Abstract
The generation of genetic mutants in Caenorhabditis elegans has long relied on the selection of mutations in large-scale screens. Directed mutagenesis of specific loci in the genome would greatly speed up analysis of gene function. Here, we adapt the CRISPR/Cas9 system to generate mutations at specific sites in the C. elegans genome.
Keywords: Caenorhabditis elegans, CRISPR, Cas9, double-strand break, genome engineering
CURRENT methods to generate mutations in the genome of Caenorhabditis elegans, including chemical mutagenesis and imprecise excision of transposons, all rely on recovering mutations in large-scale mutagenesis screens. Recently, several groups reported the use of the Streptococcus pyogenes CRISPR/Cas9 system to generate double-strand break (DSB)-induced mutations in specific genomic loci in model systems including yeast (Dicarlo et al. 2013), flies (Bassett et al. 2013; Gratz et al. 2013; Yu et al. 2013), mammalian cells (Cho et al. 2013a; Mali et al. 2013), and zebrafish (Hwang et al. 2013). Because of the enormous potential for targeted genome engineering, we here investigate the suitability of the CRISPR/Cas9 system for use in C. elegans. This article is one of six companion articles in this issue (Chiu et al. 2013; Cho et al. 2013b; Katic and Grosshans 2013; Lo et al. 2013; Tzur et al. 2013) that present different approaches to and features of Cas9-CRISPR genome editing in C. elegans.
The S. pyogenes CRISPR/Cas system effects site-specific cleavage of double-stranded DNA through a complex containing the Cas9 endonuclease and two noncoding RNAs (CRISPR RNA or crRNA, and trans-activating crRNA or tracrRNA) (Gasiunas et al. 2012; Jinek et al. 2012). Target site specificity is mediated by a 20-nt spacer region in the crRNA that is complementary to the target DNA and a 3-nt motif (NGG) following the target site in the DNA [termed protospacer adjacent motif (PAM)] (Gasiunas et al. 2012; Jinek et al. 2012). Thus a wide range of target sites can be chosen. Conveniently, a single synthetic guide RNA (sgRNA) that fuses the 3′ end of crRNA to the 5′end of tracrRNA is sufficient to target Cas9 to a specific site and generate DSBs (Jinek et al. 2012) (Figure 1A).
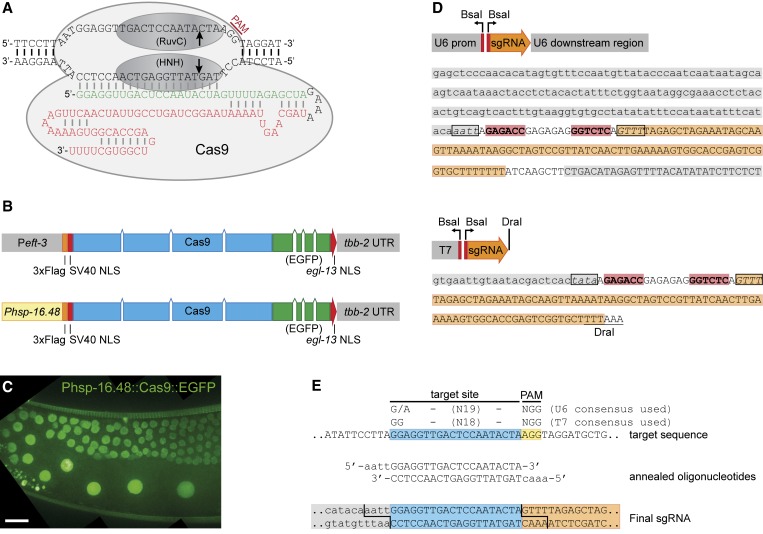
Figure 1.
