Abstract
We examined the relationship between maternal depressive symptoms in late pregnancy and Epstein-Barr virus reactivation before delivery. In this prospective observational study, prevalence of Epstein-Barr virus reactivation within one week before delivery was compared between 163 pregnant women with depressive symptoms at 33 to 34 weeks of gestation and a computer-generated control group of 163 pregnant healthy women without depressive symptoms. Depressive symptoms at 33 to 34 weeks of gestation were significantly related to the prevalence of Epstein-Barr virus reactivation before delivery after adjustment for potential confounders (adjusted OR = 2.74, 95%CI: 1.23–6.08). Compared to that in the control group, the prevalence of Epstein-Barr virus reactivation was higher in women with depressive symptoms accompanied by higher negative coping (24.2% compared with 7.9%; adjusted OR = 3.67, 95%CI: 1.47–9.16). Maternal depressive symptoms in late pregnancy are associated with Epstein-Barr virus reactivation, and this association could be moderated by maternal coping style.
Epstein-Barr virus (EBV) is a member of the herpesvirus family, and about 90% of women have serologic evidence of previous infection1. Like other herpes viruses, EBV has the ability to remain latent in the body and becomes reactivated at a later time. It has been reported that maternal EBV reactivation in pregnancy is related to the adverse offspring development including severe symmetrical fetal growth restriction, lower birthweight, and leukemia2,3,4.
Maternal depression is a mood disorder that begins before or immediately after childbirth. Between 12.7% and 23% of pregnant women will experience a depressive disorder5,6. Epidemiological evidence demonstrates that elevated levels of depressive symptoms in mothers are related to the adverse offspring development, such as lower birth weight, small for gestational age and child maladjustment7,8,9. However, the possible mechanisms underlying the association between maternal depression and adverse offspring development remain unclear. Results from animal studies suggested that maternal stress might negatively influence offspring outcomes through a process known as fetal programming10. Prenatal stress can lead to increased fetal exposure to glucocorticoids, which could permanently alter the HPA function of the offspring11,12. But the evidence from human studies about fetal programming is limited and inconsistent yet13,14,15,16,17.
Recently, Haeri et al found that women with depression had higher prevalence of EBV reactivation18. Their research seems to suggest a potential novel viral model which may elucidate biological pathways underlying how maternal psychosocial stress impacts fetal development. However, this result hasn't been repeated in heterogeneous pregnant women populations. Thus, it would be helpful to support this hypothesis by adding evidence about the relationship between prenatal psychosocial stress and EBV reactivation in pregnant women during different stages of gestation from diverse population.
The present study aims to extend current knowledge about the association between maternal psychological stress and EBV reactivation during pregnancy. We investigated the relationship between maternal depressive symptoms in late pregnancy and EBV reactivation before delivery among pregnancy cohort in Hefei, China, a typical developing country. In addition, we tested the possible moderating role of coping style in the association between depressive symptoms and EBV reactivation in pregnancy.
Results
The mean gestational age of serum collection was similar for depressed group and controls (38.7 compared with 39.1 weeks). Table 2 presents the demographic characteristics of the study sample. There were significant differences between women with depressive symptoms and controls in demographic characteristics including maternal education, family income and employment pattern. Among women with depressive symptoms, there were higher proportion in lower education attainment, medium and lower family income, mental labor and unemployment compared to controls. In addition, women with depressive symptoms were more likely to have a negative coping style to stress. In both groups, 4.3% of the women were EBV seronegative, 95.7% of the women were EBV seropositive and no acute infection was found. Women with depressive symptoms had significantly higher rates of EBV reactivation than nondepressed women (18.4% compared with 8.0%, P = 0.015).
