Abstract
Objective
To differentiate dys-synergic defaecation (DD) from normal function and slow transit constipation (STC).
Methods
The medical records of 1411 patients evaluated by a single gastroenterologist over a 16-year period at a tertiary medical centre were reviewed. DD was characterised by anorectal manometry and balloon expulsion test. There were 390 patients with DD, and 61 with STC without DD. Transit data from 211 healthy individuals served as controls. The primary endpoints were overall colonic transit (geometric centre) at 24 h and 48 h (GC24 and GC48). Regional transit was measured as ascending colon half-emptying time (AC t1/2) and residual content in descending rectosigmoid colon and stool (DRS).
Results
Age and body mass index were similar in the STC and DD groups. DD was associated with smaller perineal descent and a greater difference in rectoanal pressure than STC. Both STC and DD were associated with lower GC24 and GC48 and slower AC t1/2 than controls. GC48 differentiated DD from healthy controls (p<0.001) and DD from STC (p=0.007). AC t1/2 values differentiated healthy controls from DD (p=0.006) and STC (p<0.001) and were associated with constipation (DD vs STC, p=0.007). The regional content of DRS at 48 h discriminated DD from STC (AUC=0.82) and stool content at 48 h, increasing the odds for DD over STC (OR per 5% in stool 2.4, 95% CI 1.1 to 5.5, p=0.03).
Conclusions
DD is associated with delayed overall colonic transit at 48 h and AC t1/2 compared with healthy controls. Regional scintigraphic transit profiles differentiate DD from STC and facilitate identification of a subgroup of patients with constipation.
INTRODUCTION
Chronic constipation is a common disorder with a prevalence of 2–27% in western countries,1,2 particularly in women, elderly people and those of lower socioeconomic status.3,4 The diagnosis of functional constipation is often based on symptom criteria.5 After exclusion of structural diseases there are three large categories6: normal transit constipation, slow transit constipation (STC) and rectal evacuation disorders or dys-synergic defaecation (DD). Treatment is optimised by correct diagnosis.6 The latter may manifest as spastic pelvic floor dys-synergia, anismus or descending perineum syndrome.7 In spastic defaecatory disorders there is a lack of coordinated relaxation of the pelvic floor or paradoxical contraction of the external anal sphincter; these dysfunctions prevent rectal evacuation despite effective contraction of the colon and increases in abdominal pressure with straining.8,9 Paradoxical contraction can be observed clinically on digital rectal examination and on anorectal endosonography.10 However, the classical clinical features6 and typical findings on digital rectal examination11 may be absent. DD may be associated with delayed colonic transit.12 Anorectal manometry with balloon expulsion is commonly performed to facilitate the diagnosis. STC may be caused by a colonic myopathy or neuropathy,13 with alterations in the number of myenteric plexus neurons or interstitial cells of Cajal.14
Measurement of colonic transit may not differentiate STC from DD. Thus, DD was associated with delayed overall colonic transit by scintigraphy or radiopaque markers in 55–64% of adults with DD12,15 and 12% of adolescents with DD.16 Paradoxically, Grotz et al reported longer left colon and rectosigmoid transit times for radiopaque markers in patients with STC than in patients with pelvic floor dysfunction. Rectosigmoid transit times at 80% sensitivity had only 48% specificity for discriminating pelvic floor dysfunction from subgroups of slow transit or normal transit constipation.17
A non-invasive approach to identifying DD without the need for specialised anorectal testing would be clinically advantageous, confirm the clinical diagnosis and enhance selection of patients for specialised anorectal tests (if available) and for retraining of rectal evacuation.
We hypothesised that DD is associated with delayed left colon transit compared with healthy controls and patients with STC, whereas patients with STC have transit delay in the ascending colon (AC). The aim of this study was to compare the overall and regional transit characteristics between healthy volunteers (HV) and patients with DD or STC.
METHODS
Since this was a medical records review, the study was approved by the Mayo Clinic Institutional Review Board for patients who had given prior unrestricted consent to use their medical records for such research.
Data extraction methods are detailed in the online supplement.
Study population
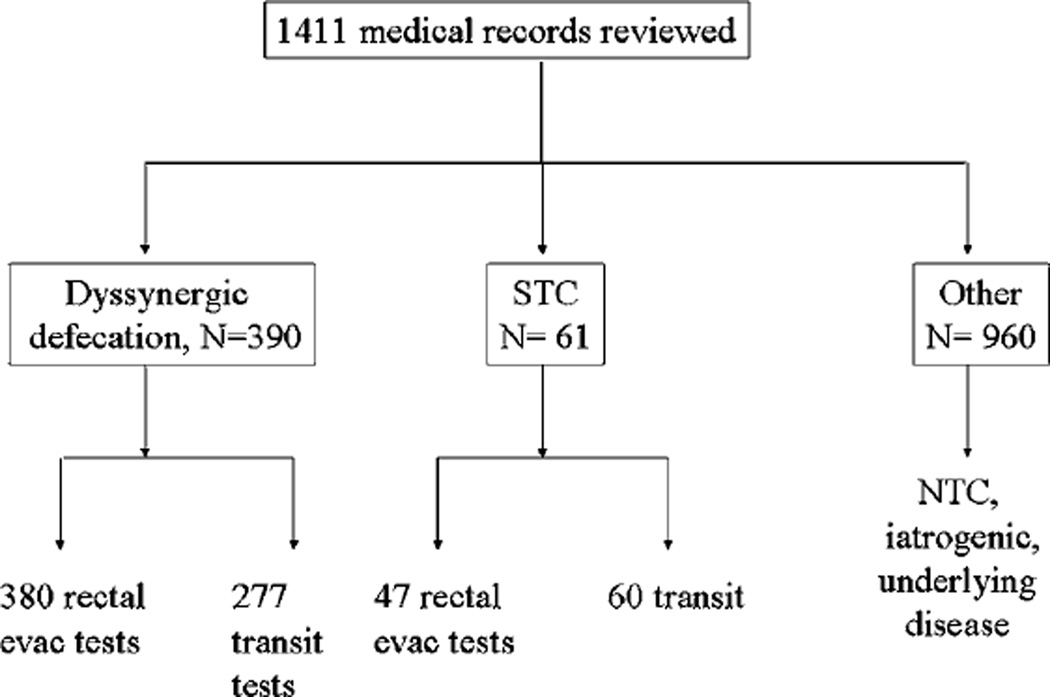
A cohort of 1411 patients was diagnosed between 1 January 1994 and 30 June 2011 by a single gastroenterologist (MC). Figure 1 shows the participants in each group and the tests performed.
Figure 1.
Participants in research study and number with measurements of transit and evacuation. NTC, normal transit constipation; STC, slow transit constipation.
