Abstract
Spectrin cytoskeleton defects produce a host of phenotypes affecting the plasma membrane, cell polarity, and secretory membrane traffic. However, many of the underlying molecular mechanisms remain unexplained by prevailing models. Here we used the larval fat body of Drosophila melanogaster as a genetic model system to further elucidate mechanisms of αβ-spectrin function. The results provide unexpected new insights into spectrin function as well as mechanisms of dietary fat uptake and storage. We show that loss of α- or β-spectrin in the fat body eliminated a population of small cortical lipid droplets and altered plasma membrane architecture, but did not affect viability of the organism. We present a novel model in which αβ-spectrin directly couples lipid uptake at the plasma membrane to lipid droplet growth in the cytoplasm. In contrast, strong overexpression of β-spectrin caused fat body atrophy and larval lethality. Overexpression of β-spectrin also perturbed transport of dietary fat from the midgut to the fat body. This hypermorphic phenotype appears to be the result of blocking secretion of the lipid carrier lipophorin from fat cells. However, this midgut phenotype was never seen with spectrin loss of function, suggesting that spectrin is not normally required for lipophorin secretion or function. The β-spectrin hypermorphic phenotype was ameliorated by co-overexpression of α-spectrin. Based on the overexpression results here, we propose that β-spectrin family members may be prone to hypermorphic effects (including effects on secretion) if their activity is not properly regulated.
Keywords: spectrin, larval fat body, lipophorin, lipid transport, perilipin
MEMBERS of the spectrin and ankyrin gene families are ubiquitous in animal cells and defects in these genes are responsible for a range of inherited human disorders, including spinocerebellar ataxia type 5 (SCA5) (Ikeda et al. 2006), anemia (Lux and Palek 1995), and Duchenne muscular dystrophy (Koenig et al. 1988). In most cases, the precise molecular mechanisms underlying the disease process are incompletely understood. Spectrin and ankyrin are most familiar as components of a subplasma membrane protein scaffold known as the spectrin cytoskeleton (Baines 2010). In one long-standing hypothesis the spectrin cytoskeleton is thought to capture and stabilize interacting membrane proteins as they arrive at the cell surface, creating domains of specialized composition and function (Dubreuil 2006). Recent genetic studies in a number of model systems suggest that spectrin and ankyrin have further roles in intracellular membrane traffic (Kizhatil et al. 2007, 2009; Ayalon et al. 2008; Stabach et al. 2008; Clarkson et al. 2010; Lorenzo et al. 2010; Tjota et al. 2011).
Given the conservation of spectrin and ankyrin genes between vertebrates and invertebrates, one would expect that their functions should also be conserved. Indeed, as is the case in vertebrates, loss-of-function mutations of α- and β-spectrin and ankyrin2 in Drosophila are lethal early in development (Lee et al. 1993; Dubreuil et al. 2000; Koch et al. 2008; Pielage et al. 2008). Lethality in Drosophila appears to be due to a critical requirement for αβ-spectrin cytoskeleton function in neurons (Mazock et al. 2010). Ankyrin1 and αβ-spectrin are also expressed ubiquitously in nonneuronal cells throughout Drosophila development; however, they do not appear to be essential (Mazock et al. 2010). Possible explanations for this unexpected observation include redundant function or a function that is not detectable under standard laboratory conditions.
There are two isoforms of spectrin in Drosophila (αβ and αβH) that are functionally distinct (reviewed by Dubreuil and Grushko 1998). The αβ-spectrin isoform (studied here) is a conventional spectrin that binds to ankyrin and is expressed in the larval fat body. The αβH isoform is a distinct, larger spectrin that does not bind to ankyrin and does not appear to be expressed in larval fat body. The α- and β-subunits of Drosophila spectrins are arranged as α2β2 tetramers that are nearly indistinguishable from vertebrate spectrin tetramers (Dubreuil et al. 1990). Tetramerization is critical for function. A point mutation in α-spectrin that blocks tetramer formation, but that does not interfere with lateral αβ-dimer formation, results in loss of function (Deng et al. 1995). Spectrin can be attached to the plasma membrane indirectly through ankyrin1 (Dubreuil et al. 1996) or independently of ankyrin (Das et al. 2006, 2008). Most of the known functional sites in the spectrin molecule (such as actin and ankyrin binding) are contained within the β-subunit. The α-subunit is composed largely of spectrin repeats with unknown function and an EF hand domain that is thought to modulate the actin-binding activity of β-spectrin (Korsgren and Lux 2010).
Here we obtained new insights into αβ-spectrin genetics and function by comparing the effects of spectrin subunit overexpression with spectrin knockdown in the larval fat body of Drosophila. We previously noted that β-spectrin knockdown produced an effect on the appearance of the fat body, but without any apparent effect on the growth or viability of the organism (Mazock et al. 2010). Following up on this observation we uncovered a novel surface architecture in the fat body that is associated with the presence of a discrete population of small lipid droplets in the cortical cytoplasm. Targeted knockdown of α- or β-spectrin in fat body dramatically perturbed surface architecture and eliminated small cortical lipid droplets, suggesting that the two are functionally connected. Targeted β-spectrin overexpression in the fat body also eliminated cortical lipid droplets in the fat body. In addition, overexpression of β-spectrin in the fat body led to abnormal accumulation of lipid droplets in the midgut epithelium. Similar phenotypes, affecting fat body and midgut lipid droplets, were described in studies of lipophorin knockdown in the fat body (Panakova et al. 2005; Palm et al. 2012). Lipophorin is the lipoprotein responsible for translocation of dietary fat from the midgut to the fat body during larval development (Arrese et al. 2001; Canavoso et al. 2001; Van Der Horst et al. 2009; Van Der Horst and Rodenburg 2010). The similarity of the β-spectrin overexpression and lipophorin knockdown phenotypes led us to examine the effect of β-spectrin over- and underexpression on the behavior of lipophorin. Remarkably, β-spectrin overexpression had an effect on lipophorin secretion that was not observed with α- or β-spectrin loss of function.
Materials and Methods
Fly stocks and transgenes
The double-strand RNA (dsRNA) line carrying two autosomal β-spectrin-specific inserts (UAS-β-SpecdsRNA) was obtained from Graeme Davis (University of California, San Francisco) (Pielage et al. 2005). The UAS-dsRNA line for Lsd-2 (stock no. 34617), the wing MS1096-Gal4 driver, the fat body and hemocyte Cg-Gal4 driver, and UAS-DSRed were obtained from the Bloomington Stock Center (Bloomington, IL). The dsRNA lines for lipophorin and α-spectrin were obtained from the Transgenic RNAi Project at Harvard Medical School. The myc-tagged UAS α- and β-spectrin transgenes were previously described (Mazock et al. 2010).
Antibodies
Rabbit anti-β-spectrin serum (KCar) (Dubreuil and Yu 1994) was used for immunofluorescence and rabbit anti-β-spectrin serum (337) (Byers et al. 1989) was used for Western blots. Myc-tag-specific antibody 9E10 was from Sigma-Aldrich (St. Louis). Immunofluorescent staining was carried out as previously described (Dubreuil et al. 2000), using Texas Red-labeled (Zymed, South San Francisco, CA) or Cy3-labeled secondary antibodies (Invitrogen, Carlsbad, CA). Guinea pig anti-lipophorin was a gift from Suzanne Eaton at the Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany (Eugster et al. 2007). Affinity-purified anti-βH-spectrin was described previously (Dubreuil et al. 1997). Western blots were performed with alkaline phosphatase-coupled secondary antibodies (Zymed) and stained with bromochloroindolyl phosphate as previously described (Dubreuil and Yu 1994).
Light microscopy
Larval tissues were dissected and fixed as previously described (Dubreuil et al. 2000) and mounted using Vectashield mounting medium (Vector Laboratories, Burlingame, CA). Images were captured using a FV500 confocal microscope (Olympus, Center Valley, PA) with a 40× Plan-Apo oil immersion objective and Fluoview 2.1 software. Images were saved as “Experiments” in Fluoview and were converted to jpeg format. Montages were assembled using Photoshop CS 4.0 (Adobe Systems, San Jose, CA). The Z-axis reconstruction of fat body was produced from a Z series of images, using the Reslice feature in ImageJ (Abramoff et al. 2004). Lipid droplets were analyzed by differential interference contrast (DIC), using a Zeiss Axioskop microscope. Images were captured using an Axiocam camera and AxioVision software.
