Abstract
Tendons are connective tissues required for motion and are frequently injured. Poor healing and inadequate return to normal tissue structure and mechanical function make tendon a prime candidate for tissue engineering, however functional tendons have yet to be engineered. The physical environment, from substrate stiffness to dynamic mechanical loading, may regulate tenogenic stem cell differentiation. Tissue stiffness and loading parameters derived from embryonic development may enhance tenogenic stem cell differentiation and tendon tissue formation. We highlight current understanding of the mechanical environment experienced by embryonic tendons and how progenitor cells may sense and respond to physical inputs. We further discuss how mechanical factors have only recently been used to induce tenogenic fate in stem cells.
Introduction
Tendons serve a critical mechanical function by transferring muscle-generated forces to bone. Unfortunately, injuries lead to disorganized tissue structure and abnormal mechanical properties, despite surgical intervention. Alternatively, tissue engineering promises the replacement of injured tendon with new, normal tissue. Efforts have focused primarily on dynamic mechanical cues to induce and guide tenogenesis, but progress has been limited, presumably due to insufficient knowledge of tenogenic mechanical factors and their roles during normal tendon development. Notably, tissue elastic modulus and dynamic mechanical forces have been shown to regulate stem cell differentiation toward other lineages [1–4]. With the goal of enhancing strategies to mechanoregulate tendon regeneration, we are interested in how tendon cell fate decisions may be influenced by embryonic mechanical factors. This review examines current understanding of the mechanical microenvironment of tendon during embryonic development. Specifically, we focus on two primary mechanical factors, tissue modulus and dynamic loading, and efforts to identify these cues and their potential influences. Furthermore, we discuss mechanically driven mechanisms that may guide tenogenic fate decisions. Finally, we review recent studies that exploit mechanical loading to direct stem cell tenogenesis (differentiation toward the tendon lineage). Characterizing the mechanical cues involved in embryonic tendon development may provide parameters for scaffold design and bioreactor culture to mechanoactively guide tenogenic stem cell differentiation and tissue formation, thereby enhancing tendon tissue engineering strategies.
Embryonic tendon elastic modulus
Substrate stiffness has been shown to regulate stem cell differentiation toward the adipogenic, myogenic, neurogenic and osteogenic lineages [1–3,5], though this has been only minimally investigated for the tenogenic lineage [5]. It is not yet known how tendon progenitor cells might sense and respond to the mechanical properties of developing tendon tissue during embryonic development because until recently, data on mechanical properties of embryonic tendon have been limited and inconsistent. For instance, reported values for tensile elastic modulus of late-stage embryonic chick tendon have varied by nearly 100-fold [6,7] (Table 1), perhaps due to difficulties with mechanically testing small and delicate embryonic tissue. Bulk tensile properties of embryonic tendon are important for understanding tissue function, but represent properties at size scales and magnitudes significantly greater than cells. In contrast, cell length-scale mechanical properties may be more relevant for mechanoregulation of cell differentiation and function. Recently, we characterized nanoscale and microscale elastic moduli in developing embryonic chick tendon using atomic force microscopy [8], finding elastic modulus to be up to 49-fold lower than previous bulk level embryonic tendon measurements and up to 40,000-fold lower than that of adult tissue [9] (Table 1). While there is still much to understand about how cell length-scale mechanical properties influence tenogenesis, these studies provide a framework from which to begin to investigate such mechanisms.
Table 1.
Elastic modulus values for embryonic and adult chicken tendon.
| Developmental stage (HH) |
Approximate embryonic days |
Measurement method |
Average elastic modulus range |
Reference |
|---|---|---|---|---|
| HH 40–43 | Day 14–18 | Bulk tensile test | 0.21–1.02 MPa | McBride et al., 1988 [6] |
| HH 39 | Day 13 | Bulk tensile test | 11 MPa | Kalson et al., 2010 [10] |
| HH 40 | Day 14 | Bulk tensile test | 20.5 MPa | Kalson et al., 2011 [7] |
| HH 28– 43 | Day 5.5–18 | Nanoscale tip indentation | 7–21 kPa | Marturano et al., 2013 [8] |
| HH 28–43 | Day 5.5–18 | Microscale tip indentation | 5–108 kPa | Marturano et al., 2013 [8] |
| Adult | Adult 8 mo. | Bulk tensile test | 210 MPa | Nakagaki et al., 2007 [9] |
Mechanical stimulation of tendon during embryonic development
In addition to mechanical cues from tissue stiffness, embryonic tendon cells (ETCs) likely experience mechanical loading via muscle contractions during development. Since muscular contractions begin relatively early in development, embryonic tendons may experience dynamic loading during important stages of differentiation and tissue formation. Embryonic motility begins early, after neuromuscular connections form [11] at developmental day 4 in chick embryos [12], embryonic day (E) 12.5–14 in mouse [11,13], and 7 weeks in human embryos [14]. Chick embryos are active 20% of the time at developmental day 6 and nearly 80% starting from day 11, based on at least one movement every 10 seconds [12]. In ovo electromyography (EMG) recordings of chick embryonic gastrocnemius muscle showed motor unit activation every 2 seconds at developmental day 7 [15] and every 0.2 seconds by day 19 [16]. EMG activity is not equivalent to force or strain, but suggests that tendons experience muscle-derived forces during development at rates of 0.5–5 Hz. Multiple muscular loads may produce complex loading regimes [17], but how patterns of muscular activity translate to mechanical forces experienced by developing tendon cells requires further investigation.