Criteria for dys-synergic defaecation (DD)
There were 390 patients with constipation and rectal evacuation disorder associated with (1) abnormal balloon expulsion test (inability to expel the balloon from the rectum with <200 g added15,18); and/or (2) high anal sphincter pressure (maximum resting pressure >90 mm Hg18–20); and/or (3) failure of the anorectal angle to open ≥15° between resting and straining.18 The criteria were developed from a review of the published data for adults studied in Minnesota20 and Iowa,19 including consideration of the age range relevant to this cohort—for example, Fox et al20 included women aged predominantly 20–70 years (six aged >70 years) and divided their normal data into those above or below a median age of 41 years.
We excluded patients with descending perineum syndrome (descent on straining >4.5 cm21) or documented denervation.
Criteria for slow transit constipation (STC)
Sixty-one patients with constipation had colonic transit geometric centre at 24 h of <1.7 or <3.0 at 48 h22 and no structural disease of the colon or presence of DD.
Healthy controls
Scintigraphic transit data from 211 HV were collected in the research laboratory during the same time period. Evacuation disorder was excluded clinically in all controls.6
Physiological measurements
Anorectal manometry and balloon expulsion studies
Anorectal manometry was performed after a sodium phosphate enema (Fleet, Lynchburg, Virginia, USA) approximately 1 h before testing. Patients were positioned in the left lateral position.
Between 1994 and 2007, anal sphincter pressures were measured by a low compliance pneumohydraulic manometric perfusion system (0.5 ml/min perfusion rate) and a polyvinyl catheter (4.8 mm outer diameter; Arndorfer Medical Specialties, Greendale, Wisconsin, USA) connected to a computerised software program (Medtronic, Minneapolis, Minnesota, USA). The method has been described elsewhere.23 Resting and squeeze pressures were recorded three times at consecutive 1 cm levels in the anal canal and referenced to intrarectal pressure. A rest of 45 s separated sequential squeeze measurements.
From 2008 we used a transanal solid-state high-resolution probe with closely spaced solid-state sensors (16 channels at each level). This allowed simultaneous high-resolution measurement of circumferential pressures in the rectum and throughout the anal canal, obviating the need to perform a station pull-through manoeuvre. The results of this technique are significantly correlated with traditional manometry24 and, furthermore, it also measures the rectoanal pressure difference.
After the manometry study, a latex balloon was inserted into the rectum and filled with 50 ml water. Additional traction weights were subsequently added if the patient was unable spontaneously to expel the balloon from the rectum.25,26
Scintigraphic defaecography
A previously described scintigraphic method was used to assess the rectoanal angle (at rest, squeeze and strain) and perineal descent.18 A difference in the rectoanal angle of at least 158 between the rest and strain positions and perineal descent of 1–4 cm are normal.
Gastrointestinal transit studies
We used our scintigraphic method22,27 to evaluate colonic transit. Our group previously evaluated colonic transit in patients with irritable bowel syndrome classified by symptoms. 28 The relationship with bowel function, performance characteristics and responsiveness to treatment using this method are well established.27,29,30
After stopping medications that could interfere with the study and overnight fasting, patients ingested a delayed-release methacrylate-coated gelatin capsule packed with 0.1 mCi 111InCl3 adsorbed on activated charcoal31 with the aid of a glass (250 ml) of water. Subjects were given instructions to standardise the caloric intake and general content of lunch 4 h and dinner 8 h after swallowing the capsule. There was no bowel preparation prior to scintigraphy and no recording of the time of last defaecation.
Data analysis
Anorectal manometry and balloon expulsion
Maximum resting and squeeze anal pressures were the highest pressures recorded in the anal canal during resting or squeezing23 and were expressed in mm Hg. The amount of weight in grams required to facilitate expulsion of the rectal balloon was recorded and censored above 576 g.
Colon transit scintigraphy
Anterior and posterior images of 2 min duration were acquired at 4, 6, 24 and 48 h, and 111In counts were quantified within a 247 keV (±10%) window and corrected for decay of the isotope and tissue attenuation (geometric mean of anterior and posterior counts).
Colonic transit can be assessed by calculating the geometric centre (GC), which is the weighted average of radioactivity in the different segments of the colon.32
GC = [(%AC*1) + (%TC*2) + (%DC*3) + (%RS*4) + (%ST*5)]/100
Ascending colon half-emptying time (AC t1/2) was calculated by linear interpolation of AC content at all times when imaging demonstrated isotope in the AC.
Statistical analysis
The results are expressed as mean±SD, with some figures reporting least squares adjusted (for covariates) means (±SEM) from the analysis of covariance (ANCOVA) models. Our primary endpoints for statistical analysis were the colonic GC at 24 h and 48 h (GC24 and GC48), the percentage of radioactive tracer retained in the different segments of the colon at 24 h and 48 h and AC t1/2.
The primary analyses used ANCOVA models to assess the association of colonic transit with group status (adjusting for age, gender and body mass index (BMI) with data summarised as least square mean±SEM). In addition, logistic regression models were used to evaluate the ability of these colonic transit measures (except AC t1/2) to discriminate between healthy controls, DD and STC. Some of the AC t1/2 values were either left-censored (eg, <8 h) or right-censored (eg, >24 h). To assess this measure of colonic transit, a Weibull regression model was used to examine the association of group status (eg, DD vs STC) with AC t1/2 values. Subject characteristics such as age, gender and BMI, as well as anorectal and defaecatory functions, were summarised separately for each group. Since BMI and gender are known to influence colonic transit time,28,33 these were also included as covariates in the logistic regression models.
The statistical analysis used SAS Version 9.2 procedures GLM, LOGISTIC and LIFEREG (SAS Institute), and SigmaPlot 12 software 2011–2012 (Systat Software) was used for plotting data.
RESULTS
Participant characteristics
Among the 1411 patients evaluated, 390 were identified with a rectal evacuation disorder and 61 with STC. The demographic characteristics of each group are shown in table 1. The average age of the three study groups was similar. Gender was not equally distributed (p<0.001) with 65.6% of HV, 83.1% of patients with DD and 91.8% of patients with STC being women. The larger proportion of men among the HV group is reflected in the higher BMI compared with the other two groups. The mean age and BMI of the DD and STC groups were virtually identical. Among the patients with constipation, previous abdominal operations recorded in the medical records are listed in table 2.
Table 1.
Demographic characteristics of healthy volunteers, patients with rectal evacuation disorders and patients with slow transit constipation
| Study group | Age (years) | BMI (kg/m2) |
|---|---|---|
| Healthy volunteers (n=211) | 33.7±0.8 (n=211) | 25.6±0.3 (n=197) |
| Men (n=73) | 30.2±1.1 (n=73) | 26.7±0.5 (n=67) |
| Women (n=138) | 35.6±1.1 (n=138) | 25.1±0.4 (n=130) |
| Evacuation disorders (n=390) | 39.8±0.8 (n=390) | 22.3±0.2 (n=362) |
| Men (n=66) | 45.5±2.3 (n=66) | 24.0±0.5 (n=61) |
| Women (n=324) | 38.6±0.8 (n=324) | 22.0±0.2 (n=301) |
| Slow transit constipation (n=61) | 42.0±1.7 (n=61) | 22.3±0.5 (n=61) |
| Men (n=5) | 54.0±8.0 (n=5) | 25.7±0.8 (n=5) |
| Women (n=56) | 40.9±1.6 (n=56) | 22.0±0.5 (n=52) |
Data shown are mean±SE.