Electron microscopy
Fat bodies were removed from third instar larvae in 2% glutaraldehyde in 0.1 M phosphate buffer, pH 7.2, at room temperature. After 10 min specimens were put on ice for an additional 50 min. Following a brief rinse with buffer, the tissues were postfixed with 2% OsO4 for 2 hr at 4°, followed by staining with uranyl acetate overnight at 4°. The tissues were dehydrated in ethanol and embedded in Dow epoxy resin 332/732 plastic, using propylene oxide as a transitional fluid. Thin sections were stained with 1% uranyl acetate and lead citrate and examined in a Technai F30 electron microscope at 300 kV.
Oil Red O staining
Larval tissues were processed as described by Gutierrez et al. (2007). Briefly, larvae were dissected and fixed in 4% paraformaldehyde in phosphate-buffered saline for 10 min. Specimens were then rinsed twice with Drosophila Ringer’s solution, incubated for 20–30 min in Oil Red O stain (6 ml of 0.1% Oil Red O in isopropanol and 4 ml distilled water: prepared fresh and passed through a 0.45-μm syringe filter), and rinsed twice with Ringer’s solution. Stained material was then transferred to glycerol mounting medium.
Rescue crosses
Wing phenotype:
Homozygous autosomal stocks with insertions of the myc-epitope-tagged α- and β-spectrin UAS transgenes were crossed to MS1096-Gal4 females, either singly or together. For rescue crosses, UAS-α-Spec37 males were first crossed with UAS-β-Spec95 females. Doubly heterozygous F1 males were then crossed with MS1096-Gal4 virgin females at 25°. Individual adult fly progeny were scored for transgenes present by Western blotting with anti-myc and anti-α-actinin antibodies (loading control).
Fat body and lipid transport defects:
Crosses between UAS-β-Spec62/Y; +/+ males and +/+; Cg-Gal4/Cg-Gal4 females resulted in 100% lethality in F1 females (UAS-β-Spec62/+; Cg-Gal4/+) but not males (+/Y; Cg-Gal4/+). For rescue crosses, UAS-α-Spec37 males were first crossed with UAS-β-Spec62 females (both parents homozygous). F1 males (UAS-β-Spec62/Y; UAS-α-Spec37/+) were then crossed with Cg-Gal4/Cg-Gal4 virgin females for fat body expression. Individual F1 flies were analyzed on Western blots to verify transgene expression, as above.
Gal4 dilution:
To ask whether the rescue of the β-spectrin overexpression phenotypes by α-spectrin overexpression was simply due to dilution of Gal4 activity by a second UAS transgene, UAS-β-Spec62/UAS-β-Spec62 females were crossed with UAS-mCD8 GFP/UAS-mCD8 GFP males. F1 males (UAS-β-Spec62/Y; UAS-CD8 GFP/+) were then crossed with Cg-Gal4/Cg-Gal4 virgin females for fat body expression. GFP-positive F1 female progeny were tested for viability (expression of UAS-β-Spec62 alone was known to be lethal in the absence of UAS-α-Spec37 coexpression).
Effect of α-spectrin gene dose on β-spectrin overexpression phenotype
The viability of flies overexpressing UAS-β-Spectrin at 22° was tested in flies that were heterozygous for a null allele of α-spectrin (αSpecrg41). Homozygous Cg-Gal4, UAS-mCD8-GFP virgin females were crossed with UAS-β-Spec95 males at 22°. F1 virgin females (X/X; Cg-Gal4, UAS mCD8-GFP/+; UAS-β-Spec95/+) were then crossed with αSpecrg41/TM3 males. Individual F1 larvae were scored for the presence of GFP fluorescence to select for the presence of the Cg-Gal4, UAS-mCD8-GFP chromosome. Larvae were grown to adulthood and then selected for the presence or absence of the αSpecrg41 chromosome by scoring the Sb-marked balancer chromosome. Finally, individual flies were analyzed on Western blots to test for UAS-β-Spec95 transgene expression.
Results
A discrete population of small cortical lipid droplets in the larval fat body
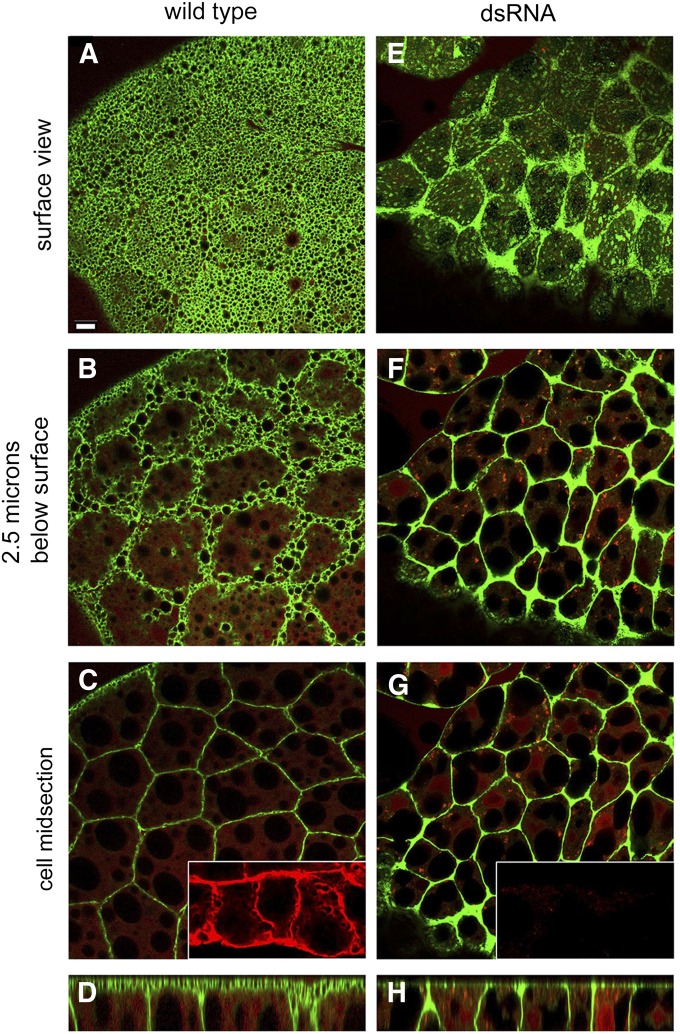
Expression of UAS-mCD8-GFP (Lee and Luo 1999) as a plasma membrane marker in the larval fat body [under control of Cg-Gal4 (Asha et al. 2003)] produced a peculiar foamy pattern of fluorescence at the “ecto” surface of cells (Figure 1A). We coined the term ecto to distinguish the fat body surfaces facing the hemolymph from regions of lateral contact between neighboring fat cells. The foamy pattern was not detected in first instar and early second instar larvae (Supporting Information, Figure S1A). There was a more typical polygonal pattern of mCD8-GFP-labeled cell–cell contacts deeper within the sheet of fat body cells (Figure 1, A–C). The foamy ecto surface pattern disappeared upon expression of dsRNA against β-spectrin, leaving primarily the pattern of labeled contacts and a diffuse speckled pattern over the ecto surface (Figure 1, E–G). β-spectrin codistributed with mCD8-GFP at both ecto surfaces (top and bottom) and at cell–cell contacts in controls (Figure 1C, inset) and β-spectrin staining was nearly eliminated after RNA interference (RNAi) (Figure 1G, inset).
Figure 1.
The foamy appearance of the larval fat body surface was lost with knockdown of β-spectrin. (A) The plasma membrane marker mCD8-GFP (green) produced an unusual foamy pattern of surface labeling in the wild-type larval fat body. (B and C) Deeper confocal sections revealed a more typical polygonal profile of cell–cell contacts. (E–G) After β-spectrin knockdown with RNAi the foamy appearance was replaced by a coarse speckled appearance, although the polygonal pattern at cell contacts remained. The β-spectrin knockdown was efficient as judged by anti-β-spectrin staining (C and G, insets, red). (D and H) A rotated view (compiled from a Z series using ImageJ) revealed a broad zone of ecto domain mCD8-GFP fluorescence in the wild type (D) that was lost after β-spectrin knockdown (H). Cells were counterstained by cytoplasmic DSRed expression, which outlines the large lipid droplets present in each cell. Bar, 10 µm.
