Abstract
EGFR inhibitors are employed in therapy of lung and pancreatic cancers, and effectively prevent cancers in multiple animal models. Although daily dosing with erlotinib is effective, weekly dosing may reduce toxicity and have advantages, particularly for prevention. We tested alternative dosing regimens for preventive/therapeutic efficacy in a rat mammary cancer model. For prevention, erlotinib was administered by gavage beginning 5 days after MNU. For therapy and biomarker studies, rats with palpable mammary cancers were treated for six weeks or for 6 days, respectively.
Prevention
Experiment A. Erlotinib (6 mg/Kg BW/day, i.g.): daily (7x/week); one day on/one day off; and two days on/two days off. All regimens decreased tumor incidence, increased tumor latency, and decreased cancer multiplicity vs controls (P<.01). However, intermittent dosing was less effective than daily dosing (P<.05). Experiment B. Erlotinib (6 mg/kg BW/day) daily or two days on/two days off; or 1x/week at 42 mg/kg BW. All regimens reduced cancer incidence and multiplicity vs controls (P<.01). Interestingly, daily and weekly dosing were equally effective (P>0.5). Experiment C. Erlotinib administered at 42 or 21 mg/kg BW, 1x/week, decreased tumor incidence and multiplicity (P<.01).
Pharmacokinetics
Erlotinib had a serum half-life of ≤8 hours, and weekly treatment yielded effective serum levels for ≤48 hours.
Therapy
Daily or weekly treatment of cancer bearing rats reduced mammary tumor size 25–35%, while control cancers increased >250%.
Biomarkers
Levels of phosphorylated ERK were strongly decreased in rats treated daily/weekly with erlotinib. Thus, altering the dosing of erlotinib retained most of its preventive and therapeutic efficacy.
Keywords: Mammary Cancer, Prevention, Therapy, Pharmacokinetics
INTRODUCTION
Rat mammary cancer models have been employed for several decades to evaluate potential chemopreventive agents. Chemically-induced models of mammary carcinogenesis were initially developed by Huggins and co-workers (1). Subsequently, female Sprague-Dawley rats treated with methylnitrosourea (MNU) were shown to develop multiple hormonally-responsive mammary cancers starting within five weeks after carcinogen administration (2). These cancers were histologically and by gene expression similar to well differentiated ER+ human breast cancers (3). As expected, treatments that altered the hormonal axis (e.g., SERMS, aromatase inhibitors) were strong chemopreventive agents in this model (4,5). In addition, the cancers were responsive to various agents, including a variety of RXR agonists and farnesyltransferase inhibitors that do not act directly on the hormonal axis (6,7).
The EGFR pathway was defined more than 20 years ago, and was quickly shown to be associated with a variety of important cellular pathways (8,9). These included cellular proliferation and the cell cycle pathway. Given its integral role in the cell cycle and that EGFR was over-expressed in a variety of cancers (head and neck, lung, etc), it was immediately recognized as a potential target for cancer therapy (10). The EGFR inhibitors are approved for treatment of lung (11) and pancreatic cancers (in conjunction with standard therapy in an advanced setting). In addition, the EGFR inhibitors have shown some efficacy in a variety of cancers in small Phase II trials in early settings. In the treatment of ER+ breast cancer (although it is not routinely used), there have been two studies which demonstrate efficacy based either on clinical outcome in a neoadjuvant setting (12) or modulation of a generally accepted biomarker (13). Furthermore, recent data have shown efficacy in advanced tamoxifen resistant ER+ breast cancer either alone on in conjunction with an aromatase inhibitor (14,15). In contrast, it has typically shown limited efficacy in advanced breast cancer patients who have undergone multiple therapies (16,17). We previously reported that the EGFR inhibitor gefitinib was highly effective in both a preventive and therapeutic setting in the methylnitrosourea (MNU)-induced model of ER+ breast cancer in rats (18). In that study, it was further observed that gefitinib strongly inhibited phosphorylation of the target molecule EGFR, as well as the immediate downstream proteins AKT and ERK.
Although this class of agents still retains significant promise, questions of toxicity and potential dosing regimens (19) inhibit their use in a prevention setting. In an attempt to address whether one might alter the dose scheduling of this class of agents while maintaining efficacy, we determined: (A) the preventive efficacy of erlotinib by daily dosing, dosing every other day, dosing two days on/two days off, and weekly dosing at higher doses; (Bxref>) the therapeutic efficacy of daily and weekly dosing; (C) the effects of daily and weekly dosing with erlotinib on the phosphorylation of ERK; and (D) the pharmacokinetics of erlotinib and an active metabolite OSI 420 in rat serum following daily or weekly dosing.
MATERIALS AND METHODS
Chemicals and Animals
Treatment of female Sprague-Dawley rats for the prevention and therapy studies were as previously described (7,18) In brief, methylnitrosourea (MNU) was obtained from the NCI Chemical Carcinogen Repository, and was injected IV (75 mg/kg BW) via the jugular vein when the animals were 50 days of age. Teklad diet and rats were obtained from Harlan Sprague-Dawley, Inc., Indianapolis, IN. Erlotinib was supplied by OSI Pharmaceuticals, LLC, and was administered by gavage either on a daily or weekly basis. Agents were administered in a volume of 0.5 ml/gavage. The vehicle for erlotinib was ethanol: polyethylene glycol 400 (10:90; v/v).
Data Collection and Analyses
In all studies, rats were palpated for mammary tumors twice each week and weighed 1x/week. Body weights of the rats did not differ more than 5% in either the prevention or therapeutic studies. Statistical analyses of cancer incidence and latency as well as final tumor multiplicity were determined using Logrank analysis as previously described (5).
Erlotinib Prevention Studies
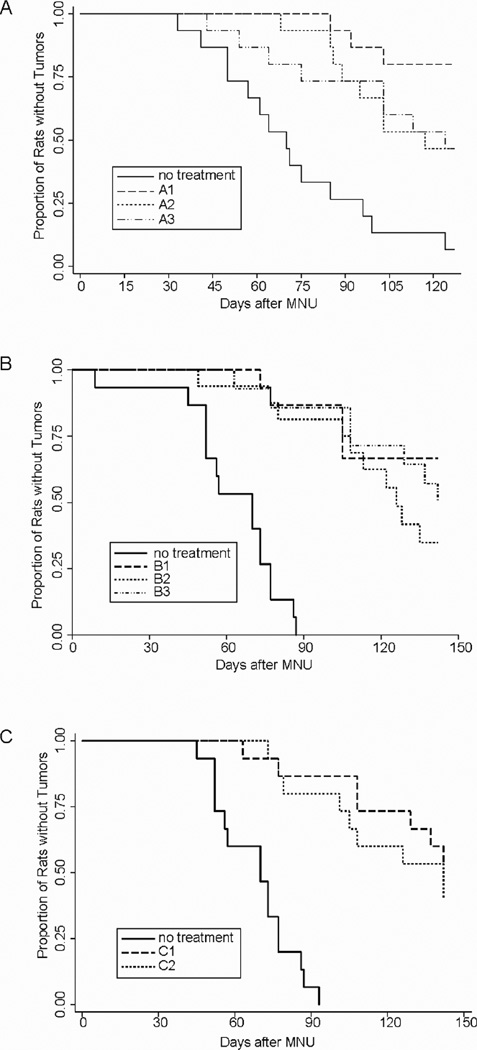
In the prevention studies, treatment of rats with erlotinib was initiated five days after MNU administration (or at 55 days of age). The number of rats/group was 15. At the end of the experiment (4 months after MNU treatment), animals were sacrificed and tumors were weighed and submitted for histological evaluation. In Experiment A (Figure 1A), erlotinib (6 mg/kg BW) was administered daily, one day on/one day off, or two days on/two days off. In Experiment B (Figure 1B), erlotinib (6 mg/kg BW) was administered daily, 2 days on/2 days off, or once per week (42 mg/kg BW). In Experiment C (Figure 1C), erlotinib was administered as a weekly dose at either 42 or 21 mg/kg BW.
Figure 1.
Effect of Erlotinib (6 mg/kg BW/day) on Time of Tumor-Free Survival in Methylnitrosourea-Induced Mammary Cancers in Female Sprage-Dawley Rats. 1A. The groups were: no treatment (solid line), erlotinib given daily (A1, dashed line), erlotinib given one day on/one day off (A2, dotted line), and erlotinib given two days on/two days off (A3, dashed/dotted line). P-values for log-rank test of survival curve equality: A1 vs control, <0.0001; A2 vs control, 0.0010; A3 vs control, 0.0031; A1 vs A2, 0.0656; A1 vs A3, 0.0617; A2 vs A3, 0.9542. 1B The various groups were: no treatment (solid line) erlotinib (6 mg/kg BW/day) given daily (B1, dashed linexref>), erlotinib (6 mg/kg BW/day) given two days on/two days off (B2, dotted line), and erlotinib (42 mg/kg BW) given 1x/week (B3, dashed/dotted line). P-values for log-rank test of survival curve equality: B1 vs control, <0.0001; B2 vs control, <0.0001; B3 vs control, <0.0001; B1 vs B2, 0.1604; B1 vs B3, 0.5016; B2 vs B3, 0.3351. 1C. The groups were: no treatment (solid line), erlotinib (42 mg/kg BW) given 1x/week (C1, dashed line), and erlotinib (21 mg/kg BW) given 1x/week (C2, dotted line). P-values for log-rank test of survival curve equality: C1 vs control, <0.0001; C2 vs control, <0.0001; C1 vs C2, 0.4372.
Erlotinib Therapeutic Study
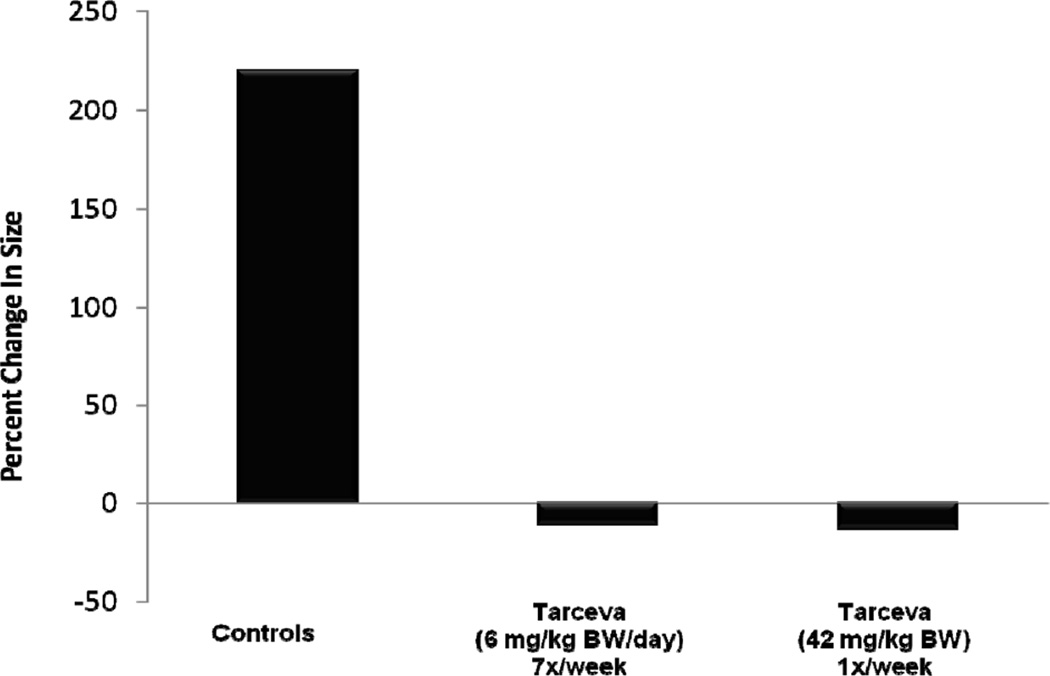
Rats received MNU at 50 days of age and were observed for the development of mammary cancers. When an animal developed a cancer of approximately 100–200 mm2, the rat received erlotinib (6 mg/kg BW/day or 42 mg/kg BW once per week) for six weeks (Figure 2). Tumor size was determined with calipers before initiation of treatment and twice each week during the course of treatment. The largest diameter of the cancer was measured and this value multiplied by the perpendicular diameter (size expressed in mm2). The erlotinib treated rats had an n of 16, while the control group had an n of 10 rats.
Figure 2.
Effect of Erlotinib on Growth of Established Mammary Cancers Induced by Methyl-nitrosourea in Female Sprague-Dawley Rats. Palpable mammary tumors were allowed to develop as described in Materials and Methods. Tumor bearing rats were treated with erlotinib daily (6 mg/kg BW), weekly (42 mg/kg BW) or vehicle for six weeks. Tumors were measured twice a week by calipers.
Erlotinib Biomarker Study
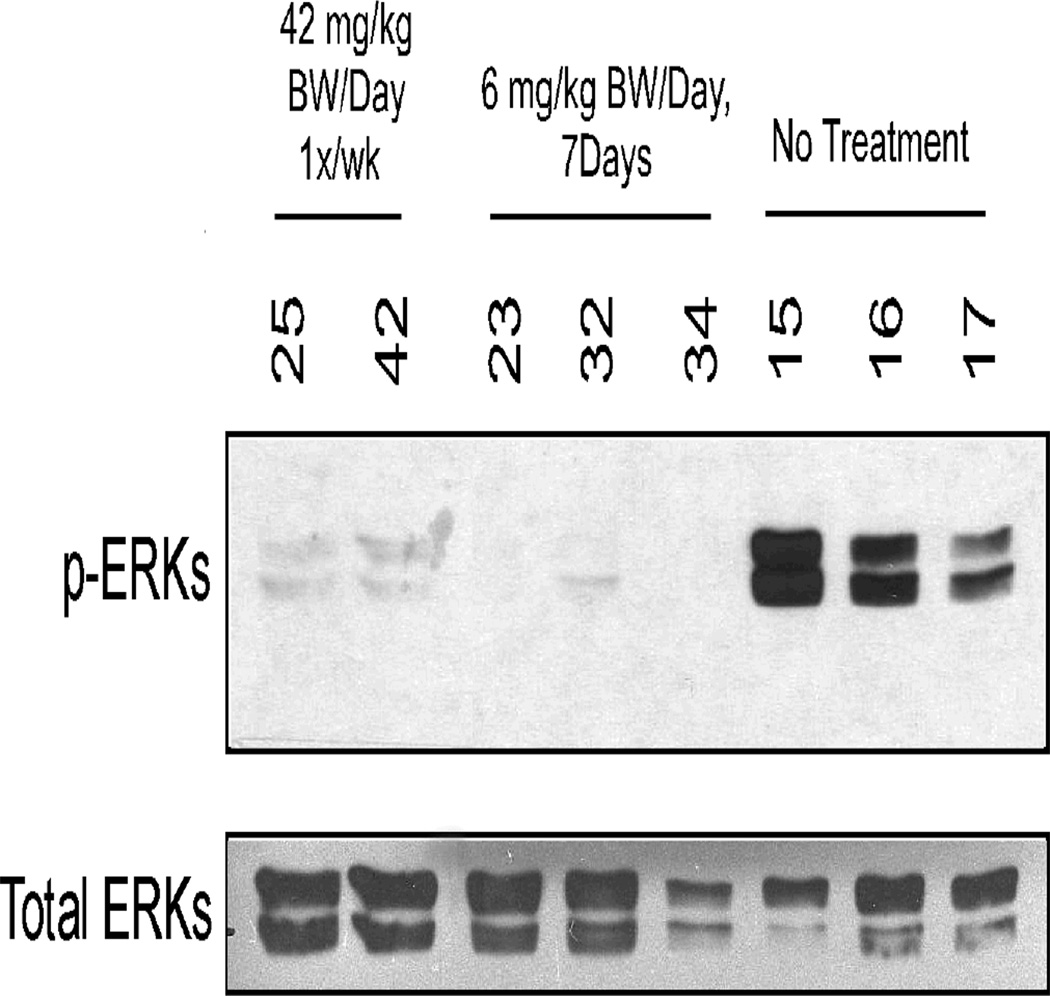
When an animal developed a palpable cancer, it was randomized into one of three groups: daily administration of erlotinib (6 mg/kg BW/day) for 7 days, administration of a single dose of erlotinib (42 mg/kg BW) on Day 0 followed by sacrifice 7 days later and vehicle control for 7 days (Figure 3). Frozen erlotinib-treated or vehicle-treated mammary cancer samples were lysed using liquid nitrogen and a pestle and mortar. A Protease Inhibitor Cocktail tablet was dissolved in 50 mL modified RIPA buffer (pH 7.4) and added to the samples. Samples were incubated on ice for 30 min, and then centrifuged twice at 13,000 rpm for 10 min at 4C; keeping the supernatant fraction each time. Protein concentration was measured. Samples were prepared using lysis buffer pH 8.0 (50 mM Tris Base, 1% NP-40, 150 mM NaCl) and 6 × SDS sample buffer, and denatured by heating at 95 C° for 5 min. