Abstract
The p53 tumour suppressor is activated in response to a wide variety of genotoxic stresses, frequently via post-translational modification. Using a knock in mouse model with a Ser312 to Ala mutation, we show here that phosphorylation of p53 on Ser312 helps to prevent tumour induction by the alkylating agent MNU, which predominantly caused T cell lymphomas. This is consistent with our previous observation that p53312A/A mice are more susceptible to X-ray induced tumourigenesis. Phosphorylation on Ser312 aids p53's interaction with E2F1, and enhances p53-mediated apoptosis. Loss of E2F1 alone does not affect tumour susceptibility to MNU, but its absence partially rescues tumour formation in p53312A/A mice, thus reflecting the oncogenic properties of E2F1. Our data confirms the participation of Ser312 phosphorylation in tumour suppression by p53.
The p53 pathway is one of the main lines of defence in response to carcinogenic stress, and p53 inactivating mutations frequently arise in tumours. Damage to DNA from radiation or carcinogens causes the stabilisation of p53, which then prevents the transmission of this damage by inducing cell cycle arrest, apoptosis or senescence. p53 is mutated in around 50% of cancers, and the familial Li-Fraumeni syndrome, which results in an increased susceptibility to cancers, is caused by the inheritance of mutations in the TP53 gene. The importance of p53 in tumour suppression is also reflected in the susceptibility of p53 null mice to spontaneous tumour formation, and the decreased resistance of p53+/− mice to γ-radiation exposure1,2,3.
E2F1, like p53, is a transcription factor that impacts upon the cell cycle, senescence, apoptosis and tumour growth, and both are pivotal in two canonical tumour suppression pathways. E2F1 drives entry of the cell into S-phase, and is restrained by its interaction with the tumour-suppressive retinoblastoma protein (Rb). Phosphorylation of Rb by cyclin dependent kinases releases E2F1, allowing it to activate genes required for entry into S-phase. The Rb/E2F pathway is frequently disrupted in tumours, either through loss of Rb, or increased phosphorylation of Rb either by inactivation of the cyclin dependent kinase inhibitor p16INK4a or amplification of cyclin D14. Because of its role in driving entry into the cell cycle, E2F1 was originally thought to be oncogenic. However, mice lacking E2F1 unexpectedly showed aberrant cell proliferation and tumour development, indicating that E2F1 also has tumour suppressive roles5,6.
There is much evidence of cross talk between the p53 and Rb/E2F1 pathways7. Both, for example, are regulated by the CDKN2A locus, which encodes the proteins p16INK4a and p19ARF. Whereas p16INK4A inhibits E2F1 by preventing phosphorylation of Rb, p19ARF stabilises p53 by inhibiting Mdm2, an E3 ligase that causes proteasomal degradation of p53. In turn, E2F1 can activate the expression of p19ARF and cause p53 stabilisation8. Co-operation between p53 and E2F1 can also occur independently of p19ARF. The N-terminus of E2F1 can interact directly with a region towards the C-terminus of p53, resulting in increased nuclear retention of p53 and p53-mediated transcription and apoptosis. This is inhibited by competition between p53 and cyclin A at the binding site within E2F19,10. The interaction between p53 and E2F1 is enhanced by phosphorylation of p53 on Ser315, a residue within the E2F1 binding region that is phosphorylated by cell cycle kinases such as cdk1, cdk2, cdk9 and Aurora kinase A11,12,13,14,15.
Ser315 is one of approximately 20 serine/threonine phosphorylation sites that have been identified within p53 that constitute part of a complex regulatory network of post-translational modifications16. Many of these sites are phosphorylated by kinases activated in response to cellular stress, and are located at the N-terminus, the region of p53 that is involved in its stability and transcriptional regulation17. Several mouse models have been made that incorporate inactivating mutations at these phosphorylation sites, and their phenotypes are generally mild17,18. Previously, we have characterised a knock in mouse model that carried a serine to alanine mutation on Ser312 (equivalent to human Ser315). Although these mice are healthy and have a normal lifespan, in response to ionising radiation they develop lymphomas and tumours in the liver19,20.
To determine the effect on tumour formation of both deleting E2F1 and preventing phosphorylation of p53 at Ser312, we crossed the p53Ser312Ala mice with E2F1 null mice and exposed them to a DNA alkylating agent, N-Methyl-N-nitrosourea (MNU). As was observed when these mice were exposed to ionising radiation, the p53312A/A mice are more susceptible to tumour formation. Loss of E2F1 alone did not render these mice more susceptible to tumours, but loss of E2F1 had a slightly protective effect upon the tumour formation seen in p53312A/A mice. Our study shows that in addition to protecting against tumours caused by ionising radiation, phosphorylation of p53 on Ser312 also contributes to the suppression of carcinogen-mediated tumourigenesis.