Table 1. Clinical Epstein-Barr Status by viral serology results.
| Clinical Status | VCA IgG | EBNA-1 IgG | EA IgG | VCA IgM |
|---|---|---|---|---|
| No previous infection | − | − | − | − |
| Acute infection | + | − | + | + |
| Previous infection | + | + | − | − |
| Reactivation | + | + | + | − |
Table 2. Demographic characteristics of women with depressive symptoms and controls.
| Characteristics | Depressed (n = 163) | Control group (n = 163) | P value |
|---|---|---|---|
| Maternal age | |||
| 20–24 years | 37(22.7) | 26(16.0) | 0.145 |
| 25–29 years | 93(57.1) | 102(62.6) | 0.342 |
| 30–34 years | 33(20.2) | 35(21.5) | 0.897 |
| Maternal education ≤ 9 years | 45(27.6) | 16(9.8) | <0.001 |
| Maternal income | |||
| <2000 RMB | 53(32.5) | 31(19.0) | 0.009 |
| 2000–4000 RMB | 90(55.2) | 121(74.2) | 0.001 |
| >4000 RMB | 20(12.3) | 11(6.7) | 0.137 |
| Interpregnancy interval | |||
| <12 months | 39(23.9) | 30(18.4) | 0.253 |
| ≥12 months | 61(37.4) | 54(33.1) | 0.505 |
| Primigravid | 63(38.7) | 79(48.5) | 0.122 |
| Antiviral drugs use | 12(7.4) | 9(5.5) | 0.648 |
| Prepregnancy BMIa | |||
| Underweigh | 31(19.0) | 29(17.8) | 0.874 |
| Normal weight | 119(72.0) | 121(75.3) | 0.892 |
| Overweight or obesity | 13(8.0) | 13(8.0) | 0.999 |
| Employment pattern | |||
| Physical labor | 64(39.3) | 51(31.3) | 0.131 |
| Mental labor | 59(36.2) | 89(54.6) | <0.001 |
| Unemployment | 40(24.5) | 23(14.1) | 0.017 |
| Higher Negative copingb | 91(55.8) | 60(36.8) | 0.001 |
| Epstein-Barr Status | |||
| No previous infection | 6(3.7) | 8(4.9) | 0.791 |
| Previous infection | 127(77.9) | 142(87.1) | 0.064 |
| Reactivation | 30(18.4) | 13(8.0) | 0.015 |
McNemar's χ2 tests were used for statistical analysis.
aBMI, body mass index. based on the BMI cut point ranges among Chinese adult: underweight(<18.5),normal weight (18.5–23.9),overweight(≥24.0).
bNegative coping style was categorized as higher negative coping and lower negative coping by using the 75th percentile.
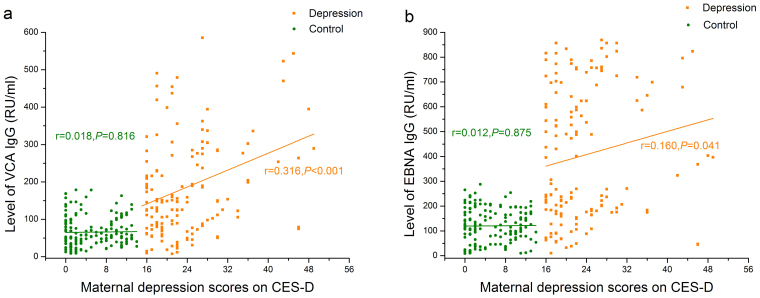
Figure 1 shows that the levels of VCA IgG (65.79 ± 39.86 VS 183.06 ± 131.01, t = 10.93, P < 0.001) and EBNA IgG (120.46 ± 68.20 VS 404.24 ± 267.08, t = 13.14, P < 0.001) in women with depressive symptoms were both significantly higher than those in the nondepressed women. Moreover, maternal depression score on CES-D was positively associated with level of both VCA IgG (r = 0.316, P < 0.001) and EBNA IgG (r = 0.160, P = 0.041) in women with depressive symptoms, but not in the control group (VCA IgG: r = 0.018, P = 0.816;EBNA IgG: r = 0.012, P = 0.875). Additionally, the levels of EA IgG and VCA IgM were not significantly related to the scores of maternal depression.
Figure 1. Correlation between maternal depression scores on CES-D and levels of IgG antibodies against EBV.
(a) Correlation between maternal depression scores and levels of VCA IgG, (b) Correlation between maternal depression scores and levels of EBNA IgG.