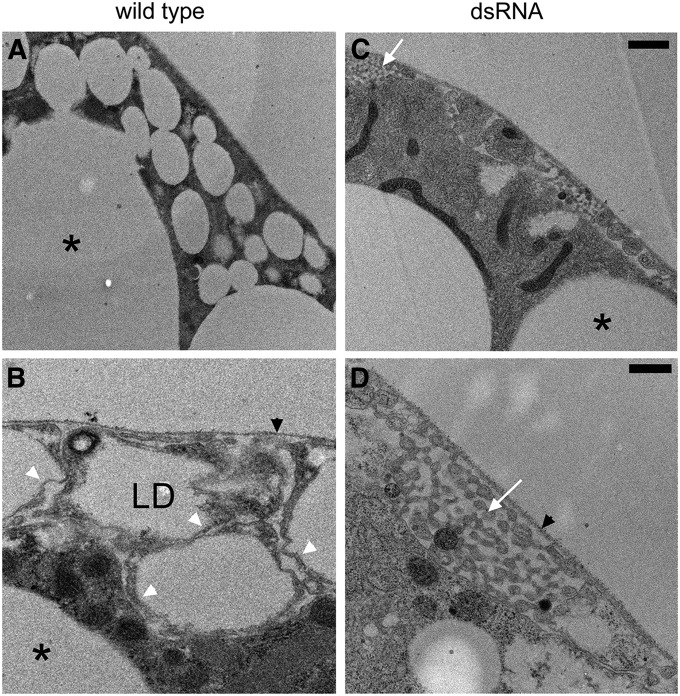
The basis for the foamy pattern of ecto surface staining became apparent after electron microscopy. There is a dense population of small cortical lipid droplets (≤4 um diameter) in the ecto region of the larval fat body cells (Figure 2A). This population is distinct from the population of much larger lipid droplets found deeper in the cytoplasm (Figure 2, A–C, *). Higher magnification views revealed a complex surface topology in which the plasma membrane is sculpted in a pattern that is intimately associated with the cortical lipid droplets (Figure 2B). The lipid droplets were found enclosed within small protrusions of the cell surface. In many sections (including Figure 2B) the protrusions appeared to be disconnected from the rest of the cell because they were connected in another plane. Typically there was a very thin rim of cytoplasm between the lipid droplet surface and the surrounding plasma membrane (Figure 2B, white arrowheads). Because of this complex topology, the plasma membrane occupies a relatively broad zone at the cell cortex. Thus, the foamy appearance of fluorescent markers (Figure 1) is most likely due to negative staining of lipid droplets within this broad zone.
Figure 2.
Electron microscopy revealed that a population of small, cortical lipid droplets in larval fat body explains the foamy appearance with fluorescent markers. (A and B) In wild type, small lipid droplets (LD) were found closely apposed to the plasma membrane. The small cortical lipid droplets (1–4 μm) were distinct from the much larger lipid droplets (*) found deeper in the cytoplasm. (C and D) The population of small cortical LD was absent after knockdown of β-spectrin. Higher magnification views revealed that the cortical lipid droplets were housed within small protuberances of the plasma membrane, tightly packed between the prominent extracellular matrix that surrounds the fat body (black arrowheads) and the rest of the cell. White arrowheads mark the thin rim of cytoplasm found between LD and the plasma membrane. (D) The lipid droplets and protuberances were no longer visible after β-spectrin knockdown. Instead there was a peculiar pattern of smaller, interconnected tubular structures (white arrows) in pockets formed between the extracellular matrix and the cell body. Bars, A and C, 1 µm; B and D, 0.5 µm.
The loss of the foamy pattern after β-spectrin knockdown was also explained by electron microscopy of the fat body (Figure 2, C and D). The population of small cortical lipid droplets was nearly eliminated and in its place there was a peculiar pattern of small interconnected tubules (Figure 2, C and D, white arrows) in the space between the extracellular matrix (Figure 2D, black arrowhead) and the plasma membrane. This residual structure may account for the speckled mCD8-GFP pattern observed by fluorescence microscopy after β-spectrin knockdown. In the absence of the small cortical population of lipid droplets, the large lipid droplets were often found in close proximity to the plasma membrane, which was not the case in controls. An overexposed pattern of mCD8-GFP fluorescence (also color inverted in Photoshop) highlighted the pattern of large lipid droplets in the cytoplasm, helping to establish that their size and number were unaltered by β-spectrin knockdown (Figure S1B). All of these parameters were similarly affected by knockdown of α-spectrin (Figure S2), indicating that loss of αβ-spectrin tetramer function is responsible for the observed phenotypes. Remarkably, the changes brought about by targeted α- or β-spectrin knockdown in the fat body did not otherwise affect the development or longevity of knockdown animals.
The physiological significance of the small cortical lipid droplets is not apparent from their morphology. To distinguish whether they represent a stable or a transient compartment, we examined the third instar larval fat body by DIC after starvation (Figure S3). The prominent layer of cortical lipid droplets found in controls (Figure S3A) was no longer visible in starved animals (Figure S3B), as was the case after β-spectrin knockdown (Figure S3C). Thus, the small cortical lipid droplets appear to be a transient intermediate in the transport of dietary fat to large lipid droplets.
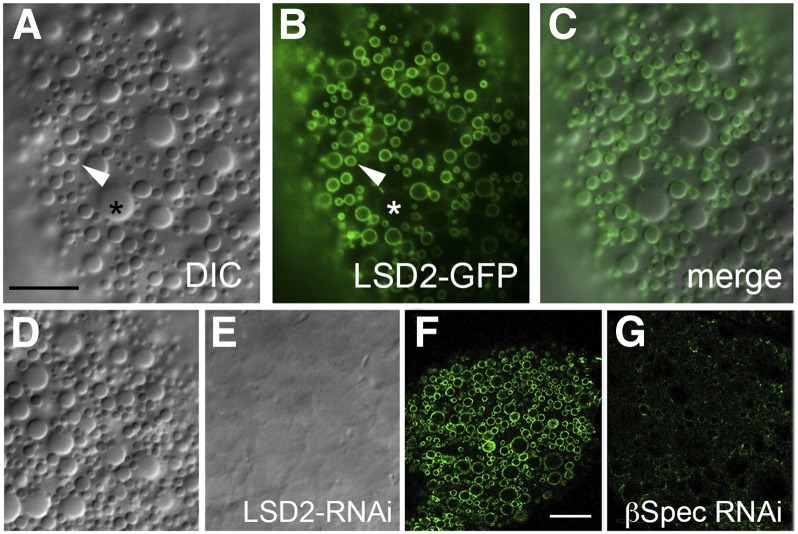
We also addressed the relationship of the small cortical lipid droplets to two classes of lipid droplets described in previous studies. There are small lipid droplets (∼1–4 μm) that express the markers Lsd-2 and Lsd-1 and larger lipid droplets (>4 μm) that express only Lsd1 (Bi et al. 2012). Expression of UAS-Lsd2-GFP in the fat body specifically labeled the spectrin-dependent population of small cortical lipid droplets described above (Figure 3, A–C). Knockdown of Lsd2 with RNAi eliminated the cortical lipid droplet population (Figure 3, D and E), including the >4-μm diameter cortical droplets that were not labeled with Lsd2-GFP (Figure 3C). Thus there may be a precursor product relationship between the two sizes. Knockdown of β-spectrin eliminated the population of small lipid droplets that were labeled with Lsd2-GFP (Figure 3, F and G). Thus, it appears that the spectrin-dependent population of lipid droplets described here corresponds to the Lsd-2-bearing lipid droplets described previously. But, unlike that in Lsd-2 knockouts (Gronke et al. 2003), loss of spectrin function did not lead to a deficit in stored triacylglycerol as measured by susceptibility of adults to starvation (Figure S4).
Figure 3.
The small cortical lipid droplets are Lsd-2 positive. (A,D) Lipid droplets in the ecto domain of fat body cells can be detected by DIC microscopy. (B and F) Expression of UAS‐Lsd-2-GFP in the fat body (via Cg-Gal4) produces a pattern of small lipid droplets labeled on their surface (B) that exactly coincides with the DIC pattern (merge in C). Larger lipid droplets (>4 μm) were not labeled by Lsd-2‐GFP (B, *). (E) Knockdown of Lsd-2 by RNAi resulted in disappearance of the cortical lipid droplets. (G) Likewise, knockdown of β-spectrin by RNAi eliminated the population of Lsd‐2‐GFP-labeled vesicles in the cortex. Bars, A–E, 10 μm; F and G, 20 μm.