Results
We generated the mice for this study by intercrossing p53312A/+; E2F1+/− mice. The mice were born at the expected Mendelian frequency and showed no gross abnormalities (Table 1). Both males and females were born at close to the predicted numbers. None of the mice developed spontaneous tumours within the time frame examined.
Table 1. Birth ratios following intercross between p53312A/+; E2F1+/− mice. Figures in brackets represent predicted numbers based on Mendelian segregation.
| E2F1 | MALE | FEMALE | TOTAL | |
|---|---|---|---|---|
| p53WT | WT | 10 (9) | 8 (9) | 18 (17) |
| +/− | 24 (17) | 13 (18) | 37 (35) | |
| −/− | 6 (9) | 9 (9) | 15 (17) | |
| p53312A/+ | WT | 19 (17) | 20 (18) | 39 (35) |
| +/− | 41 (35) | 35 (35) | 76 (70) | |
| −/− | 16 (17) | 10 (18) | 26 (35) | |
| p53312A/A | WT | 12 (9) | 15 (9) | 27 (17) |
| +/− | 8 (17) | 18 (18) | 26 (35) | |
| −/− | 5 (9) | 10 (9) | 15 (17) | |
| 141 | 138 | 279 |
We previously observed that p53312A/A mice are more susceptible to tumour formation than wild type mice following exposure to ionising radiation19. To determine if these mice are susceptible to other carcinogens, and whether the presence or absence of E2F1 affects this susceptibility, we gave 4–6 week old mice a single dose of the alkylating agent N-Methyl-N-nitrosourea (MNU). The study group consisted of 18 wild type (7 females and 11 males), 13 p53312A/A (9 females and 4 males), 13 E2F1−/− (6 females and 7 males) and 12 p53312A/A; E2F1−/− (6 females and 6 males). The mice were culled either when they showed signs of poor health or at 20 weeks post-injection when the study was terminated.
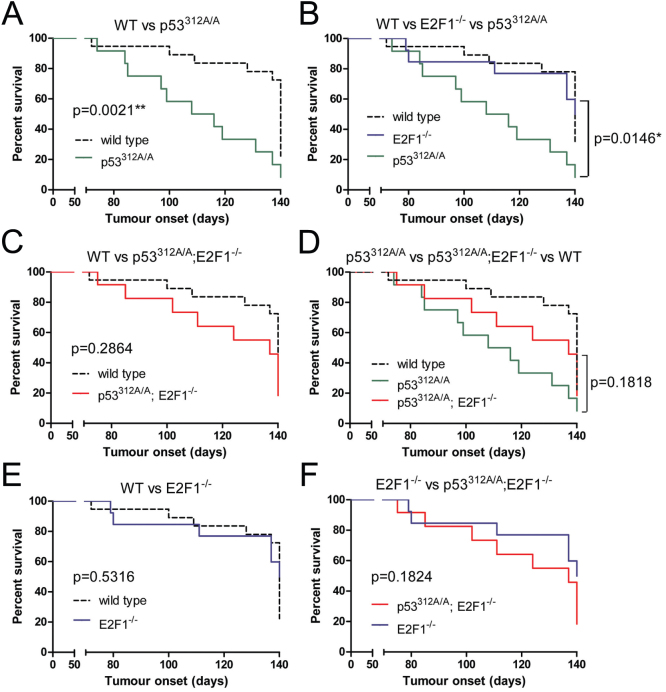
The mice began to develop tumours at 10–11 weeks post injection (Figure 1). Mice of all genotypes developed tumours, but p53312A/A mice were significantly more susceptible to tumours than both wild type and E2F1−/− mice, with p values of 0.0021 and 0.0146 respectively (Figure 1A and B). p53312A/A mice developed tumours within a shorter time frame than either wild type and E2F1−/− mice, and in the wild type mice in particular, the existence of a tumour was asymptomatic and only detected post-mortem at 20 weeks. Not all mice developed tumours within the time frame of this study, although only one p53312A/A mouse was tumour-free at 20 weeks. p53312A/A; E2F1−/− mice developed tumours earlier than wild type mice, although this was not significant (Figure 1C). Although fewer p53312A/A; E2F1−/− mice than p53312A/A mice developed tumours, again this difference was not significant (Figure 1D). There was little difference in tumour susceptibility between E2F1−/− and wild type mice (Figure 1E).