BMI, body mass index.
Table 2.
Previous abdominal operations in patients with constipation
| Evacuation disorders | Slow transit constipation | |||
|---|---|---|---|---|
| Women (n=324) |
Men (n=66) |
Women (n=56) |
Men (n=5) |
|
| Pregnancies | 1.9±0.1 (n=143) | NA | 1.5±0.1 (n=47) | NA |
| Appendectomy | 50 | 6 | 4 | 0 |
| Cholecystectomy | 28 | 4 | 7 | 1 |
| Caesarian section | 7 | NA | 2 | NA |
| Pelvic surgery | 29 | 0 | 4 | 0 |
| Rectocoele repair | 2 | 0 | 1 | 0 |
| Colonic resection: p/t | 12 | 0 | 1 | 1 |
| Small bowel resection | 6 | 2 | 3 | 0 |
| Ileostomy | 2 | 0 | 0 | 0 |
| Other abdominal surgery | 9 | 9 | 2 | 0 |
p/t, partial/total.
There were 960 patients with normal transit constipation who did not fulfil the criteria for DD or STC. In a randomly selected sample of 10% of this cohort (13 men and 82 women) the mean±SEM age was 42.1±1.7 years, mean BMI was 23.5±0.5 kg/m2 and colonic GC24 was 2.1±0.1. Other than the difference in colonic transit, we noted that the gender distribution, age and BMI were similar to those of the two other constipation groups.
Defaecatory functions in the groups presenting with constipation
The results of anorectal manometry and other defaecatory function tests are summarised in table 3. Maximum resting sphincter pressure was >90 mm Hg in 94 patients; all required >200 g weight to expel the rectal balloon. Among the 390 patients with evacuation disorders, the weight required to expel the balloon was >200 g in 317 patients, 188 g in 14, 94 g in four patients and 0 g in 40 patients; 15 patients did not undergo the test. Perineal descent was <1.5 cm in 48 patients; all required >200 g to expel the balloon. The change in the rectoanal angle was <158 in 81 patients; all required >200 g to expel the balloon. There were more than one positive ‘criteria’ in the vast majority. In four patients with highly consistent clinical features, evacuation disorder was associated only with a maximum squeeze anal sphincter pressure of >180 mm Hg (double the threshold for abnormal resting pressure).
Table 3.
Anorectal manometry characteristics and evacuation parameters of groups with constipation
| Study group | Evacuation disorders (n=390) |
Slow transit constipation (n=61) |
|---|---|---|
| Maximum anal resting pressure (mm Hg) | 84.0±29.1 (n=382) | 71.3±24.2 (n=44) |
| 10–90th percentile | 49.3–118.5 | 41.9–100.2 |
| Men | 92.1±32.6 (n=66) | 68.2±24.0 (n=4) |
| Women | 82.3±28.1 (n=316) | 71.6±24.5 (n=40) |
| Maximum anal squeeze pressure (mm Hg) | 144.3±60. 3 (n=379) | 149.4±66.0 (n=44) |
| 10–90th percentile | 79.4–218.1 | 82.4–250.7 |
| Men | 202.5±79.0 (n=66) | 230.5±85.3 (n=4) |
| Women | 132.0±47.4 (n=313) | 141.2±59.2 (n=40) |
| Rectoanal pressure difference (mm Hg) | −36.3±34.2 (n=92) | 3.4±46.4 (n=12) |
| 10–90th percentile | −79.3 to −3.0 | −38.2 to 31.6 |
| Men | −42.0±52.1 (n=23) | (n=0) |
| Women | −34.4±26.0 (n=69) | 3.4±46.4 (n=12) |
| Added balloon weight (g) | 447.8±192.3 (n=371) | 25.3±67.0 (n=47) |
| 10–90th percentile | 0–586.0 | 0–188.0 |
| Men | 386.5±238.4 (n=66) | 47.0±94.0 (n=4) |
| Women | 461.0±178.5 (n=305) | 23.2±65.1 (n=43) |
| Perineal descent (cm) | 1.6±1.4 (n=94) | 2.1±0.7 (n=5) |
| 10–90th percentile | 0.3–3.5 | 1.4–3.0 |
| Men | 1.5±1.1 (n=11) | 1.4 (n=1) |
| Women | 1.7±1.4 (n=83) | 2.2±0.8 (n=4) |
| Change in anorectal angle (°) | 7.0±13.4 (n=97) | 1.0 (n=1) |
| 10–90th percentile | −5.0–20.0 | |
| Men | 8.5±9.5 (n=11) | (n=0) |
| Women | 6.8±13.9 (n=88) | 1.0 (n=1) |
Data shown are mean±SD.
In accordance with the inclusion criteria, the selection process identified DD and STC groups that were clinically different with reference to anal sphincter pressure at rest and the additional weight required to expel the rectal balloon (table 3). In addition, there was a greater negative rectoanal pressure difference in patients with DD than in those with STC (p=0.003), which was not in the definition of DD.
Characterisation of the colon transit profile in the three groups
Overall colonic transit in both genders, including adjustment for covariates
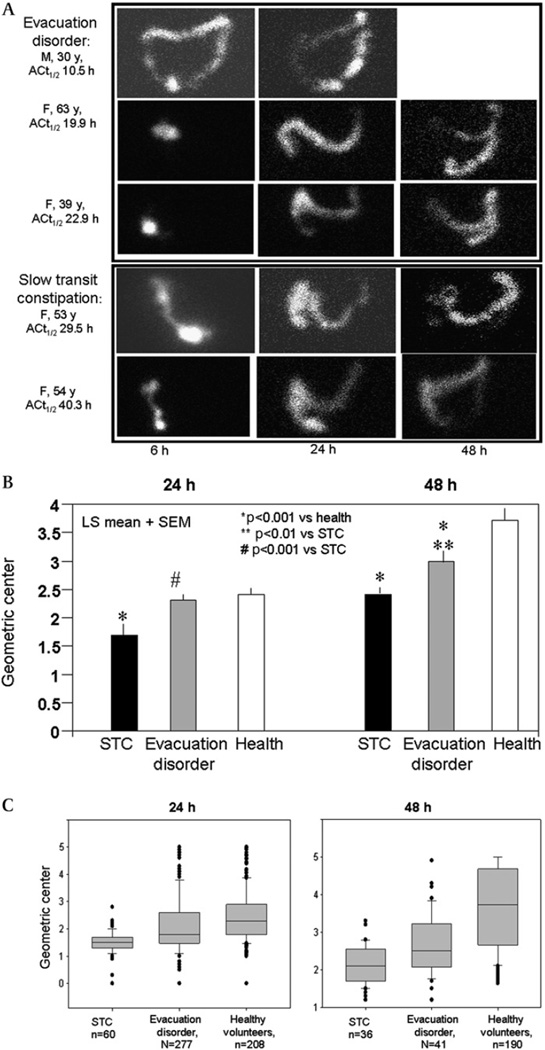
Examples of colonic transit profiles in patients with DD and STC are shown in figure 2A. Overall colonic transit in all study groups combined was faster in men than in women (see table A in online supplement); however, gender differences in GC24 and GC48 were not observed in the DD or STC groups.
