Abstract
Background
Monitoring of HIV viral load in patients on combination antiretroviral therapy (ART) is not generally available in resource-limited settings. We examined the cost-effectiveness of qualitative point-of-care viral load tests (POC-VL) in sub-Saharan Africa.
Design
Mathematical model based on longitudinal data from the Gugulethu and Khayelitsha township ART programmes in Cape Town, South Africa.
Methods
Cohorts of patients on ART monitored by POC-VL, CD4 cell count or clinically were simulated. Scenario A considered the more accurate detection of treatment failure with POC-VL only, Scenario B also considered the effect on HIV transmission. Scenario C further assumed that the risk of virologic failure is halved with POC-VL due to improved adherence. We estimated the change in costs per quality-adjusted life-year gained (incremental cost-effectiveness ratios, ICER) of POC-VL compared to CD4 and clinical monitoring.
Results
POC-VL tests with detection limits <1000 copies/ml increased costs due to unnecessary switches to second-line ART, without improving survival. Assuming POC-VL unit costs between US$5–US$20 and detection limits between 1000 and 10000 copies/ml, the ICER of POC-VL was US$4010–US$9230 compared to clinical and US$5960–US$25540 compared to CD4 monitoring. In Scenario B the corresponding ICERs were US$2450–US$5830 and US$2230–US$10380. In Scenario C the ICER ranged between US$960–US$2500 compared to clinical monitoring and between cost-saving and US$2460 compared to CD4 monitoring.
Conclusions
The cost-effectiveness of POC-VL for monitoring ART is improved by a higher detection limit, by taking the reduction in new HIV infections into account and when assuming that failure of first-line ART is reduced due to targeted adherence counselling.
Keywords: Antiretroviral therapy, cost-effectiveness, mathematical model, sub-Saharan Africa, viral load monitoring
Introduction
Despite the rapid scale-up of combination antiretroviral therapy (ART) in HIV-infected patients during the past decade, the capacity to monitor treatment response remains limited in many settings [1]. Routine viral load (VL) monitoring is standard to detect virologic failure and inform decisions on switching patients to second-line ART in industrialised countries. In contrast, in most resource-limited settings these decisions are based on clinical symptoms and CD4 cell counts, which correlate poorly with VL [2]. The main barriers for the implementation of VL monitoring in low-income settings are the need for centralised laboratory facilities [3] and the required scale-up of expensive second-line ART [4].
A recent randomised controlled trial (RCT) evaluating the effect of routine laboratory monitoring on clinical outcomes among patients on ART in Uganda showed that compared to clinical monitoring alone outcomes were more favourable with laboratory monitoring, but there was no significant difference between the CD4 arm and the VL arm [5]. The setting of an RCT may not, however, reflect routine care in settings without access to VL monitoring, where patients may stay on failing first-line regimens for prolonged periods of time, which may increase the risk of onward transmission of HIV [6], lead to multi-drug resistance [7] and increase mortality [8]. Lack of VL monitoring hampers the detection of poor adherence to ART and the targeted counselling of patients [9]. Finally, a substantial number of patients may switch unnecessarily with suppressed VL [10].
In sub-Saharan Africa only South Africa [11] and Botswana [12] have implemented VL monitoring in their national ART programmes. Point-of-care (POC) tests are being developed for monitoring both CD4 cell counts and VL. UNITAID and the Bill & Melinda Gates Foundation are supporting the development and implementation of POC tests [13, 14]. POC testing can improve the monitoring of ART by making results available rapidly and providing access to clinics in remote settings [13].
The aim of our study was to explore the cost-effectiveness of routine POC-VL monitoring in settings in sub-Saharan Africa where only CD4 or clinical monitoring is currently available. We assumed that the VL test would be qualitative and available to all patients on ART. We varied the assumptions regarding the costs and detection limits of the test, and the costs of second-line therapy.
Methods
Data sources
The International epidemiologic Databases to Evaluate AIDS in Southern Africa (IeDEA-SA) is a collaboration of ART programmes in seven countries in Southern Africa [15]. Data are collected at ART initiation (baseline) and each follow-up visit using standardised instruments. All sites have ethical approval to collect data and participate in IeDEA-SA. We restricted our analyses to the Gugulethu [16] and Khayelitsha [17] township ART programmes in Cape Town, South Africa, where VL and CD4 cell counts are measured regularly (Table 1). All treatment-naïve patients aged at least 16 years, who had started ART with at least two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI) were included. Second-line ART was defined as a switch from an NNRTI-based regimen to a protease inhibitor-based regimen, with at least one NRTI changed.
Table 1. Patient characteristics in the Gugulethu and Khayelitsha programmes used to parameterise the mathematical model.
A total of 9888 patients were followed for 16278 person-years on first-line therapy and 435 person-years on second-line therapy.
| Unit | Value | |
|---|---|---|
| Age at baseline* (n=9888) | Years, median (IQR) | 33 (29–39) |
| Gender (n=9888) | Number (percentage) | |
| Male | 3240 (32.8%) | |
| Female | 6648 (67.2%) | |
| Cohort (n=9888) | Number (percentage) | |
| Gugulethu | 2658 (26.9%) | |
| Khayelitsha | 7230 (73.1%) | |
| CD4 cell count at baseline* (n=7259) | Cells/μl; median (IQR) | 93 (41–158) |
| HIV-1 viral load at baseline* (n=5274) | Log10 copies/ml; median (IQR) | 5.0 (4.5–5.5) |
| First-line regimens (n=9888) | Number (percentage) | |
| d4T 3TC EFV | 4985 (50.4%) | |
| d4T 3TC NVP | 3182 (32.2%) | |
| ZDV 3TC NVP | 1031 (10.4%) | |
| ZDV 3TC EFV | 680 (6.9%) | |
| d4T ddI EFV | 7 (0.1%) | |
| ZDV ddI EFV | 3 (0.0%) | |
| Second-line regimens (n=353) | Number (percentage) | |
| ZDV ddI LPV/r | 244 (69.1%) | |
| ZDV 3TC EFV LPV/r | 34 (9.6%) | |
| d4T ddI LPV/r | 22 (6.2%) | |
| ZDV 3TC LPV/r | 9 (2.6%) | |
| Other | 44 (12.5%) | |
| Follow-up time on ART (n=9888) | Months, median (IQR) | 17.8 (7.5–29.8) |
| Time from ART start to switch to 2nd-line (n=353) | Months, median (IQR) | 21.2 (13.7–30.3) |
IQR, interquartile range; 3TC, lamivudine; d4T, stavudine; ZDV, zidovudine; ddI, didanosine; NVP, nevirapine; EFV, efavirenz; LPV/r, ritonavir-boosted lopinavir.
Baseline variables were defined as the closest value within 3 months before up to 2 weeks after antiretroviral therapy initiation.
Definitions of treatment failure
Criteria for clinical and immunologic failure were those of the World Health Organization (WHO) [18]. Virologic failure was defined with five alternative thresholds (125, 400, 1000, 5000 or 10000 copies/ml). Failures have to be confirmed in a second measurement within one year (usually 3 months after the first measurement). True treatment failure was defined as a rebound in VL after suppression to at least 125 copies/ml, with VL remaining elevated until the patient switches therapy. Whereas virologic, immunologic and clinical failures are observations, true treatment failure is an underlying event which cannot be observed exactly. Detectable VL of unknown origin (DVU) was defined as VL above 125 copies/ml, which returns below 125 copies/ml while on the same regimen. We calculated the probabilities of observing a DVU with all five thresholds (Supplementary Table 1).
Mathematical model
The IeDEA-SA mathematical model of ART has been described in detail elsewhere [6]. We adapted this model to compare quality-adjusted life-years (QALYs), costs and cost-effectiveness between different monitoring strategies in cohorts of patients receiving ART. In brief, the model simulates cohorts of patients who are followed from starting ART until death. The properties of the individual patient and the timing of events are calculated probabilistically based on a series of rules and parametric distributions [6]. The model was parameterised with data from the South African township ART programmes and estimates published in the literature (Supplementary Table 2). For the present study we additionally modelled VL trajectories (Web Appendix 1).
Each simulated patient is at risk of true treatment failure, clinical failure, immunologic failure and death. The observation of the failures depends on the chosen monitoring strategy. If VL monitoring is available, virologic failure is observed if the VL trajectory is above the limit of detection (LOD) at the time of the measurement. Virologic failure may also be observed if the trajectory is below the LOD but DVU is present, typically due to blips [19, 20] or non-adherence. After an immunologic or virologic failure is observed, another measurement is taken 3 months later. If failure is observed again, the patient switches to second-line ART. On second-line ART the patient continues to be at risk of failure. Finally, the number of expected HIV transmissions is calculated. Each patient is assigned a frequency of partner change and sex acts. The times of virologic failures and switching determine the value of the VL at each sex act. The expected number of new infections is calculated using a relationship between VL and infectiousness based on the results of the Rakai study in Uganda [21, 22] (Web Appendix 2).
Costs, QALYs and ICERs
Costs of appointments, CD4 and VL measurements and ART were considered (Supplementary Table 3). Cost estimates of the antiretroviral drugs were based on the ceiling price list of the Clinton Health Access Initiative (CHAI) [23]. We used the average of the two most common first-line (zidovudine/lamivudine/nevirapine or tenofovir/lamivudine/efavirenz, $146.50/year) and second-line (zidovudine or tenofovir/lamivudine/ritonavir-boosted lopinavir, $465.50/year) regimens. Based on discussions with experts and organisations developing and implementing POC-VL tests, a range of prices per test were assumed. With the currently available laboratory-based VL tests, the cost of the consumables is about US$28 and the cost of the machine between US$100000 and US$225000 [13]. The cost-effectiveness study [24] of the Ugandan trial [5] estimated the total cost per test to be US$29.64, but higher estimates have also been reported [25, 26]. The Bill & Melinda Gates Foundation is currently funding the development of a qualitative POC-VL test costing US$3 to US$5 per cartridge and <US$1000 per machine [14]. We assumed that a plausible minimum for the unit cost of the POC-VL test (including consumables and per-test costs of the machine) would be US$5 with a LOD of 1000 copies/ml or higher. For lower thresholds, we assumed a minimum of US$10 per test. Finally, we assumed that the cost would not exceed US$20 per test for any LOD.
Quality of life weights were derived from the disability weights according to the Global Burden of Disease project [27]. For asymptomatic HIV, the weight was 0.865. For symptomatic HIV, we took the weight of the most common disease, TB, and multiplied it with the weight of asymptomatic HIV: 0.865*0.729=0.631. We used the same weights for all patients irrespective of age. We also estimated costs and lost QALYs in the partners infected by the modelled patients. We assumed that the infected partners would have a life expectancy of 40 years at the time of infection, which would be reduced to 30 years because of HIV. We calculated incremental cost-effectiveness ratios (ICER), which are defined as the ratio of the change in costs divided by the change in QALYs. Both QALYs and costs were discounted by 3% per year.