Figure 1. p53312A/A mice are more susceptible to tumour development following exposure to MNU than both wild type and E2F1−/− mice.
Kaplan-Meier survival curves illustrating the time taken to develop tumours following injection with MNU. p53312A/A mice are significantly more liable to develop tumours than both wild type (A) and E2F1−/− (B) mice (p < 0.05). In contrast p53312A/A; E2F1−/− mice are neither significantly more susceptible to tumours than wild type mice (C) nor significantly more resistant than p53312A/A mice (p > 0.05) (D). Loss of E2F1 alone had little effect upon tumour susceptibility compared with both wild type (E) and p53312A/A; E2F1−/− mice (F).
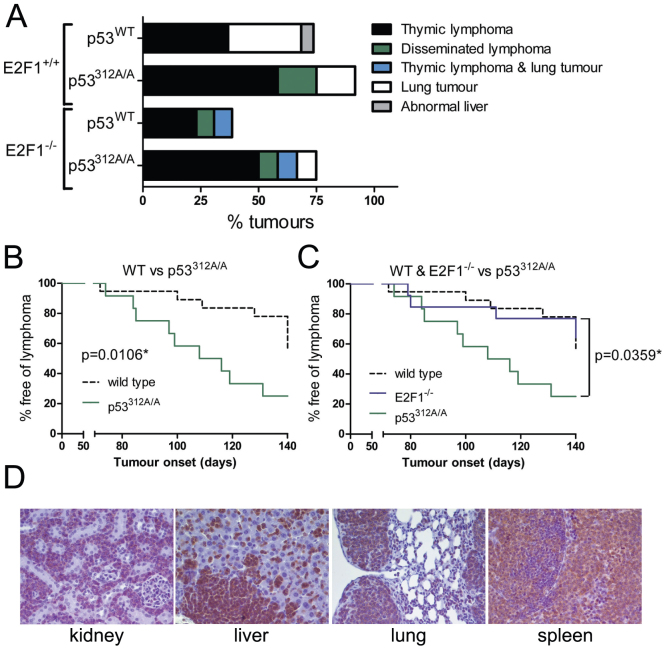
The predominant tumour type developed by mice of all genotypes was lymphoma, observable mainly as a grossly enlarged thymus that had lost its normal corticomedullary architecture, but also disseminated lymphomas that infiltrated organs such as the spleen, liver, kidney and lung (Figure 2A). This was observed as enlargement, pallor and mottling of the affected organs, with the thymus remaining unaffected. 8/19 (42.1%) wild type mice developed lymphomas, and 6/19 (31.6%) developed lung tumours which were almost always found at 20 weeks, with 5/19 (26.3%) showing no tumour development. One wild type mouse was found to have an abnormal liver at the end of the study. Of the p53312A/A cohort, 9/12 mice (75.0%) developed lymphomas, 2/12 (16.6%) had lung tumours and one mouse was tumour-free. 5/13 (38.5%) E2F1−/− mice developed lymphomas (including one mouse that had both thymic lymphoma and lung tumour), but 8/13 (61.5%) finished the study without developing tumours. 8/12 p53312A/A; E2F1−/− mice (66.7%) developed lymphomas, 2/12 (16.6%) developed lung tumours (again one mouse had both thymic lymphoma and a lung tumour) and 3/12 (25.0%) mice carried no tumours. Almost all the lung tumours (bronchioalveolar adenoma) were found at the end of the study and the incidence of lung tumours between the four groups was not significantly different (data not shown). Therefore, nearly all the p53312A/A mice developed tumours (91.6%), compared with 73.1% of wild type mice, 75% of p53312A/A; E2F1−/− mice and only 38.5% E2F1−/− mice.
Figure 2. p53312A/A mice are more susceptible to lymphomas following exposure to MNU.
(A) Tumour types found in mice exposed to MNU. p53312A/A mice are significantly more susceptible to MNU-induced lymphomas compared with both wild type (B) and E2F1−/− mice (C) (p < 0.05). (D) Infiltration of CD3- positive T cell lymphomas into the lung, kidney, liver and spleen in mice exposed to MNU.
