Abstract
Acute asthma exacerbations are one of the most common reasons for pediatric emergency department (PED) visits and hospitalizations, and relapse frequently necessitates repeat urgent care. While care plans exist, there are no acute asthma prediction rules (APR)to assess severity and predict outcome. The primary objective of the Acute Asthma Severity Assessment Protocol (AASAP) study is to develop a multivariable APR for acute asthma exacerbations in the pediatric patient.
We are enrolling a prospective, convenience sample aged 5 to 17 years with acute asthma exacerbations who present to an urban, academic, tertiary PED. The study protocol and data analysis plan conform to accepted biostatistical and clinical standards for clinical prediction rule development. Modeling of the APR will be performed once the entire sample size of 1,500 has accrued. We anticipate that the APR will improve resource utilization in the emergency department, aid in standardization of disease assessment, and allow physician and non-physician providers to participate in earlier objective decision making. The objective of this report is to describe the study objectives and detailed methodology of the AASAP study.
Keywords: MeSH:, Asthma, Dyspnea, Treatment outcome, Non-MeSH:, Asthma severity, pediatric, spirometry
Introduction
Asthma is the most common serious chronic disease of childhood and is the most frequent reason for childhood hospitalization in the United States.1-3 It accounts for almost two million ED admissions annually and an ED relapse rate of 7-15%.4 The economic costs of asthma are substantial and were responsible for direct health care costs of $11.5 billion and indirect costs of $4.6 billion in 2004 dollars, second only to expenditures for mental disorders in children.1,5,6 The Centers for Disease Control considers asthma to be a “critical clinical and public health problem,” and it is the focus of multiple objectives of Healthy People 2010, including reduction of asthma deaths, hospitalizations and ED visits.2,7
A challenging feature of this complex genetic and environmental disease is the great variability of clinical expression and response to treatment.8 Across the spectrum of acute asthma severity, those with severe disease are readily identified. In this regard, Geelhoed and colleagues have demonstrated that oxygen saturation on presentation for emergent care is highly predictive of poor outcome.9 However, for the remaining majority of patients there is great variability, with few objective measures to assess severity and predict outcome.
The challenge of assessing acute asthma is substantial in the ED practice environment, one in which clinicians encounter undifferentiated problems of varying acuity, high decision density, and cognitive loading.10-13 Additionally, there is frequent variability in diagnosis and management and a lack of standardization in decision making for asthma and other commonly encountered but potentially complex and high-risk diseases.14 This results in variability of management that may result in variable length of stay and disposition decisions.
Emergency Medicine physicians recognize the need for heuristics and other approaches to improve clinical performance in the ED environment. Croskerry has described these approaches and has developed cognitive forcing strategies for specific situations to optimize decision making and to avoid error.11 These deliberate strategies include the clinical prediction rule (CPR), defined by Laupacis and colleagues as “a decision-making tool, which is derived from original research and incorporates three or more variables from the history, physical examination, or simple tests.”15 A CPR assists clinicians in dealing with uncertainty in clinical decision making as well as in predicting prognosis and enhancing the efficiency of resource utilization. The acute asthma exacerbation is a condition that fulfills each of five criteria that Stiell identified to determine whether there is a need for a CPR.16
A CPR is developed by applying statistical techniques to determine combinations of predictors that describe heterogeneous groups of patients as risk groups. The development, validation and use of CPRs have increased in tandem with the evolution of evidence-based medicine, patient safety, and the need to improve resource allocation. Significant contributions to the refinement of CPRs were clinical and biostatistical methodological standards for their development.17-27
The primary aim of the Acute Asthma Severity Assessment Protocol (AASAP)study is to develop a multivariable APR conforming to these standards.21,25,28 The APR will be a CPR and will be developed in accordance with the CPR biostatistical and clinical standards using candidate predictor variables. These will include participant demographic and asthma characteristics, physical exam findings, and measures of lung function and inflammation. Certain of these measures are not yet widely available in the ED environment. However, if they are found to be predictive of outcome efforts to implement their use may be warranted because the use of a CPR will serve to improve patient care and resource utilization.
Study Objectives
The primary objective of the Acute Asthma Severity Assessment Protocol (AASAP) study is to develop a multivariable APR for acute asthma exacerbations in the pediatric patient. The objective of this report is to describe the study objectives and detailed methodology of the AASAP study.
Study Rationale
There is an overall lack of validated, accurate and objective methods for assessment of acute asthma exacerbations. A multivariable APR may improve severity assessment and outcome prediction, improve management and resource utilization, and facilitate disposition decisions. Development of an APR might be of greatest use for patients with moderate exacerbations, as these patients present the greatest management dilemma in terms of the decisions for hospital admission or discharge.
Material and Methods
Setting, Study Population and Participant Recruitment
We are conducting a prospective study of pediatric participants with acute asthma presenting to a tertiary care pediatric emergency department (PED), in order to develop an APR. Inclusion criteria are age 5 to 17 years, doctor-diagnosed asthma, signs or symptoms of an asthma exacerbation (cough, dyspnea, wheezing and/or chest pain),29 need for treatment with systemic corticosteroid (CCS) and inhaled bronchodilator as determined by the attending physician, and availability for phone follow-up if discharged to home. We exclude participants with chronic lung disease other than asthma, foreign body aspiration, or other reason for pertinent signs and symptoms. The clinical team is comprised of physicians (emergency medicine and pediatric emergency medicine faculty; pediatric and emergency medicine resident; and pediatric emergency medicine fellows), pediatric nurses and respiratory therapists.