Model scenarios
The following monitoring strategies were modelled: clinical monitoring, 6-monthly CD4 count monitoring and 6-monthly VL monitoring with a qualitative POC-VL test with a LOD of 125, 400, 1000, 5000 or 10000 copies/ml. We assumed that the results of the VL test would be immediately available to the caregiver and the patient. We used the model to calculate the ICERs of VL monitoring with different LODs compared to either CD4 cell count or clinical monitoring. We investigated three possible benefits of routine VL monitoring: in Scenario A we included only the more accurate detection of treatment failure and more timely and potentially more appropriate switching of patients to second-line ART. In Scenario B we additionally considered the effect on HIV transmission. In Scenario C we also assumed that the risk of virologic failure is twice as high with clinical or CD4 monitoring than with VL monitoring, because VL monitoring detects non-adherence and improves adherence by making targeted interventions possible [28, 29].
Results
We present key model outcomes, including the number of unnecessary switches to second-line ART, the number of missed true treatment failures, the QALYs expected at the start of ART, the costs of patient management on ART (in total and broken down by costs of appointments, tests and ART), and outcomes related to HIV transmission (QALYs lost due to new infections and total costs due to new infections). These outcomes are given in Table 2 per lifetime of one patient or 100 patients on ART, by monitoring strategy and the five different LODs for the POC-VL test.
Table 2.
Key outcomes of simulated strategies.
| Outcome | Clinical monitoring Adherence scenario: |
CD4 monitoring Adherence scenario: |
POC viral load monitoring LOD (copies/ml): |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| A/B | C | A/B | C | 125 | 400 | 1000 | 5000 | 10000 | |
| Monitoring | |||||||||
| Unnecessary switches to second-line ART (per 100 patients) | 9 | 7 | 5 | 5 | 15 | 8 | 5 | 3 | 2 |
| Missed true treatment failure (per 100 failures) | 76 | 77 | 37 | 36 | 5 | 5 | 5 | 5 | 6 |
| Life expectancy at start of ART (QALYs) | |||||||||
| Mean quality-adjusted life years | 12.78 | 12.60 | 12.89 | 12.80 | 12.93 | 12.93 | 12.93 | 12.93 | 12.93 |
| Costs (US$ per person) | |||||||||
| Appointments | 577 | 569 | 582 | 577 | 583 | 583 | 584 | 584 | 583 |
| Diagnostic tests | 0 | 0 | 159 | 159 | 476–952* | 470–941* | 234–935** | 232–928** | 231–926** |
| First-line ART | 2004 | 1952 | 1940 | 1800 | 1698 | 1799 | 1838 | 1865 | 1873 |
| Second-line ART | 456 | 527 | 720 | 1117 | 1506 | 1187 | 1066 | 977 | 951 |
| Total costs of ART | 3037 | 3047 | 3400 | 3654 | 4263–4739* | 4038–4510* | 3721–4422** | 3658–4354** | 3639–4334** |
| HIV transmission (per 100 patients) | |||||||||
| New infections | 7.1 | 11.1 | 5.7 | 9.3 | 4.3 | 4.0 | 3.9 | 3.9 | 4.0 |
| QALYs lost | 22.6 | 32.3 | 20.2 | 29.2 | 15.5 | 14.9 | 14.7 | 14.7 | 15.0 |
| Costs in US$ | 14500 | 20600 | 12900 | 18700 | 9900 | 9500 | 9400 | 9400 | 9600 |
Each simulation is based on 10 000 patients starting antiretroviral therapy, followed up until death.
QALY, quality-adjusted life years
Adherence scenario A/B: Risk of virologic failure is equal in all monitoring strategies
Adherence scenario C: Risk of virologic failure is twice as high with clinical and CD4 monitoring compared to viral load monitoring
Depending on unit cost of viral load test, ranging from $10 to $20
Depending on unit cost of viral load test, ranging from $5 to $20
POC, point-of-care; LOD, limit of detection; ART, antiretroviral therapy; QALY, quality-adjusted life year
Five to six out of 100 true treatment failures remained unobserved over the entire lifetime with VL monitoring strategies, compared to 37 with CD4 and 76 with clinical monitoring. The number of unnecessary switches to second-line ART per 100 patients was 9 with clinical monitoring and 5 with CD4 count monitoring. With VL monitoring it ranged from 15 (lowest LOD, 125 copies/ml) to 5 or fewer (LODs ≥1000 copies/ml). Despite these differences in the accuracy of monitoring and switching, only small differences in the quality-adjusted life expectancy emerged, with mean QALYs expected at the start of ART ranging from 12.78 with clinical monitoring to 12.93 with POC-VL monitoring. Expressed per 100 patients, the difference in QALYs was 15. If we assumed that the true treatment failure rate was twice as high with clinical monitoring compared to VL monitoring (Scenario C), this difference increased to 33 QALYs. Depending on the monitoring strategy, the number of new HIV infections ranged from around 4 with POC-VL monitoring to 7 per 100 patients with clinical monitoring, leading to a loss of 15 to 23 QALYs per 100 patients (Table 2). The number of new infections was higher in VL monitoring with LOD 125 copies/ml compared to higher LODs. This is due to the large number of unnecessary switches to second-line ART, which will move second-line failure forward in time and, in the absence of further treatment options, increase the total number of patients on failing regimens.