Effects of muscle activity on tendon development
Reduced or altered skeletal muscle contraction during embryonic development produces significant skeletal abnormalities [18–25]. Chick embryo paralysis by D-tubocurarine from developmental days 10–18 inhibited formation of the tendon synovial sheath and fibrocartilaginous regions of embryonic digital flexor tendons [25]. Other studies using neuromuscular blocker decamethonium bromide to induce paralysis during embryonic chick development demonstrated tendon degeneration and a reduction in tendon size [22,23], and decreases in tenascin-C gene and protein expression [24]. In adult mice, botulinum toxin A-induced muscle paralysis decreased the number of scleraxis-positive (basic helix-loop-helix transcription factor specific for tendon [26]) tendon cells by nearly 80%, along with decreases in collagen fibril density and stiffness [27]. In the same study, isolated primary tendon cells cultured under static conditions reduced scleraxis expression over time, whereas mechanical stimulation via fluid shear stress rescued scleraxis expression, which may have been mediated by the transforming growth factor (TGF-β) type I receptor and Smad 2/3 [27]. Chick ETCs express the TGF-β type I receptor [28], however the role of this receptor in mechanoregulation of embryonic tendon is unknown. Taken together, muscle contractions seem important for normal embryonic tendon development and homeostasis, however a potential non-mechanical confounding factor may be altered biochemical signaling from muscle tissues with paralysis. Other approaches to study effects of muscle loading in vivo have assessed embryonic tendon development in muscleless limbs. In the absence of muscle, initial tenogenic induction and progenitor cell distribution are unaffected in chick, but further tendon development is unable to proceed in the absence of muscle [29,30]. It is unknown if this dependency on muscle is a function of muscle-derived mechanical stimulation, secreted soluble (e.g., growth) factors, or both. It may be a combinatorial effect, as scleraxis expression is rescued with application of fibroblast growth factor (FGF)-4 in the absence of muscle [31]. Future studies are needed to delineate individual muscle-derived mechanical and biochemical effects on tendon development.
Quasi-static tension and compressive loading
Developing embryonic tendons may also experience quasi-static tension associated with limb lengthening. For example, the embryonic chick toe increases in length from HH 36–43 at a rate of 1.9 mm/day [32]. Recently, slow mechanical stretch was applied to chick ETCs seeded in fibrin gels in vitro, leading to increased collagen type I gene expression, collagen fibril size, volume fraction, ultimate tensile stress, elastic modulus and cell nuclei length [7].
Typically associated with tension, tendons also experience compressive and shear strains when wrapping around joints and contain fibrocartilaginous tissue in these regions [33,34]. Compressive loading of adult bovine flexor tendons upregulated synthesis of large proteoglycans in vitro [35]. Similarly, cyclically compressed embryonic bovine tendons upregulated aggrecan and biglycan gene expression in vitro [36]. TGF-β1 treatment enhanced expression of proteoglycans and TGF-β1, indicating positive feedback between loading and TGF-β1 [36]. These studies suggest specific mechanical stimuli can direct tendon progenitor cells toward distinct phenotypes within tendon tissue.
Mechanosensing mechanisms in tendon cells
Cells have force sensors to receive and transmit mechanical stimuli as biological signals. Though minimally characterized in ETCs, studies with adult tendon cells provide insight into potential mechanisms of mechanotransduction. Mechanosensing and mechanotransducing mechanisms in ETCs may occur through direct cell-to-cell connections (e.g., gap junctions and cadherins) or cell-to-extracellular matrix (ECM) adhesion molecules (e.g., integrins) (Figure 1). Downstream, cell cytoskeletal components may also respond to the mechanical environment.
Figure 1. Mechanotransductive components of embryonic tendon cells.