Experimental design and germline Cas9 expression. (A) Cas9/sgRNA in complex with a target site. RuvC and HNH endonuclease domains together generate a double-strand break. In the sgRNA sequence, green bases are crRNA derived and red bases tracrRNA derived. (B) Schematic of the Cas9 expression vectors used in this study, placing Cas9 or Cas9::EGFP under control of the eft-3 promoter or the hsp-16.48 heat-shock promoter. Versions lacking EGFP are not shown. (C) Germline expression and nuclear localization of Cas9::EGFP expressed from the hsp-16.48 heat-shock promoter. Shown is a maximum-intensity projection of a Z-stack. Bar, 10 μm. (D) Diagrams and sequences of the U6::sgRNA and T7::sgRNA vectors. Gray background, promoter or downstream regions; orange background, sgRNA sequence downstream of the target recognition sequence; red background, BsaI recognition sites; boxed nucleotides, sequences left as 5′ overhang after BsaI digestion. (E) Example of cloning a target sequence into the U6::sgRNA vector. The 20-bp target site is outlined in blue and the PAM in yellow. The consensus sequences we used for target site selection are also indicated. Detailed materials and methods are available in File S1.
To promote expression of Cas9, we codon optimized the S. pyogenes Cas9 coding sequence for C. elegans, introduced artificial introns, and attached SV40 and egl-13 nuclear localization signals to the N and C termini, respectively, of the encoded Cas9 protein (Figure 1B). To express Cas9 in the germline, we placed the Cas9 coding sequence under control of the eft-3 or hsp-16.48 promoters and the tbb-2 3′-UTR, each of which has been shown to be compatible with germline expression (Bessereau et al. 2001; Merritt et al. 2008; Frøkjær-Jensen et al. 2012). To visualize expression of Cas9, we also generated Cas9::EGFP fusion vectors. We did not detect EGFP expression after injection of Peft-3::Cas9::EGFP (>20 animals examined). Injection of Phsp-16.48::Cas9::EGFP did result in visible EGFP expression, 5 hr after heat-shock induction for 1 hr at 34°. Expression did vary between experiments: one series of injections resulted in high expression in 5/5 animals examined (Figure 1C), while a second series of injections showed only weak expression in 1/12 animals examined. Because even low expression levels may provide sufficient enzymatic activity, in further experiments we tested both Peft-3- and Phsp-16.48-containing constructs for activity.
To provide the sgRNA, we tested two different approaches. First, we generated a vector containing a T7 promoter upstream of the sgRNA sequence for in vitro transcription of the sgRNA. Second, we generated a vector expressing the sgRNA under control of the regulatory sequences of an RNA polymerase III transcribed U6 snRNA on chromosome III, to enable in vivo transcription (Thomas et al. 1990). Both vectors contain BsaI restriction sites for simple insertion of the target recognition sequence as an oligomer linker (Figure 1, D and E).
As a first test of functional activity, we generated a reporter construct carrying an out-of-frame copy of EGFP and lacZ downstream of the myo-2 promoter. Imprecise repair of a DSB in a linker region between the first ATG and EGFP can result in a frameshift, leading to EGFP expression. We co-injected the reporter (15 ng/µl) with Peft-3::Cas9 or Phsp-16.48::Cas9 (50 ng/µl), a U6-driven sgRNA targeting the linker region (50 ng/µl), and a Pmyo-3::mCherry co-injection marker (5 ng/µl). We also tested injection of lower Cas9/sgRNA concentrations (20 ng/µl both) together with PstI-digested λ DNA (20 ng/µl), to promote generation of more complex extrachromosomal arrays. Per condition we injected 10 animals, and Phsp expression was induced by a 1-hr heat shock at 34° after the injection. None of the injections with Peft-3::Cas9 yielded viable transgenic F1’s. Instead, we observed mCherry-expressing dead embryos, indicating a deleterious effect of this construct. A series of test injections showed that the embryonic lethality is concentration dependent, ranging from 30% at 1 ng/µl to 100% at 20 ng/µl (see Supporting Information, Table S1). In contrast, 89% of the transgenic lines obtained from the injections with Phsp-16.48::Cas9 expressed EGFP in the pharynx, indicating the presence of an extrachromosomal array with at least one frame-shifted copy of the reporter (Table 1). The injection of a lower concentration Phsp::Cas9 diluted with λ DNA resulted in a higher number of transgenic offspring, although the fraction expressing EGFP was similar (90% and 84%, respectively, Table 1). Control injections lacking the sgRNA did not show EGFP expression, demonstrating specific Cas9/sgRNA activity (50 transgenic F1’s examined).
