpos cells) e αEβ7 and α1β1 staining among tetramerneg cells and f tetramerpos cells. Panels display representative flow cytometry from TCD8 from vaccine site biopsies and time-matched PBMC for a representative group 2C patient
The proportion of CD8+ T cells expressing α1β1 was higher in the VSME than PBMC, for both tetramerneg (p < 0.001, Fig. 5c) and tetramerpos CD8+ T cells (p = 0.001, Fig. 5d). Group 2 VSME had highly elevated α1β1 expression among tetramerpos cells, with means of 74 % expressing it at week 3 (Fig. 5d) and 78 % at week 7. In matched PBMC, α1β1+ proportions of tetramerpos CD8+ T cells averaged 24 % at week 3 and 30 % at week 7. In all patients, 5 % or less of tetramerneg cells in PBMC were α1β1+ at any time point.
Differences between study groups and time points
For most measures evaluated, there were no statistically significant differences between patients in Groups 1 and 2. However, expression of retention integrins was higher in group 2 VSME samples (IFA and peptides) than in group 1 (IFA alone), among tetramerneg CD8+ T cells, for αEβ7 (p = 0.008) and for α1β1 (p = 0.015). Elevated VSME expression of α1β1 among tetramerpos CD8+T cell trended toward a greater effect in group 2 (p = 0.120).
Similarly, the effect size of the difference between VSME and PBMC did not differ significantly from week 3 to 7 for most measures, except that greater numbers of ELISpot+ T cells were observed in the VSME week 7 than week 3 (p = 0.017). Comparing ELISpot+ to tetramerpos ratios, a smaller proportion of functional cells was observed in the vaccine site at week 3 than at week 7 (p = 0.059).
Discussion
We have previously reported features of lymphoid neogenesis in the VSME after IFA-based vaccines [10]. However, it is not clear if the resulting lymphoid aggregates are fully functional TLO. The findings of the present study suggest that they are not fully functional. TLO are characterized by high endothelial venules expressing peripheral node addressin [PNAd, which binds CD62L (L-selectin) on lymphocytes] and also by high levels of CCL19 and CCL21, ligands for CCR7. In the VSME lymphoid aggregates, we might anticipate an enrichment of T cell populations expressing CCR7 and CD62L, including naïve T cells and TCM. Instead, we find that in all patients, including those receiving only IFA at the vaccine site biopsied, the VSME CD8+ T cells are dominated by a large population of TEM phenotype with moderate proportions of TEMRA and scant Tnaive and Tcm (Fig. 2, Supplemental Fig. SF1). Similarly, cells bearing CD62L are significantly reduced or absent in the VSME (Supplemental Fig. SF3).
Low numbers of Tnaïve in the vaccine site may reflect a return of Tnaïve to circulation after encountering the VSME or in situ conversion to a memory phenotype in the VSME after exposure to antigen. Despite loss of CCR7, TEM cells typically display other chemokine receptors and adhesion molecules required for homing to inflamed tissues; thus, TEM in the vaccine site may also be recruited from circulation and retained. The dominance of memory cells in the VSME of group 2 is counterintuitive, as peptide antigens administered in IFA emulsion might be expected to activate memory cells and to convert them to effectors. However, most T cells in the VSME are not immediately adjacent to vaccine emulsion depot, which resides in the tissue as small, loculated islands evident in histologic sections [10]. Also, the half-lives of peptides in our vaccine are short [15]; they may be degraded before encountering dermal perivascular lymphocytes. Whether from conversion or lack of retention, the marked absence of Tnaïve in the VSME lymphoid aggregates suggests that these sites are not fully functional TLO, since higher proportions of naïve cells are found in normal nodes.
In a murine model of vaccination for melanoma, activated, antigen-specific T cells traffic to vaccine sites containing IFA plus peptide, and the vaccine site acts as a sink or graveyard for these tumor-specific T cells [11]. Several findings from the present study corroborate, in humans, what has been observed in the murine model: the VSME manifests a marked, long-lasting accumulation of activated (CD69+) CD8+ T cells, with concentration of antigen-specific (tetramerpos) cells in the VSME in group 2 patients, in whom the vaccine site also contains peptide antigen. Our study differs from the murine model in that we observe T cell recruitment to the site injected with IFA alone. This may reflect that our examination takes place after repeated vaccination in the same site rather than observation after just one vaccine in the animal model. In previous immunohistochemical analysis of vaccine sites, we have demonstrated that single injections with adjuvant alone induce inflammatory dermal infiltrates which are transient and disorganized compared to those induced by repeated vaccination [10]. Though T cells in the VSME are clearly activated, evidence from the present study suggests that they are dysfunctional in their cytokine and proliferative responses to antigen, raising concerns about their ability to survive. In melanoma patients receiving vaccines in IFA, accumulation and persistent retention of TEM with subsequent depletion of these potential effectors at the vaccine site may limit vaccine-induced tumor control.