Figure 2.
(A) Examples of scintiscans at 6 h, 24 h and 48 h in patients with evacuation disorder (DD) and slow transit constipation (STC). Note that delayed transit is also demonstrated at 48 h in the patients with STC and the retention of isotope in the left colon in patients with evacuation disorder. (B) Comparison of least square means (adjusted for gender and body mass index) of colonic geometric centre at 24 h and 48 h. The DD group is best distinguished from healthy controls at 48 h; transit is slower in the STC than DD groups at both 24 h and 48 h. (C) Distribution of geometric centres (median, IQR, 5th and 95th percentiles) at 24 h and 48 h in the different subgroups (n provided for each group). Note, however, that there is considerable overlap of overall transit in the DD and STC groups.
Overall colonic transit was associated with group status at 24 h and 48 h (figure 2B and table A in online supplement), with significant differences between the STC and HV groups at 24 h and 48 h and between the DD and HV groups at 48 h (figure 2B).
GC24 was not a significant discriminator between DD and HV in the entire study population (p=0.29). However, there were differences in GC24 between DD and HV groups for those with BMI >25 kg/m2. Thus, an ANCOVA model indicated no differential association of DD versus HV groups with GC24 due to gender (p>0.5), but a differential association depending on BMI (overall test for interaction effect in ANCOVA model, p=0.016). In particular, different colonic transit in DD versus HV was observed in subjects with BMI >25 kg/m2 (p=0.003) but not for BMI >25 kg/m2 (p>0.9).
The distribution of colonic transit data is illustrated in figure 2C. GC24 was still a significant discriminator between DD and STC (p<0.0001). Similarly, in those subjects with GC48 data (N=41 DD, N=177 HV and N=38 STC), GC48 was a significant discriminator between DD and HV (p<0.001) and between DD and STC (p=0.007).
Regional colonic transit at 24 h and 48 h
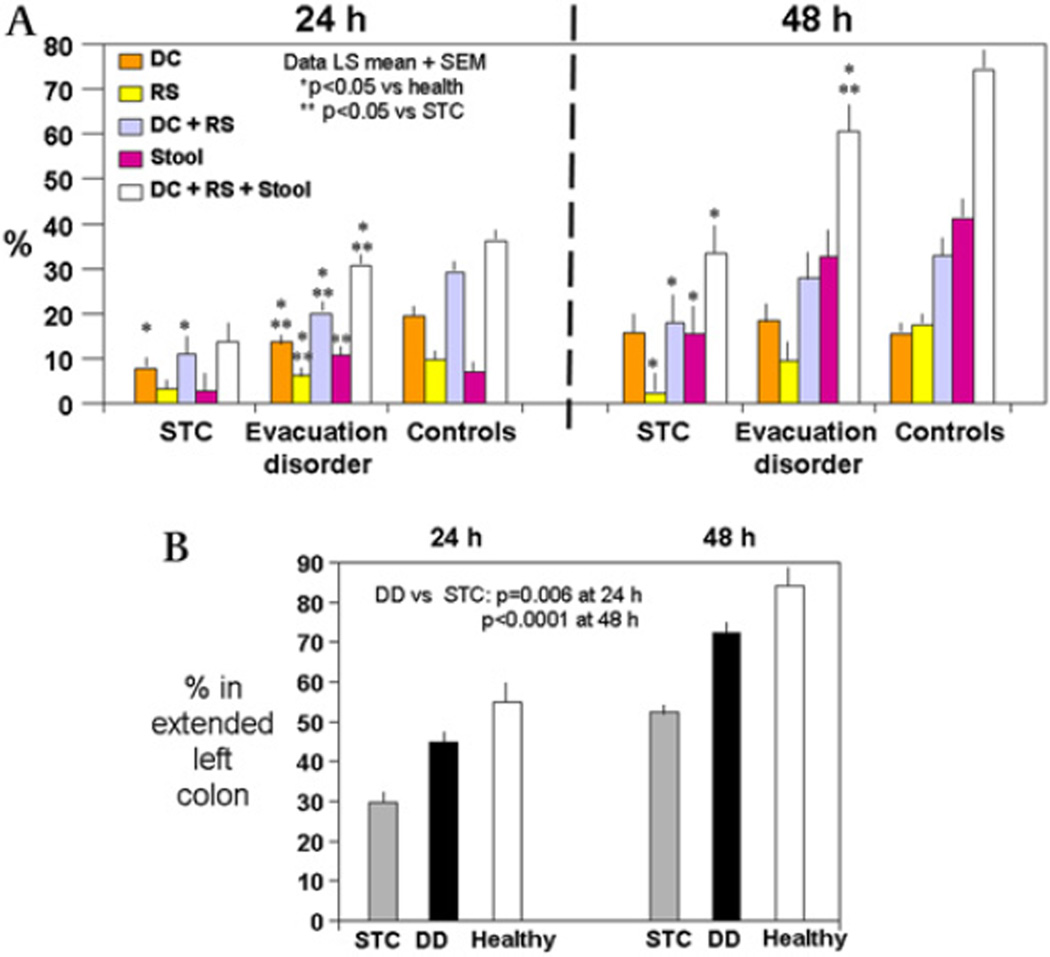
The radioactivity in each colonic segment and stool at different time points is shown in table A in the online supplement. A summary of mean left colon regional percentages (descending, rectosigmoid and stool (DRS)) is shown in figure 3.
Figure 3.
(A) Percentage of radioactivity in left colonic regions and stool at 24 h and 48 h. The analysis at 48 h includes data from 31 patients with slow transit constipation (STC), 35 patients with evacuation disorder (DD) and 171 healthy volunteers. (B) Content of extended left colon in three groups (least square means±SEM). Note the higher proportion of isotope in the left colon (including 50% of the counts in the transverse colon) in the DD than in the STC group. There were significant differences in all pairwise comparisons among the three groups (all p<0.001).
At 24 h there were significant associations of segmental and regional percentages of radioactivity with overall three-group status (descending colon (p<0.001), rectosigmoid colon (p<0.01), DRS (p<0.001)). The most dramatic difference was the small percentage of radioactive counts in DRS in the STC group; as a result, there were significant differences in each individual region and DRS between the STC and DD groups (figure 3A).