Participants are recruited from 7am to 10pm on each weekday and on approximately every third weekend day. An electronic patient logis programmed to immediately notify us by pager when a patient meeting screening criteria is triaged or admitted to the PED. All pharmacologic treatments administered and in-hospital management information (for participants admitted to hospital) are ascertained through an electronic medical record and order system (EMR).Data collection time points include baseline, 2- and 4-hour assessments, and phone follow-up at 48 to 72 hours for participants discharged to home to determine 48 hour relapse rate and parental perception of participant improvement.
All study data are collected by the principal investigator or research assistant. The research assistant was trained by the principal investigator in the study protocol. Both investigators have received training from and have ongoing supervision in the PED from a pediatric pulmonary function lab technician in the performance of portable spirometry
Human Subjects Protection
The institutional review board (IRB) reviewed and approved this as an expedited, minimal risk study protocol. The IRB has granted a waiver of immediate informed consent that enables the investigators to acquire baseline data prior to initiation of systemic corticosteroid and bronchodilator treatments. After baseline data acquisition, informed consent from the legal guardian and assent from the participant are obtained. If consent/assent are not obtained, the participant is not enrolled. The clinical team (i.e., physicians, nurses, respiratory therapists) maintains exclusive, primary decision making capacity in order to assure that patient care is not compromised.
Study data is not made available to the clinical team. This is necessary to avoid influencing clinical decisions made by the clinical team. As such, there is no immediate benefit to the participant.
Examination Components at Baseline, 2-hours and 4-hours and Phone Follow-up
Study time points
Predictor variables are acquired at baseline, defined as after triage but before systemic CCS treatment, and at 2 to 4 hours after administration of systemic CCS.30-32 The 4-hour time point was chosen because beneficial physiologic effects from systemic CCS treatment are likely to become apparent by 4 hours post administration.31-33
Baseline
At baseline we record past medical history, family asthma history, social and demographic information, coexisting illness, medications in use, asthma symptom history, Global Initiative for Asthma (GINA) chronic asthma control for the preceding 3-months,34 prior PICU admission or prior endotracheal intubation for asthma, second-hand smoke exposure, dog or cat exposure, insurance coverage, type of health care provider managing the participant's asthma, parent preference regarding in-hospital or outpatient management of the exacerbation, and parent education level. Clinical variables measured and recorded at baseline include the participant's degree of breathlessness as determined by ability to speak (complete, short or partial sentences, words, or no speech) and calculation of the Pediatric Respiratory Assessment Measure (PRAM). The12-point PRAM has been validated against airway resistance by forced oscillation in preschool children and had good performance characteristics in children aged 2 to 17 years with acute asthma exacerbations.35,36
Baseline, 2- and 4-hour variables
The following clinical variables are measured and recorded at each time point. Oxygen saturation (Sp02) is determined on room air as a 10 minute mean using a Masimo Radical-7 pulse co-oximeter (Masimo Corp., Irvine, CA). Methemoglobin, carboxyhemoglobin, and plethysmograph variability index (PVI) are also recorded as 10 minute means.37
We measure respiratory rate with the participant at rest and on room air by counting the number of capnometry waveforms for a full minute using an Oridion Pediatric Microstream end-tidal carbon dioxide (EtC02) sensor (Oridion Corp, Needham MA) and Philips MP60 bedside monitor (Philips Corp, Boeblingen, Germany).For subjects who cannot tolerate capnometry, respiratory rate is determined by a 1-minute count of respirations during auscultation of the chest. ETCO2 is also recorded by the investigator from a 1-minute capnometry output.
Auscultation of the chest is performed using a Littman Master Classic II stethoscope (3M Corp., St. Paul, MN). Auscultatory findings recorded included inspiratory to expiratory ratio, air entry, and wheezing. Accessory muscle use is defined as any visible use of the scalene, sternocleidomastoid, suprasternal, intercostal or subcostal muscles and is recorded by individual muscle group.35
Airway resistance is measured using a MicroDirect MRT6000 module (Micro Medical, Kent, England). We apply a nose clip and instruct the participant to perform comfortable, tidal volume breathing through the mouthpiece (SpiroSafe, Micro Medical) and to keep the tongue against the floor of the mouth. We support the cheeks and submental tissue and extend the neck slightly to achieve the ‘sniffing’ position.38 Five measurements are made during exhalation and the median value recorded. The device outputs percent predicted values for participants ages 2 to 10 years (%Rint) using the McKenzie standards and absolute values (kPa/l/sec) for participants 11 years of age and above (aRint).39
Exhaled nitric oxide (eNO), an indirect measure of airway inflammation, is measured using a NIOX MINO Airway Inflammation Monitor (Aerocrine AB, Solna, Sweden). The participant is instructed to inhale through the device mouthpiece from residual volume to total lung capacity and to then exhale through the device at a steady flow rate. The device provides user feedback with audible and light signals to enable the participant to exhale at an appropriate and steady flow rate.40 This also closes the soft palate to prevent contamination with nasal air. A 6-second exhalation time is used.41 One determination is obtained at each time point.42The aforementioned measurements are made prior to spirometry because the forced vital capacity (FVC) maneuvers required for spirometry may alter airway tone and airway resistance measures.43
Spirometry is performed using a MicroDirect MicroLoop spirometer (Micro Medical, Kent, England). We calibrate the spirometer each day using a 3-liter syringe. A nose clip is applied, and we instruct each participant to perform forced vital capacity(FVC) maneuvers in accordance with American Thoracic Society (ATS) 1994 spirometry standards.44 Participants are studied in the sitting position. Each series of sequential FVC maneuvers performed at a study time point is termed a trial. %FEV1 and other parameters are calculated based on Knudson standards.45,46
We attempt to obtain three FVC maneuvers that meet ATS acceptability criteria for each trial.44 ATS guidelines also include reproducibility criteria for these maneuvers but note that “use of data from maneuvers with poor reproducibility is left to the discretion of the interpreter.” This is because patients with airways obstruction may have greater coefficients of variation and for this reason may not meet full reproducibility criteria.47 Eliminating trials from these participants may result in systematic bias in the results reported.