We have previously reported that both CD4+ and CD8+ cells in the VSME induced with IFA are actively dividing, as manifest by Ki67 staining, and that T cells and mature DC are closely associated in the vaccine sites [10], raising the possibility of in situ priming in the VSME, though such priming may be dysfunctional. To the extent that peptide may also bind to other cells (e.g. fibroblasts), in situ priming may be ineffective or even toleragenic [16]. On the other hand, when activated T cells reactive to peptides in the vaccine are evident in VSME without peptide, it is likely that they have been recruited to the site after activation. Ongoing recruitment of CD4+ and CD8+ T cells to all vaccine sites containing IFA may be mediated in part by chemokines attracting the high proportion of CXCR3+ cells observed in the VSME compared to blood (Fig. 4). However, CLA is not consistently increased despite the skin location of the vaccines.
Even in VSME containing peptide, well over 95 % of CD8+ T cells in the VSME are tetramerneg, indicating that the IFA-containing VSME appears to recruit and to stimulate T cells reactive to antigens other than those in the vaccine. The identity of those targets is not known, but we can hypothesize potential explanations: (1) recruitment of circulating T cells by cytokine-activated endothelium in the VSME, (2) decreased skin barrier function due to inflammation, with sensitization to environmental haptens and/or protein antigens in contact with the skin surface, and (3) reactivity to normal host skin antigens or melanocytic antigens due to injection of adjuvant into the skin.
T cell recruitment appears to lead to long-term retention, as substantial T cell aggregates in the VSME persist 6 weeks after the final vaccination [6]. Retention integrins provide a likely mechanism for the observed T cell sequestration within the VSME, as both αEβ7 and α1β1 are significantly higher in sites where peptide antigen is present, with the most elevated expression in tetramerpos T cells in group 2 (Fig. 5). αEβ7 (CD103) binds E-cadherin on epithelial cells, and triggers CCR5 mediated recruitment and retention of T cells [17]. α1β1 (VLA-1) binds type IV collagen, and is critical for the development and maintenance of memory CD8+ T cells in peripheral tissues [18]. The significant elevation of αEβ7 in group 2 vaccine sites over group 1 is likely related to the presence of antigen, with αEβ7 upregulation dependent upon T cell receptor engagement with cognate antigen [19]. Systemic antitumor immunity may be compromised by recruitment and retention of T cells to the VSME, limiting circulation and homing to tumor.
Despite evident activation (CD69 positivity) of CD8+ T cells in the VSME and increased antigen-specific cells in the group 2 VSME, lymphocytes retained in the VSME are strikingly dysfunctional (Fig. 3). Consistent with previous murine model findings after similar vaccination with short peptides in IFA [11], T cells in our human study appear refractory to correction with IL-2 and antigen stimulation, suggesting that activated CD8+ T cells within the VSME are in a refractory or exhausted state.
Vaccines containing IFA can be highly effective inducers of antibody responses; however, IFA may not be an optimal adjuvant for induction of T cell responses to peptides. Though sample size in these pilot data precludes definitive conclusions regarding the role of antigen in dysfunction of lymphoid aggregates, the study provides evidence that vaccine sites with IFA recruit and retain activated, dysfunctional CD8+ T cells, predominantly of effector memory phenotype. Selective retention of antigen-specific CD8+ T cells within the VSME is suggested by increased tetramerpos cells in the VSME of group 2 and high proportions of cells expression retention integrins. This may point to a significant mechanism underlying transient immune responses and low clinical response rates to peptide vaccines seen in the melanoma population to date. The same mechanism may interfere with combinations of T cell directed therapies (e.g. vaccines with adoptive T cell therapy, IL-2, or checkpoint blockade). This challenge may well be overcome by use of newer adjuvants and/or different vaccine formulations that avoid chronic inflammation in the VSME.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We greatly appreciate the work of Patrice Neese, Carmel Nail, and Priscilla Merrill in care of the patients on this trial, and Cheryl F.Murphy, Nadejda V. Galeassi, Kelly Smith and Elizabeth Coleman in immune analyses and data management. Funding support was provided by the National Institutes of Health Research Training Grant T32HL007849 (PI- Irving Kron) to EPS and the University of Virginia Rebecca Clay Harris Memorial Fellowship to SMS. Additional funding for this study was provided by the University of Virginia Cancer Center Support Grant (NIH/NCI P30 CA44579, Biorepository and Tissue Research Facility) and the University of Virginia General Clinical Research Center (NIH M01 RR00847). Peptides used in this vaccine were prepared with philanthropic support from the Commonwealth Foundation for Cancer Research and Alice and Bill Goodwin. Additional philanthropic support was provided by the James and Rebecca Craig Foundation, George S. Suddock, Richard and Sherry Sharp, and the Patients and Friends Research Funds of the University of Virginia Cancer Center. Montanide ISA-51 (produced by Seppic, Inc.) was used in the vaccines of this trial, but paid for by the University of Virginia. No corporate funding support was provided for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- CLA
Cutaneous leukocyte antigen
- HEV
High endothelial venule
- IFA
Incomplete Freund’s adjuvant
- PBMC
Peripheral blood mononuclear cell
- TCM
Central memory T cell (CD45RAneg/CCR7+)
- TEM
Effector memory T cell (CD45RAneg/CCR7neg)
- TEMRA
CD45RApos effector memory T cell (CD45RAneg/CCR7neg)
- TLO
Tertiary lymphoid organ
- Tnaive
Naïve T cell (CD45RA+/CCR7+)
- VSME
Vaccine site microenvironment
Footnotes
Elise P. Salerno and Sofia M. Shea contributed equally to this work.