At 48 h, only 17.3±26.4% of radioactivity was in the stool of patients with DD compared with 37.3±40.2% in the stool of HV; this contrasts with the lack of differences in radioactivity in the descending and rectosigmoid colon in these two groups. The overall transit delay in the STC group was demonstrated with only 2.2%±6.7% in the stool at 48 h. In addition, patients in the STC group had lower percentages of radioactivity in both the rectosigmoid colon and stool compared with HV (figure 3A).
Similar to the observation at 24 h, there was more radioactivity located at 48 h in the DRS in patients with DD than in those with STC (figure 3).
The colonic retention of isotope in patients with DD is also evident from the counts in the transverse colon at 24 h and 48 h (36.8±23.6% at 24 h and 38.1±25.2% at 48 h) compared with 46.2±24.9% at 24 h and 53.6±21.0% at 48 h in those with STC.
In patients with STC, small amounts of radioactivity were present in the rectosigmoid colon (5.8±10.4%) at 48 h, in contrast to significant retention in the combined ascending colon and transverse colon regions (91±25% and 74±20% at 24 h and 48 h, respectively). Figure 3 also shows that, at 48 h, the amount of radioactivity in the entire left colon was considerably lower in the STC group (overall association with group status, p<0.0001). In a comparison of the DD and HV groups at 48 h, the radioactive content of the DRS between the two groups was significantly different (p=0.026), the difference in the content of the rectosigmoid colon was of borderline significance (p=0.06) and the content of the descending colon was not different.
Extended left colon content at 24 h and 48 h
Since previous literature has evaluated left and right colon transit based on intracolonic markers to the left and right of the midline, we estimated the cumulative content at 24 h and 48 h of the DRS and half the content of the transverse colon, and we refer to this as the ‘extended left colon’. Overall, associations of extended left colon content at both 24 h and 48 h and group were observed (both p<0.0001). Figure 3B shows significantly higher amounts of isotope in the left colon in patients with DD than in those with STC (p=0.006 at 24 h and p<0.0001 at 48 h) and significantly larger amounts in both constipation groups than in HV. This is consistent with the overall delay of transit documented by the GC24 and GC48.
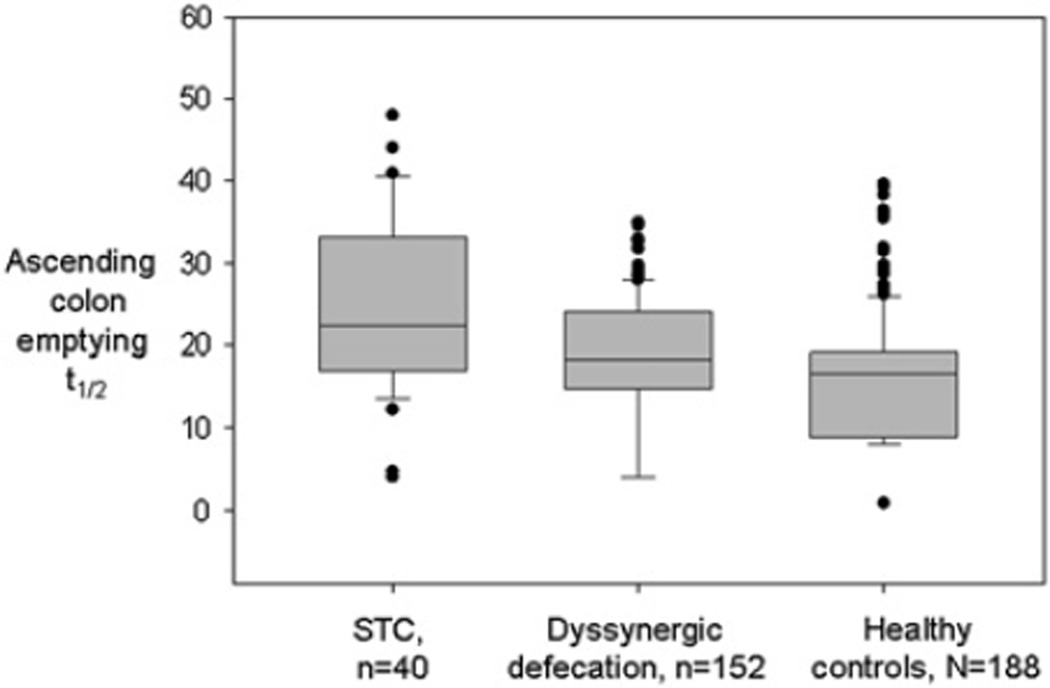
Ascending colon (AC) half-emptying time
Using an analysis that accommodated the left and right censoring in AC t1/2 values (either <4 or <8 and >24 or >48, respectively), the AC t1/2 values (figure 4) were associated with group status, both constipation groups having significantly longer AC t1/2 than controls (DD vs HV, p=0.006; DD vs STC, p=0.007). In an assessment of the regional content of AC at 48 h in 35 patients with DD, 172 HV and 27 patients with STC, there was a significant association of group status with AC content (p<0.0001) with observed differences for DD (least square mean±SEM 10.9±1.7) versus HV (2.7±0.8; p<0.001) and DD versus STC (20.2±2.0; p<0.001).
Figure 4.
Ascending colon half-emptying time in the three study groups showing significant difference between healthy controls and the two constipation groups, and between the dys-synergic defaecation (DD) and slow transit constipation (STC) groups (ANOVA on ranks p<0.001); all pairwise comparison p<0.05 by Dunn method.