Some participants cannot perform trials with 3 FVC maneuvers that meet full ATS criteria. Some of these trials include at least one FVC maneuver with flow-volume and volume-time curves meeting ATS criteria for start-of-test and end-of-test. A standing pulmonary function test oversight committee reviews these trials to determine whether the data should be included for analysis. This committee is comprised of a pulmonologist-pulmonary physiologist and a respiratory therapist-pediatric pulmonary function technician. Each member records their determination whether a non-ATS trial should be retained for analysis based on the flow-volume and volume-time curves of the available FVC maneuvers. Committee members are blinded to the other member's determination and to all other participant data. A trial is included if both members determined independently that it is of high quality.
Asthma medication administration
We record all treatment administered during each time interval (0-2hr, 2-4hr, 4-6hr). These include systemic CCS, albuterol (MDI, one-time/continuous nebulization), magnesium, terbutaline, epinephrine, aminophylline, heliox, bilevel positive airway pressure (BiPAP), continuous positive pressure (CPAP), and/or endotracheal intubation and mechanical ventilation. For participants admitted to hospital, site of admission (PICU or floor) is recorded.
Ascertainment of disposition
For participants admitted to hospital, site of admission (PICU or floor) is recorded. Hospital length of stay (LOS)is ascertained in 0.5 hour units from the EMR. For participants admitted to the PICU this is determined from the time a critical care consultation order is placed to the time when a discharge to home order is placed. Initiating this time period from the time of the consultation allows the LOS to begin when the PED attending determines that the participant requires critical care. For participants admitted to the floor, LOS is the time from the admission order to the discharge order.
Ascertainment of relapse
For participants discharged to home, follow-up phone calls are made by one of the investigators at 48 to 72 hours after discharge from the PED. If the parent cannot be contacted after 3 phone calls, a letter requesting that the parent call the investigator is sent to the recorded home address. If there is no response to this letter the participant's data will not be included in analyses.
Scripted phone questions included whether the parent thinks the participant's asthma is improved, if the parent and/or PED physicians requested a recheck visit with the participant's physician, if the participant required an unscheduled recheck to a physician or emergency department and, if so, if this was because the participant was not improved or worse. Finally, if the participant was been prescribed an oral CCS in the PED, we query whether the prescription had been filled and if the participant was taking the medication.
Standardization of Pediatric Emergency Department and In-hospital Management
Acute asthma management in our PED is administered collaboratively by nurses, respiratory therapists (RTs), and physicians using a protocol derived from national guidelines and current best evidence.48,49 The PED has a minimum of 2 dedicated RTs at all times who perform assessments and adjustment of albuterol treatment Q 1-2 hours. In-hospital albuterol treatment and weaning are administered by RTs using a similar protocol.
Selection of Candidate Predictor Variables
Predictor variables for an APR must be well-defined, available in the clinical setting, and enter the scoring system consistent with the manner in which each predictor becomes available clinically.15,16,20,26 It is widely accepted that the best approach is to choose a set of candidate predictor variables based upon clinical and biological plausibility and on information from previous studies, referred to as fitting a pre-specified model.20,50,51 We have identified candidate predictor variables for APR development that are consistent with these standards (Table 1).
Table 1. Candidate predictor and outcome variables for asthma prediction rule modeling.