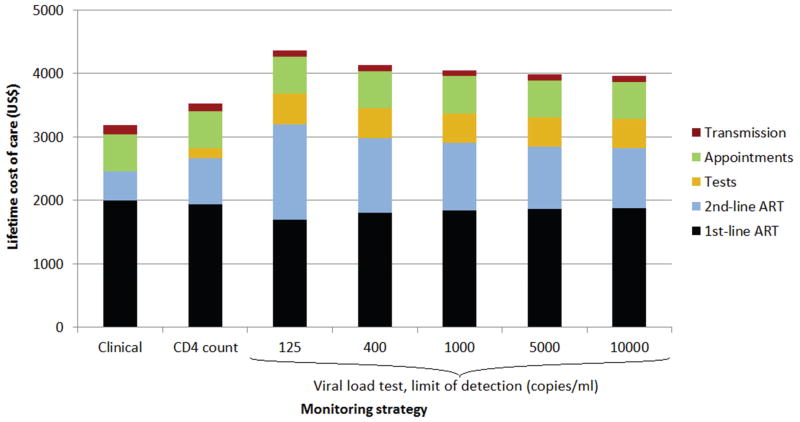
Total (lifetime) cost of ART ranged between US$3037 per patient (clinical monitoring) and US$4739 per patient (VL monitoring, US$20/VL test, LOD 125 copies/ml). The most important determinant of total costs was second-line ART, which increased over three-fold from US$456 per patient (clinical monitoring) to US$1506 per patient (VL monitoring, LOD 125 copies/ml) (Figure 1). The costs of tests were substantially higher in strategies with VL monitoring compared to CD4 monitoring even when assuming a low unit cost of US$5 per VL test. New HIV infections caused additional costs between US$9400 (VL monitoring, LOD 1000 or 5000 copies/ml) and US$14500 (clinical monitoring) per 100 patients on ART (Table 2) but were not an important contributor to costs over the lifetime of the index patients on ART (Figure 1). Since VL monitoring using a LOD of 125 or 400 copies/ml was more expensive, did not improve survival and caused slightly more transmissions compared to VL monitoring with a higher LOD, these two strategies were excluded from the cost-effectiveness analyses.
Figure 1. Breakdown of costs of antiretroviral therapy care per patient in different monitoring strategies.
Unit cost of viral load test was assumed to be US$10.
ART, antiretroviral therapy
The cost-effectiveness of POC-VL monitoring compared to CD4 monitoring was poor in Scenario A (Table 3): even the most cost-effective VL monitoring scenario (US$5 per POC-VL test, LOD 10000 copies/ml) had an incremental cost-effectiveness ratio (ICER) of US$5960 per QALY. Compared to clinical monitoring, CD4 count monitoring (ICER US$3300/QALY) was more cost-effective than VL monitoring (ICER US$4010 per QALY) under the same assumptions (US$5/test, LOD 10000 copies/ml). Including the effect on transmission (Scenario B) improved the cost-effectiveness of routine VL monitoring. Compared to CD4 count monitoring, the ICER of routine VL monitoring with the same assumptions as above decreased from US$5960 under Scenario A to US$2230 per QALY. Compared to clinical monitoring, POC-VL monitoring became more cost-effective than CD4 count monitoring (US$2450/QALY versus US$2590/QALY). Finally, in Scenario C routine POC-VL monitoring became a clearly cost-effective strategy. Compared to CD4 count monitoring, VL monitoring was cost-saving if the cost of the VL test was US$5. The ICER compared to either CD4 or clinical monitoring remained below US$2500 per QALY with all LODs and costs of POC-VL tests.
Table 3.
Cost-effectiveness of POC routine viral load monitoring compared to CD4 or clinical monitoring in three scenarios.
| Unit cost of viral load test (US$) | CD4 monitoring | POC viral load monitoring LOD (copies/ml): |
|||
|---|---|---|---|---|---|
| 1 000 | 5 000 | 10 000 | |||
| Scenario A | |||||
| Compared to CD4 monitoring | 5 | n/a | 8010 | 6430 | 5960 |
| 10 | n/a | 13860 | 12230 | 11740 | |
| 20 | n/a | 25540 | 23830 | 23340 | |
| Compared to clinical monitoring | 5 | 3300 | 4560 | 4140 | 4010 |
| 10 | 3300 | 6120 | 5690 | 5550 | |
| 20 | 3300 | 9230 | 8780 | 8650 | |
| Scenario B | |||||
| Compared to CD4 monitoring | 5 | n/a | 3010 | 2340 | 2230 |
| 10 | n/a | 5470 | 4780 | 4740 | |
| 20 | n/a | 10380 | 9670 | 9790 | |
| Compared to clinical monitoring | 5 | 2590 | 2760 | 2490 | 2450 |
| 10 | 2590 | 3790 | 3500 | 3470 | |
| 20 | 2590 | 5830 | 5530 | 5520 | |
| Scenario C | |||||
| Compared to CD4 monitoring | 5 | n/a | c/s | c/s | c/s |
| 10 | n/a | 760 | 520 | 460 | |
| 20 | n/a | 2460 | 2210 | 2170 | |
| Compared to clinical monitoring | 5 | 2540 | 1110 | 990 | 960 |
| 10 | 2540 | 1570 | 1440 | 1420 | |
| 20 | 2540 | 2500 | 2360 | 2340 | |
Cost-effectiveness is presented as incremental cost-effectiveness ratio (US$/quality adjusted life year) with 3% annual discounting. Cost-effectiveness of CD4 versus clinical monitoring is also shown.