Gain-of-function effects of β-spectrin overexpression
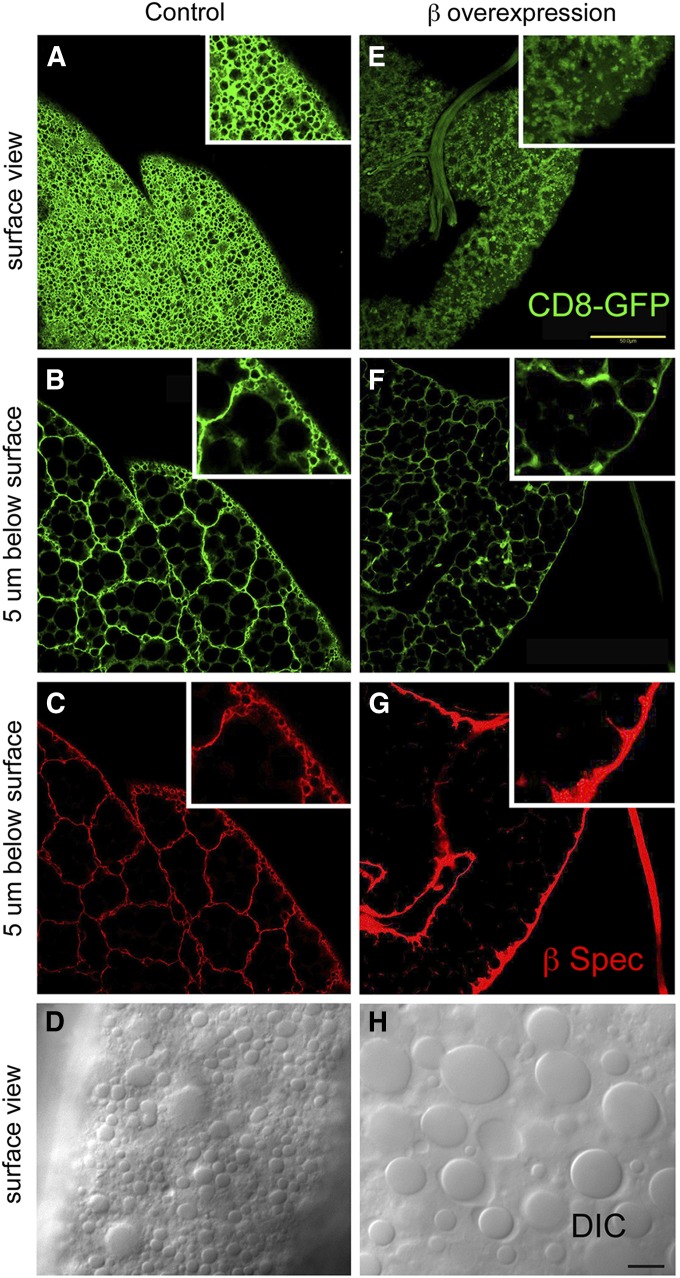
It was previously shown that overexpression of a UAS β-spectrin transgene (UAS-β-Spec95) in many larval tissues (including fat body) had a toxic effect, resulting in larval lethality at 25° (Mazock et al. 2010). Using mCD8-GFP as a marker (as above), β-spectrin overexpression produced a loss of foaminess and a speckled pattern similar to what was observed with α- or β-spectrin knockdown (Figure 4E). Antibody staining revealed a dramatic increase in β-spectrin staining at the ecto surface of fat body cells (Figure 4G). There was a concomitant loss of the foamy character seen in control cells by fluorescence staining (Figure 4, A and E) and by DIC light microscopy (Figure 4, D and H).
Figure 4.
Overexpression of β-spectrin in the larval fat body altered the surface mCD8GFP pattern and eliminated the population of small, cortical lipid droplets. UAS-β-Spec95 expression in fat body was driven by Cg-Gal4. Overexpression resulted in a dramatic increase of β-spectrin antibody staining at the ecto domain (G) compared to wild type (C). There was also an increase in mCD8-GFP intensity, making it necessary to lower the photomultiplier tube (PMT) setting during image capture (E and F) relative to that for controls (A and B). By lowering the PMT it was possible to see loss of the foamy pattern, which was replaced by a speckled pattern, similar to what was seen with β-spectrin knockdown (Figure 1). The pattern of small, cortical lipid droplets (1–5 μm) detected by DIC microscopy in controls (D) was also largely eliminated by β overexpression (H). Bars, E, 50 µm; H, 10 µm.
The lethal effect of β-spectrin overexpression was associated with a gradual disappearance of the fat body (Figure 5). After several days of development, larvae overexpressing β-spectrin acquired an unusual empty appearance due to the nearly complete disappearance of fat tissue (Figure 5, right). The consequences of β-spectrin overexpression were first noted with Cg-Gal4-driven expression of UAS-β-Spec95 at 25°. When the level of transgene expression was reduced by lowering the growth temperature to 22°, lethality and fat body atrophy were no longer observed (described below).
Figure 5.
Overexpression of β-spectrin in the fat body caused fat body atrophy and lethality. Much of the space within a wild-type larva is occupied by lobes of fat tissue (left). Larvae overexpressing β-spectrin (UAS-β-spec95) at 25° ultimately die by third instar, at which time the fat body has substantially atrophied, leaving larvae with large empty spaces (*).
A hypermorphic effect of β-spectrin overexpression in the fat body
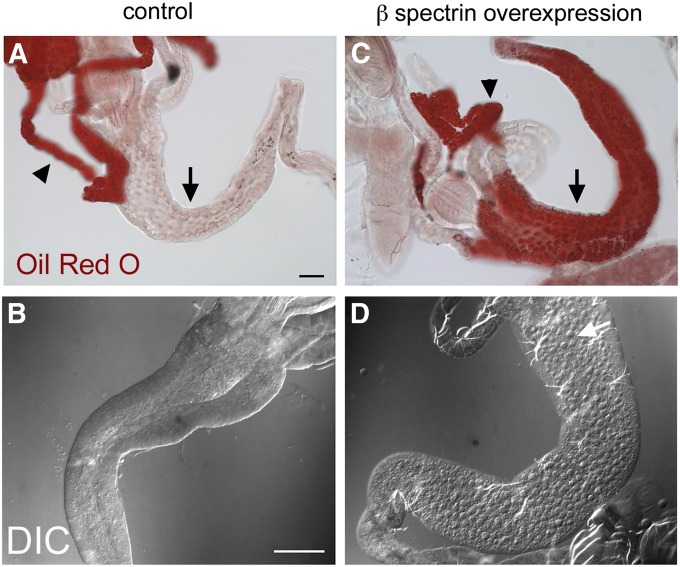
Lipid droplets can also be detected by light microscopy, using Oil Red O as a hydrophobic dye (Gutierrez et al. 2007). Oil Red O staining in dissected control preparations produced a striking pattern in the fat body (Figure 6A, arrowhead), but there was scant staining in the midgut (Figure 6A, arrow). There was little effect of β-spectrin overexpression on Oil Red O staining in the fat body, although over time there was progressively less detectable fat body tissue (in larvae reared at 25°; Figure 5). In contrast, there was a dramatic and unexpected increase in Oil Red O staining of the midgut epithelium (Figure 6C, arrow). By DIC microscopy, lipid droplets were relatively rare in control preparations of midgut (Figure 6B), but they became conspicuous in both the anterior (Figure 6D) and the posterior midgut (not shown) of β-spectrin overexpressers at 25° and in the posterior midgut at 22°. It is noteworthy that the abnormal accumulation of lipid droplets was never observed in β-spectrin loss-of-function mutants (not shown). Thus abnormal accumulation of lipid in the midgut is a nonautonomous gain-of-function effect of β-spectrin overexpression in the fat body. We describe this gain-of-function effect as hypermorphic, noting that the phenotype is distinct from that observed with loss of function.
Figure 6.