Receiver operating characteristic curves to differentiate STC and DD
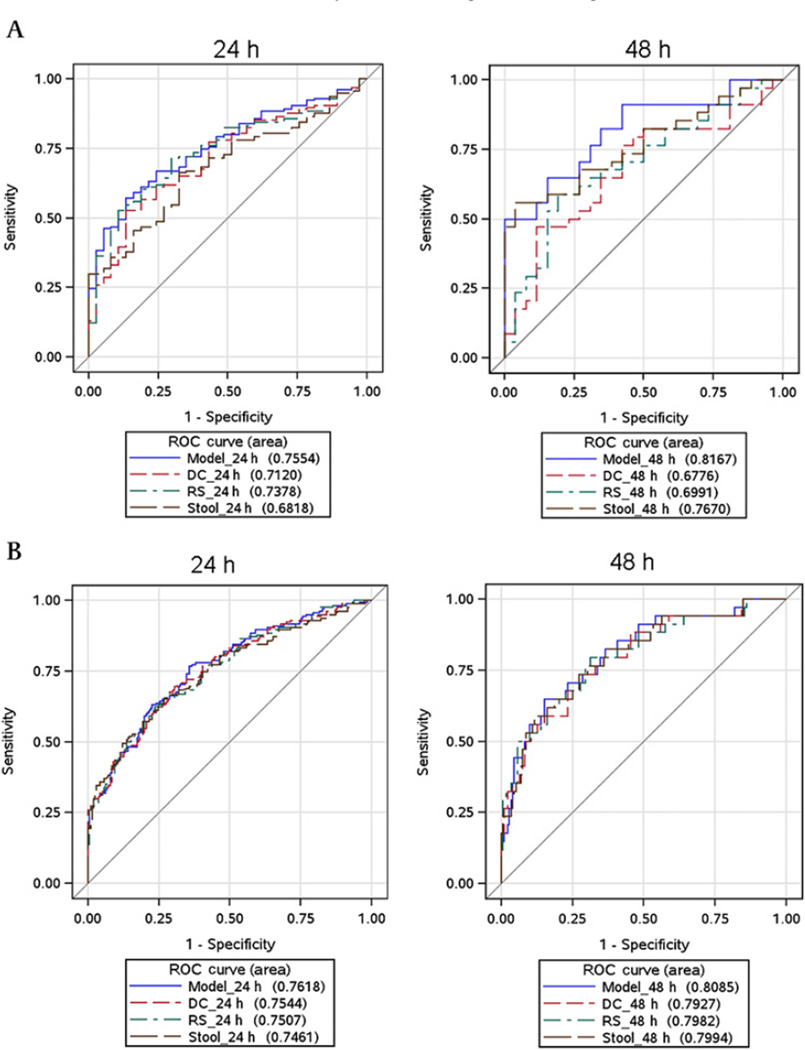
We assessed the ability of regional counts in the left colon (descending colon, rectosigmoid colon and stool individually and in combination) at 24 h and 48 h to differentiate DD from STC (figure 5A) and, separately, DD from HV (figure 5B) using logistic regression. A summary of the discriminant ability of these values was generated based on receiver operating characteristic curves (ROC) and the corresponding area under the ROC curve (AUC values). The models that yielded the largest AUC values to discriminate DD from STC and, separately, DD from HV incorporated the cumulative DRS at 48 h. The logistic discriminant model for DD versus STC (which included gender, BMI, descending colon, rectosigmoid colon and stool percentages at 48 h) provided an AUC of 0.82. Using a cut-off that provided 82% sensitivity and 65% specificity, the negative likelihood ratio (LR−) for this combined model to discriminate STC from DD was 0.27 and the positive LR (LR+) was 2.38. In addition, the model using only stool radioactivity at 48 h as well as gender and BMI (see figure 5A right panel) resulted in an LR− of 0.35 and LR+ of 1.65 using a cut-off yielding 82% sensitivity and 50% specificity.
Figure 5.
ROC curves of content of the descending colon (DC), rectosigmoid colon (RS) or combined regions at 24 h, and of the same regions and stool individually and in combinations at 48 h. The largest area under the curve for discriminating evacuation disorder (DD) from the two other groups is provided by the model incorporating DC, RS and stool content at 48 h. (A) Discrimination between slow transit constipation (STC) and DD transit. (B) Discrimination between DD and healthy volunteer transit.
The ORs for DD (vs STC) per 5% increase in segmental percentages were 1.19 (95% CI 0.96 to 1.48; p=0.11) for descending colon, 1.04 (95% CI 0.81 to 1.34; p=0.76) for rectosigmoid colon and 2.44 (95% CI 1.09 to 5.48; p=0.03) for stool. However, the sensitivity of 60–70% at 80% specificity to differentiate DD from STC or HV shows that there is only moderate discrimination between these groups.
DISCUSSION
This study shows that regional colonic transit measurement identifies a transit profile that can differentiate DD from STC. Thus, AC t1/2 (figure 4), the content of the descending colon and stool at 24 h (figure 3A), the cumulative content of the DRS and extended left colon at 24 h and 48 h (figure 3A, B) were significantly different in patients with STC than in those with DD.
There are group differences in the gender (and associated BMI) of the healthy controls and patients with constipation. However, it is relevant to note that, in people who are not elderly, there are no significant differences between the genders in maximal resting pressure19 or perineal descent34 or in colonic transit.22 The two constipation groups were well matched for age, gender and BMI.
Our study confirms that overall colonic transit is delayed in patients with DD and STC relative to HV, and overall transit is slower in STC than in DD. The data in DD confirm that, even in the presence of slow colonic transit, it is still essential to exclude an evacuation disorder.6
Although differences between STC and DD were previously reported based on radiopaque marker colonic transit by Grotz et al,17 our observations are very different. Grotz et al observed more delayed left colon and rectosigmoid transit in patients with STC than in patients with pelvic floor dysfunction, whereas we observed a higher percentage of counts in the descending colon and stool in patients with DD than in those with STC at 24 h. Grotz et al observed that 24 of 54 patients with DD had slow overall transit and their right colon transit time was no different from that of patients with STC. Conversely, we showed slower AC emptying in patients with STC than in those with DD.
There are differences in the methods of measuring transit in our study and that of Grotz et al.17 First, scintigraphy tracks the movement of radiolabelled solids several times over 48 h whereas the technique of Metcalf et al35 x-rays the number of markers remaining in the colon on day 4 (or day 4 and 7) and may be less sensitive to detect the retardation of AC emptying. Second, Grotz et al defined the left colon as the colon located to the left of the midline and included the left half of the transverse colon.17 When we included 50% of the counts in the transverse colon (see figure 3B) with the descending colon, rectosigmoid colon and stool, we observed higher isotope counts in the extended left colon in the DD group than in the STC group at 24 h and 48 h. This reflects both retention of isotope in the left colon in DD and slower AC emptying in STC. The ability of the content of the left colon to discriminate DD from the other groups (figure 5) is limited, with sensitivities around 60–70% at 80% specificity.
On the other hand, AC t1/2 was significantly different for STC and DD, suggesting that AC transit delay due to a primary motor disorder in STC is more profound than in DD. The slower AC t1/2 in patients with DD than in HV may be explained either by ‘functional’ obstruction to entire colon emptying or by reflex inhibition of proximal colonic propulsion, both resulting from the retention of stool in the left colon. This reflex inhibition is consistent with colo-colonic inhibition through reflexes that relay at the prevertebral ganglia36 or at the spine37 and are mediated by cholecystokinin, opioids and neurokinins.38,39 Treatment of rectal evacuation disorder by biofeedback in DD normalises postprandial sigmoid tone.40
In the tertiary referral practice of a single gastroenterologist, about 28% were diagnosed with DD, 5% with STC, and the remainder with normal transit constipation. These data over a 15-year period are similar to those reported by Pemberton et al26 in 1991 at the Mayo Clinic and show that evacuation disorders account for a sizeable proportion of patients with chronic constipation in referral practice in many countries including the USA,15 Italy,41 Scandinavia,42 the UK43 and Turkey.44 Questionnaire-based studies of people in the community also show that symptoms suggestive of evacuation disorders are endorsed in almost one-third of patients who have symptoms of chronic constipation.45
The limitations of our study are the retrospective nature and the limited number of patients with DD who underwent colonic transit scanning at 48 h. However, the analysis of the detailed regional 48 h data is based on 35 patients with DD, 31 with STC and 171 healthy controls (see table A in online supplement). Another limitation is that scintigraphy was stopped at 48 h, so we were unable to assess fully the transit of isotope through the left colon. We are therefore unable to refute the observation of Grotz et al that transit through the left colon was slower in patients with STC than in those with DD when assessed at 4 or 7 days.17
The strengths of the study include the large patient cohort from the practice of one gastroenterologist and the standardised validated scintigraphic measurement of colonic transit in the clinical environment and research laboratory.22,27,30 The clinical implications of our findings are: (1) the non-invasive measurement of colonic transit may confirm a clinical diagnosis of DD at centres that do not have specialised tests of defaecatory function; (2) identification of a transit profile typical of STC will facilitate selection of patients for treatment with colonic prokinetic agents; and (3) identification of a transit profile suggestive of DD will avoid unnecessary and potentially deleterious colectomy in patients with DD.