| Candidate Predictor Variable | df1 | Ascertainment & Definition |
|---|---|---|
| Age | 2 | Years (calculated to 0.01 yr) |
| Gender | 1 | Male/female |
| Race2 | 2 | White; Black; Asian; American indian/Alaskan; Native Hawaiian/Pacific islander |
| Asthma control (3-Month) | 1 | GINA guidelines (Yes/No) |
| Prior adverse event | 1 | Prior PICU admission (Yes/No) |
| Respiratory rate | 1 | Breaths/minute (>97.5% ile for age) |
| Degree of breathlessness | 3 | Complete sentences; short sentences; partial sentences; words; no speech |
| Accessory muscle use | 1 | Present/Absent |
| Oxygen saturation by pulse oximetry | 2 | % value |
| Oximeter plethysmograph variability index | 2 | % value |
| Airway resistance | 2 | % predicted or kPa/L/s |
| Forced expiratory volume in 1 second | 2 | % predicted |
| End-tidal carbon dioxide | 2 | mm Hg |
| Exhaled nitric oxide | 2 | Parts per billion |
| Total df | 24 | |
| Primary Outcome Variable | ||
| Need for hospitalization | Length of stay > 24 hours (for admitted subjects) or unscheduled return for asthma care to a physician or hospital within 48 hours (for discharged subjects) | |
| Secondary Outcome Variables3 | ||
| Length of stay > 24 hours | Yes/No | |
| Length of stay | Hours | |
| Value-added elements of in-hospital care | ||
| Total daily dose of albuterol | Milligrams | |
| Total daily duration of continuous albuterol | Hour units | |
| Time to q 4 hr albuterol (hours) | Hour units | |
| For participants discharged to home | ||
| 48 hour relapse3 | Yes/No | |
| Parental perception of improvement | Yes/No |
Degrees of freedom. For continuous variables, 2 df are designated to account for possible non-linearity using restricted cubic splines
Categorized in accordance with NIH guidelines
Secondary outcomes for prediction rule modeling if insufficient occurrence of primary outcome variable and for internal validation of derived prediction rule
Selection of Outcome Variables
The APR must predict an outcome that is both clinically important and clearly defined.15,27 The most definitive, objective outcome is death from respiratory failure. Although of great concern, deaths from asthma are too infrequent to be of use as an outcome measure for developing the APR. Patients who are appropriately admitted to the PICU or hospital might include those at greatest risk of dying from asthma.52,53 However, decisions to admit a pediatric patient to the PICU or hospital are variable, in part because they are influenced by social and staffing circumstances and local practice. For example, the most frequent criterion for PICU admission in our facility is need for albuterol treatment at least every 2 hours. Because of these sources of selection bias, hospital or PICU admission alone are not appropriate outcome measures for development of the APR.
A refinement of hospital or PICU length of stay (LOS) may at least partially account for this variability. Hospital LOS for all diagnoses in children's hospitals are right-skewed, with median and mean LOS of 2 and 4 days.54 Mean LOS for asthma was 3 days and did not vary by hospital type (children's/community and teaching/nonteaching) in New York state.55 Median and mean LOS for children with asthma were 2 days in both a children's hospital and 17 community general hospitals in King County, WA.56 Moreover, 42% of these children were hospitalized for only 1 day, and these children are likely those in whom the decision to admit to hospital is strongly influenced by social and situational circumstances rather than medical indications for admission.56
The APR must also predict those patients in whom discharge from the PED is not appropriate. Inappropriate discharge to home may be identified by relapse, defined as return for unscheduled asthma care to a physician or hospital within 48 hours.14 Investigators have noted difficulty in predicting relapse after evaluation and treatment of asthma in EDs.4 Indeed, relapse generally indicates the need for hospitalization at the initial visit and meets criteria as a primary outcome measure for APR modeling. With these considerations in mind, need for hospitalization, defined as LOS of greater than 24 hours (for those admitted to hospital) or relapse (for those discharged to home) is the primary outcome measure for APR modeling (Table 1).
Because the decision to return for care is most often that of the parent or caregiver, parental perception of improvement ascertained by phone follow-up at 48 – 72 hours is a relevant secondary outcome variable. Finally, value-added elements of in-hospital care are relevant secondary outcome variables because they indicate need for hospitalization. These include LOS as a continuous variable (hours), dose and duration of albuterol treatments, as well as time to Q 4-hr albuterol. These variables may be sensitive to dynamic change in asthma severity because they are determined by an asthma treatment protocol administered by RTs. In addition, these outcome variables are measurable in every participant admitted to hospital. These secondary outcome measures are thus informative and may be of use if the primary outcome measure occurs too infrequently for robust APR modeling or for validation analyses of the APR.
Statistical Analysis for Prediction Rule Modeling
Sample Size Calculation
For development of the APR, the number of predictor variables must be sufficiently conservative or the sample size must be sufficiently large for the model to be reliable and accurate on a future stream of similar patients. In addition, the standards for development and validation of prediction rules include 100% follow-up of enrolled participants and exclusion of duplicate enrollments.57 Finally, in order to expect validation and reliability of predictive discrimination for the APR on a new sample, there must be at least 10 to 15 participant events per the total degrees of freedom (df) of all candidate predictor variables.
Based on the 24 df of our candidate predictor variables (Table 1), there must be at least [15 × 24] = 360 primary outcome events to provide ample power for our multiple regression models.20,23 The historic asthma admission rate is 24% from our PED. With these considerations in mind, and to assure a sufficiently rich dataset, we have calculated sample size as [number of participant events needed]/[hospital admission rate] = 360/0.24 or 1,500 participants. This sample size will account for possible non-linear associations using regression splines.23 A planned recruitment period of five years will ensure a representative sample and account for seasonal variations in asthma precipitants and severity.
Descriptive Statistics
We will report continuous variables as means (standard deviation, SD) or medians (interquartile ranges, IQR), as appropriate and categorical variables as frequencies and proportions. Race and ethnicity will be categorized in accordance with NIH guidelines.58
We will compare demographic characteristics of study participants with those of all patients ages 5 to 17 years admitted to the PED with a primary diagnosis of asthma exacerbation (ICD code 493). This population data will be extracted from a database designed primarily for billing purposes. While not directly comparable to our study sample, this data might provide a sense of how representative the demographic characteristics of our convenience sample are of the overall population of patients ages 5 to 17 years with acute asthma exacerbations cared for in our PED during the enrollment period. All analyses will be performed using Stata v. 10.1 (StataCorp, College Station, TX) and R version 2.10.1 (www.r-project.org).