Table 1. Number of transgenic and EGFP-expressing F1 animals obtained using Cas9/sgRNA directed against an EGFP frameshift reporter.
| Results | ||||
|---|---|---|---|---|
| sgRNA concentrationa | Phsp-16.48::Cas9 concentrationa | No. P0 injected | Transgenic F1 | F1 expressing EGFP |
| 20 | 20 | 10 | 126 | 114 (90%) |
| 50 | 50 | 10 | 32 | 27 (84%) |
All concentrations are in nanograms per microliter. Injections with 20 ng/µl Cas9/sgRNA are supplemented with 20 ng/µl of PstI-digested λ DNA. All injections include 5 ng/µl of the Pmyo-3::mCherry marker to identify transgenic animals and 15 ng/µl of the out-of-frame EGFP reporter.
We also examined 18 stable transgenic lines obtained from EGFP-expressing F1 animals. Of these, 15 expressed EGFP in most (>90%) of the F2 transgenic animals. The small fraction of EGFP-negative transgenics could be due to mosaic inheritance of the extrachromosomal reporter array. Since Cas9 expression is induced by heat shock only in the injected P0 animals, these findings may indicate that DSBs were generated in the germline of the P0. Taken together, Cas9/sgRNA appears to efficiently generate DSBs in our plasmid-based reporter.
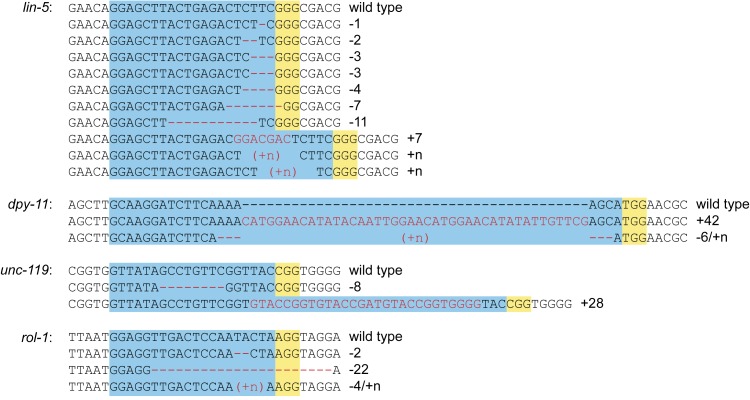
We next wanted to determine whether Cas9/sgRNA can be used to generate heritable mutations at a specific genomic locus in C. elegans. For this purpose, we generated sgRNA constructs targeting the lin-5 coding sequence near the known ev571 mutation. We injected Phsp::Cas9 together with either in vitro transcribed sgRNA or the U6::sgRNA plasmid, as well as the Pmyo-3::mCherry co-injection marker (Table 2). For each combination we injected 20 P0 animals, selected individual F1 animals expressing mCherry, and examined their F2 progeny for the presence of Lin-5 offspring. Animals injected with in vitro-produced sgRNA failed to produce lin-5 mutants (Table 2). In contrast, injections with U6::sgRNA yielded a total of 10 F1 animals that produced approximately one-quarter Lin-5 offspring (Table 2). We confirmed the presence of mutations at the lin-5 locus by sequence analysis, identifying several deletions and a 7-bp insertion (Figure 2). For each F1 line we sequenced two mutant F2 animals independently, and in each case both animals harbored exactly the same mutation, strongly suggesting that the mutations were inherited from the parent and were not generated de novo by somatic events. Two mutations could not be resolved: Sanger sequencing traces from both sides degrade into double peaks at the sgRNA target site. This can result from the presence of a repeated sequence, and we speculate that DSB repair resulted in the duplication of a short DNA sequence. Injections with the lower concentration of Cas9 and sgRNA expression plasmids coupled with λ DNA yielded higher numbers of transgenic F1 animals, but ultimately produced the same number of lin-5 mutants (Table 2).