Reflecting the trend of overall tumour incidence, p53312A/A mice were significantly more susceptible to lymphoma development than both wild type and E2F1−/− mice (p = 0.0106 and p = 0.0359 respectively) (Figures 2B and C). The median time of onset for lymphoma in p53312A/A mice was 112 days compared with over 140 days for wild type mice (fewer than 50% wild type mice had developed lymphomas at the end of the study). More p53312A/A; E2F1−/− mice developed lymphomas than wild type mice (Fig. 2A), although this was not significant (p = 0.194). All lymphomas were CD3 positive T cell lymphomas (Figure 2D): staining for the B cell marker CD45R/B220 was only found at a low level (data not shown).
In addition to thymic hyperplasia, lymphocytes were also frequently found in organs such as the kidneys, liver, lungs and spleen. Table 2 summarises according to genotype the organs where lymphocytes were found in mice carrying a thymic lymphoma. The extent of metastasis was similar for lymphomas from wild type, p53312A/A and p53312A/A; E2F1−/− mice. In addition to the organs listed in the table, lymphoma was found to have spread to the lymph nodes in two wild type mice and two p53312A/A mice.
Table 2. The extent of infiltration of peripheral organs by lymphocytes in mice that developed thymic lymphomas following exposure to MNU. An asterisk indicates sacrifice of the animal at the end of the study (20 weeks).
| Genotype | Mouse | Time of tumour onset | Lung | Liver | Kidney | Spleen |
|---|---|---|---|---|---|---|
| wild type | 458 | 128 | Y | Y | Y | Y |
| 483 | 100 | - | - | - | - | |
| 497 | 140* | Y | - | - | - | |
| 527 | 72 | Y | Y | Y | Y | |
| 555 | 140* | Y | Y | Y | Y | |
| 600 | 140* | - | - | - | - | |
| 616 | 140* | Y | - | Y | - | |
| 619 | 140* | Y | Y | Y | Y | |
| 670 | 109 | Y | Y | Y | Y | |
| p53312A/A | 467 | 115 | - | Y | - | Y |
| 470 | 116 | Y | Y | Y | Y | |
| 489 | 108 | Y | Y | Y | Y | |
| 511 | 85 | Y | - | - | - | |
| 520 | 97 | - | - | - | Y | |
| 537 | 131 | Y | Y | Y | Y | |
| 584 | 74 | Y | Y | Y | - | |
| E2F1−/− | 479 | 80 | Y | Y | Y | Y |
| 545 | 111 | - | - | - | - | |
| 562 | 140* | - | - | - | - | |
| 590 | 140* | Y | Y | Y | Y | |
| p53312A/A; E2F1−/− | 456 | 137 | - | - | - | - |
| 506 | 102 | Y | Y | Y | Y | |
| 521 | 75 | Y | Y | Y | Y | |
| 567 | 140* | Y | - | - | Y | |
| 575 | 111 | Y | Y | Y | Y | |
| 580 | 124 | Y | Y | Y | Y | |
| 663 | 85 | - | - | Y | - |
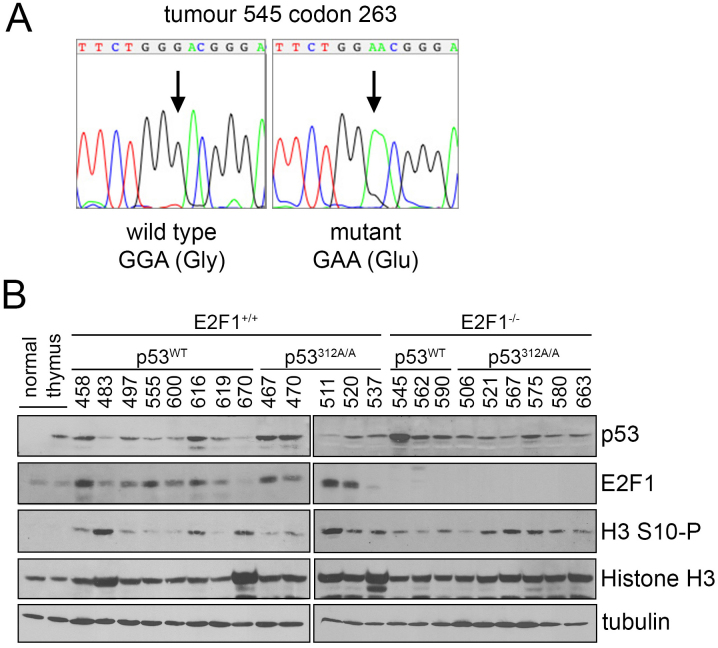
The p53 cDNAs from 22 of the tumours isolated in this study were extracted and sequenced, and tumour 545 (E2F1−/−) was the only tumour found to have a mutation in the p53 open reading frame (GGA → GAA/Gly → Glu at codon 263) (Figure 3A). To analyse p53 levels in MNU-induced lymphomas, lysates were prepared from these tumours for analysis by immunoblotting (Figure 3B). p53 expression was retained in all the tumours analysed. The levels of total p53 were not especially high, except for tumour 545, which correlates with it carrying an inactivating mutation. Phosphorylation on p53 serine 312 could not be detected (data not shown), as found previously when trying to detect phosphoserine 312 in mouse embryonic fibroblasts19. E2F1 levels were also examined. E2F1 levels were low in normal thymus, but were elevated in some of the tumours derived from mice with wild type E2F1, indicating that E2F1 conferred a growth advantage in these circumstances, especially where p53 was mutant. Those tumours from the p53312A/A cohort that expressed high levels of E2F1 (e.g. numbers 467, 511 and 520) tended to die at an earlier time point and also with less lymphocyte spread to peripheral organs than those with lower levels of E2F1 (e.g. number 537) (Table 2). Other than where p53 was mutant (545), loss of E2F1 did not substantially elevate p53 levels.