Statistical APR Modeling
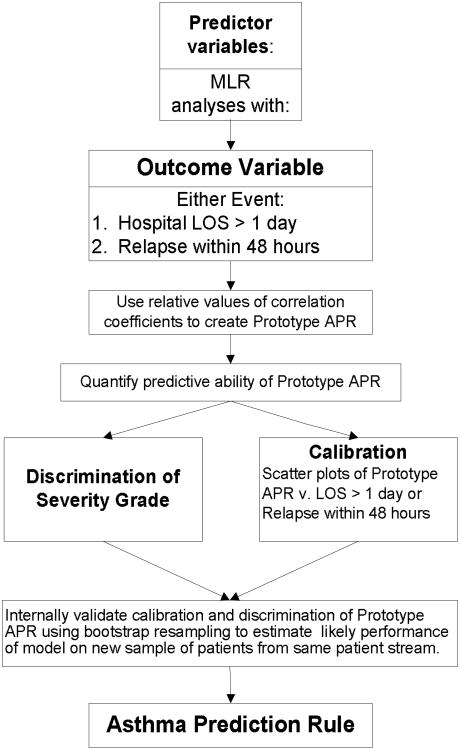
For all analyses we will use only first enrollment data for participants with duplicate enrollments. We will not perform APR modeling until the entire sample size of 1,500 has been studied because this will expend available df for statistical analysis. We will model the APR (Figure 1) using multivariable logistic regression analyses that will allow us to calculate probability estimates for the outcome given the combination of predictors.20,23 As a sensitivity analysis in which we can maximize the power of the analysis, we will also examine a regression model using LOS as continuous variable. The discriminatory power of the regression model can be assessed from the explained variance (R2).
Figure 1. Flow Chart for Asthma Prediction Rule Development.
We will assess for statistical assumptions of multivariable logistic regression.59 These include assessing for non-linear relationships of candidate predictor variables with the outcome using regression splines and a check for the normality of residuals with data transformation as necessary.23
The relative point-weighting of adjusted regression coefficients will then be used to determine which candidate predictors to include in a prototype APR.20,24,25,50 A final series of multiple regression analyses for the outcome will be used to determine if this prototype model is statistically appropriate as the APR. Coefficients will be converted to integers on a scale of 1 to 10 in order to enhance ease of use of the APR.
The predictive accuracy of the prototype model will be assessed using discrimination and calibration. Discrimination is the ability of a model to separate or rank patients with different outcomes and will be measured using the c-index (equivalent to area under the receiver operating curve), the proportion of all usable subject pairs in which predictions and outcomes are concordant.20,60 Calibration measures the accuracy of the predicted probability of the outcome by assessing the bias of predicted values when plotted against observed outcomes.
Clinical Sensibility of the Prototype Model
Clinical standards for development of clinical prediction rule include assessment for “sensibility” and content validity.15,18 We will review the prototype model with clinicians who are masked to the predictive discrimination (c-index). Alternatively, several scoring systems may be derived based on the regression coefficients, and these will then be presented to clinicians to select the most “sensible” APR. This will maximize the potential impact factor of the final APR.
Validation of the APR
Internal validation of the prototype APR will be determined by calibration and discrimination using bootstrap resampling. There are multiple advantages of bootstrapping as compared with data-splitting and cross-validation for internal validation, especially preserving the sample size.20 This will estimate the likely performance of the model on future similar patients and will facilitate identification of over-fitting of the model. Secondary outcome measures (e.g., time to q 4 hour albuterol for participants admitted to hospital) may be used to internally validate the APR by determining concordance of prediction of primary and secondary outcomes by the APR. In addition, external validation will be necessary to assess generalizability to persons with acute asthma exacerbations who have different baseline characteristics.60 To do so, the APR developed and internally validated will be applied prospectively to subjects at another center and/or patient stream (e.g., adult ED, pulmonary clinic).
Data Management
Data are acquired at the bedside and recorded using paper forms. This information is then entered into a secure, web-based database.61 All electronic data entry is performed by one investigator and ascertained for accuracy by the other investigator. Accuracy of data entry is enhanced by a database auto-validation feature and by quarterly data cleaning, facilitated using a database graphical and descriptive statistics feature. Cleaned data are exported directly to the statistical software for analysis. An additional spirometry database is maintained on a password protected computer.
Missing Data and Quality Control
We anticipate that some values for future APR modeling will be missing. Missing values most often do not occur at random, and we will perform analyses to understand the reasons for missing data by quantifying the extent of missing data and by identifying participant characteristics associated with missingness. Further, it has been demonstrated that excluding subjects with a missing value from analysis (complete case analysis) leads to bias and loss of power. It is widely accepted that it is better to impute missing values based on known subject characteristics.20,62,63
As such, we will perform multiple imputation of missing values for certain candidate predictor variables. These variables will be carefully selected to include only those that have limited missing values and that can be appropriately imputed with terms having complete data such as age, gender, respiratory rate and lung function. We will then perform a sensitivity analysis by comparing the effect sizes obtained after multiple imputation with those obtained with complete case analysis.