POC, point-of-care; LOD, limit of detection; n/a, not applicable; c/s, cost-saving Scenario A (equal failure rates, HIV transmission not considered), Scenario B (equal failure rates, HIV transmission considered) and Scenario C (true treatment failure rate twice as high with CD4 or clinical compared to viral load monitoring, HIV transmission considered).
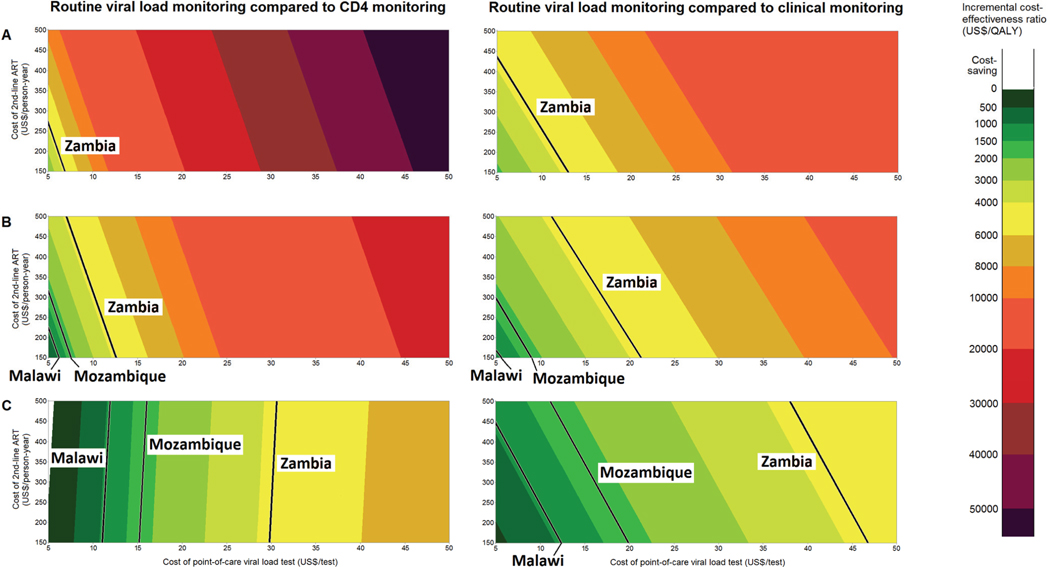
Figure 2 shows the cost-effectiveness of POC-VL monitoring compared to CD4 monitoring (left panel) and clinical monitoring (right panel) for Scenarios A, B and C. The LOD was 1000 copies/ml and the costs of POC-VL tests and of second-line ART ranged from US$5 to US$50 and US$150 to US$500, respectively. The limits for cost-effectiveness (3 times per-capita gross domestic product) are shown for three sub-Saharan African countries: Zambia (US$4242/QALY), Mozambique (US$1749/QALY) and Malawi (US$1053/QALY) [30]. In Scenarios A and B the cost-effectiveness of routine VL monitoring was highly sensitive to the cost of second-line therapy: the lower the cost of the second-line therapy, the more cost-effective VL monitoring was. In Scenario C, the cost of the POC-VL test was the most important determinant when comparing with CD4 count monitoring whereas the cost of second-line ART was again the determining factor in the comparison with clinical monitoring (Figure 2).
Figure 2. Cost-effectiveness of point-of-care routine viral load monitoring compared to CD4 (left panel) or clinical monitoring (right panel) as a function of the unit cost of a viral load test and the annual costs of second-line antiretroviral therapy in three scenarios: A (equal failure rates, no transmission), B (equal failure rates, transmission included) and C (failure rate 2× higher with clinical or CD4 monitoring compared to viral load monitoring, transmission included).
Limit of detection of the viral load test was assumed to be 1 000 copies/ml. The black lines show the maximum limit of cost-effectiveness (3× per-capita gross domestic product) for selected southern African countries.
ART, antiretroviral therapy; QALY, quality adjusted life years
In Scenario A, VL monitoring could be considered cost-effective in Zambia if both VL and second-line costs were minimised. In Scenario B, cost-effectiveness in Mozambique could be reached under the same conditions. The pattern changed in Scenario C when VL monitoring was compared to CD4 monitoring: since VL monitoring now decreased the need for second-line therapy, cost-effectiveness increased with higher second-line therapy costs. Assuming a fixed price of US$500 per year for second-line therapy (corresponding to current levels), a unit cost of US$12 per VL test would render VL monitoring cost-effective in Malawi. A unit cost of US$5 would be cost-saving.
Discussion
This mathematical modelling study found that POC-VL monitoring likely improves survival slightly and prevents new HIV infections, but also increases the costs of ART. VL monitoring more accurately detects treatment failure, but since the risk of failure is low, the resulting benefit on survival was small. The cost-effectiveness was thus poor when, in Scenario A, we only considered this benefit of POC-VL monitoring. When we also included effects on adherence and HIV transmission, our estimates of cost-effectiveness improved. Nevertheless, POC-VL monitoring remained more expensive than CD4 cell monitoring even when reducing the costs of POC tests to US$5, since in most scenarios VL monitoring increased the need for second-line ART and required additional tests to confirm failure. To minimise unnecessary switches to second-line ART, the LOD of the POC-VL test should be 1000 copies/ml or higher.