Figure 3. Variations in protein expression in thymic lymphomas derived from mice exposed to MNU.
(A) Chromatogram showing the Gly to Glu point mutation found in tumour 545. (B) Immunoblots of tumour lysates derived from thymic lymphomas arising in mice exposed to MNU. Larger versions of these blots are shown in Supplementary Figure 1.
To analyse proliferation levels in this panel of tumours, phosphorylation of Histone H3 on serine 10 was used as a marker of mitosis (Figure 3B). There appeared to be little correlation between levels of histone phosphorylation and genotype, other than lower p53 levels correspond with higher levels of phosphorylation as would be predicted from p53's role in preventing cell cycle progression.
Taken together, this data reinforces our previous finding that preventing phosphorylation of p53 on serine 312 reduces its efficacy as a tumour suppressor, as alteration of this residue to alanine both increases the proportion of mice that develop tumours in response to the carcinogen MNU and reduces time of onset.
Discussion
In this study we analysed the susceptibility to carcinogen-mediated tumour formation of mice lacking the ability to phosphorylate p53 on Ser312, and the impact upon this of E2F1 loss. We found that p53312A/A mice are significantly more susceptible to tumour development than both wild type and E2F1−/− mice in response to the alkylating agent MNU. While p53312A/A mice are more susceptible than either wild type or E2F1−/− mice, this is not significant, and they are less vulnerable to tumours than p53312A/A mice.
MNU has been widely used in rodent tumour models and has been reported to cause tumours in a range of tissues including the gastrointestinal tract, lung, nervous system, pancreas and mammary glands21. The tumours observed in this study were predominantly lymphomas. Lymphomas, especially of the thymus, are a common tumour type found in mice carrying mutations in the p53 pathway1,2, and in a previous study such lymphomas were found in p53312A/A mice exposed to ionising radiation19. Lymphomas are also the predominant tumour type arising in p53+/− mice dosed with MNU22,23,24,25. As in the present study, the lymphomas in p53+/− mice were CD3-positive and B220 negative22. Although the predominant cause of death in p53+/− mice was lymphoma, other tumour types that were not seen in our study were also found, principally adenomas and adenocarcinomas of the intestine. Conversely, no lung neoplasms were observed in these other studies22,23,24,25. This may be attributable to strain differences, as these studies were all carried out using mice with a C57BL/6 background, whereas the mice used in this study were on a mixed genetic background.