Table 3 shows the relation of maternal demographic characteristics and EBV reactivation among seropositive women. Significantly higher rates of EBV reactivation were only found in women with interpregnancy intervals longer than one year, higher negative coping and depressive symptoms. Conditional logistic regression model demonstrated that the OR of EBV reactivation in relation to maternal depressive symptoms is 2.31(95%CI:1.21–4.42, P = 0.012) without adjustment and 2.74(95%CI: 1.23–6.08, P = 0.013) with adjustment for maternal age, education, family income, interpregnancy interval, antiviral drug use, prepregnancy BMI, employment patterns and negative coping style.
Table 3. Epstein-Barr reactivation according to maternal characteristics among seropositive women (N = 312).
| Characteristics | n(%) | Previous infection | Reactivation | χ2 | P value |
|---|---|---|---|---|---|
| Maternal age | |||||
| 20–24 years | 63(20.2) | 52(82.5) | 11(17.5) | 1.11 | 0.573 |
| 25–29 years | 186(59.6) | 161(86.6) | 25(13.4) | ||
| ≥30 years | 63(20.2) | 56(88.9) | 7(11.1) | ||
| Maternal education | |||||
| ≤9 years | 61(19.6) | 50(82.0) | 11(18.0) | 1.15 | 0.283 |
| >9 years | 251(80.4) | 219(87.3) | 32(12.7) | ||
| Maternal income | |||||
| <2000 RMB | 76(24.4) | 62(81.6) | 14(18.4) | 1.83 | 0.400 |
| 2000–4000 RMB | 205(65.7) | 180(87.8) | 25(12.2) | ||
| >4000 RMB | 31(9.9) | 27(87.1) | 4(12.9) | ||
| Interpregnancy interval | |||||
| <12 months | 62(19.9) | 59(95.2) | 3(4.8) | 9.19 | 0.010 |
| ≥12 months | 110(35.3) | 87(79.1) | 23(20.9) | ||
| Primigravid | 140(44.9) | 123(87.9) | 17(12.1) | ||
| Antiviral drugs use | |||||
| Yes | 20(6.4) | 16(80.0) | 4(20.0) | 0.70 | 0.404 |
| No | 292(93.6) | 253(86.6) | 39(13.4) | ||
| Prepregnancy BMIa | |||||
| Underweigh | 60(19.2) | 51(85.0) | 9(15.0) | 0.13 | 0.935 |
| Normal weight | 232(74.4) | 201(86.6) | 31(13.4) | ||
| Overweight or obesity | 20(6.4) | 17(85.0) | 3(15.0) | ||
| Employment pattern | |||||
| Physical labor | 115(36.9) | 96(83.5) | 19(16.5) | 2.95 | 0.229 |
| Mental labor | 140(44.9) | 120(85.7) | 20(14.3) | ||
| Unemployment | 57(18.3) | 53(93.0) | 4(7.0) | ||
| Negative Coping styleb | |||||
| Higher | 145(46.5) | 118(81.4) | 27(18.6) | 5.34 | 0.021 |
| Lower | 167(53.5) | 151(90.4) | 16(9.6) | ||
| Depressive symptoms | |||||
| Yes | 157(50.3) | 127(80.9) | 30(19.1) | 7.55 | 0.006 |
| No | 155(49.7) | 142(91.6) | 13(8.4) |
Pearson's χ2 tests were used for statistical analysis.
aBMI, body mass index. based on the BMI cut point ranges among Chinese adult: underweight(<18.5), normal weight (18.5–23.9), overweight(≥24.0).
bNegative coping style was categorized as higher negative coping and lower negative coping by using the 75th percentile.
Table 4 presents the effect of interaction of maternal depressive symptoms and coping style on Epstein-Barr virus reactivation. Compared to that in the control group, the prevalence of Epstein-Barr virus reactivation was higher in women with depressive symptoms accompanied by higher negative coping (24.2% compared with 7.9%; adjusted OR = 3.67, 95%CI: 1.47–9.16), but not in women with depressive symptoms accompanied by lower negative coping (12.1% compared with 7.9%; adjusted OR = 1.61, 95%CI: 0.56–4.64).