Standards for Reporting of Diagnostic Accuracy (STARD) Compliance
The Standards for Reporting of Diagnostic Accuracy (STARD) initiative formulated guidelines for improved accuracy and completeness of reports of diagnostic tests to facilitate comprehension of the meaning of these studies.64,65 Because an APR may be considered a diagnostic test, when reporting APR modeling we will report which of the 25 STARD checklist items we address and will include a flow diagram to clarify participant recruitment and study implementation.
Limitations
This study protocol has limitations. First, there is great phenotypic variability and heterogeneity of acute asthma expression and of response to treatment.8 Indeed, use of a convenience sample and not recruiting participants after 10PM may result in spectrum bias. This is because the asthma and demographic characteristics of our sample might not be comparable to the overall population of patients admitted to our PED with asthma exacerbations. Notwithstanding this concern, spirometry and other variables we acquire are effort dependent on the part of the participant, and we have found it difficult to obtain these studies late at night.
Second, the population admitted to our PED may not be representative of that managed in other EDs or acute care settings. Although there is great phenotypic expression of asthma, we believe that our cohort will include a broad spectrum of acute severity as measured by the presenting PAS values and lung function measures. Additionally, our tertiary children's hospital PED manages most pediatric patients with acute asthma in an urban, suburban and rural region. Although it is difficult to precisely capture the full clinical variability of acute asthma, we believe our cohort will represent a wide spectrum of both patient demographics and asthma presentations. We recognize that the APR will need to be externally validated in different clinical settings.
Conclusions
The APR made possible by the AASAP study will facilitate standardization of disease assessment and enhance resource utilization in the emergency department and other acute care settings. Our methods may be of use to other investigators contemplating studies of pediatric patients with acute asthma exacerbations and are applicable for development of clinical prediction rules for other diseases or injuries.
Acknowledgments
The authors gratefully acknowledge the nurses, respiratory therapists and other staff of the Vanderbilt Children's Hospital Emergency Department.
Funding: This study was funded by NIH/NHLBI K23 HL80005 (Dr. Arnold), by NIH/NCRR UL1 RR024975 (Vanderbilt CTSA), and NIAID K24 AI77930 (Dr. Hartert). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, NIAID, NCRR, or the National Institutes of Health.
Footnotes
Author Contributions: Dr. Arnold designed the study, enrolls participants and is the primary author of this manuscript. Ms. Gebretsadik assisted with study design and will perform statistical analyses. Dr. Sheller assisted with study design and performs spirometry review and quality control. Dr. Abramo assisted with manuscript preparation. Mr. Resha enrolls participants and assisted with preparation of this manuscript. Dr. Hartert serves as Dr. Arnold's NHLBI Career Development Award mentor, assisted with study design and with manuscript preparation.
Competing Interests: Aerocrine Corporation provided the NIOX MINO nitric oxide analyzer, Masimo Corporation the pulse cooximeter, and Oridion Corporation the end-tidal CO2 sensors used in the study.
Copyright License Statement: The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in JNL and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
References
- 1.American Lung Association. Trends in asthma morbidity and mortality. American Lung Association; New York: 7-1-2006. 1-18-2007 Ref Type: Electronic Citation. [Google Scholar]
- 2.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980-1999. MMWR Surveill Summ. 2002;51:1–13. [PubMed] [Google Scholar]
- 3.Asthma prevalence and control characteristics by race/ethnicity---United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;53:145–148. [PubMed] [Google Scholar]
- 4.McFadden ER., Jr Acute severe asthma. Am J Respir Crit Care Med. 2003;168:740–759. doi: 10.1164/rccm.200208-902SO. [DOI] [PubMed] [Google Scholar]
- 5.Weiss KB, Sullivan SD, Lyttle CS. Trends in the cost of illness for asthma in the United States, 1985-1994. J Allergy Clin Immunol. 2000;106:493–499. doi: 10.1067/mai.2000.109426. [DOI] [PubMed] [Google Scholar]
- 6.AHRQ Medical Expenditure Panel Survey. The five most costly children's conditions, 2006: Estimates for the U.S. civilian non institutionalized children, ages 0-17. Statistical Brief # 242. 2009 Ref Type: Electronic Citation. [Google Scholar]
- 7.US Department of Health and Human Services. Healthy People 2010 US Department of Health and Human Services Respiratory Diseases, Goal 24. US Department of Health and Human Services; 2004. 1-30-2004 Ref Type: Electronic Citation. [Google Scholar]
- 8.Lemanske RF, Jr, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125:S95–102. doi: 10.1016/j.jaci.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geelhoed GC, Landau LI, Le Souef PN. Evaluation of SaO2 as a predictor of outcome in 280 children presenting with acute asthma. Ann Emerg Med. 1994;23:1236–1241. doi: 10.1016/s0196-0644(94)70347-7. [DOI] [PubMed] [Google Scholar]