We studied the cost-effectiveness of POC-VL monitoring under a range of assumptions with results covering a wide range of ICERs from cost-saving to over US$25000/QALY saved. We did not highlight any particular scenario, since the key determinants, i.e. the unit cost of the POC-VL test and the overall benefit of VL monitoring, remain unknown. The assumed benefits in Scenarios B and C will also vary across different settings. In particular, the effect on HIV transmission will depend on the sexual behaviour, and the effect on adherence on the type of adherence intervention in place in a given programme.
Our study consistently supports the use of VL tests with detection limits of 1000 copies/ml or above. This is at odds with current practice: a systematic review found that out of 39 studies from sub-Saharan Africa reporting a single virologic failure threshold, only two used 10000 copies/ml as recommended by WHO at that time [31], and 12 used a threshold of 500 copies/ml or below [32]. Since then, WHO reduced the threshold from 10000 to 5000 copies/ml [18]. Using a threshold below 1000 copies/ml is problematic because it results in a large number of DVUs. Additional measurements are required to determine if a detectable VL has returned to undetectable levels before switching to second-line ART. Moreover, some patients will have two consecutive DVUs and therefore switch to second-line therapy unnecessarily.
The cost-effectiveness of routine VL compared to CD4 monitoring has been investigated in several modelling studies [25, 26, 33–37] as well as a randomised controlled trial [5] (Supplementary Table 4). The results from these studies vary and are difficult to compare because monitoring frequency, failure criteria, time horizon, costs and other parameters differed substantially between studies. Only Phillips et al modelled adherence [35], and Vijayaraghavan et al was the only study considering HIV transmission [34]. Moreover, this study, together with the recent analysis by Hamers et al [26], were the only two studies that replaced VL monitoring with CD4 monitoring, rather than combining them. The results of Hamers et al were clearly in favour of VL monitoring: the ICER was $86 per life-year when compared to CD4 monitoring. However, Hamers et al investigated a laboratory-based, quantitative VL test and assumed 100% sensitivity and specificity. We found that the poor specificity with low LODs led to many unnecessary switches. We feel that a specificity of 100% is unrealistic even with a quantitative test, and that the high cost-effectiveness reported by Hamers et al is questionable.
Our study has several limitations. The lack of empirical data on the effect of VL monitoring on adherence and consequently the rate of virologic failure is one of them. In the Khayelitsha and Gugulethu township cohorts less than 5% of all patients failed virologically in the first year of ART. We assumed that without VL monitoring failure rates would be twice as high. Some support for a higher rate of virologic failure in the absence of VL monitoring comes from cross-sectional studies of virologic failure from settings without routine VL monitoring. For example, in the HIV/AIDS outpatient clinic of the Central Hospital in Yaoundé, Cameroon, the percentage of patients with detectable VL at one year was 16% [38]. Similarly, 26% of individuals receiving ART in Luanda, Angola had detectable VL after a median of one year of follow up [39]. In a rural district of Malawi, 13% of patients on ART had detectable VL at 10 months [40]. In all three studies virologic failure was defined as a VL above 1000 copies/ml. However, the key question – to what extent routine VL monitoring prevents treatment failure – remains unanswered. More research is urgently needed to address this question.
Another limitation is the lack of data on long-term outcomes: simulations were not limited to a fixed time window, but modelled costs and benefits over the entire lifespan. We thus had to extrapolate the progression of the disease from the available data, which was restricted to, at most, 10 years of follow-up. Our results may therefore be sensitive to long-term outcomes, although their influence on model outcomes was reduced by annual discounting. Also, the data on disease progression from the two township ART programmes in Cape Town, which have electronic medical records and access to routine VL and CD4 monitoring and second-line ART, may not be applicable to other settings in sub-Saharan Africa. We did not explicitly model all potential benefits of POC-VL testing, for example improved retention in care [41] or the prevention of viral resistance. The effect on adherence was modelled by varying virologic failure rates but costs of adherence interventions were not included. Finally, our estimates of new infections do not take into account transmission dynamics at the population level or differences in risk behaviours. We will expand the present model to address these shortcomings.
Conclusions
Our study demonstrates that the impact of POC-VL monitoring on adherence and HIV transmission remains poorly understood despite the fact that these are the key factors that determine whether or not POC-VL tests will be cost-effective. To minimise unnecessary switches, the detection limit of the test should not be less than 1000 copies/ml, which has important implications for the design of these tests. In general, lowering the cost of any POC-VL test, and of second-line ART regimens, are the most promising strategies to maximise cost-effectiveness of monitoring ART with POC-VL tests.
Supplementary Material
Acknowledgments
J.E., M.E. and O.K. designed the study. J.E., T.H. and O.K. developed the mathematical model. J.E., N.B. and L.S. performed the statistical analyses. D.G. and R.W. were involved in data acquisition and data management. J.C. provided expertise on health economics and cost data. J.E. wrote the first version of the manuscript, which was revised by M.E. and O.K. All authors contributed to the interpretation of the results and to the final version of the manuscript.
We thank Christine Rousseau, Russell Wada, George Maltezos and Graham S. Cooke for helpful discussions and suggestions and Kali Tal for commenting and editing the manuscript.