Table 4. Effect of interaction of maternal depressive symptoms and coping style on Epstein-Barr virus reactivation (N = 312).
| n | Reactivation n(%) | Crude OR(95%CI) | P value | Adjustede OR(95%CI) | P value | |
|---|---|---|---|---|---|---|
| Control groupa | 101 | 8(7.9) | 1.0 | 1.0 | ||
| Case group 1b | 54 | 5(9.3) | 1.17(0.37–3.82) | 0.775 | 1.27(0.38–4.18) | 0.698 |
| Case group 2c | 66 | 8(12.1) | 1.60(0.57–4.51) | 0.371 | 1.61(0.56–4.64) | 0.381 |
| Case group 3d | 91 | 22(24.2) | 3.71(1.56–8.82) | 0.003 | 3.67(1.47–9.16) | 0.005 |
Abbreviation: CI, confidence interval; OR, odds ratio.
aControl group was identified as women without depressive symptoms accompanied by lower negative coping.
bCase group 1 was identified as women without depressive symptoms accompanied by higher negative coping.
cCase group 2 was identified as women with depressive symptoms accompanied by lower negative coping.
dCase group 3 was identified as women with depressive symptoms accompanied by higher negative coping.
eAdjustment for maternal age, education, family income, interpregnancy interval, antiviral drug use, prepregnancy BMI and employment pattern.
Discussion
Although studies have indicated that stress promotes latent Epstein-Barr virus reactivation19,20, evidence for this association in pregnancy is limited. In this study, we found women with depressive symptoms during 32 gestational weeks had significant higher prevalence of EBV reactivation before delivery than nondepressed healthy women, and maternal depression score on CES-D was positively associated with level of both VCA IgG and EBNA IgG in women with depressive symptoms. These findings suggested the positive correlationship of prenatal depressive symptoms with the increase of both latent EBV reactivation risk and the antibody titers, which might be interpreted as a response to the increase in viral antigens synthesized due to reactivation. Though carried out in developing country, the current evidence supports the results of Haeri's study, which was from developed country.
However, there are some differences between our study and Haeri's. Firstly, we focused on maternal depressive symptoms rather than depression. Maternal depressive symptoms are more common during pregnancy5,6. According to our results, even general depressive symptoms can also increase the risk of EBV reactivation during pregnancy. Secondly, we put attention on the late-pregnancy period while Haeri's study concentrated on the depression before pregnancy. A systematic review of the prevalence of depression during pregnancy demonstrated that the prevalence of depression during the second and the third trimester is higher than that observed in the first trimester21. The increasing prevalence of maternal depression from early to late pregnancy accompanies by the rapid increasing of maternal cortisol22. Thus, maternal depression was significantly associated with EBV reactivation not only in first trimester during which the pregnant women were under low level of cortisol18, but also in late pregnancy, during which the serum cortisol concentration was increased. Additionally, besides the demographic characteristics including age, race, BMI and insurance type, other potential important confounders, especially coping style, which was regarded as an important modified factor for perceived stress23, were taken into consideration in the present study.
Our results also indicated that coping style could moderate the effect of maternal depressive symptoms on the risk of EBV reactivation. Compared with controls, women with depressive symptoms accompanied by higher negative coping were significantly related to an increased risk for EBV reactivation, while women with depressive symptoms accompanied by lower negative coping did not have a significantly increased risk for EBV reactivation. Virus reactivation reflects a deficiency in the cellular immune response. Chronic stress affected by emotional disorder was associated with suppression of both cellular and humoral measures24. However, effective coping style such as participating in recreation activities, aerobic and resistance exercise programmes could significantly alleviate depressive symptoms and psychological stress23,25. Researches have demonstrated that effective coping strategies can protect the stress-induced immune regulation, while negative/defensive coping style is negatively correlated to the immune system26. The above facts may contribute to the mechanisms of moderating effect of coping style on the association between maternal depressive symptoms and the risk of EBV reactivation.
Studies of biological pathway underlying the psychosocial stress during pregnancy influences fetal development focused most on the fetal programming of glucocorticoids exposure during pregnancy. However, the rapidly increased cortisol during pregnancy and the circadian rhythm confound the association of psychosocial stress during pregnancy and fetal development, and findings from human studies about the fetal programming effects of prenatal glucocorticoids exposure were limited and inconsistent yet8,13,14,15,16,17. Our results, together with that of previous study18, indicated that prenatal depression was associated with maternal EBV reactivation. In view of the link between EBV reactivation during pregnancy and adverse offspring development2,3,4, it is plausible that prenatal psychosocial stress may promote maternal latent EBV reactivation, which increases the risk of fetal exposure to the virus, and contributes to adverse offspring development. However, EBV reactivation during pregnancy was also considered as a non-causal marker of impaired cellmediated immune function, which could contribute to adverse perinatal health outcomes27. Thus, more attention should be paid to the role of EBV and other herpes viruses reactivation in the association between prenatal depressive symptoms and adverse fetal development. The viral hypothesis might throw new light into the interpretation of the potential biological mechanisms underlying how prenatal psychosocial stress influences fetal developments.