- 10.Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775–780. doi: 10.1097/00001888-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Croskerry P. Cognitive forcing strategies in clinical decision making. Ann Emerg Med. 2003;41:110–120. doi: 10.1067/mem.2003.22. [DOI] [PubMed] [Google Scholar]
- 12.Croskerry P. Achieving quality in clinical decision making: cognitive strategies and detection of bias. Acad Emerg Med. 2002;9:1184–1204. doi: 10.1111/j.1553-2712.2002.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 13.Croskerry P, Sinclair D. Emergency medicine: a practice prone to error? CJEM. 2001;3:271–276. doi: 10.1017/s1481803500005765. [DOI] [PubMed] [Google Scholar]
- 14.Schenkel S. Promoting patient safety and preventing medical error in emergency departments. Acad Emerg Med. 2000;7:1204–1222. doi: 10.1111/j.1553-2712.2000.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 15.Laupacis A, Sekar N, Stiell IG. Clinical prediction rules. A review and suggested modifications of methodological standards. JAMA. 1997;277:488–494. [PubMed] [Google Scholar]
- 16.Stiell IG. The Development of Clinical Decision Rules for Injury Care. In: Rivara FP, editor. Injury control: research and program evaluation. Cambridge, UK: Cambridge University Press; 2001. pp. 217–235. [Google Scholar]
- 17.Wasson JH, Sox HC, Neff RK, Goldman L. Clinical prediction rules. Applications and methodological standards. N Engl J Med. 1985;313:793–799. doi: 10.1056/NEJM198509263131306. [DOI] [PubMed] [Google Scholar]
- 18.Feinstein AR. Clinimetrics. New Haven, CT: Yale University Press; 1987. [Google Scholar]
- 19.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users' guides to the medical literature: XXII: how to use articles about clinical decision rules.Evidence-Based Medicine Working Group. JAMA. 2000;284:79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 20.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 21.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modeling with logistic regression analysis: in search of a sensible strategy in small data sets. Med Decis Making. 2001;21:45–56. doi: 10.1177/0272989X0102100106. [DOI] [PubMed] [Google Scholar]
- 22.Harrell FE, Jr, Shih YC. Using full probability models to compute probabilities of actual interest to decision makers. Int J Technol Assess Health Care. 2001;17:17–26. doi: 10.1017/s0266462301104034. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE. Regression Modeling Strategies. New York: Springer; 2001. [Google Scholar]
- 24.Harrell FE, Jr, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80:1198–1202. doi: 10.1093/jnci/80.15.1198. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FE, Jr, Lee KL, Matchar DB, Reichert TA. Regression models for prognostic prediction: advantages, problems, and suggested solutions. Cancer Treat Rep. 1985;69:1071–1077. [PubMed] [Google Scholar]
- 26.Moons KGM. Diagnostic Research, Theory and Application. Erasmus Universiteit Rotterdam; Oct 30, 1996. pp. 1–152. Ref Type: Thesis/Dissertation. [Google Scholar]
- 27.Stiell IG, Wells GA. Methodologic standards for the development of clinical decision rules in emergency medicine. Ann Emerg Med. 1999;33:437–447. doi: 10.1016/s0196-0644(99)70309-4. [DOI] [PubMed] [Google Scholar]
- 28.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–1079. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Sanders DL, Gregg W, Aronsky D. Identifying asthma exacerbations in a pediatric emergency department: A feasibility study. Int J Med Inform. 2006 doi: 10.1016/j.ijmedinf.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Schuh S, Johnson D, Stephens D, Callahan S, Canny G. Hospitalization patterns in severe acute asthma in children. Pediatr Pulmonol. 1997;23:184–192. doi: 10.1002/(sici)1099-0496(199703)23:3<184::aid-ppul3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 31.Scarfone RJ, Fuchs SM, Nager AL, Shane SA. Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518. [PubMed] [Google Scholar]
- 32.Keogh KA, Macarthur C, Parkin PC, Stephens D, Arseneault R, Tennis O, Bacal L, Schuh S. Predictors of hospitalization in children with acute asthma. J Pediatr. 2001;139:273–277. doi: 10.1067/mpd.2001.116282. [DOI] [PubMed] [Google Scholar]
- 33.Lin RY, Pesola GR, Bakalchuk L, Heyl GT, Dow AM, Tenenbaum C, Curry A, Westfal RE. Rapid improvement of peak flow in asthmatic patients treated with parenteral methylprednisolone in the emergency department: A randomized controlled study. Ann Emerg Med. 1999;33:487–494. doi: 10.1016/s0196-0644(99)70334-3. [DOI] [PubMed] [Google Scholar]
- 34.Global Initiative for Asthma. O'Byrne Paul., MD Chair . Global strategy for asthma management and prevention. Global Initiative for Asthma; 12-10-0007. 1-28-2008 Ref Type: Report. [Google Scholar]
- 35.Chalut DS, Ducharme FM, Davis GM. The Preschool Respiratory Assessment Measure (PRAM): a responsive index of acute asthma severity. J Pediatr. 2000;137:762–768. doi: 10.1067/mpd.2000.110121. [DOI] [PubMed] [Google Scholar]
- 36.Ducharme FM, Chalut D, Plotnick L, Savdie C, Kudirka D, Zhang X, Meng L, McGillivray D. The Pediatric Respiratory Assessment Measure: a valid clinical score for assessing acute asthma severity from toddlers to teenagers. J Pediatr. 2008;152:476–80. 480. doi: 10.1016/j.jpeds.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 37.Masimo Corporation. Masimo Technical Bulletin 3: Pleth Variability Index (PVI) Masimo Corporation; Aug 10, 0009. pp. 1–3. Ref Type: Electronic Citation. [Google Scholar]
