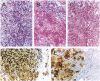
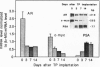
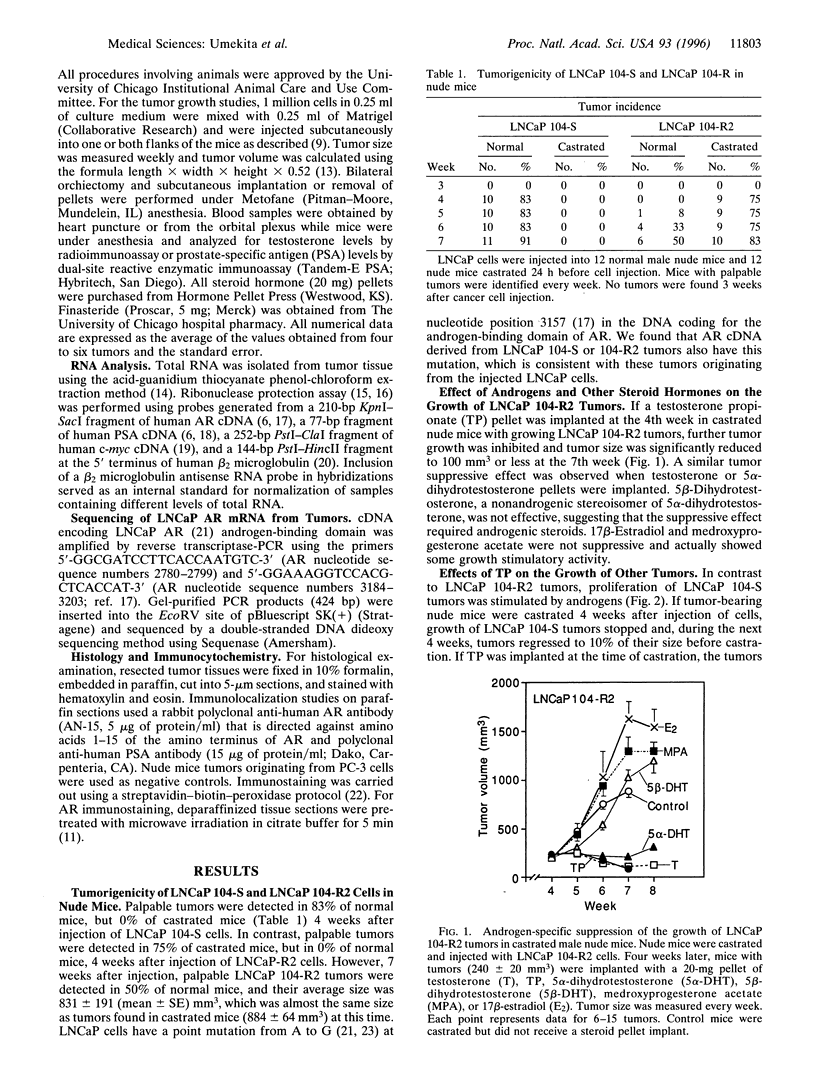
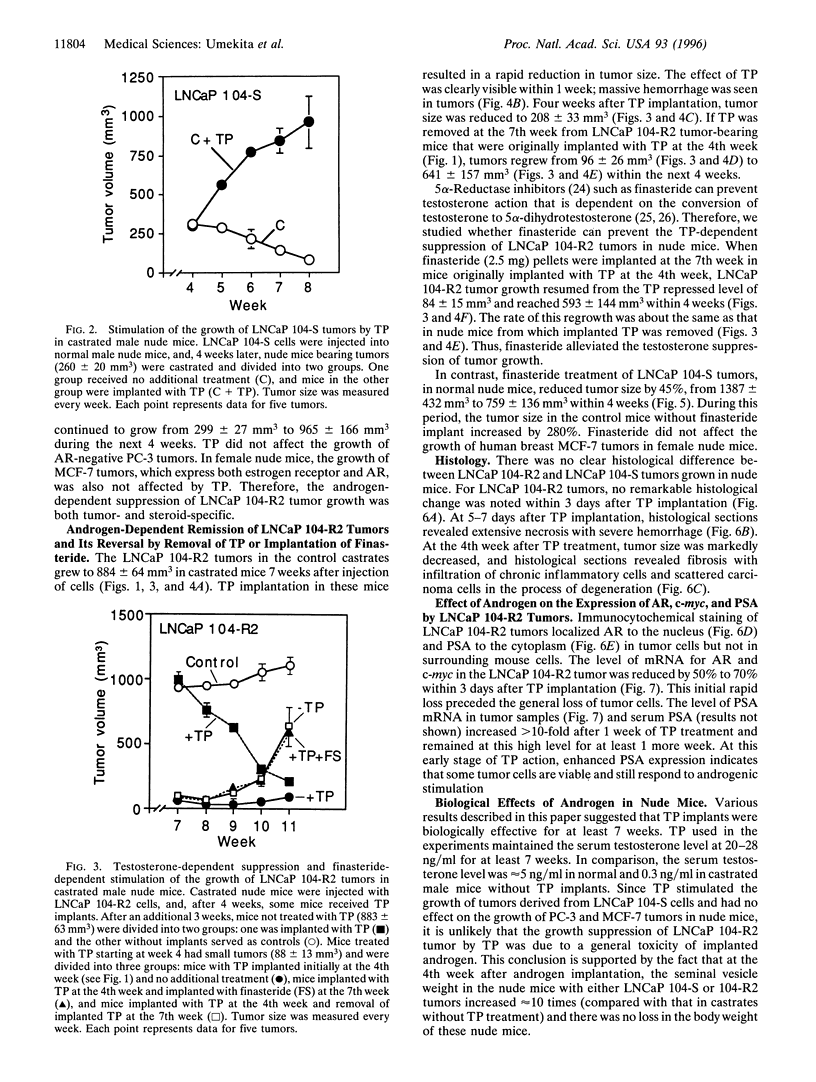
Abstract
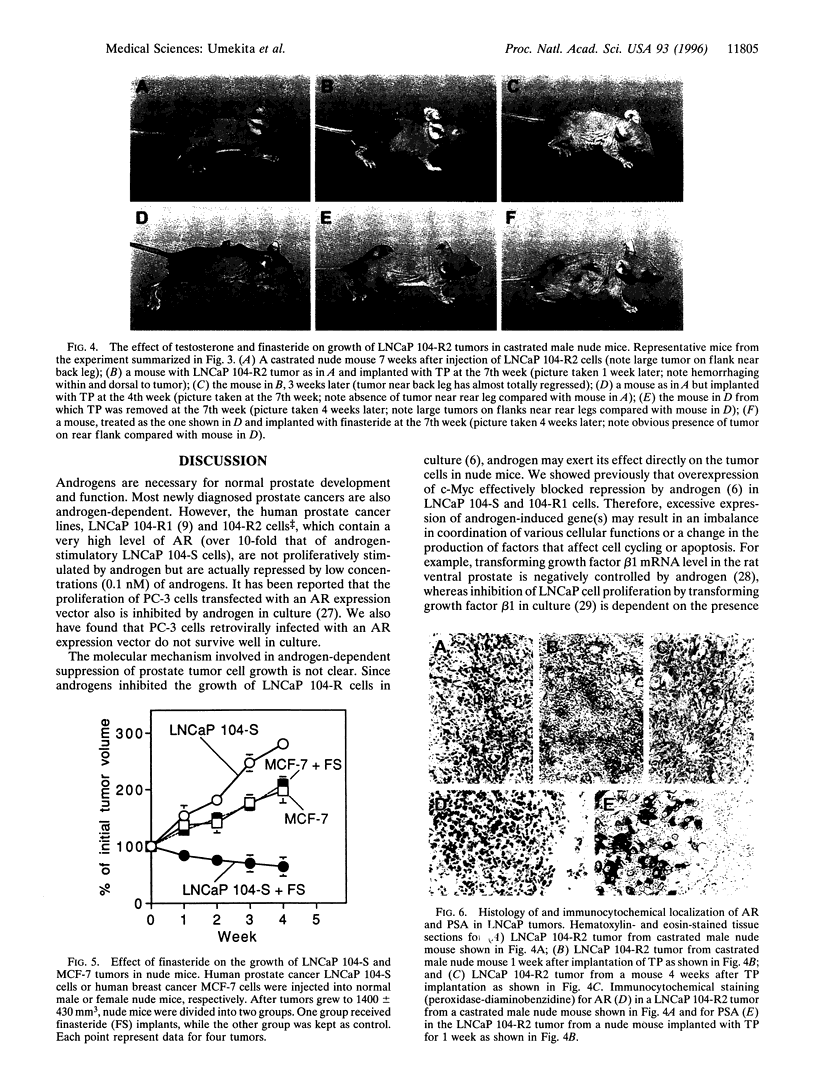
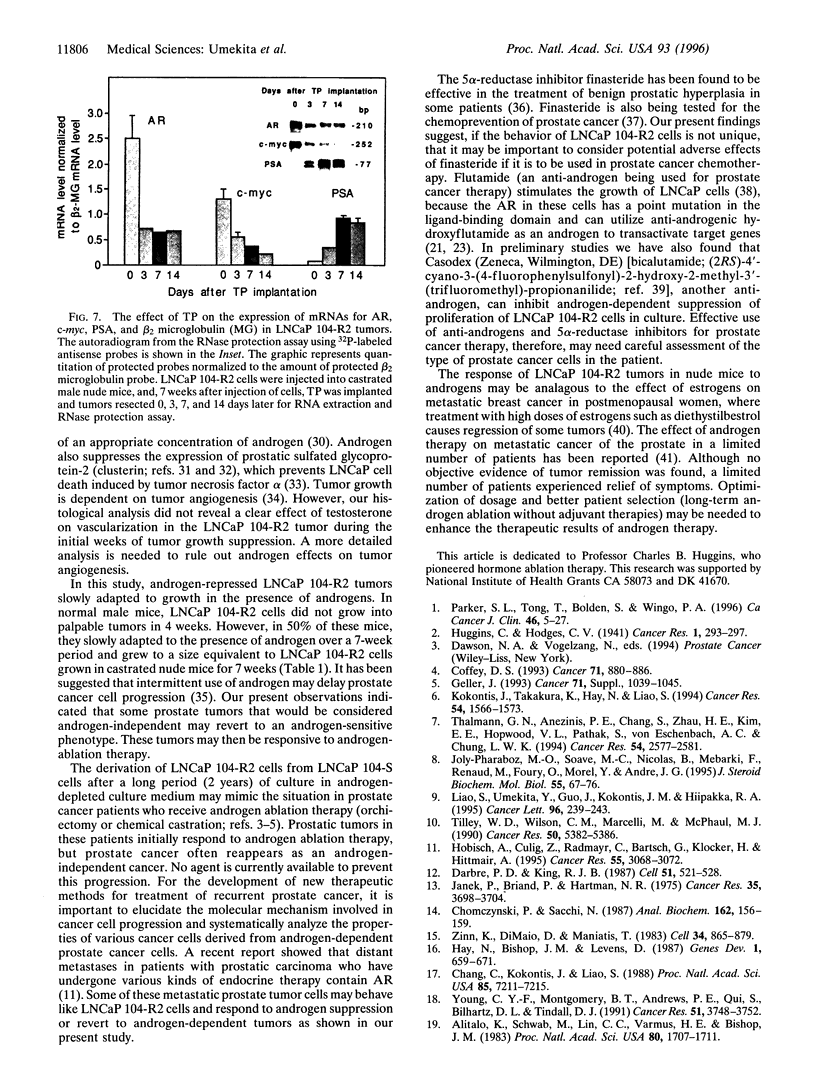
When the human prostate cancer cell line, LNCaP 104-S, the growth of which is stimulated by physiological levels of androgen, is cultured in androgen-depleted medium for > 100 passages, the cells, now called LNCaP 104-R2, are proliferatively repressed by low concentrations of androgens. LNCaP 104-R2 cells formed tumors in castrated male athymic nude mice. Testosterone propionate (TP) treatment prevented LNCaP 104-R2 tumor growth and caused regression of established tumors in these mice. Such a tumor-suppressive effect was not observed with tumors derived from LNCaP 104-S cells or androgen receptor-negative human prostate cancer PC-3 cells. 5 alpha-Dihydrotestosterone, but not 5 beta-dihydrotestosterone, 17 beta-estradiol, or medroxyprogesterone acetate, also inhibited LNCaP 104-R2 tumor growth. Removal of TP or implantation of finasteride, a 5 alpha-reductase inhibitor, in nude mice bearing TP implants resulted in the regrowth of LNCaP 104-R2 tumors. Within 1 week after TP implantation, LNCaP 104-R2 tumors exhibited massive necrosis with severe hemorrhage. Three weeks later, these tumors showed fibrosis with infiltration of chronic inflammatory cells and scattered carcinoma cells exhibiting degeneration. TP treatment of mice with LNCaP 104-R2 tumors reduced tumor androgen receptor and c-myc mRNA levels but increased prostate-specific antigen in serum- and prostate-specific antigen mRNA in tumors. Although androgen ablation has been the standard treatment for metastatic prostate cancer for > 50 years, our study shows that androgen supplementation therapy may be beneficial for treatment of certain types of human prostate cancer and that the use of 5 alpha-reductase inhibitors, such as finasteride or anti-androgens, in the general treatment of metastatic prostate cancer may require careful assessment.