Oil Red O staining of lipid droplets in dissected preparations of wild-type and β-spectrin-overexpressing larvae. (A) Most of the Oil Red O staining in wild type was confined to the fat body (arrowhead) with only a trace of staining visible in the midgut epithelium (arrow). (C) There was a dramatic increase in anterior midgut staining in larvae that overexpressed UAS‐β‐Spec95 at 25°. (B and D) The change was also visible by DIC: lipid droplets were rarely detectable in controls (B) and there was a dramatic increase in lipid droplets in the anterior midgut (white arrow) upon UAS‐β-Spec95 overexpression (D). Bars, A and C, 50 µm; B and D, 20 µm.
Hypermorphic effects of β-spectrin overexpression are relieved by coexpresssion of α-spectrin
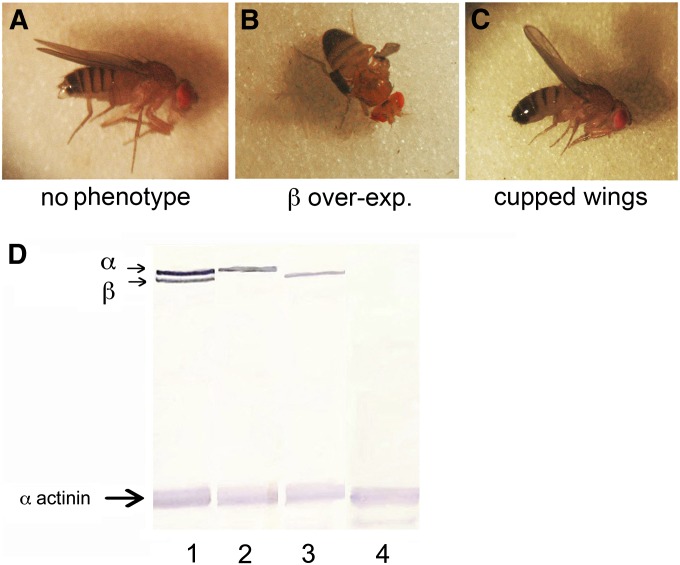
We previously observed a severe disruption of normal wing development with overexpression of UAS-β-Spec95, using the wing-specific Gal4 driver MS1096 (Mazock et al. 2010). In contrast, overexpression of α-spectrin alone in the wing had no effect (Figure 7). However, when it was coexpressed with β-spectrin, α-spectrin largely ameliorated the detrimental effects of overexpressing β-spectrin alone (Figure 7C). A class of flies with a mildly cupped wing shape corresponded to overexpression of both the α- and β-spectrin transgenes.
Figure 7.
Rescue of the β-spectrin overexpression phenotype in the wing by coexpression of α-spectrin. Doubly heterozygous males carrying autosomal UAS-α-Spec37 and UAS-β-Spec95 transgenes were crossed to homozygous MS1096-Gal4 females to drive their expression during wing development. (A–C) Three classes of wing phenotypes were distinguished in the adult progeny. (D) Western blot analysis with anti-myc tag antibody detected the expected progeny classes expressing transgene alone (lanes 2 and 3), both transgenes (lane 1), or neither transgene (lane 4). When reared at 25°, adults expressing UAS-β-Spec95 under control of MS1096-Gal4 produced a severe wing phenotype (B). Flies expressing neither transgene (A) or expressing just UAS-α-Spec37 were indistinguishable with no detectable wing phenotype (no transgene shown). A third class of flies was observed in which wings had a mildly cupped phenotype, corresponding to the class overexpressing both α- and β-spectrin (C).
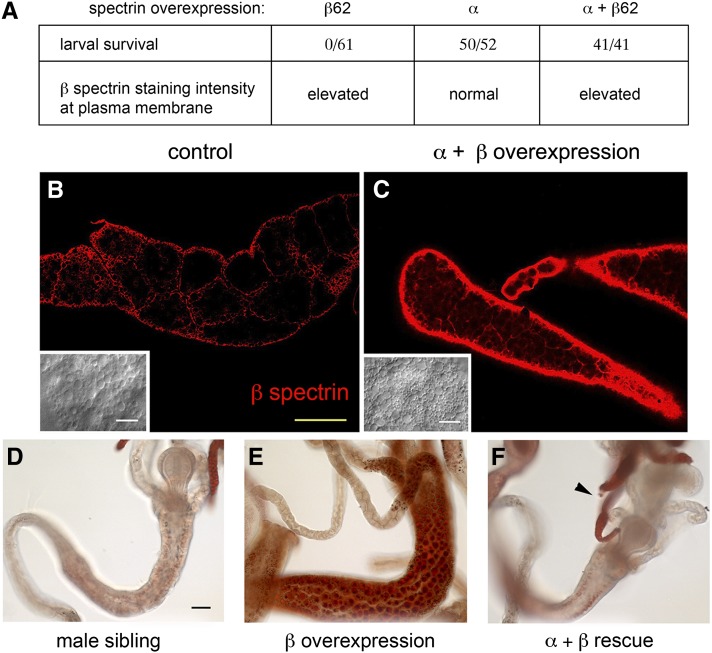
We asked whether α-spectrin coexpression could also rescue the lethality of β-spectrin overexpression in the fat body. Here we used an X-linked β-spectrin transgene (UAS-β-Spec62) that exhibits a higher level of expression than UAS-β-Spec95 (Mazock et al. 2010). Expression of the UAS-β-Spec62 transgene driven by Cg-Gal4 at 25° resulted in consistent early second instar larval lethality of female progeny. Male siblings did not express the X-linked transgene in this cross and thus provided a useful control group that survived to adulthood. When UAS-α-Spec37 was coexpressed with UAS-β-Spec62, lethality was overcome and all female progeny survived to adulthood (Figure 7A). Western blots confirmed that the female progeny expressed both the α- and β-spectrin transgenes (not shown). The possibility that rescue of the β-spectrin overexpression phenotype was due to a trivial Gal4 dilution effect caused by introduction of the UAS-α spectrin transgene was ruled out by coexpressing another UAS transgene (UAS-mCD8-EGFP) together with UAS-β-spectrin, with no change in outcome (not shown).
It appears that α-spectrin exerts its effect by altering the activity of β-spectrin, as opposed to changing its distribution or rate of turnover. Anti-β-spectrin antibody staining of the larval fat body from rescued females showed the same high-density accumulation of spectrin at the ecto domain (Figure 8C) as in larvae overexpressing β-spectrin alone (Figure 4G), in contrast to the much weaker staining detected in control sibling males (Figure 8B, same microscope settings as in Figure 8C). Oil Red O staining showed that, in addition to rescuing the lethality of β-spectrin overexpression at 25°, α-spectrin coexpression also prevented the abnormal accumulation of lipid droplets in the midgut (Figure 8F).
Figure 8.
Rescue of the β-spectrin overexpression phenotype in the fat body. Males carrying X-linked UAS-β-Spec62 and heterozygous for autosomal UAS‐α‐Spec37 (UAS-β-Spec62/Y; UAS‐α-Spec37/+) were crossed to females homozygous for autosomal Cg-Gal4 (+/+; Cg‐Gal4/Cg-Gal4) at 25°. (A) In control crosses, expression of UAS‐β-Spec62 alone at 25° resulted in 100% larval lethality at second instar and expression of UAS‐α-Spec37 alone had no effect on larva viability. However, in rescue crosses with α- and β-spectrin transgenes together, female progeny could be recovered as third instar larvae and they were 100% viable to adulthood. (B and C) These third instar female progeny exhibited dramatically elevated β-spectrin staining in the fat body (C) compared to their male siblings that did not express the β-spectrin transgene (B, same microscope setting as in C). Insets show lipid droplets in wild-type fat body by DIC (B) and their return after rescue of β overexpression by α-spectrin (C). (D–F) Oil Red O staining of dissected second instar larvae. The midgut lipid droplet accumulation observed with β-spectrin overexpression alone (E) was not seen in larvae expressing both β- and α-spectrin (F), although lipid staining was apparent in the fat body (arrowhead). A male sibling from the rescue cross not expressing excess β-spectrin is shown as a negative control (D). Bar, 50 µm.
Further evidence for a regulatory interaction between α-spectrin and hypermorphic β-spectrin was obtained by lowering the α-spectrin gene dose. Survival of β-spectrin overexpressers at 22° in this genetic background was somewhat reduced (7/56 total progeny) compared to other backgrounds where there was 100% survival. In contrast, no surviving overexpressers were observed in heterozygotes carrying a null α-spectrin allele (0/56 total progeny). Thus the hypermorphic β-spectrin phenotype could be made more severe by either reducing the dose of α-spectrin or increasing the level of β-spectrin overexpression.