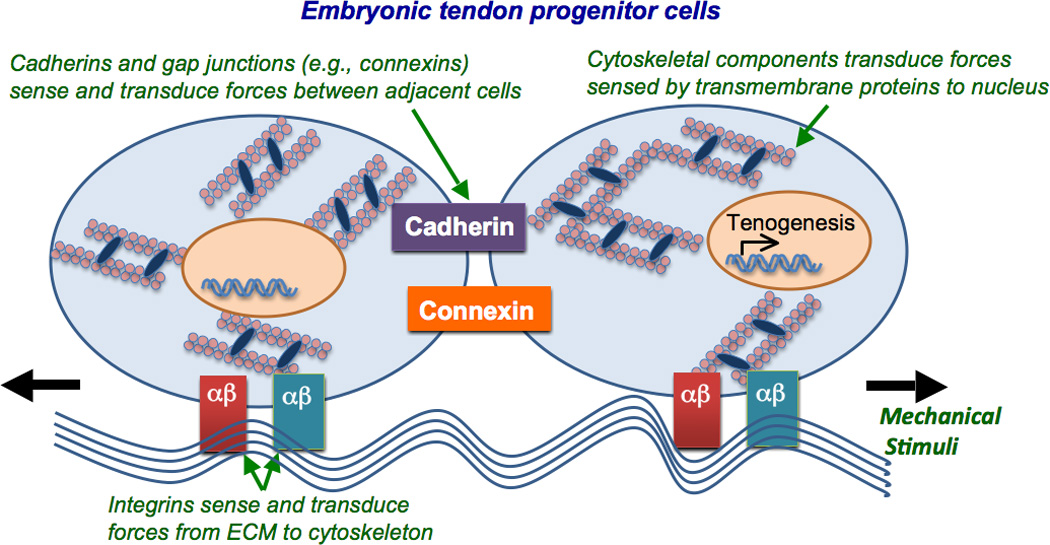
ETCs may sense and transduce mechanical signals between cells via direct cell-to-cell contacts such as cadherin (purple) and connexin (orange), and from the surrounding ECM via integrins (red and green). Downstream, cytoskeletal components that link to these transmembrane proteins may transduce forces to the nucleus to regulate gene and protein expression.
Adult tendon cells in vivo maintain a network of gap junction proteins, connexins (Cx) 32 and 43 [37,38]. Gap junctions connect the cytoplasm of adjacent cells, allowing direct cellular communication and the transport of small molecules. Cx 32 and 43 are also present in cells throughout the limb bud and tendon during embryonic development [38–40]. Cx 32 appears to link tendon cells longitudinally, while Cx 43 links all adjacent cells, laterally and longitudinally [37]. Blocking gap junctions with octanol treatment in chicken tendon cells inhibited stimulation of DNA and collagen synthesis by cyclic loading, suggesting gap junctions play a role in mechanotransduction [41]. A more recent study demonstrated Cx 32 and 43 in chicken tendon cells respond differentially to mechanical stimuli. Antisense downregulation of Cx 32 reduced the stimulatory effect of mechanical loading on collagen synthesis, while antisense downregulation of Cx 43 enhanced collagen synthesis [42], suggesting they work in opposition. Cx 32 only links tendon cells along the tendon long axis [37], therefore the authors suggested that load in this direction may stimulate collagen synthesis, though Cx 43 co-activation may mitigate collagen production in response to mechanical signals [42]. Taken together, gap junctions and the molecules they transport may play mechanoregulatory roles in tendon development to produce a coordinated and directed cellular response to mechanical stimuli.
Cadherins are cell-to-cell adhesion proteins, which may also function as force sensors and mechanotransducers [43]. Cadherin-11, in particular, is highly expressed in embryonic tendon [44]. Downregulation of cadherin-11 with siRNA in chick embryonic tendon at developmental day 13 resulted in a loss of contact between adjacent ETCs and disrupted collagen fibril organization [44]. These results demonstrated that cadherin-11 maintains cell-to-cell contact and plays a role in collagen fibril organization during embryonic tendon development.
In addition to cell-to-cell connections, ETCs express ECM-specific integrins, which may play a role in mechanosensing and subsequent mechanotransduction pathways [45]. During early stages (developmental day 4) of embryonic chick development, integrin α5β1 was found throughout the limb mesenchyme and at later stages localized in the developing connective tissues [46]. Mesenchymal cells express integrin α11β1 during embryonic development [47,48], which, interestingly, was seen to be expressed in a similar pattern as scleraxis [48], suggesting a role in tenogenesis. As integrins have been shown to act as mechanosensors in other cell types and tissue systems, future studies should focus on whether and how tendon progenitor cells interrogate their physical environment via these transmembrane proteins.