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. M., Liao S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature. 1968 Jul 20;219(5151):277–279. doi: 10.1038/219277a0. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S., Hiipakka R. A., Gilna P., Liao S. T. Identification of an androgen-repressed mRNA in rat ventral prostate as coding for sulphated glycoprotein 2 by cDNA cloning and sequence analysis. Biochem J. 1989 Jan 1;257(1):293–296. doi: 10.1042/bj2570293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchovsky N., Wilson J. D. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968 Apr 25;243(8):2012–2021. [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coffey D. S. Prostate cancer. An overview of an increasing dilemma. Cancer. 1993 Feb 1;71(3 Suppl):880–886. doi: 10.1002/1097-0142(19930201)71:3+<880::aid-cncr2820711403>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Darbre P. D., King R. J. Progression to steroid insensitivity can occur irrespective of the presence of functional steroid receptors. Cell. 1987 Nov 20;51(4):521–528. doi: 10.1016/0092-8674(87)90121-8. [DOI] [PubMed] [Google Scholar]
- Fowler J. E., Jr, Whitmore W. F., Jr The response of metastatic adenocarcinoma of the prostate to exogenous testosterone. J Urol. 1981 Sep;126(3):372–375. doi: 10.1016/s0022-5347(17)54531-0. [DOI] [PubMed] [Google Scholar]
- Furr B. J. Casodex: preclinical studies and controversies. Ann N Y Acad Sci. 1995 Jun 12;761:79–96. doi: 10.1111/j.1749-6632.1995.tb31371.x. [DOI] [PubMed] [Google Scholar]
- Geller J. Basis for hormonal management of advanced prostate cancer. Cancer. 1993 Feb 1;71(3 Suppl):1039–1045. doi: 10.1002/1097-0142(19930201)71:3+<1039::aid-cncr2820711423>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Goldenberg S. L., Bruchovsky N., Gleave M. E., Sullivan L. D., Akakura K. Intermittent androgen suppression in the treatment of prostate cancer: a preliminary report. Urology. 1995 May;45(5):839–845. doi: 10.1016/s0090-4295(99)80092-2. [DOI] [PubMed] [Google Scholar]