The midgut lipid accumulation phenotype is due to an effect on lipophorin secretion from the fat body
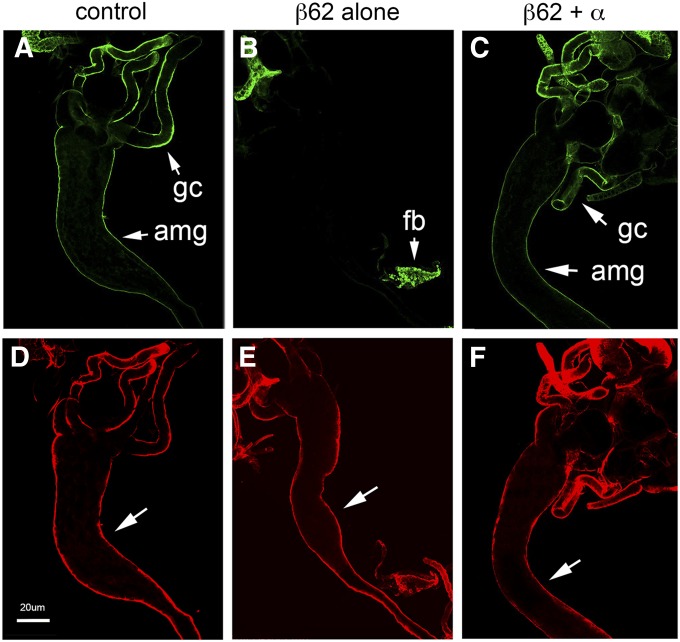
The accumulation of dietary fat in the midgut in response to β-spectrin overexpression in the fat body was similar to what had been previously observed after knockdown of lipophorin in the fat body with RNAi (Panakova et al. 2005). Staining experiments with an anti-lipophorin antibody were performed to ask whether β-spectrin overexpression had an effect on lipophorin behavior. Overexpression of β-spectrin in the fat body resulted in an increase in lipophorin staining within the fat body (Figure S6, C and D) relative to that in wild-type controls (Figure S6, A and B). Overexpression of β-spectrin in the fat body also eliminated lipophorin staining in the midgut (Figure 9B) to the same extent as lipophorin knockdown (Figure S5G). Midgut lipophorin staining was restored in β-spectrin overexpressers that coexpressed UAS-α-spectrin (Figure 9C). Together these results support the conclusion that overexpressed β-spectrin exerts a hypermorphic effect on lipophorin secretion from the fat body, thereby interfering with transport of dietary fat from larval midgut to fat body.
Figure 9.
Overexpression of β-spectrin alters the behavior of lipophorin. (A–F) Dissected larvae expressing UAS‐β‐Spec62 alone (B and E) or together with UAS‐α-Spec37 (C and F, as in Figure 8) in the fat body (Cg‐Gal4) were double labeled with anti‐lipophorin antibody and FITC secondary antibody (A–C, green) and anti-β-spectrin antibody and TR secondary antibody as a staining control (D–F, red). Lipophorin antibody stained the outer surface of the anterior midgut (amg) and gastric caeca (gc) of wild-type larvae (A). Midgut lipophorin staining was absent when β-spectrin was overexpressed in the fat body (B), although it was still abundantly detected within the fat body (fb). Lipophorin staining of the midgut was restored in rescued larvae expressing both α- and β-spectrin transgenes (C). Bar, 20 µm.
Discussion
Here we identified a population of plasma membrane-associated lipid droplets whose presence relies on αβ-spectrin function. These lipid droplets were previously recognized by their small size and distinct protein composition relative to other lipid droplets in the same cells (Bi et al. 2012). The physiological properties of these lipid droplets can now be considered in light of their intimate association with the plasma membrane via spectrin. We also demonstrated that spectrin is prone to significant hypermorphic effects that are distinct from loss of function. There has been a tacit assumption in previous studies that genetic perturbations of the spectrin cytoskeleton exert their effects through a loss of function or, alternatively, as dominant negatives. The results here establish for the first time that hypermorphic effects are also possible, and they can lead to disruption of secretory traffic.
A model for lipid uptake in the fat body
Genetic studies have established that lipophorin plays a key role in dietary fat uptake in Drosophila (Panakova et al. 2005; Palm et al. 2012). The lipophorin apoprotein is related to Apolipoprotein B (ApoB) in mammals (Van Der Horst et al. 2009). It is secreted from the fat body into the hemolymph of Drosophila larvae. It acquires diet-derived lipid cargo (principally diacylglycerol and esterified sterols) from the digestive tract. Once loaded, it can circulate back to the fat body for unloading and recycling. Some of the associated cofactors required for loading and unloading of lipophorin are beginning to be elucidated (Palm et al. 2012), but the picture at present is far from complete. There is evidence that lipophorin can be endocytosed by fat body cells (Dantuma et al. 1998). But there is also evidence that lipophorin can unload its lipid cargo without endocytosis (Canavoso et al. 2001). In either case there is a requirement for (a) lipid cargo crossing the lipid bilayer (plasma membrane or endosome) and (b) lipid packaging into the hydrophobic core of cytoplasmic lipid droplets. The mechanisms responsible have yet to be determined.
Our new data lead us to propose a novel model for the fat body cell surface in which α2β2-spectrin tetramers and cortical Lsd-2-positive lipid droplets are components of a lipid uptake apparatus (Figure 10). The model accounts for unloading of lipophorin that is docked outside the cell, without the need for endocytosis (Canavoso et al. 2001). The Lsd-2-positive cortical lipid droplets are intimately linked to the plasma membrane over much of their surface, leaving only a thin rim of cytoplasm between the lipid droplet surface and the plasma membrane (Figure 2). This intimate association raises the possibility that there is an active mechanism linking the two together. The α2β2-spectrin and perilipin Lsd-2 are both positioned in such a way that they could link the small cortical lipid droplets to the plasma membrane. We speculate that spectrin is attached to lipid uptake proteins in the plasma membrane such as fatty acid and/or sterol transporters that mediate uptake of lipid cargo from extracellular lipophorin. Spectrin may further serve as an adapter that links these transporters to the lipid droplet surface by binding to perilipin (e.g., Lsd-2). This physical linkage would be expected to facilitate the direct translocation of nonpolar lipid molecules from outside the cell to the lipid droplet interior. The presence of cortical lipid droplets is dependent on lipophorin activity (Panakova et al. 2005), suggesting that the droplets are lost during starvation because they are a plasma membrane-associated intermediate in the lipid uptake process.
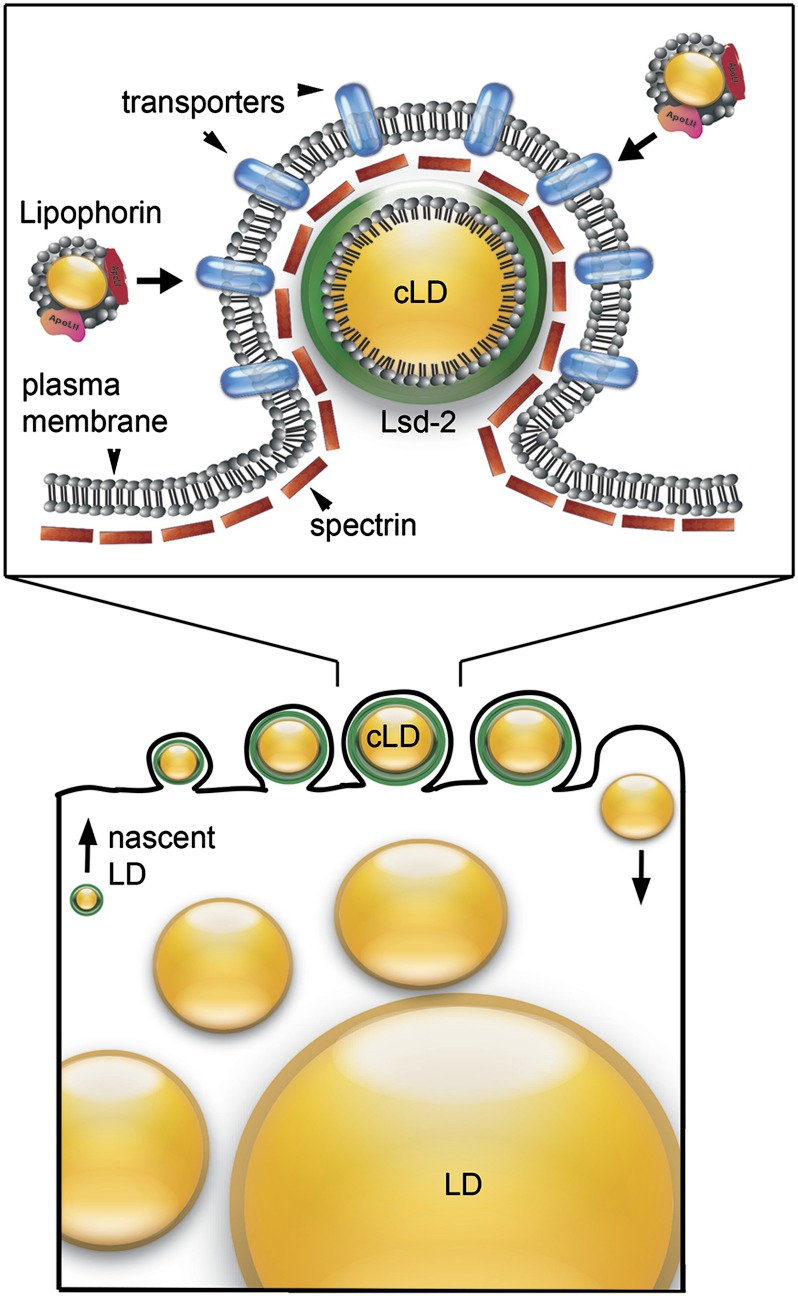
Figure 10.
Model for lipid uptake at the fat body surface. Cortical lipid droplets (cLD) in the larval fat body are intimately associated with the plasma membrane in surface protuberances (top). We propose that this structure represents a lipid uptake apparatus that carries lipid cargo from extracellular lipophorin to the hydrophobic core of the cytoplasmic lipid dropet. The plasma membrane is uniformly lined with αβ-spectrin (red rectangles) and the surface of the small cortical lipid droplets is covered with the perilipin Lsd-2 (green). Since formation of cLD is dependent on the presence of both αβ-spectrin and Lsd-2, we suggest that these proteins are part of a stable complex that links lipid transport proteins in the plasma membrane to the cLD. Nascent lipid droplets are thought to arise from the ER membrane (bottom). We speculate that Lsd-2 on these lipid droplets directly or indirectly triggers initial association with spectrin on the plasma membrane and ultimately zips the two surfaces together into a tightly associated complex. LD growth may proceed until the lipid droplet reaches a size that causes dissociation of Lsd-2, allowing the lipid droplet to fall away from the plasma membrane and fuse with larger lipid droplets in the cytoplasm.
Relatively little is known about the origin or growth of lipid droplets. They are believed to originate within the endoplasmic reticulum membrane from what has been described as a lens structure intermediate (Martin and Parton 2006; Walther and Farese 2012). We speculate that cortical lipid droplets first appear in the fat cell cytoplasm as a progenitor from the ER with perilipins on its surface. Lsd-2 itself, or perhaps another Lsd-2-dependent lipid droplet protein, may then initiate contact with the plasma membrane by binding directly or indirectly to α2β2-spectrin. Lsd-2 associates primarily with small (<4 μm) cortical lipid droplets. We suggest that the surface-associated lipid droplets grow in size through lipid uptake until they reach a threshold that triggers dissociation of Lsd-2 (Figure 10, bottom). Loss of Lsd-2 would be expected to cause dissociation of the lipid droplet from the plasma membrane, presumably making it available for fusion with larger lipid droplets deeper in the cytoplasm.
Implications for redundant function
It was previously suggested that spectrin function is redundant in nonneuronal cells of Drosophila (Mazock et al. 2010). Thus, one might suspect that there is a compensating protein that performs a similar function. However, based on current results, that does not appear to be the case in the Drosophila fat body. Loss of α- or β-spectrin produced a dramatic transformation of plasma membrane morphology. Given that loss of spectrin function in the fat body had no detectable effect on growth or viability of the organism, it appears to be the peculiar morphology of the fat body surface that is dispensable. Therefore spectrin appears to be a nonredundant component of a cellular system that is redundant.
Multiple mechanisms may contribute to the unloading of lipophorin and transfer of its lipid contents to lipid droplets. Loss of lipophorin function leads to an abnormal accumulation of lipid droplets in the midgut epithelium (Panakova et al. 2005; Palm et al. 2012; this study). Significantly, no such accumulation occurred with loss of spectrin function, indicating that lipophorin continues to transport dietary lipid in the absence of cortical lipid droplets in the fat body. Alternative mechanisms may include (1) endocytosis of lipophorin (Rodenburg and Van Der Horst 2005), (2) a pathway that feeds directly into large lipid droplets, or (3) delivery of dietary lipids to an alternate site [e.g., oenocytes were previously shown to be a lipophorin destination during starvation (Gutierrez et al. 2007)]. Further experiments will be needed to address this issue.
Distinguishing gain-of-function and loss-of-function effects of spectrin mutations
While the hypermorphic phenotype described here results from overexpression of a recombinant transgene, we speculate that some spectrin gene mutations could potentially produce comparable effects (that are distinct from loss of function). Our results are consistent with a mechanism in which functional sites in β-spectrin (e.g., actin or ankyrin binding) may exert toxic effects in the absence of appropriate dynamic regulation by α-spectrin. If so, then any mutation compromising intersubunit regulation could potentially lead to a hypermorphic phenotype.
Based largely on overlapping phenotypes between dominant SCA5 alleles and βIII-spectrin knockouts (Perkins et al. 2010) it has been suggested (but not proved) that the SCA5 alleles are dominant negatives. Muller (1932) described simple genetic tests to discriminate between dominant negative (antimorphic) mutations and hypermorphs by increasing or decreasing the wild-type gene dose. Accordingly, dominant negative SCA5 phenotypes would be expected to become less severe as wild-type gene dose is increased, but the phenotypes would become worse if mutations are hypermorphic.
Supplementary Material
Acknowledgments
We thank Suzanne Eaton for providing the anti-lipophorin antibody used in these studies. This work was supported in part by National Institutes of Health grant GM49301 (to R.R.D.).