However, there are several potential limitations in the present study. Most importantly, the statistical power to identify the mediating role of coping style in association between prenatal depression and the risk of EBV reactivation was limited. Further research is needed to determine the reliability of these findings in larger cohort. Simultaneously evaluation of maternal depression and serum collection during late pregnancy could not clarify the causal relationship between depression and EBV activation. Compared with the research conducted in America18 and Norway3, our research using population based cohort in China observed a lower rate of EBV reactivation. This discrepancy may be due to the enhanced ability of the cellular immune response in late gestation compared to early stage and the racial difference. Thus, more caution should be warranted in generalizing our findings beyond the region/culture being studied. Extra study is necessary to be conducted in various races and regions to further validate the association between maternal depressive symptoms during pregnancy and EBV reactivation.
In conclusion, our research suggested that maternal depressive symptoms in late pregnancy significantly increased the risk of the reactivation of latent EBV, and maternal coping style could moderate this effect. Our results, together with that of previous study, might provide a novel perspective in interpreting the potential biological mechanisms underlying how prenatal depressive symptoms influence fetal developments.
Methods
The participants of this prospective observational study were recruited from 3316 married pregnant women who received prenatal check-ups after 32 completed weeks in the Department of Gynecology and Obstetrics of Hefei Maternal and Child Health Hospital from March to November 2008. A total of 2552 pregnant women participated in the project willingly. With informed consent, they completed a structured questionnaire. Nonfasting blood samples were routinely collected within one week before delivery, and serum aliquots were bar-coded and frozen at −80°C. Demographic characteristics and pregnancy history were assessed through the interview, and delivery outcomes were obtained from medical charts after delivery. This study was approved by the ethical committee of the Anhui Medical University, and informed consent was obtained from the participants.
At 33 to 34 weeks of gestation, maternal depressive symptoms were assessed with the validated China version of the 20-item Center for Epidemiological Studies Depression Scale (CES-D) with well-established reliability28, designed for using among the general population, that measures the level of depressive symptomatology within last week with a cutoff score of 16 or more. Each item is scored on a 4-point scale of frequency of occurrence of the symptom ranging from rarely or none of the time (0) to most or all the time (3). Internal consistency (Cronbach's alpha) of the scales was 0.86.
Coping refers to the cognitive and behavioral efforts used when faced with specific demands appraised as taxing or exceeding one's resources. The Chinese Revised Edition of Trait Coping Style Questionnaire (TCSQ) including 10 items with the total score range from 10 to 50, which has previously been validated in sample form China29, assessed negative coping style to stress. The scale was designed to assess the using of strategies involving avoiding thinking about a problem, avoiding dealing with a stressor, avoiding relationships or social activities. For analysis, a global score is used which is calculated based on all 10 items. The more scores, the more tendency to adopt avoidance coping style to stress. Because the cut-off was not available for this instrument, we categorized higher negative coping and lower negative coping by using the 75th percentile, the score being 32, as cutoff in present study. Internal consistency (Cronbach's alpha) of the scales was 0.88.
Maternal sociodemographic information was obtained from interview, including maternal age, education, income, prepregnancy weight and employment pattern. Maternal age was categorized as 20–24, 25–29 and 30–34 years. Maternal income was categorized as low, medium and high according to income level per month (less than 2000, 2000–4000 and more than 4000 RMB). Educational attainment was similarly categorized as middle school and less (≤9 years of completed schooling), or high school and beyond (>9 years of completed schooling). Maternal employment pattern was categorized as physical labor, mental labor and unemployment.