In conclusion, DD retards overall colonic transit at 48 h and AC t1/2 compared with healthy controls. DD can be differentiated from STC by AC t1/2 and the retention of content in the descending colon at 48 h. The measurement of regional transit by scintigraphy may be helpful for the diagnosis of DD when anorectal manometry and defaecation testing are not available.
Supplementary Material
Significance of this study.
What is already known about this subject?
-
►
Constipation may result from slow colonic transit (STC) or disorders of rectal evacuation (ie, dys-synergic defaecation (DD)).
-
►
Anorectal manometry, functional studies of defaecation and colonic radiopaque marker transit measurement are used to differentiate STC and DD.
-
►
There is considerable overlap in the transit results of patients with STC and DD.
What are the new findings?
-
►
DD is associated with delayed overall colonoc transit at 48 h and ascending colon half-emptying time measured by radioscintigraphy compared with health.
-
►
Regional scintigraphic transit profiles differentiate DD from STC.
-
►
Regional scintigraphic transit profiles facilitate identification of subgroups in patients with constipation.
How might it impact on clinical practice in the foreseeable future?
-
►
Non-invasive measurement of colonic transit may confirm a clinical diagnosis of DD at centres that do not have specialised tests of defaecatory function.
-
►
Identification of the transit profile typical of STC will facilitate selection of patients for treatment with colonic prokinetic agents.
-
►
Identification of the transit profile suggestive of DD will identify patients for retraining of evacuation disorder.
Acknowledgements
We thank Doris Harvey who conducted most of the anorectal tests over the study period and Cindy Stanislav for her excellent secretarial support.
Funding MCamilleri is funded by grant RO1-DK079866, R01-DK092179 and 1RC1-DK086182 from National Institutes of Health.
Footnotes
Additional data are published online only. To view these files please visit the journal online (http://gut.bmj.com/content/61/8.toc).
Competing interests None.
Ethics approval Ethics approval was provided by Mayo Clinic Institutional Review Board.
Contributors SN analysed the patient records and wrote the manuscript. TN analysed the patient records and critically reviewed the paper. MC was the sole clinician who managed the patients, developed the study protocol, identified aims and hypotheses, helped in interpreting the statistical analysis and wrote and finalised the manuscript. DB assisted in analysing patient records, calculated the transit times, critically reviewed the paper and participated with MC in the clinical appraisal and management of the patients. JI constructed the different databases, aided in selecting suitable patients and critically reviewed the paper. MV-R constructed the different databases, aided in correctly selecting patients and critically reviewed the paper. ARZ performed the statistical analysis and critically reviewed the paper.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001;96:3130–3137. doi: 10.1111/j.1572-0241.2001.05259.x. [DOI] [PubMed] [Google Scholar]
- 2.Stewart WF, Liberman JN, Sandler RS, et al. Epidemiology of constipation (EPOC) study in the United States: relation of clinical subtypes to sociodemographic features. Am J Gastroenterol. 1999;94:3530–3540. doi: 10.1111/j.1572-0241.1999.01642.x. [DOI] [PubMed] [Google Scholar]
- 3.Suares NC, Ford AC. Prevalence of, and risk factors for, chronic idiopathic constipation in the community: systematic review and meta-analysis. Am J Gastroenterol. 2011;106:1582–1591. doi: 10.1038/ajg.2011.164. quiz 1581, 1592. [DOI] [PubMed] [Google Scholar]
- 4.Tack J, Müller-Lissner S, Stanghellini V, et al. Diagnosis and treatment of chronic constipation–a European perspective. Neurogastroenterol Motil. 2011;23:697–710. doi: 10.1111/j.1365-2982.2011.01709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Lembo A, Camilleri M. Chronic constipation. N Engl J Med. 2003;349:1360–1368. doi: 10.1056/NEJMra020995. [DOI] [PubMed] [Google Scholar]
- 7.Bharucha AE, Fletcher JG, Seide B, et al. Phenotypic variation in functional disorders of defecation. Gastroenterology. 2005;128:1199–1210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Rao SS, Welcher KD, Leistikow JS. Obstructive defecation: a failure of rectoanal coordination. Am J Gastroenterol. 1998;93:1042–1050. doi: 10.1111/j.1572-0241.1998.00326.x. [DOI] [PubMed] [Google Scholar]
- 9.Whitehead WE, Wald A, Diamant NE, et al. Functional disorders of the anus and rectum. Gut. 1999;45(Suppl 2):II55–II59. doi: 10.1136/gut.45.2008.ii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Outryve SM, Van Outryve MJ, De Winter BY, et al. Is anorectal endosonography valuable in dyschesia? Gut. 2002;51:695–700. doi: 10.1136/gut.51.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tantiphlachiva K, Rao P, Attaluri A, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8:955–960. doi: 10.1016/j.cgh.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 12.Rao SS, Mudipalli RS, Stessman M, et al. Investigation of the utility of colorectal function tests and Rome II criteria in dyssynergic defecation (anismus) Neurogastroenterol Motil. 2004;16:589–596. doi: 10.1111/j.1365-2982.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 13.Wong S, Lubowski D. Slow-transit constipation: evaluation and treatment. ANZ J Surg. 2007;77:320–328. doi: 10.1111/j.1445-2197.2007.04051.x. [DOI] [PubMed] [Google Scholar]
- 14.He CL, Burgart L, Wang L, et al. Decreased interstitial cell of cajal volume in patients with slow-transit constipation. Gastroenterology. 2000;118:14–21. doi: 10.1016/s0016-5085(00)70409-4. [DOI] [PubMed] [Google Scholar]
- 15.Surrenti E, Rath DM, Pemberton JH, et al. Audit of constipation in a tertiary referral gastroenterology practice. Am J Gastroenterol. 1995;90:1471–1475. [PubMed] [Google Scholar]
- 16.Chitkara DK, Bredenoord AJ, Cremonini F, et al. The role of pelvic floor dysfunction and slow colonic transit in adolescents with refractory constipation. Am J Gastroenterol. 2004;99:1579–1584. doi: 10.1111/j.1572-0241.2004.30176.x. [DOI] [PubMed] [Google Scholar]