Footnotes
Communicating editor: I. K. Hariharan
Literature Cited
- Abramoff M. D., Magelhaes P. J., Ram S. J., 2004. Image processing with ImageJ. Biophoton Int. 11: 36–42 [Google Scholar]
- Arrese E. L., Canavoso L. E., Jouni Z. E., Pennington J. E., Tsuchida K., et al. , 2001. Lipid storage and mobilization in insects: current status and future directions. Insect Biochem. Mol. Biol. 31: 7–17 [DOI] [PubMed] [Google Scholar]
- Asha H., Nagy I., Kovacs G., Stetson D., Ando I., et al. , 2003. Analysis of ras-induced overproliferation in Drosophila hemocytes. Genetics 163: 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayalon G., Davis J. Q., Scotland P. B., Bennett V., 2008. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135: 1189–1200 [DOI] [PubMed] [Google Scholar]
- Baines A. J., 2010. The spectrin-ankyrin-4.1-adducin membrane skeleton: adapting eukaryotic cells to the demands of animal life. Protoplasma 244: 99–131 [DOI] [PubMed] [Google Scholar]
- Bi J., Xiang Y., Chen H., Liu Z., Gronke S., et al. , 2012. Opposite and redundant roles of the two Drosophila perilipins in lipid mobilization. J. Cell Sci. 125: 3568–3577 [DOI] [PubMed] [Google Scholar]
- Byers T. J., Husain-Chishti A., Dubreuil R. R., Branton D., Goldstein L. S. B., 1989. Drosophila β spectrin: sequence similarity to the amino-terminal domain of α-actinin and dystrophin. J. Cell Biol. 109: 1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavoso L. E., Jouni Z. E., Karnas K. J., Pennington J. E., Wells M. A., 2001. Fat metabolism in insects. Annu. Rev. Nutr. 21: 23–46 [DOI] [PubMed] [Google Scholar]
- Clarkson Y. L., Gillespie T., Perkins E. M., Lyndon A. R., Jackson M., 2010. Beta-III spectrin mutation L253P associated with spinocerebellar ataxia type 5 interferes with binding to Arp1 and protein trafficking from the Golgi. Hum. Mol. Genet. 19: 3634–3641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantuma N. P., Pijnenburg M. A. P., Diederen J. H. B., Vanderhorst D. J., 1998. Multiple interactions between insect lipoproteins and fat body cells: extracellular trapping and endocytic trafficking. J. Lipid Res. 39: 1877–1888 [PubMed] [Google Scholar]
- Das A., Base C., Dhulipala S., Dubreuil R. R., 2006. Spectrin functions upstream of ankyrin in a spectrin cytoskeleton assembly pathway. J. Cell Biol. 175: 325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Base C., Manna D., Cho W., Dubreuil R. R., 2008. Unexpected complexity in the mechanisms that target assembly of the spectrin cytoskeleton. J. Biol. Chem. 283: 12643–12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H., Lee J. K., Goldstein L. S. B., Branton D., 1995. Drosophila development requires spectrin network formation. J. Cell Biol. 128: 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R. R., 2006. Functional links between membrane transport and the spectrin cytoskeleton. J. Membr. Biol. 211: 151–161 [DOI] [PubMed] [Google Scholar]
- Dubreuil R. R., Grushko T., 1998. Genetic studies of spectrin: new life for a ghost protein. BioEssays 20: 825–828 [DOI] [PubMed] [Google Scholar]
- Dubreuil R. R., Yu J., 1994. Ankyrin and beta spectrin accumulate independently of alpha spectrin in Drosophila. Proc. Natl. Acad. Sci. USA 91: 10285–10289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R. R., Byers T. J., Stewart C. T., Kiehart D. P., 1990. A β spectrin isoform from Drosophila (βH) is similar in size to vertebrate dystrophin. J. Cell Biol. 111: 1849–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R. R., Macvicar G. R., Dissanayake S., Liu C., Homer D., et al. , 1996. Neuroglian-mediated adhesion induces assembly of the membrane skeleton at cell contact sites. J. Cell Biol. 133: 647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R. R., Maddux P. B., Grushko T., Macvicar G. R., 1997. Segregation of two spectrin isoforms: polarized membrane binding sites direct polarized membrane skeleton assembly. Mol. Biol. Cell 8: 1933–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil R. R., Wang P., Dahl S. C., Lee J. K., Goldstein L. S. B., 2000. Drosophila β spectrin functions independently of α spectrin to polarized the Na,K ATPase in epithelial cells. J. Cell Biol. 149: 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster C., Panakova D., Mahmoud A., Eaton S., 2007. Lipoprotein-heparan sulfate interactions in the Hh pathway. Dev. Cell 13: 57–71 [DOI] [PubMed] [Google Scholar]
- Gronke S., Beller M., Fellert S., Ramakrishnan H., Jackle H., et al. , 2003. Control of fat storage by a Drosophila PAT domain protein. Curr. Biol. 13: 603–606 [DOI] [PubMed] [Google Scholar]
- Gutierrez E., Wiggins D., Fielding B., Gould A. P., 2007. Specialized hepatocyte-like cells regulate Drosophila lipid metabolism. Nature 445: 275–280 [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Dick K. A., Westherspoon M. R., Gincel D., Armbrust K. R., et al. , 2006. Spectrin mutations cause spinocerebellar ataxia type 5. Nat. Genet. 38: 184–190 [DOI] [PubMed] [Google Scholar]
- Kizhatil K., Davis J. Q., Davis L., Hoffman J., Hogan B. L. M., et al. , 2007. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J. Biol. Chem. 282: 26552–26561 [DOI] [PubMed] [Google Scholar]
- Kizhatil K., Baker S. A., Arshavsky V. Y., Bennett V., 2009. Ankyrin-G promotes cyclic nucleotide-gated channel transport to rod photoreceptor sensory cilia. Science 323: 1614–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch I., Schwarz H., Beuchle D., Goellner B., Langegger M., et al. , 2008. Drosophila ankyrin 2 is required for synaptic stability. Neuron 58: 210–222 [DOI] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M., 1988. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell 53: 219–228 [DOI] [PubMed] [Google Scholar]
- Korsgren C., Lux S. E., 2010. The carboxyterminal EF domain of erythroid alpha-spectrin is necessary for optimal spectrin-actin binding. Blood 116: 2600–2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Coyne R., Dubreuil R. R., Goldstein L. S. B., Branton D., 1993. Cell shape and interaction defects in alpha-spectrin mutants of Drosophila melanogaster. J. Cell Biol. 123: 1797–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., Luo L., 1999. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22: 451–461 [DOI] [PubMed] [Google Scholar]
- Lorenzo D. N., Li M.-G., Mische S. E., Armbrust K. R., Ranum L. P. W., et al. , 2010. Spectrin mutations that cause spinocerebellar ataxia type 5 impair axonal transport and induce neurodegeneration in Drosophila. J. Cell Biol. 189: 143–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E., Palek J., 1995. Disorders of the red cell membrane, pp. 1701–1818 in Blood: Principles and Practice of Hematology, edited by Handin R. I., Lux S. E., Stossel T. P. J. B. Lippincott, Philadelphia [Google Scholar]
- Martin S., Parton R. G., 2006. Lipid droplets: a unified view of a dynamic organelle. Nat. Rev. Mol. Cell Biol. 7: 373–378 [DOI] [PubMed] [Google Scholar]
- Mazock G. H., Das A., Base C., Dubreuil R. R., 2010. Transgene rescue identifies an essential function for Drosophila β spectrin in the nervous system and a selective requirement for ankyrin-2 binding activity. Mol. Biol. Cell 21: 2860–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, H. J., 1932 Further studies on the nature and causes of gene mutations. Int. Congr. Genet., 213–255. [Google Scholar]
- Palm W., Sampaio J. L., Brankatschk M., Carvalho M., Mahmoud A., et al. , 2012. Lipoproteins in Drosophila melanogaster-assembly, function, and influence on tissue lipid composition. PLoS Genet. 8: e1002828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panakova D., Sprong H., Marois E., Thiele C., Eaton S., 2005. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435: 58–65 [DOI] [PubMed] [Google Scholar]
- Perkins E. M., Clarkson Y. L., Sabaatier N., Longhurst D. M., Millward C. P., et al. , 2010. Loss of β-III spectrin leads to purkinje cell dysfunction recapitulating the behavior and neurophathology of spinocerebellar ataxia type 5 in humans. J. Neurosci. 30: 4857–4867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J., Fetter R. D., Davis G. W., 2005. Presynaptic spectrin is essential for synapse stabilization. Curr. Biol. 15: 918–928 [DOI] [PubMed] [Google Scholar]
- Pielage J., Cheng L., Fetter R., Carlton P. M., Sedat J. W., et al. , 2008. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron 58: 195–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg K. W., Van Der Horst D. J., 2005. Lipoprotein-mediated lipid transport in insects: analogy to the mammalian lipid carrier system and novel concepts for the functioning of LDL receptor family members. Biochim. Biophys. Acta 1736: 10–29 [DOI] [PubMed] [Google Scholar]
- Stabach P. R., Devarajan P., Stankewich M. C., Bannykh S., Morrow J. S., 2008. Ankyrin facilitates intracellular trafficking of apha1-Na-ATPase in polarized cells. Am. J. Physiol. 295: 1202–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjota M., Le S.-K., Wu J., Williams J. A., Khanna M. R., et al. , 2011. Annexin B9 binds to βH-spectrin and is required for multivesicular body function in Drosophila. J. Cell Sci. 124: 2914–2926 [DOI] [PubMed] [Google Scholar]
- Van Der Horst D. J., Rodenburg K. W., 2010. Lipoprotein assembly and function in an evolutionary perspective. BioMol. Concepts 1: 165–183 [DOI] [PubMed] [Google Scholar]
- Van Der Horst D. J., Roosendaal S. D., Rodenburg K. W., 2009. Circulatory lipid transport: lipoprotein assembly and function from an evolutionary perspective. Mol. Cell. Biochem. 326: 105–119 [DOI] [PubMed] [Google Scholar]
- Walther T. C., Farese R. V., Jr, 2012. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 81: 687–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.