Table 2. Number of transgenic F1 and mutant F2 progeny produced using Cas9/sgRNA directed against genomic loci.
| sgRNA | Transgenic F1 | ||||
|---|---|---|---|---|---|
| Method/target | Concentrationa | Phsp-16.48::Cas9 concentrationa | No. P0 injected | No. selected | With mutant progeny |
| U6 × lin-5 | 20 | 20 | 20 | 92 | 5 |
| U6 × lin-5 | 50 | 50 | 20 | 24 | 5 |
| T7 × lin-5 | 10 | 50 | 20 | 29 | 0 |
| T7 × lin-5 | 150 | 50 | 20 | 124 | 0 |
| U6 × rol-1 | 20 | 20 | 40 | 144 | 1 |
| U6 × rol-1 | 50 | 50 | 20 | 140 | 2 |
| U6 × dpy-11 | 50 | 50 | 20 | 20 | 2 |
| U6 × unc-119 | 50 | 50 | 20 | 41 | 2 |
All concentrations are in nanograms per microliter. Injections with 20 ng/µl Cas9/sgRNA are supplemented with 35 ng/µl of PstI-digested λ DNA. All injections include 5 ng/µl of the Pmyo-3::mCherry marker to identify transgenic animals.
Figure 2.
Genomic mutations generated by Cas9/sgRNA. Mutations are shown relative to the wild-type sequences. Three mutations could not be resolved by sequencing and may correspond to insertion of a repeated sequence. Blue indicates sgRNA target site, and yellow is the PAM motif.
Finally, we targeted three additional loci—dpy-11, rol-1, and unc-119—using Phsp-16.48::Cas9 and U6::sgRNA (Table 2). As for lin-5, we selected transgenic F1 animals and looked for the presence of visible mutants in the F2 generation. For dpy-11 and unc-119, we identified two transgenic F1’s each that segregated approximately one-quarter mutant progeny, from a total of 20 and 41 transgenic F1 animals selected, respectively (Table 2). Homozygous mutations in dpy-11 or unc-119 were readily identified in all cases (Figure 2). For rol-1, from 284 transgenic F1’s, we observed three plates with only a single Rol F2 animal. Sequencing of these mutants did confirm the presence of mutations at the target site (Figure 2). It appears therefore that the rol-1 phenotypes generated by our sgRNA are only partially penetrant. Together, these results confirm the ability of our approach to generate mutations at specific loci in the genome.
Here, we adapted the CRISPR/Cas9 system for use in C. elegans and demonstrate its ability to efficiently generate genomic mutations. For dpy-11, lin-5, and unc-119, we obtained on average one mutant from every 5 or 6 P0 animals injected. For rol-1, the frequency was much lower (three mutants of 60 P0 injections), but the partial penetrance of the phenotype likely caused us to miss several mutations. The approach is not only efficient but also fast: cloning, mutant isolation, and sequencing of mutations can be completed in 10 days.
A recently published CRISPR/Cas9 method for C. elegans uses Peft-3 to drive Cas9 expression (Friedland et al. 2013). However, we found that expression of Cas9 from the eft-3 promoter causes embryonic lethality. This contrasting result may be due to differences in the exact Cas9 protein produced. While the reason for the observed lethality is unclear, use of the heat-shock promoter to provide a pulse of expression only in the injected animal circumvents this problem.
Five companion articles (Chiu et al. 2013; Cho et al. 2013b; Katic and Grosshans 2013; Lo et al. 2013; Tzur et al. 2013) also report the successful application of CRISPR/Cas9 in C. elegans. These groups use various approaches to provide Cas9 and sgRNA, including injection of Cas9 RNA or protein and in vitro-produced sgRNA. Thus, although in our case heat-shock-induced Cas9 coupled with U6-driven sgRNA proved most efficient, it appears that the methodology to provide these two components can be highly flexible.