The actin cytoskeleton provides structural integrity to tendon cells [49] and may participate in mechanotransductive signaling pathways [50]. In vivo, actin fibers in adult tendon cells follow collagen crimp patterns along the longitudinal axis (stretch direction) of the tendon [51]. When chicken tendon cells were mechanically strained in culture, tropomyosin protein content increased, suggesting enhanced actin fiber assembly [51]. Recent work with isolated primary chick ETCs demonstrated these cells contain actin fiber motors, nonmuscle myosin II (NMMII) heavy chain proteins, IIA and IIB [10]. Prior studies have shown that NMMII regulates cellular tension and mechanotransduction, affecting stem cell fate decisions [52,53]. Interestingly, transcripts for nonmuscle myosin heavy chain proteins IIA and IIB were higher in ETCs on tissue culture plastic than in those in soft 3D fibrin gels [10]. However, collagen type I gene expression was elevated in soft fibrin gels, compared to hard tissue culture plastic. These changes in gene expression may reflect a dependence on substrate stiffness, but there were confounding factors such as 2D vs 3D culture or altered integrin binding due to substrate material. In the same study, both NMMII and actin inhibition abolished expected increases in fibrin gel elastic modulus and ultimate tensile stress in vitro [10], suggesting that actin and NMMII interactions in ETCs are required for the development of engineered tissue mechanical properties. More work is needed to identify specific mechanotranductive signaling pathways that regulate tenogenesis during development.
Mechanoregulation of stem cell tenogenesis
In vitro studies have examined how dynamic tensile loading influences tenogenic gene expression in stem cells (Figure 2). Cyclic strain applied to C3H10T1/2 murine mesenchymal stem cells in collagen gels upregulated scleraxis expression levels over static conditions [54]. In a number of studies, cyclically strained human mesenchymal stem cells (MSCs) maintained or upregulated tenogenic genes (scleraxis, collagen types I and III, tenascin-C) and increased matrix production [55–57]. Dynamic strain also increased focal adhesion kinase (FAK) phosphorylation in MSCs [55,56]. When FAK phosphorylation was inhibited, expression levels of collagen types I and III, tenascin-C, and scleraxis were reduced [55,56]. Similarly, actin disassembly and RhoA/ROCK signaling inhibition abolished the tenogenic response to dynamic strain [56]. Taken together, stretch-induced tenogenic gene expression in stem cells may be mediated by FAK, the actin cytoskeleton and RhoA/Rock signaling pathways. Further work is needed to characterize these and other potential mechanisms of mechanotransduction in tenogenically differentiating cells.
Figure 2. Mechanical factors may influence tenogenic differentiation of mesenchymal stem cells.
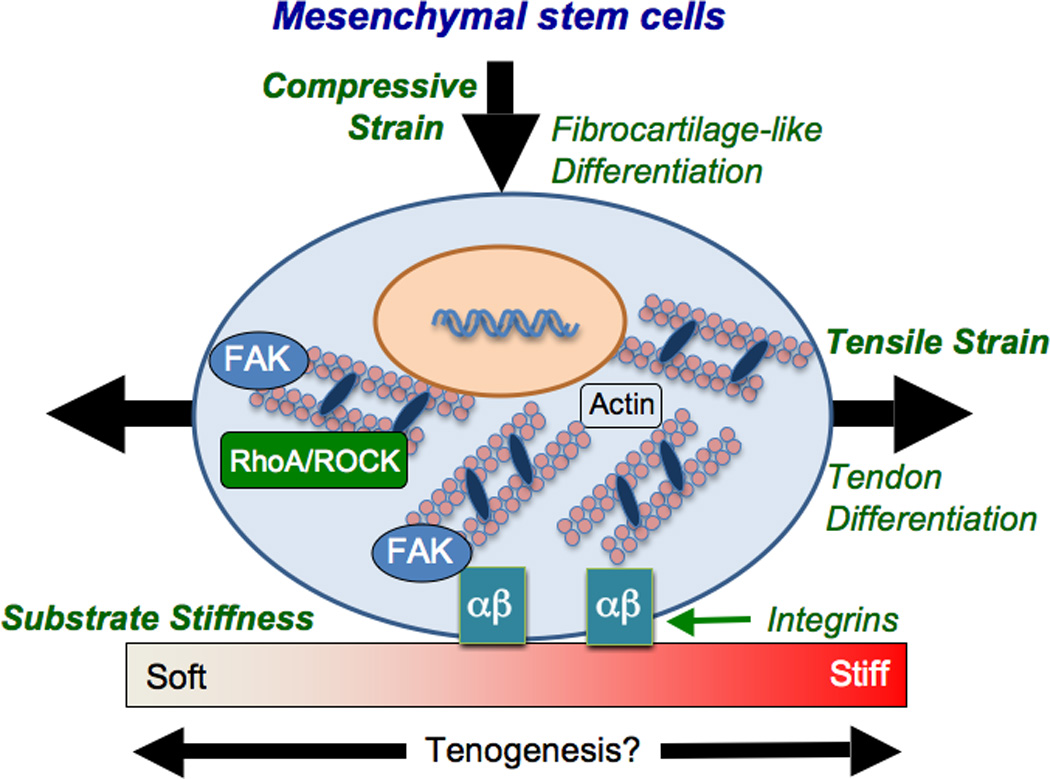
The tenogenic effect of dynamic tensile strain may be mediated through FAK, RhoA/ROCK and the actin cytoskeleton in MSCs. While tension is tenogenic, compressive loading may induce a fibrocartilage-like phenotype. Substrate stiffness may provide an additional mechanical signal to influence stem cell tenogenesis.
MSCs isolated from rat bone marrow were examined in collagen constructs that experienced regions of either tension or compression [58]. MSCs experiencing tension became aligned and elongated, and upregulated scleraxis and collagen type I gene expression, relative to compressed regions. TGF-β3 supplementation abolished scleraxis expression in either tensile or compressive regions, and increased aggrecan expression [58]. These results demonstrate that while tensile loading is tenogenic, compressive loading enhances a fibrocartilage-like phenotype in MSCs (Figure 2), and TGF-βs may play a role in this process. Currently it is unclear through what mechanisms tendon progenitor cells sense and respond differentially to tensile and compressive loads.
Recent work has also demonstrated that substrate stiffness may influence tenogenic stem cell differentiation (Figure 2). Human bone marrow stromal cells had increased scleraxis, tenomodulin, tenascin-C and collagen III gene expression on collagen-coated substrates with an elastic modulus of 40 kPa, relative to 20 kPa and 80 kPa [5], suggesting that substrate stiffness may mediate tenogenesis. Elastic modulus may be an important cue for tenogenesis by adult stem cells and deserves further investigation.
Conclusions and future directions
The complex mechanical environment that embryonic tendon cells experience encompasses physical factors from tissue stiffness to dynamic loading. By studying the mechanical microenvironment and mechanically regulated mechanisms involved in embryonic tendon development we may identify physical requirements for tenogenic stem cell differentiation. Toward that end, additional studies are needed to identify mechanical stimuli that elicit robust tenogenic signaling in differentiating ETCs. The chick has been the dominant embryonic animal model with which to study the role of mechanical influences in tendon development, presumably for its accessibility and relatively short developmental program (~21 days). However, while mechanisms of chick tendon formation overlap significantly with that of mouse [26], discovery and validation of mechanical cues with mammalian animal models and engineered systems will be necessary to advance human stem cell-based tendon regeneration strategies. This review focused on embryonic development, the period when stem and progenitor cell lineage commitment and differentiation occur, though understanding early postnatal events will be important in directing the continued development and maturation of engineered tendon as a functional tissue [59]. Characterizing mechanically regulated pathways during embryonic development may provide cues to guide engineered tissue formation and regeneration with stem cells and improve tissue engineering outcomes.
Highlights.
Mechanical influences on tendon cells during embryonic development are reviewed.
Potential mechanisms of mechanotransduction in embryonic tendon cells are discussed.
Mechanoregulation strategies to induce tenogenesis of stem cells are examined.
Acknowledgements
The authors would like to thank Jeffrey Brown, Ph.D. of Tufts University for critically reading this review. We would also like to thank our funding support by Research Grant 5-FY11-153 from the March of Dimes Foundation (to C.K.K.) and Award Number R03AR061036 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (to C.K.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nathan R. Schiele, Email: Nathan.Schiele@tufts.edu.
Joseph E. Marturano, Email: Joseph.Marturano@tufts.edu.
Catherine K. Kuo, Email: CatherineK.Kuo@tufts.edu.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert PM, Havenstrite KL, Magnusson KEG, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate Elasticity Regulates Skeletal Muscle Stem Cell Self-Renewal in Culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guvendiren M, Burdick JA. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat Commun. 2012;3:792. doi: 10.1038/ncomms1792. [DOI] [PubMed] [Google Scholar]
- 4.Huang AH, Farrell MJ, Kim M, Mauck RL. Long-term dynamic loading improves the mechanical properties of chondrogenic mesenchymal stem cell-laden hydrogel. Eur Cell Mater. 2010;19:72–85. doi: 10.22203/ecm.v019a08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma RI, Snedeker JG. Biochemical and biomechanical gradients for directed bone marrow stromal cell differentiation toward tendon and bone. Biomaterials. 2010;31:7695–7704. doi: 10.1016/j.biomaterials.2010.06.046. [DOI] [PubMed] [Google Scholar]
- 6.McBride DJ, Trelstad RL, Silver FH. Structural and Mechanical Assessment of Developing Chick Tendon. International Journal of Biological Macromolecules. 1988;10:194–200. [Google Scholar]
- 7. Kalson NS, Holmes DF, Herchenhan A, Lu Y, Starborg T, Kadler KE. Slow stretching that mimics embryonic growth rate stimulates structural and mechanical development of tendon-like tissue in vitro. Dev Dyn. 2011;240:2520–2528. doi: 10.1002/dvdy.22760. This paper studied the influence of a slow tensile stretch on ETCs and the resulting mechanical properties of tendon-like constructs during culture in vitro.