- 38.Hadjikoumi I, Hassan A, Milner AD. Effects of respiratory timing and cheek support on resistance measurements, before and after bronchodilation in asthmatic children using the interrupter technique. Pediatr Pulmonol. 2003;36:495–501. doi: 10.1002/ppul.10384. [DOI] [PubMed] [Google Scholar]
- 39.McKenzie SA, Chan E, Dundas I, Bridge PD, Pao CS, Mylonopoulou M, Healy MJ. Airway resistance measured by the interrupter technique: normative data for 2-10 year olds of three ethnicities. Arch Dis Child. 2002;87:248–251. doi: 10.1136/adc.87.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–930. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 41.Koopman M, Arets HG, Uiterwaal CS, van der Ent CK. Comparing 6 and 10 sec exhalation time in exhaled nitric oxide measurements in children. Pediatr Pulmonol. 2009;44:340–344. doi: 10.1002/ppul.21006. [DOI] [PubMed] [Google Scholar]
- 42.McGill C, Malik G, Turner SW. Validation of a hand-held exhaled nitric oxide analyzer for use in children. Pediatr Pulmonol. 2006;41:1053–1057. doi: 10.1002/ppul.20491. [DOI] [PubMed] [Google Scholar]
- 43.Black j, Baxter-Jones ADG, Gordon J, Findlay AL, Helms PJ. Assessment of airway function in young children with asthma: comparison of spirometry, interrupter technique, and tidal flow by inductance plethsmography. Pediatr Pulmonol. 2004;37:548–553. doi: 10.1002/ppul.20046. [DOI] [PubMed] [Google Scholar]
- 44.Standardization of Spirometry, 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 45.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113:587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 46.Sherrill DL, Lebowitz MD, Knudson RJ, Burrows B. Methodology for generating continuous prediction equations for pulmonary function measures. Comput Biomed Res. 1991;24:249–260. doi: 10.1016/0010-4809(91)90047-z. [DOI] [PubMed] [Google Scholar]
- 47.Pennock BE, Rogers RM, McCaffree DR. Changes in measured spirometric indices. What is significant? Chest. 1981;80:97–99. doi: 10.1378/chest.80.1.97. [DOI] [PubMed] [Google Scholar]
- 48.National Heart, Lung and Blood Institute National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma NIH. 2007. Ref Type: Electronic Citation. [Google Scholar]
- 49.Dexheimer JW, Arnold DH, Abramo TJ, Aronsky D. Development of an asthma management system in a pediatric emergency department. AMIA Annu Symp Proc. 2009;2009:142–146. [PMC free article] [PubMed] [Google Scholar]
- 50.Harrell FE, Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med. 1984;3:143–152. doi: 10.1002/sim.4780030207. [DOI] [PubMed] [Google Scholar]
- 51.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19:1059–1079. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 52.Turner MO, Noertjojo K, Vedal S, Bai T, Crump S, Fitzgerald JM. Risk factors for near-fatal asthma. A case-control study in hospitalized patients with asthma. Am J Respir Crit Care Med. 1998;157:1804–1809. doi: 10.1164/ajrccm.157.6.9708092. [DOI] [PubMed] [Google Scholar]
- 53.Getahun D, Demissie K, Rhoads GG. Recent trends in asthma hospitalization and mortality in the United States. J Asthma. 2005;42:373–378. doi: 10.1081/JAS-62995. [DOI] [PubMed] [Google Scholar]
- 54.Merenstein D, Egleston B, Diener-West M. Lengths of stay and costs associated with children's hospitals. Pediatrics. 2005;115:839–844. doi: 10.1542/peds.2004-1622. [DOI] [PubMed] [Google Scholar]
- 55.Huang ZJ, LaFleur BJ, Chamberlain JM, Guagliardo MF, Joseph JG. Inpatient childhood asthma treatment: relationship of hospital characteristics to length of stay and cost: analyses of New York State discharge data, 1995. Arch Pediatr Adolesc Med. 2002;156:67–72. doi: 10.1001/archpedi.156.1.67. [DOI] [PubMed] [Google Scholar]
- 56.Samuels BN, Novack AH, Martin DP, Connell FA. Comparison of length of stay for asthma by hospital type. Pediatrics. 1998;101:E13. doi: 10.1542/peds.101.4.e13. [DOI] [PubMed] [Google Scholar]
- 57.McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS. Users' guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284:79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 58.National Institutes of Health. NIH policy on reporting race and ethnicity data: Subjects in clinical research NIH web site. 8-1-2001. 3-8-2008 Ref Type: Electronic Citation. [Google Scholar]
- 59.Katz MH. Multivariable Analysis. 2. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- 60.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130:515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 61.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Donders AR, van der Heijden GJ, Stijnen T, Moons KG. Review: a gentle introduction to imputation of missing values. J Clin Epidemiol. 2006;59:1087–1091. doi: 10.1016/j.jclinepi.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 63.van der Heijden GJ, Donders AR, Stijnen T, Moons KG. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. J Clin Epidemiol. 2006;59:1102–1109. doi: 10.1016/j.jclinepi.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 64.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Standards for Reporting of Diagnostic Accuracy. Clin Chem. 2003;49:1–6. doi: 10.1373/49.1.1. [DOI] [PubMed] [Google Scholar]
- 65.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Lijmer JG, Moher D, Rennie D, de Vet HC. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Fam Pract. 2004;21:4–10. doi: 10.1093/fampra/cmh103. [DOI] [PubMed] [Google Scholar]