In addition to generating mutants, the DSBs produced by Cas9/sgRNA enable several other applications of genome engineering, including insertion of exogenous DNA through homologous recombination (Katic and Grosshans 2013; Tzur et al. 2013), and are likely to become an important tool for C. elegans researchers.
Supplementary Material
Acknowledgments
We thank M. Harterink for critical reading of the manuscript. Some strains were provided by the Caenorhabditis Genetics Center (CGC), which is funded by the National Institutes of Health Office of Research Infrastructure Programs (P40 OD010440). This work is supported by Innovational Research Incentives Scheme Vidi grant 864.09.008 and research grant 81902016, financed by The Netherlands Organization for Scientific Research.
Footnotes
Communicating editor: O. Hobert
Literature Cited
- Bassett A. R., Tibbit C., Ponting C. P., Liu J.-L., 2013. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 4: 220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessereau J. L., Wright A., Williams D. C., Schuske K., Davis M. W., et al. , 2001. Mobilization of a Drosophila transposon in the Caenorhabditis elegans germ line. Nature 413: 70–74 [DOI] [PubMed] [Google Scholar]
- Chiu H., Schwartz H. T., Antoshechkin I., Sternberg P. W., 2013. Transgene-free genome editing in Caenorhabditis elegans using CRISPR-Cas. Genetics 195: 1167–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. W., Kim S., Kim J. M., Kim J.-S., 2013a Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31: 230–232 [DOI] [PubMed] [Google Scholar]
- Cho S. W., Lee J., Carroll D., Kim J., Lee J., 2013b Heritable gene knockout in C. elegans by direct injection of Cas9-sgRNA ribonucleoproteins. Genetics 195: 1177–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCarlo J. E., Norville J. E., Mali P., Rios X., Aach J., et al. , 2013. Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems. Nucleic Acids Res. 41: 4336–4343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland A. E., Tzur Y. B., Esvelt K. M., Colaiácovo M. P., Church G. M., et al. , 2013. Heritable genome editing in C. elegans via a CRISPR-Cas9 system. Nat. Methods 10: 741–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjær-Jensen C., Davis M. W., Ailion M., Jorgensen E. M., 2012. Improved Mos1-mediated transgenesis in C. elegans. Nat. Methods 9: 117–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiunas G., Barrangou R., Horvath P., Siksnys V., 2012. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 109: E2579–E2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratz S. J., Cummings A. M., Nguyen J. N., Hamm D. C., Donohue L. K., et al. , 2013. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics 194: 1029–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W. Y., Fu Y., Reyon D., Maeder M. L., Tsai S. Q., et al. , 2013. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31: 227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinek M., Chylinski K., Fonfara I., Hauer M., Doudna J. A., et al. , 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337: 816–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katic I., Grosshans H., 2013. Targeted heritable mutation and gene conversion by Cas9-CRISPR in Caenorhabditis elegans. Genetics 195: 1173–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo T.-W., Pickle C. S., Lin S., Ralston E. J., Gurling M., et al. , 2013. Heritable genome editing using TALENs and CRISPR/Cas9 to engineer precise insertions and deletions in evolutionarily diverse nematode species. Genetics 195: XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mali P., Yang L., Esvelt K. M., Aach J., Guell M., et al. , 2013. RNA-guided human genome engineering via Cas9. Science 339: 823–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt C., Rasoloson D., Ko D., Seydoux G., 2008. 3′ UTRs are the primary regulators of gene expression in the C. elegans germline. Curr. Biol. 18: 1476–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J., Lea K., Zucker-Aprison E., Blumenthal T., 1990. The spliceosomal snRNAs of Caenorhabditis elegans. Nucleic Acids Res. 18: 2633–2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzur Y. B., Friedland A. E., Nadarajan S., Church G. M., Calarco J. A., et al. , 2013. Heritable custom genomic modifications in C. elegans via a CRISPR-Cas9 system. Genetics 195: 1181–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z., Ren M., Wang Z., Zhang B., Rong Y. S., et al. , 2013. Highly efficient genome modifications mediated by CRISPR/Cas9 in Drosophila. Genetics 195: 289–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.