Also, the time of tumour onset for the p53+/− mice is comparable to that seen with the p53312A/A mice used in this study: the earliest onset with the p53+/− mice is between 56 days and 75 days22,23 versus 74 days for the p53312A/A mice, and a median survival of 106 days24 compared with 112 days with the p53312A/A mice. This is akin to the similarity in time of tumour onset for irradiated p53+/− and p53312A/A mice3,19. p53 is thought to suppress tumours in the thymus by inducing apoptosis, as p53 null mice develop spontaneous lymphomas, and thymocytes derived from these mice are resistant to apoptosis induced by DNA damage1,2,26,27. However, thymocytes derived from p53312A/A mice are not resistant to DNA damage and p53312A/A mice do not develop lymphomas spontaneously19. A previous study that exposed p53+/− mice to MNU found that many of the resulting tumours had lost the remaining p53 allele25, however all the tumours analysed in the current study retained p53 expression and p53 was only found to be mutated in one instance, a G263E mutation in the DNA binding domain (Figure 3). This suggests that p53Ser312 phosphorylation is suppressing tumours by other means. Likewise, thymocytes from E2F1−/− mice are also sensitive to DNA damage-induced apoptosis28. It should be noted that mice carrying mutations at other p53 phosphorylation sites, e.g. Ser18, Ser23, or Ser389, have not been reported to develop T cell lymphomas29,30,31. However, it is very rare to find p53 phosphorylation sites mutated in tumours. These phosphorylation sites, as well as Ser312, lie outside the DNA binding domain of p53 which is the region where most tumour-derived mutations occur. Missense mutations within this region prevent p53 from binding to DNA and altering gene expression, whereas p53 phosphorylation mutants all retain some transcriptional activity19,31,32,33,34. Mice that have had inactivating tumour-derived mutations introduced e.g. p53R172H and p53R270H mice, spontaneously display a range of tumour types even when heterozygous35,36, reflecting the important contribution made by p53 mutations in the DNA binding domain towards tumour growth. Interestingly, the G263E mutation found in our study (equivalent to human G266E) is a deleterious mutation that has been found in tumours derived from tissues including lung and breast, although not at a high rate37.
Deletion of E2F1 in the mice used in this study did not affect their susceptibility to tumour development in comparison with wild type mice (Figures 1E, 2C). p53312A/A; E2F1−/− mice developed more tumours than E2F1−/− mice, but fewer than p53312A/A mice (Figures 1D, 1F), although not to a significant degree. p53, when phosphorylated on Ser312, has been shown to compete with cyclin A in its interaction with E2F19. In the wild type scenario, phosphorylation of p53 on Ser312 enables interaction with E2F1 which leads to increased nuclear retention of p53, cell death and tumour suppression10. In the absence of Ser312 phosphorylation in p53312A/A mice, this death pathway should fail to activate, facilitating cell survival and promoting tumour development. With E2F1−/− mice, wild type p53 is still able to cause apoptosis by E2F1-independent means, as the Ser312Ala mutation is only subtle and does not abrogate the majority of p53's functions19. However while the p53-phosphoSer312-E2F1 death pathway cannot activate in p53312A/A; E2F1−/− mice, the loss of E2F1 may also restrict proliferation and counteract tumour formation when compared to p53312A/A mice, where E2F1 is present. This is indicated by the relationship between E2F1 levels and lymphocyte infiltration with or without E2F1 in p53312A/A mice. The primary symptom of thymic lymphoma that requires an animal to be sacrificed is laboured respiration due to constriction of the lungs by the tumour. As shown in Figure 3B and Table 2, p53312A/A mice with tumours with high E2F1 levels tended to have shorter lifespans and thymic lymphomas that did not infiltrate other organs (e.g. 511 and 520), perhaps suggesting that the tumours in these mice grew to a critical size within the thoracic cavity before lymphocyte migration to other organs was able to occur. In contrast p53312A/A mice with low E2F1 levels (e.g. 537) died at a later stage with increased lymphocyte infiltration. This is reflected in the double mutant p53312A/A; E2F1−/− mice, most of which also displayed extensive lymphocyte infiltration.
Data generated from studies that have crossed p53 null mice with E2F1 null mice have proved inconsistent. In one study, the deletion of E2F1 abolished the susceptibility of p53−/− mice to spontaneous thymic lymphomas28, whereas another study found that the loss of E2F1 had no effect38. One likely explanation is that two different E2F1 knockout mouse strains were used in these studies. The strain used by Wikonkal et al5,28. is also the strain used in the present study, so the partial rescue of the tumour susceptibility of p53312A/A mice by E2F1 deletion is consistent with their observations. Genetic background may also play a part, as another study crossing p53−/− mice with the E2F1−/− mice described by Field et al. but on a different strain background found that although overall survival was not affected in p53−/−;E2F1−/− mice, the number of mice developing lymphomas was reduced with a corresponding increase in sarcomas and carcinomas5,39.
In conclusion, we observed an increased susceptibility to lymphoma formation induced by MNU in p53 knock in mice carrying a Ser312 to Ala mutation. This strengthens our previous observation that these mice are susceptible to tumourigenesis after exposure to ionising radiation. Thus, phosphorylation on Ser312 enables p53 to fully function as a tumour suppressor.