This work was supported by the National Institutes of Health (grant number 5U01-AI069924-05), Bill&Melinda Gates Foundation, UNITAID as well as a PROSPER fellowship to O.K. (grant 32333B_131629) and a PhD fellowship to O.K. and M.E. for N.B. (PDFMP3_137106) from the Swiss National Science Foundation.
Conflicts of interest
Part of the salary of J.C. is paid by grants supporting the expansion of point-of-care testing in seven countries.
References
- 1.Keiser O, Orrell C, Egger M, Wood R, Brinkhof M, Furrer H, et al. Public-health and individual approaches to antiretroviral therapy: township South Africa and Switzerland compared. PLoS Med. 2008;5:e148. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keiser O, Macphail P, Boulle A, Wood R, Schechter M, Dabis F, et al. Accuracy of WHO CD4 cell count criteria for virological failure of antiretroviral therapy. Trop Med Int Health. 2009;14:1220–1225. doi: 10.1111/j.1365-3156.2009.02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Undetectable - How viral load monitoring can improve HIV treatment in developing countries. [Accessed 19 December 2012];Médecins Sans Frontières. 2012 http://www.msfaccess.org/sites/default/files/MSF_assets/HIV_AIDS/Docs/MSF_ViralLoad_Report._FINAL_Sept2012_webres.pdf.
- 4.Walensky RP, Ciaranello AL, Park JE, Freedberg KA. Cost-effectiveness of laboratory monitoring in sub-Saharan Africa: a review of the current literature. Clin Infect Dis. 2010;51:85–92. doi: 10.1086/653119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mermin J, Ekwaru JP, Were W, Degerman R, Bunnell R, Kaharuza F, et al. Utility of routine viral load, CD4 cell count, and clinical monitoring among adults with HIV receiving antiretroviral therapy in Uganda: randomised trial. BMJ. 2011;343:d6792. doi: 10.1136/bmj.d6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estill J, Aubrière C, Egger M, Johnson L, Wood R, Garone D, et al. Viral load monitoring of antiretroviral therapy, cohort viral load and HIV transmission in Southern Africa: A mathematical modelling analysis. AIDS. 2012;26:1403–1413. doi: 10.1097/QAD.0b013e3283536988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sungkanuparph S, Manosuthi W, Kiertiburanakul S, Piyavong B, Chumpathat N, Chantratita W. Options for a second-line antiretroviral regimen for HIV type 1-infected patients whose initial regimen of a fixed-dose combination of stavudine, lamivudine, and nevirapine fails. Clin Infect Dis. 2007;44:447–452. doi: 10.1086/510745. [DOI] [PubMed] [Google Scholar]
- 8.Keiser O, Chi BH, Gsponer T, Boulle A, Orrell C, Phiri S, et al. Outcomes of antiretroviral treatment in programmes with and without routine viral load monitoring in Southern Africa. AIDS. 2011;25 :1761–1769. doi: 10.1097/QAD.0b013e328349822f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman CJ, Charalambous S, Sim J, Ledwaba J, Schwikkard G, Chaisson RE, et al. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis. 2009;49:1928–1935. doi: 10.1086/648444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigaloff KCE, Hamers RL, Wallis CL, Kityo C, Siwale M, Ive P, et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr. 2011;58:23–31. doi: 10.1097/QAI.0b013e318227fc34. [DOI] [PubMed] [Google Scholar]
- 11.Clinical Guidelines for the Management of HIV & AIDS in Adults and Adolescents. National Department of Health; South Africa: 2010. [Accessed 19 December 2012]. http://www.who.int/hiv/pub/guidelines/south_africa_art.pdf. [Google Scholar]
- 12.Botswana National HIV/AIDS Treatment Guidelines: 2008 Version. Ministry of Health; Botswana: 2008. [Accessed 19 December 2012]. http://www.moh.gov.bw/templates/moh/File/BOTSWANA%20HIVAIDS%20TREATMENT%20%20GUIDELINES%20(November%201%202008).pdf. [Google Scholar]
- 13.UNITAID. HIV/AIDS Diagnostic Technology Landscape. 2. UNITAID; 2012. [Google Scholar]
- 14.Rousseau C. Diagnostics Innovation in China - Bringing Chinese TB and HIV Molecular Diagnostics to Market. Shanghai, China: 2012. [Accessed 19 December 2012]. HIV Point of Care Viral Load Test Development - Request for Applications. http://mat1.gtimg.com/gongyi/2012/enzhenduandahui/16ChristineRousseau.pdf. [Google Scholar]
- 15.Egger M, Ekouevi DK, Williams C, Lyamuya RE, Mukumbi H, Braitstein P, et al. Cohort Profile: The international epidemiological databases to evaluate AIDS (IeDEA) in sub-Saharan Africa. Int J Epidemiol. 2012;41:1256–1264. doi: 10.1093/ije/dyr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–2148. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 17.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization; 2010. [Accessed 19 December 2012]. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a public health approach. 2010 revision. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. [PubMed] [Google Scholar]
- 19.Nettles RE, Kieffer TL, Kwon P, Monie D, Han Y, Parsons T, et al. Intermittent HIV-1 Viremia (Blips) and Drug Resistance in Patients Receiving HAART. JAMA. 2005;293:817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 20.Gallant JE. Making Sense of Blips. J Infect Dis. 2007;196:1729–1731. doi: 10.1086/523705. [DOI] [PubMed] [Google Scholar]
- 21.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral Load and Heterosexual Transmission of Human Immunodeficiency Virus Type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 22.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008;372:314–320. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 23.Antiretroviral (ARV) Ceiling Price List. [Accessed 19 December 2012];Clinton Health Access Initiative. 2012 http://www.clintonhealthaccess.org/files/CHAI_ARV_Ceiling_Price_List_May_2012.pdf.