- Gormley G. J., Brawley O., Thompson I. The potential application of finasteride for chemoprevention of prostate cancer. Ann N Y Acad Sci. 1995 Sep 30;768:163–169. doi: 10.1111/j.1749-6632.1995.tb12119.x. [DOI] [PubMed] [Google Scholar]
- Hay N., Bishop J. M., Levens D. Regulatory elements that modulate expression of human c-myc. Genes Dev. 1987 Sep;1(7):659–671. doi: 10.1101/gad.1.7.659. [DOI] [PubMed] [Google Scholar]
- Hobisch A., Culig Z., Radmayr C., Bartsch G., Klocker H., Hittmair A. Distant metastases from prostatic carcinoma express androgen receptor protein. Cancer Res. 1995 Jul 15;55(14):3068–3072. [PubMed] [Google Scholar]
- Janik P., Briand P., Hartmann N. R. The effect of estrone-progesterone treatment on cell proliferation kinetics of hormone-dependent GR mouse mammary tumors. Cancer Res. 1975 Dec;35(12):3698–3704. [PubMed] [Google Scholar]
- Joly-Pharaboz M. O., Soave M. C., Nicolas B., Mebarki F., Renaud M., Foury O., Morel Y., Andre J. G. Androgens inhibit the proliferation of a variant of the human prostate cancer cell line LNCaP. J Steroid Biochem Mol Biol. 1995 Oct;55(1):67–76. doi: 10.1016/0960-0760(95)00155-s. [DOI] [PubMed] [Google Scholar]
- Kim I. Y., Kim J. H., Zelner D. J., Ahn H. J., Sensibar J. A., Lee C. Transforming growth factor-beta1 is a mediator of androgen-regulated growth arrest in an androgen-responsive prostatic cancer cell line, LNCaP. Endocrinology. 1996 Mar;137(3):991–999. doi: 10.1210/endo.137.3.8603613. [DOI] [PubMed] [Google Scholar]
- Kokontis J., Ito K., Hiipakka R. A., Liao S. Expression and function of normal and LNCaP androgen receptors in androgen-insensitive human prostatic cancer cells. Altered hormone and antihormone specificity in gene transactivation. Receptor. 1991;1(4):271–279. [PubMed] [Google Scholar]
- Kokontis J., Takakura K., Hay N., Liao S. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 1994 Mar 15;54(6):1566–1573. [PubMed] [Google Scholar]
- Kyprianou N., Isaacs J. T. Expression of transforming growth factor-beta in the rat ventral prostate during castration-induced programmed cell death. Mol Endocrinol. 1989 Oct;3(10):1515–1522. doi: 10.1210/mend-3-10-1515. [DOI] [PubMed] [Google Scholar]
- Liang T., Hoyer S., Yu R., Soltani K., Lorincz A. L., Hiipakka R. A., Liao S. Immunocytochemical localization of androgen receptors in human skin using monoclonal antibodies against the androgen receptor. J Invest Dermatol. 1993 May;100(5):663–666. doi: 10.1111/1523-1747.ep12472330. [DOI] [PubMed] [Google Scholar]
- Liao S., Umekita Y., Guo J., Kokontis J. M., Hiipakka R. A. Growth inhibition and regression of human prostate and breast tumors in athymic mice by tea epigallocatechin gallate. Cancer Lett. 1995 Sep 25;96(2):239–243. doi: 10.1016/0304-3835(95)03948-v. [DOI] [PubMed] [Google Scholar]
- Montpetit M. L., Lawless K. R., Tenniswood M. Androgen-repressed messages in the rat ventral prostate. Prostate. 1986;8(1):25–36. doi: 10.1002/pros.2990080105. [DOI] [PubMed] [Google Scholar]
- Parker S. L., Tong T., Bolden S., Wingo P. A. Cancer statistics, 1996. CA Cancer J Clin. 1996 Jan-Feb;46(1):5–27. doi: 10.3322/canjclin.46.1.5. [DOI] [PubMed] [Google Scholar]
- Russell D. W., Wilson J. D. Steroid 5 alpha-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- Sensibar J. A., Sutkowski D. M., Raffo A., Buttyan R., Griswold M. D., Sylvester S. R., Kozlowski J. M., Lee C. Prevention of cell death induced by tumor necrosis factor alpha in LNCaP cells by overexpression of sulfated glycoprotein-2 (clusterin). Cancer Res. 1995 Jun 1;55(11):2431–2437. [PubMed] [Google Scholar]
- Stoll B. A. Hypothesis: breast cancer regression under oestrogen therapy. Br Med J. 1973 Aug 25;3(5877):446–450. doi: 10.1136/bmj.3.5877.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoner E. Maintenance of clinical efficacy with finasteride therapy for 24 months in patients with benign prostatic hyperplasia. The Finasteride Study Group. Arch Intern Med. 1994 Jan 10;154(1):83–88. [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann G. N., Anezinis P. E., Chang S. M., Zhau H. E., Kim E. E., Hopwood V. L., Pathak S., von Eschenbach A. C., Chung L. W. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994 May 15;54(10):2577–2581. [PubMed] [Google Scholar]
- Tilley W. D., Wilson C. M., Marcelli M., McPhaul M. J. Androgen receptor gene expression in human prostate carcinoma cell lines. Cancer Res. 1990 Sep 1;50(17):5382–5386. [PubMed] [Google Scholar]
- Veldscholte J., Ris-Stalpers C., Kuiper G. G., Jenster G., Berrevoets C., Claassen E., van Rooij H. C., Trapman J., Brinkmann A. O., Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990 Dec 14;173(2):534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- Weidner N., Carroll P. R., Flax J., Blumenfeld W., Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993 Aug;143(2):401–409. [PMC free article] [PubMed] [Google Scholar]
- Wilding G., Chen M., Gelmann E. P. Aberrant response in vitro of hormone-responsive prostate cancer cells to antiandrogens. Prostate. 1989;14(2):103–115. doi: 10.1002/pros.2990140204. [DOI] [PubMed] [Google Scholar]
- Wilding G. Response of prostate cancer cells to peptide growth factors: transforming growth factor-beta. Cancer Surv. 1991;11:147–163. [PubMed] [Google Scholar]
- Young C. Y., Montgomery B. T., Andrews P. E., Qui S. D., Bilhartz D. L., Tindall D. J. Hormonal regulation of prostate-specific antigen messenger RNA in human prostatic adenocarcinoma cell line LNCaP. Cancer Res. 1991 Jul 15;51(14):3748–3752. [PubMed] [Google Scholar]
- Yuan S., Trachtenberg J., Mills G. B., Brown T. J., Xu F., Keating A. Androgen-induced inhibition of cell proliferation in an androgen-insensitive prostate cancer cell line (PC-3) transfected with a human androgen receptor complementary DNA. Cancer Res. 1993 Mar 15;53(6):1304–1311. [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]