References
- 1.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P. gp100 Peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slingluff CL., Jr The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J. 2011;17(5):343–350. doi: 10.1097/PPO.0b013e318233e5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Hibbitts S, Murphy C, Johansen N, Grosh WW, Yamshchikov GV, Neese PY, Patterson JW, Fink R, Rehm PK. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res. 2007;13(21):6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 5.Freund J, Thomson KJ. A simple, rapid technic of preparing water-in-oil emulsions of penicillin, drugs and biologics. Science. 1945;101(2627):468–469. doi: 10.1126/science.101.2627.468-a. [DOI] [PubMed] [Google Scholar]
- 6.Schaefer JT, Patterson JW, Deacon DH, Smolkin ME, Petroni GR, Jackson EM, Slingluff CL., Jr Dynamic changes in cellular infiltrates with repeated cutaneous vaccination: a histologic and immunophenotypic analysis. J Transl Med. 2010;8:79. doi: 10.1186/1479-5876-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lang A, Nikolich-Zugich J. Development and migration of protective CD8+ T cells into the nervous system following ocular herpes simplex virus-1 infection. J Immunol. 2005;174(5):2919–2925. doi: 10.4049/jimmunol.174.5.2919. [DOI] [PubMed] [Google Scholar]
- 8.Shomer NH, Fox JG, Juedes AE, Ruddle NH. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infect Immun. 2003;71(6):3572–3577. doi: 10.1128/IAI.71.6.3572-3577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170(9):4638–4648. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 10.Harris RC, Chianese-Bullock KA, Petroni GR, Schaefer JT, Brill LB, 2nd, Molhoek KR, Deacon DH, Patterson JW, Slingluff CL., Jr The vaccine-site microenvironment induced by injection of incomplete Freund’s adjuvant, with or without melanoma peptides. J Immunother. 2012;35(1):78–88. doi: 10.1097/CJI.0b013e31823731a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, Liu C, Lou Y, Wang Z, Ma W, Rabinovich B, Schluns KS, Davis RE, Hwu P, Overwijk WW. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med. 2013;19(4):465–472. doi: 10.1038/nm.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Ross MI, Haas NB, von Mehren M, Grosh WW. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol. 2011;29(21):2924–2932. doi: 10.1200/JCO.2010.33.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slingluff CL, Jr, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, Grosh WW, Boisvert ME, Kirkwood JM, Chianese-Bullock KA. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15(22):7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 15.Brinckerhoff LH, Kalashnikov VV, Thompson LW, Yamshchikov GV, Pierce RA, Galavotti HS, Engelhard VH, Slingluff CL., Jr Terminal modifications inhibit proteolytic degradation of an immunogenic MART-1(27–35) peptide: implications for peptide vaccines. Int J Cancer. 1999;83(3):326–334. doi: 10.1002/(SICI)1097-0215(19991029)83:3<326::AID-IJC7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179(8):5033–5040. doi: 10.4049/jimmunol.179.8.5033. [DOI] [PubMed] [Google Scholar]
- 17.Le Floc’h A, Jalil A, Franciszkiewicz K, Validire P, Vergnon I, Mami-Chouaib F. Minimal engagement of CD103 on cytotoxic T lymphocytes with an E-cadherin-Fc molecule triggers lytic granule polarization via a phospholipase Cgamma-dependent pathway. Cancer Res. 2011;71(2):328–338. doi: 10.1158/0008-5472.CAN-10-2457. [DOI] [PubMed] [Google Scholar]
- 18.Chapman TJ, Topham DJ. Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1. J Immunol. 2010;184(7):3841–3849. doi: 10.4049/jimmunol.0902281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franciszkiewicz K, Le Floc’h A, Jalil A, Vigant F, Robert T, Vergnon I, Mackiewicz A, Benihoud K, Validire P, Chouaib S, Combadiere C, Mami-Chouaib F. Intratumoral induction of CD103 triggers tumor-specific CTL function and CCR5-dependent T-cell retention. Cancer Res. 2009;69(15):6249–6255. doi: 10.1158/0008-5472.CAN-08-3571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.