Prepregnancy BMI (weight in kilograms divided by height in meters squared) was calculated based on height routinely measured at the clinic visit and on self-reported pregravid weight obtained at interview. Based on the BMI cut point ranges among Chinese adult30, the participants could be categorized into underweight (<18.5), normal weight (18.5–23.9) and overweight or obesity (≥24.0).
Interpregnancy interval, antiviral drug use and delivery outcomes including gestational age at birth and birthweight were obtained from medical charts after delivery. Gestational age was determined as the difference between the date of the last menstrual period and the date of delivery. In cases of uncertain menstrual dates, ultrasound estimates of gestational age were used.
Of the original 204 women with depressive symptoms, 163 were eligible and identified as depressed group. These women were matched in 1:1 ratio to a random computer-generated control group of 163 healthy women without depressive symptoms. Exclusion criteria for both case and control participants included maternal age greater than 35 years, delivery before 37 weeks of gestation, pregnancy with assisted reproductive technology, superfetation, complications with pregnancy including diabetes mellitus, hypertension, glandula thyreoidea disease, and intrahepatic cholestasis of pregnancy, history of abnormal pregnant outcome including fetal death, stillbirth, birth defect, and neonatal death, stillbirth, and birth defect.
Participants' serum were assayed to determine viral capsid antigen (VCA) immunoglobulin (Ig) G, VCA IgM, Epstein-Barr virus nuclear antigen-1(EBNA) IgG, early antigen (EA) IgG levels using commercial enzyme-linked immunosorbent assay (ELISA) kits (Euroimmun, Lübeck, Germany) according to manufacturer's instructions, and quantitative outcomes were obtained. The results were based on the following index value cutoffs: 0.79 or less considered negative, 0.80–1.09 considered equivocal, and 1.10 or more considered positive. The differential serodiagnosis is presented in Table 1 for healthy cases, acute infection, previous infection and reactivation.
McNemar's χ2 tests analysis were adopted to test the differences in the characteristics of depressive women and controls. Correlation between maternal depression scores on CES-D and level of IgG antibodies against EBV were assessed applying Pearson correlations. The differences of prevalence of EBV reactivation according to maternal characteristics were test by using the χ2 tests. Crude and adjusted odds ratios (OR) with 95% confidence intervals were generated for associations of maternal depressive symptoms with EBV reactivation by using conditional logistic regression. The effect of interaction of maternal depressive symptoms and coping style on EBV reactivation was tested by using binary logistic regression. All analyses were 2-tailed and P < 0.05 was considered significant. SPSS version 13.0 was used to perform analyses.
Author Contributions
F.B.T. and P.Z. designed the study, carried out the statistical analyses and wrote the manuscript. Y.J.C., J.H.H., K.H., R.X.T. and X.M.J. participated in data collection and analysis. Y.J.C. and J.F.G. revised the manuscript.
Acknowledgments
We gratefully acknowledge contributions made by staff members of Hefei Maternal and Child Health Hospital and the whole CABCD team. We also thanks all of the participants. This study was supported by grants from the Key Projects in the National Key Technologies R&D Program (No. 2006BAI05A03), the National Natural Science Foundation of China (No. 81072310, 30901203 and 30901202), the Grants for Key Member in Academy of AMU and the Grants for Scientific Research of BSKY from AMU (No. XJ201019).