Methods
Mice, tumour induction and analysis
E2F1 null mice described by Field et al. were obtained from The Jackson Laboratory5. p53312A/A knock in mice were described previously19. The mice were on a mixed C57BL/6; 129/Sv; FVB strain background. All animal breeding, maintenance and procedures were approved by the University of Oxford ethical committee and licensed by the U.K. Home Office.
4–6 week old mice were given a single intraperitoneal dose of N-Methyl-N-nitrosourea (MNU) (Sigma) in citrate buffered saline at 50 mg/kg. The mice were sacrificed either upon ill health or at 20 weeks post-injection. Following sacrifice, tissues were fixed and embedded in paraffin for histological analysis as described previously40 or frozen on liquid nitrogen for biochemical analysis. Statistical significance was determined using the Log-rank (Mantel-Cox) test and GraphPad Prism software.
Immunohistochemistry and immunoblotting
Antibodies used were: anti-CD3 and β-tubulin (Abcam); anti-CD45R/B220 (BD Pharmingen); anti-p53 CM5 (Leica); anti-E2F1 and Histone H3 (Cell Signalling Technologies); Histone H3 phospho-Ser10 (Millipore).
Blocking Reagent (Roche) was used to block tissue sections and as antibody vehicle. Signal was developed using the ABC system and diaminobenzidine (Vector Laboratories). Tumour lysates were prepared by homogenising tissue samples in NETN buffer (50 mM Tris pH8.0, 150 mM NaCl, 1 mM EDTA, 1% NP40) containing protease and phosphatase inhibitors.
Author Contributions
The study was designed by E.A.S. and X.L. E.A.S. performed the experiments and analysed the data. The manuscript was written by E.A.S.
Supplementary Material
Supplementary Figure 1
Acknowledgments
This research was funded by the Ludwig Institute for Cancer Research. We would like to thank Indrika Ratnayaka for technical assistance.
References
- Donehower L. A. et al. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356, 215–221 (1992). [DOI] [PubMed] [Google Scholar]
- Jacks T. et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 4, 1–7 (1994). [DOI] [PubMed] [Google Scholar]
- Kemp C. J., Wheldon T. & Balmain A. p53-deficient mice are extremely susceptible to radiation-induced tumorigenesis. Nat Genet 8, 66–69 (1994). [DOI] [PubMed] [Google Scholar]
- Burkhart D. L. & Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer 8, 671–682 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field S. J. et al. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell 85, 549–561 (1996). [DOI] [PubMed] [Google Scholar]
- Yamasaki L. et al. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell 85, 537–548 (1996). [DOI] [PubMed] [Google Scholar]
- Polager S. & Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer 9, 738–748 (2009). [DOI] [PubMed] [Google Scholar]
- Bates S. et al. p14ARF links the tumour suppressors RB and p53. Nature 395, 124–125 (1998). [DOI] [PubMed] [Google Scholar]
- Hsieh J. K. et al. Novel function of the cyclin A binding site of E2F in regulating p53-induced apoptosis in response to DNA damage. Molecular and cellular biology 22, 78–93 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogal V., Hsieh J. K., Royer C., Zhong S. & Lu X. Cell cycle-dependent nuclear retention of p53 by E2F1 requires phosphorylation of p53 at Ser315. Embo J 24, 2768–2782 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff J. R., Friedman P. N., Marshak D. R., Prives C. & Beach D. Human p53 is phosphorylated by p60-cdc2 and cyclin B-cdc2. Proc Natl Acad Sci U S A 87, 4766–4770 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. & Prives C. Increased and altered DNA binding of human p53 by S and G2/M but not G1 cyclin-dependent kinases. Nature 376, 88–91 (1995). [DOI] [PubMed] [Google Scholar]
- Katayama H. et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet 36, 55–62 (2004). [DOI] [PubMed] [Google Scholar]
- Radhakrishnan S. K. & Gartel A. L. CDK9 phosphorylates p53 on serine residues 33, 315 and 392. Cell Cycle 5, 519–521 (2006). [DOI] [PubMed] [Google Scholar]
- Qu L. et al. Endoplasmic reticulum stress induces p53 cytoplasmic localization and prevents p53-dependent apoptosis by a pathway involving glycogen synthase kinase-3beta. Genes Dev 18, 261–277 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray-Zmijewski F., Slee E. A. & Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat Rev Mol Cell Biol 9, 702–712 (2008). [DOI] [PubMed] [Google Scholar]