- 24.Kahn JG, Marseille E, Moore D, Bunnell R, Were W, Degerman R, et al. CD4 cell count and viral load monitoring in patients undergoing antiretroviral therapy in Uganda: cost effectiveness study. BMJ. 2011;343:d6884. doi: 10.1136/bmj.d6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braithwaite RS, Nucifora KA, Yiannoutsos CT, Musick B, Kimaiyo S, Diero L, et al. Alternative antiretroviral monitoring strategies for HIV-infected patients in east Africa: opportunities to save more lives? J Int AIDS Soc. 2011;14:38. doi: 10.1186/1758-2652-14-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamers RL, Sawyer AW, Tuohy M, Stevens WS, de Wit TFR, Hill AM, et al. Cost-effectiveness of laboratory monitoring for management of HIV treatment in sub-Saharan Africa: a model-based analysis. AIDS. 2012;26:1663. doi: 10.1097/QAD.0b013e3283560678. [DOI] [PubMed] [Google Scholar]
- 27.Global Burden of Disease 2004 update: Disability weights for diseases and conditions. World Health Organization; 2004. [Accessed 19 December 2012]. http://www.who.int/healthinfo/global_burden_disease/GBD2004_DisabilityWeights.pdf. [Google Scholar]
- 28.Orrell C, Harling G, Lawn SD, Kaplan R, McNally M, Bekker LG, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antivir Ther. 2007;12:83–88. [PubMed] [Google Scholar]
- 29.Maggiolo F, Airoldi M, Kleinloog HD, Callegaro A, Ravasio V, Arici C, et al. Effect of Adherence to HAART on Virologic Outcome and on the Selection of Resistance-Conferring Mutations in NNRTI- or PI-Treated Patients. HIV Clinical Trials. 2007;8:282–292. doi: 10.1310/hct0805-282. [DOI] [PubMed] [Google Scholar]
- 30.World Economic Outlook Database. [Accessed 19 December 2012];International Monetary Fund. 2012 http://www.imf.org/external/pubs/ft/weo/2012/01/weodata/index.aspx.
- 31.World Health Organisation. Antiretroviral therapy for HIV Infection in adults and adolescents in resource-limited settings: towards universal access. [Accessed 19 December 2012];Recommendations for a public health approach. http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf.
- 32.Barth RE, van der Loeff MFS, Schuurman R, Hoepelman AIM, Wensing AMJ. Virological follow-up of adult patients in antiretroviral treatment programmes in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2010;10:155–166. doi: 10.1016/S1473-3099(09)70328-7. [DOI] [PubMed] [Google Scholar]
- 33.Bishai D, Colchero A, Durack DT. The cost effectiveness of antiretroviral treatment strategies in resource-limited settings. AIDS. 2007;21:1333–1340. doi: 10.1097/QAD.0b013e328137709e. [DOI] [PubMed] [Google Scholar]
- 34.Vijayaraghavan A, Efrusy MB, Mazonson PD, Ebrahim O, Sanne IM, Santas CC. Cost-effectiveness of alternative strategies for initiating and monitoring highly active antiretroviral therapy in the developing world. J Acquir Immune Defic Syndr. 2007;46:91–100. doi: 10.1097/QAI.0b013e3181342564. [DOI] [PubMed] [Google Scholar]
- 35.Phillips AN, Pillay D, Miners AH, Bennett DE, Gilks CF, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet. 2008;371:1443–1451. doi: 10.1016/S0140-6736(08)60624-8. [DOI] [PubMed] [Google Scholar]
- 36.Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GD, Owens DK. Cost-effectiveness of hiv monitoring strategies in resource-limited settings: A southern african analysis. Arch Intern Med. 2008;168:1910–1918. doi: 10.1001/archinternmed.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimmel AD, Weinstein MC, Anglaret X, Goldie SJ, Losina E, Yazdanpanah Y, et al. Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness. J Acquir Immune Defic Syndr. 2010;54:258–268. doi: 10.1097/QAI.0b013e3181d0db97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kouanfack C, Montavon C, Laurent C, Aghokeng A, Kenfack A, Bourgeois A, et al. Low levels of antiretroviral-resistant HIV infection in a routine clinic in Cameroon that uses the World Health Organization (WHO) public health approach to monitor antiretroviral treatment and adequacy with the WHO recommendation for second-line treatment. Clin Infect Dis. 2009;48:1318–1322. doi: 10.1086/597779. [DOI] [PubMed] [Google Scholar]
- 39.Garrido C, Zahonero N, Fernándes D, Serrano D, Silva AR, Ferraria N, et al. Subtype variability, virological response and drug resistance assessed on dried blood spots collected from HIV patients on antiretroviral therapy in Angola. J Antimicrob Chemother. 2008;61:694–698. doi: 10.1093/jac/dkm515. [DOI] [PubMed] [Google Scholar]
- 40.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 41.Jani IV, Sitoe NE, Alfai ER, Chongo PL, Quevedo JI, Rocha BM, et al. Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet. 2011;378:1572–1579. doi: 10.1016/S0140-6736(11)61052-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.