- 8. Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci U S A. 2013;110:6370–6375. doi: 10.1073/pnas.1300135110. This paper quantitatively characterized cell length-scale mechanical properties and biochemical composition of embryonic tendon throughout development and investigated potential mechanisms of mechanical property elaboration.
- 9.Nakagaki WR, Biancalana A, Benevides GP, Gomes L. Biomechanical and biochemical properties of chicken calcaneal tendon under effect of age and nonforced active exercise. Connect Tissue Res. 2007;48:219–228. doi: 10.1080/03008200701492136. [DOI] [PubMed] [Google Scholar]
- 10.Kalson NS, Holmes DF, Kapacee Z, Otermin I, Lu Y, Ennos RA, Canty-Laird EG, Kadler KE. An experimental model for studying the biomechanics of embryonic tendon: Evidence that the development of mechanical properties depends on the actinomyosin machinery. Matrix Biol. 2010;29:678–689. doi: 10.1016/j.matbio.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzue T, Shinoda Y. Highly reproducible spatiotemporal patterns of mammalian embryonic movements at the developmental stage of the earliest spontaneous motility. Eur J Neurosci. 1999;11:2697–2710. doi: 10.1046/j.1460-9568.1999.00686.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamburger V, Balaban M, Oppenheim R, Wenger E. Periodic motility of normal and spinal chick embryos between 8 and 17 days of incubation. J Exp Zool. 1965;159:1–13. doi: 10.1002/jez.1401590102. [DOI] [PubMed] [Google Scholar]
- 13.Kodama N, Sekiguchi S. The development of spontaneous body movement in prenatal and perinatal mice. Dev Psychobiol. 1984;17:139–150. doi: 10.1002/dev.420170205. [DOI] [PubMed] [Google Scholar]
- 14.de Vries JI, Visser GH, Prechtl HF. Fetal behaviour in early pregnancy. Eur J Obstet Gynecol Reprod Biol. 1986;21:271–276. doi: 10.1016/0028-2243(86)90003-1. [DOI] [PubMed] [Google Scholar]
- 15.Bekoff A, Stein PS, Hamburger V. Coordinated motor output in the hindlimb of the 7-day chick embryo. Proc Natl Acad Sci U S A. 1975;72:1245–1248. doi: 10.1073/pnas.72.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bekoff A. Ontogeny of leg motor output in the chick embryo: a neural analysis. Brain Res. 1976;106:271–291. doi: 10.1016/0006-8993(76)91025-8. [DOI] [PubMed] [Google Scholar]
- 17.Roddy KA, Kelly GM, van Es MH, Murphy P, Prendergast PJ. Dynamic patterns of mechanical stimulation co-localise with growth and cell proliferation during morphogenesis in the avian embryonic knee joint. J Biomech. 2010;44:143–149. doi: 10.1016/j.jbiomech.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 18.Nemec SF, Hoftberger R, Nemec U, Bettelheim D, Brugger PC, Kasprian G, Amann G, Rotmensch S, Graham JM, Jr, Rimoin DL, et al. : Fetal akinesia and associated abnormalities on prenatal MRI. Prenat Diagn. 2011;31:484–490. doi: 10.1002/pd.2724. [DOI] [PubMed] [Google Scholar]
- 19.Lamb KJ, Lewthwaite JC, Lin JP, Simon D, Kavanagh E, Wheeler-Jones CP, Pitsillides AA. Diverse range of fixed positional deformities and bone growth restraint provoked by flaccid paralysis in embryonic chicks. Int J Exp Pathol. 2003;84:191–199. doi: 10.1046/j.1365-2613.2003.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall BK, Herring SW. Paralysis and growth of the musculoskeletal system in the embryonic chick. Journal of Morphology. 1990;206:45–56. doi: 10.1002/jmor.1052060105. [DOI] [PubMed] [Google Scholar]
- 21. Roddy KA, Prendergast PJ, Murphy P. Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PLoS One. 2011;6:e17526. doi: 10.1371/journal.pone.0017526. This paper characterized the effects of reduced embryonic movement on tissue morphology and gene expression in the knee joint during morphogenesis.
- 22.Germiller JA, Lerner AL, Pacifico RJ, Loder RT, Hensinger RN. Muscle and tendon size relationships in a paralyzed chick embryo model of clubfoot. J Pediatr Orthop. 1998;18:314–318. [PubMed] [Google Scholar]
- 23.Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J Rehabil Res Dev. 2000;37:127–133. [PubMed] [Google Scholar]
- 24.Mikic B, Wong M, Chiquet M, Hunziker EB. Mechanical modulation of tenascin-C and collagen-XII expression during avian synovial joint formation. J Orthop Res. 2000;18:406–415. doi: 10.1002/jor.1100180312. [DOI] [PubMed] [Google Scholar]
- 25.Beckham C, Dimond R, Greenlee TK., Jr The role of movement in the development of a digital flexor tendon. Am J Anat. 1977;150:443–459. doi: 10.1002/aja.1001500306. [DOI] [PubMed] [Google Scholar]
- 26.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 27. Maeda T, Sakabe T, Sunaga A, Sakai K, Rivera AL, Keene DR, Sasaki T, Stavnezer E, Iannotti J, Schweitzer R, et al. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr Biol. 2011;21:933–941. doi: 10.1016/j.cub.2011.04.007. This paper demonstrated mechanical signals in tendon cells may be transduced via TGF-β-mediated signaling pathways to maintain tissue homeostasis.
- 28.Kuo CK, Petersen BC, Tuan RS. Spatiotemporal protein distribution of TGF-betas, their receptors, and extracellular matrix molecules during embryonic tendon development. Dev Dyn. 2008;237:1477–1489. doi: 10.1002/dvdy.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieny M, Chevallier A. Autonomy of tendon development in the embryonic chick wing. J Embryol Exp Morphol. 1979;49:153–165. [PubMed] [Google Scholar]
- 30.Kardon G. Muscle and tendon morphogenesis in the avian hind limb. Development. 1998;125:4019–4032. doi: 10.1242/dev.125.20.4019. [DOI] [PubMed] [Google Scholar]
- 31.Edom-Vovard F, Schuler B, Bonnin MA, Teillet MA, Duprez D. Fgf4 positively regulates scleraxis and tenascin expression in chick limb tendons. Dev Biol. 2002;247:351–366. doi: 10.1006/dbio.2002.0707. [DOI] [PubMed] [Google Scholar]
- 32.Hamburger V, Hamilton HL. A Series of Normal Stages in the Development of the Chick Embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- 33.Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat. 2008;212:211–228. doi: 10.1111/j.1469-7580.2008.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel KG, Ordog A, Pogany G, Olah J. Proteoglycans in the compressed region of human tibialis posterior tendon and in ligaments. J Orthop Res. 1993;11:68–77. doi: 10.1002/jor.1100110109. [DOI] [PubMed] [Google Scholar]
- 35.Koob TJ, Clark PE, Hernandez DJ, Thurmond FA, Vogel KG. Compression loading in vitro regulates proteoglycan synthesis by tendon fibrocartilage. Arch Biochem Biophys. 1992;298:303–312. doi: 10.1016/0003-9861(92)90127-i. [DOI] [PubMed] [Google Scholar]
- 36.Robbins JR, Evanko SP, Vogel KG. Mechanical loading and TGF-beta regulate proteoglycan synthesis in tendon. Arch Biochem Biophys. 1997;342:203–211. doi: 10.1006/abbi.1997.0102. [DOI] [PubMed] [Google Scholar]
- 37.McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Anat. 1996;189(Pt 3):593–600. [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley RL, Fleck RA, Becker DL, Goodship AE, Ralphs JR, Patterson-Kane JC. Gap junction protein expression and cellularity: comparison of immature and adult equine digital tendons. J Anat. 2007;211:325–334. doi: 10.1111/j.1469-7580.2007.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makarenkova H, Becker DL, Tickle C, Warner AE. Fibroblast growth factor 4 directs gap junction expression in the mesenchyme of the vertebrate limb Bud. J Cell Biol. 1997;138:1125–1137. doi: 10.1083/jcb.138.5.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman CM, Loredo GA, Lo CW, Tuan RS. Correlation of GDF5 and connexin 43 mRNA expression during embryonic development. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1117–1121. doi: 10.1002/ar.a.10125. [DOI] [PubMed] [Google Scholar]
- 41.Banes AJ, Weinhold P, Yang X, Tsuzaki M, Bynum D, Bottlang M, Brown T. Gap junctions regulate responses of tendon cells ex vivo to mechanical loading. Clin Orthop Relat Res. 1999:S356–S370. doi: 10.1097/00003086-199910001-00034. [DOI] [PubMed] [Google Scholar]
- 42.Waggett AD, Benjamin M, Ralphs JR. Connexin 32 and 43 gap junctions differentially modulate tenocyte response to cyclic mechanical load. Eur J Cell Biol. 2006;85:1145–1154. doi: 10.1016/j.ejcb.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Leckband DE, le Duc Q, Wang N, de Rooij J. Mechanotransduction at cadherin-mediated adhesions. Curr Opin Cell Biol. 2011;23:523–530. doi: 10.1016/j.ceb.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 44.Richardson SH, Starborg T, Lu Y, Humphries SM, Meadows RS, Kadler KE. Tendon development requires regulation of cell condensation and cell shape via cadherin-11-mediated cell-cell junctions. Mol Cell Biol. 2007;27:6218–6228. doi: 10.1128/MCB.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwartz MA. Integrins and extracellular matrix in mechanotransduction. Cold Spring Harb Perspect Biol. 2010;2:a005066. doi: 10.1101/cshperspect.a005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muschler JL, Horwitz AF. Down-regulation of the chicken alpha 5 beta 1 integrin fibronectin receptor during development. Development. 1991;113:327–337. doi: 10.1242/dev.113.1.327. [DOI] [PubMed] [Google Scholar]
- 47.Popova SN, Rodriguez-Sanchez B, Liden A, Betsholtz C, Van Den Bos T, Gullberg D. The mesenchymal alpha11beta1 integrin attenuates PDGF-BB-stimulated chemotaxis of embryonic fibroblasts on collagens. Dev Biol. 2004;270:427–442. doi: 10.1016/j.ydbio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 48.Tiger CF, Fougerousse F, Grundstrom G, Velling T, Gullberg D. alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Dev Biol. 2001;237:116–129. doi: 10.1006/dbio.2001.0363. [DOI] [PubMed] [Google Scholar]
- 49.Qi J, Fox AM, Alexopoulos LG, Chi L, Bynum D, Guilak F, Banes AJ. IL-1beta decreases the elastic modulus of human tenocytes. J Appl Physiol. 2006;101:189–195. doi: 10.1152/japplphysiol.01128.2005. [DOI] [PubMed] [Google Scholar]
- 50.Arnoczky SP, Tian T, Lavagnino M, Gardner K. Ex vivo static tensile loading inhibits MMP-1 expression in rat tail tendon cells through a cytoskeletally based mechanotransduction mechanism. J Orthop Res. 2004;22:328–333. doi: 10.1016/S0736-0266(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 51.Ralphs JR, Waggett AD, Benjamin M. Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002;21:67–74. doi: 10.1016/s0945-053x(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 52.Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 2007;17:178–186. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 53.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 54.Scott A, Danielson P, Abraham T, Fong G, Sampaio AV, Underhill TM. Mechanical force modulates scleraxis expression in bioartificial tendons. J Musculoskelet Neuronal Interact. 2011;11:124–132. [PubMed] [Google Scholar]
- 55.Xu B, Song G, Ju Y. Effect of Focal Adhesion Kinase on the Regulation of Realignment and Tenogenic Differentiation of Human Mesenchymal Stem Cells by Mechanical Stretch. Connect Tissue Res. 2011 doi: 10.3109/03008207.2010.541961. [DOI] [PubMed] [Google Scholar]
- 56. Xu B, Song G, Ju Y, Li X, Song Y, Watanabe S. RhoA/ROCK, cytoskeletal dynamics, and focal adhesion kinase are required for mechanical stretch-induced tenogenic differentiation of human mesenchymal stem cells. J Cell Physiol. 2011;227:2722–2729. doi: 10.1002/jcp.23016. This paper examined potential mechanotransduction pathways involved in mechanical stretch-induced tenogenic differentiation of adult human stem cells.
- 57.Kuo CK, Tuan RS. Mechanoactive tenogenic differentiation of human mesenchymal stem cells. Tissue Eng Part A. 2008;14:1615–1627. doi: 10.1089/ten.tea.2006.0415. [DOI] [PubMed] [Google Scholar]
- 58. Thomopoulos S, Das R, Birman V, Smith L, Ku K, Elson EL, Pryse KM, Marquez JP, Genin GM. Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression. Tissue Eng Part A. 2011;17:1039–1053. doi: 10.1089/ten.tea.2009.0499. This paper examined the influence of tensile and compressive mechanical loading on the tenogenic phenotype of MSCs in a tissue engineered construct.
- 59. Connizzo BK, Yannascoli SM, Soslowsky LJ. Structure-function relationships of postnatal tendon development: A parallel to healing. Matrix Biol. 2013;32:106–116. doi: 10.1016/j.matbio.2013.01.007. This paper reviewed research on structure-function relationships of developing and healing postnatal tendon, commented on parallels between the two processes, and discussed recent repair strategies.