References
- Haeri S., Baker A. M. & Boggess K. A. Prevalence of Epstein-Barr virus reactivation in pregnancy. Am J Perinatol. 27, 715–719 (2010). [DOI] [PubMed] [Google Scholar]
- Tomai X. H. Stillbirth following severe symmetric fetal growth restriction due to reactivation of Epstein-Barr virus infection in pregnancy. J Obstet Gynaecol Res. 37, 1877–1882 (2011). [DOI] [PubMed] [Google Scholar]
- Eskild A. et al. Epstein-Barr virus infection during pregnancy and the risk of adverse pregnancy outcome. BJOG. 112, 1620–1624 (2005). [DOI] [PubMed] [Google Scholar]
- Tedeschi R. et al. Activation of maternal Epstein-Barr virus infection and risk of acute leukemia in the offspring. Am J Epidemiol. 165, 134–137 (2007). [DOI] [PubMed] [Google Scholar]
- Gavin N. I. et al. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 106, 1071–1083 (2005). [DOI] [PubMed] [Google Scholar]
- Faisal-Cury A. & Rossi Menezes P. Prevalence of anxiety and depression during pregnancy in a private setting sample. Arch Womens Ment Health. 10, 25–32 (2007). [DOI] [PubMed] [Google Scholar]
- Grote N. K. et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 67, 1012–1024 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diego M. A. et al. Prenatal depression restricts fetal growth. Early Hum Dev. 85, 65–70 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker E. D. et al. The contribution of prenatal and postnatal maternal anxiety and depression to child maladjustment. Depress Anxiety. 28, 696–702 (2011). [DOI] [PubMed] [Google Scholar]
- Seckl J. R. & Meaney M. J. Glucocorticoid programming. Ann N Y Acad Sci. 1032, 63–84 (2004). [DOI] [PubMed] [Google Scholar]
- Kapoor A. et al. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 572, 31–44 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V., O'Connor T. G. & O'Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 35, 17–22 (2010). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Late gestational maternal serum cortisol is inversely associated with fetal brain growth. Neurosci Biobehav Rev. 36, 1085–1092 (2012). [DOI] [PubMed] [Google Scholar]
- Brand S. R. et al. The effect of maternal PTSD following in utero trauma exposure on behavior and temperament in the 9-month-old infant. Ann N Y Acad Sci. 1071, 454–458 (2006). [DOI] [PubMed] [Google Scholar]
- Gutteling B. M. et al. Does maternal prenatal stress adversely affect the child's learning and memory at age six? J Abnorm Child Psychol. 34, 789–798 (2006). [DOI] [PubMed] [Google Scholar]
- Gutteling B. M. et al. The effects of prenatal stress on temperament and problem behavior of 27-month-old toddlers. Eur Child Adolesc Psychiatry. 14, 41–51 (2005). [DOI] [PubMed] [Google Scholar]
- Sarkar P. et al. Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: possible implications for foetal programming. J Neuroendocrinol. 20, 489–496 (2008). [DOI] [PubMed] [Google Scholar]
- Haeri S. et al. Maternal depression and Epstein-Barr virus reactivation in early pregnancy. Obstet Gynecol. 117, 862–866 (2011). [DOI] [PubMed] [Google Scholar]
- McClure H. H. et al. Discrimination-related stress, blood pressure and epstein-barr virus antibodies among latin american immigrants in Oregon, us. J Biosoc Sci. 42, 433–461 (2010). [DOI] [PubMed] [Google Scholar]
- Pierson D. L. et al. Epstein-Barr virus shedding by astronauts during space flight. Brain Behav Immun. 19, 235–242 (2005). [DOI] [PubMed] [Google Scholar]
- Bennett H. A. et al. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 103, 698–709 (2004). [DOI] [PubMed] [Google Scholar]
- Carr B. R. et al. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am J Obstet Gynecol. 139, 416–422 (1981). [DOI] [PubMed] [Google Scholar]
- Kerr J. L., Dattilo J. & O'Sullivan D. Use of recreation activities as positive coping with chronic stress and mental health outcomes associated with unemployment of people with disabilities. Work. 43, 279–292 (2012). [DOI] [PubMed] [Google Scholar]
- Segerstrom S. C. & Miller G. E. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull. 130, 601–630 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rethorst C. D., Wipfli B. M. & Landers D. M. The antidepressive effects of exercise: a meta-analysis of randomized trials. Sports Med. 39, 491–511 (2009). [DOI] [PubMed] [Google Scholar]
- Olff M. Stress, depression and immunity: the role of defense and coping styles. Psychiatry Res. 85, 7–15 (1999). [DOI] [PubMed] [Google Scholar]
- Christian L. M. et al. Epstein-Barr virus reactivation during pregnancy and postpartum: Effects of race and racial discrimination. Brain Behav Immun. 26, 1280–1287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. et al. Development of the Chinese age norms of CES-D in urban area. Chin Ment Health. 24, 139–143 (2010). [Google Scholar]
- Jiang Q. J. & Zhu Y. H. Further explorations for a coping style questionnaire. Chin J Behav Med. 8, 167–169 (1999). [Google Scholar]
- Zhou B. F. Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Chin J Epidemiol. 23, 5–10 (2002). [PubMed] [Google Scholar]