- 17.Grotz RL, Pemberton JH, Talley NJ, et al. Discriminant value of psychological distress, symptom profiles, and segmental colonic dysfunction in outpatients with severe idiopathic constipation. Gut. 1994;35:798–802. doi: 10.1136/gut.35.6.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pezim ME, Pemberton JH, Levin KE, et al. Parameters of anorectal and colonic motility in health and in severe constipation. Dis Colon Rectum. 1993;36:484–491. doi: 10.1007/BF02050015. [DOI] [PubMed] [Google Scholar]
- 19.Rao SS, Hatfield R, Soffer E, et al. Manometric tests of anorectal function in healthy adults. Am J Gastroenterol. 1999;94:773–783. doi: 10.1111/j.1572-0241.1999.00950.x. [DOI] [PubMed] [Google Scholar]
- 20.Fox JC, Fletcher JG, Zinsmeister AR, et al. Effect of aging on anorectal and pelvic floor functions in females. Dis Colon Rectum. 2006;49:1726–1735. doi: 10.1007/s10350-006-0657-4. [DOI] [PubMed] [Google Scholar]
- 21.Harewood GC, Coulie B, Camilleri M, et al. Descending perineum syndrome: audit of clinical and laboratory features and outcome of pelvic floor retraining. Am J Gastroenterol. 1999;94:126–130. doi: 10.1111/j.1572-0241.1999.00782.x. [DOI] [PubMed] [Google Scholar]
- 22.Cremonini F, Mullan BP, Camilleri M, et al. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 23.Bharucha AE, Seide B, Fox JC, et al. Day-to-day reproducibility of anorectal sensorimotor assessments in healthy subjects. Neurogastroenterol Motil. 2004;16:241–250. doi: 10.1111/j.1365-2982.2004.00499.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones MP, Post J, Crowell MD. High-resolution manometry in the evaluation of anorectal disorders: a simultaneous comparison with water-perfused manometry. Am J Gastroenterol. 2007;102:850–855. doi: 10.1111/j.1572-0241.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 25.Preston DM, Lennard-Jones JE, Thomas BM. The balloon proctogram. Br J Surg. 1984;71:29–32. doi: 10.1002/bjs.1800710109. [DOI] [PubMed] [Google Scholar]
- 26.Pemberton JH, Rath DM, Ilstrup DM. Evaluation and surgical treatment of severe chronic constipation. Ann Surg. 1991;214:403–411. doi: 10.1097/00000658-199110000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423. e95. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manabe N, Wong BS, Camilleri M, et al. Lower functional gastrointestinal disorders: evidence of abnormal colonic transit in a 287 patient cohort. Neurogastroenterol Motil. 2010;22 doi: 10.1111/j.1365-2982.2009.01442.x. 293–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bharucha AE, Low PA, Camilleri M, et al. Pilot study of pyridostigmine in constipated patients with autonomic neuropathy. Clin Auton Res. 2008;18:194–202. doi: 10.1007/s10286-008-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camilleri M. Scintigraphic biomarkers for colonic dysmotility. Clin Pharmacol Ther. 2010;87:748–753. doi: 10.1038/clpt.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton DD, Camilleri M, Mullan BP, et al. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med. 1997;38:1807–1810. [PubMed] [Google Scholar]
- 32.Camilleri M, Zinsmeister AR. Towards a relatively inexpensive, noninvasive, accurate test for colonic motility disorders. Gastroenterology. 1992;103:36–42. doi: 10.1016/0016-5085(92)91092-i. [DOI] [PubMed] [Google Scholar]
- 33.Delgado-Aros S, Camilleri M, Garcia MA, et al. High body mass alters colonic sensory-motor function and transit in humans. Am J Physiol Gastrointest Liver Physiol. 2008;295:G382–G388. doi: 10.1152/ajpgi.90286.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bannister JJ, Abouzekry L, Read NW. Effect of aging on anorectal function. Gut. 1987;28:353–357. doi: 10.1136/gut.28.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metcalf AM, Phillips SF, Zinsmeister AR, et al. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 36.Kreulen DL, Szurszewski JH. Reflex pathways in the abdominal prevertebral ganglia: evidence for a colo-colonic inhibitory reflex. J Physiol. 1979;295:21–32. doi: 10.1113/jphysiol.1979.sp012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Groat WC, Krier J. The central control of the lumbar sympathetic pathway to the large intestine of the cat. J Physiol. 1979;289:449–468. doi: 10.1113/jphysiol.1979.sp012746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gué M, del Rio C, Junien JL, et al. Interaction between CCK and opioids in the modulation of the rectocolonic inhibitory reflex in rats. Am J Physiol. 1995;269:G240–G245. doi: 10.1152/ajpgi.1995.269.2.G240. [DOI] [PubMed] [Google Scholar]
- 39.Julia V, Morteau O, Buéno L. Involvement of neurokinin 1 and 2 receptors in viscerosensitive response to rectal distension in rats. Gastroenterology. 1994;107:94–102. doi: 10.1016/0016-5085(94)90065-5. [DOI] [PubMed] [Google Scholar]
- 40.Mollen RM, Salvioli B, Camilleri M, et al. The effects of biofeedback on rectal sensation and distal colonic motility in patients with disorders of rectal evacuation: evidence of an inhibitory rectocolonic reflex in humans? Am J Gastroenterol. 1999;94:751–756. doi: 10.1111/j.1572-0241.1999.00947.x. [DOI] [PubMed] [Google Scholar]
- 41.Iantorno G, Cinquetti M, Mazzocchi A, et al. Audit of constipation in a gastroenterology referral centre. Dig Dis Sci. 2007;52:317–320. doi: 10.1007/s10620-006-9486-5. [DOI] [PubMed] [Google Scholar]
- 42.Glia A, Lindberg G, Nilsson LH, et al. Constipation assessed on the basis of colorectal physiology. Scand J Gastroenterol. 1998;33:1273–1279. doi: 10.1080/00365529850172359. [DOI] [PubMed] [Google Scholar]
- 43.Ragg J, McDonald R, Hompes R, et al. Isolated colonic inertia is not usually the cause of chronic constipation. Colorectal Dis. 2011;13:1299–1302. doi: 10.1111/j.1463-1318.2010.02455.x. [DOI] [PubMed] [Google Scholar]
- 44.Öncü K, Özel AM, Demırtürk L, et al. Determination of the frequency of dyssynergic defecation and patient characteristics in patients with functional constipation. Turk J Gastroenterol. 2010;21:372–380. [PubMed] [Google Scholar]
- 45.Talley NJ, Weaver AL, Zinsmeister AR, et al. Functional constipation and outlet delay: a population-based study. Gastroenterology. 1993;105:781–790. doi: 10.1016/0016-5085(93)90896-k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.