- Jenkins L. M., Durell S. R., Mazur S. J. & Appella E. p53 N-terminal phosphorylation: a defining layer of complex regulation. Carcinogenesis 33, 1441–1449 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo F. & Wahl G. M. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 6, 909–923 (2006). [DOI] [PubMed] [Google Scholar]
- Slee E. A. et al. Phosphorylation of Ser312 contributes to tumor suppression by p53 in vivo. Proc Natl Acad Sci U S A 107, 19479–19484 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slee E. A. & Lu X. In the right place at the right time: analysis of p53 serine 312 phosphorylation in vivo. Cell Cycle 10, 1345–1346 (2011). [DOI] [PubMed] [Google Scholar]
- National Institute of Environmental Health Sciences. Report on Carcinogens: Carcinogen Profiles (National Institute of Environmental Health Sciences, Durham, N.C., 2000). [Google Scholar]
- Morton D. et al. N-Methyl-N-Nitrosourea (MNU): A positive control chemical for p53+/− mouse carcinogenicity studies. Toxicol Pathol 36, 926–931 (2008). [DOI] [PubMed] [Google Scholar]
- Hoivik D. J. et al. Studies evaluating the utility of N-methyl-N-nitrosourea as a positive control in carcinogenicity studies in the p53+/− mouse. Int J Toxicol 24, 349–356 (2005). [DOI] [PubMed] [Google Scholar]
- Reese J. S., Allay E. & Gerson S. L. Overexpression of human O6-alkylguanine DNA alkyltransferase (AGT) prevents MNU induced lymphomas in heterozygous p53 deficient mice. Oncogene 20, 5258–5263 (2001). [DOI] [PubMed] [Google Scholar]
- Ohgaki H. et al. Effect of intragastric application of N-methylnitrosourea in p53 knockout mice. Mol Carcinog 28, 97–101 (2000). [DOI] [PubMed] [Google Scholar]
- Clarke A. R. et al. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature 362, 849–852 (1993). [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A. & Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362, 847–849 (1993). [DOI] [PubMed] [Google Scholar]
- Wikonkal N. M. et al. Inactivating E2f1 reverts apoptosis resistance and cancer sensitivity in Trp53-deficient mice. Nat Cell Biol 5, 655–660 (2003). [DOI] [PubMed] [Google Scholar]
- Hoogervorst E. M. et al. Lack of p53 Ser389 phosphorylation predisposes mice to develop 2-acetylaminofluorene-induced bladder tumors but not ionizing radiation-induced lymphomas. Cancer Res 65, 3610–3616 (2005). [DOI] [PubMed] [Google Scholar]
- Chao C., Herr D., Chun J. & Xu Y. Ser18 and 23 phosphorylation is required for p53-dependent apoptosis and tumor suppression. Embo J 25, 2615–2622 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson D. et al. Defective apoptosis and B-cell lymphomas in mice with p53 point mutation at Ser 23. Embo J 23, 3689–3699 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao C. et al. Cell type- and promoter-specific roles of Ser18 phosphorylation in regulating p53 responses. J Biol Chem 278, 41028–41033 (2003). [DOI] [PubMed] [Google Scholar]
- Wu Z. et al. Mutation of mouse p53 Ser23 and the response to DNA damage. Molecular and cellular biology 22, 2441–2449 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruins W. et al. The absence of Ser389 phosphorylation in p53 affects the basal gene expression level of many p53-dependent genes and alters the biphasic response to UV exposure in mouse embryonic fibroblasts. Molecular and cellular biology 28, 1974–1987 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G. A. et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 119, 861–872 (2004). [DOI] [PubMed] [Google Scholar]
- Olive K. P. et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 119, 847–860 (2004). [DOI] [PubMed] [Google Scholar]
- Petitjean A. et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat 28, 622–629 (2007). [DOI] [PubMed] [Google Scholar]
- Wloga E. H., Criniti V., Yamasaki L. & Bronson R. T. Lymphomagenesis and female-specific lethality in p53-deficient mice occur independently of E2f1. Nat Cell Biol 6, 565–567; author reply 567–568 (2004). [DOI] [PubMed] [Google Scholar]
- Palacios G., Talos F., Nemajerova A., Moll U. M. & Petrenko O. E2F1 plays a direct role in Rb stabilization and p53-independent tumor suppression. Cell Cycle 7, 1776–1781 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vives V. et al. ASPP2 is a haploinsufficient tumor suppressor that cooperates with p53 to suppress tumor growth. Genes Dev 20, 1262–1267 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1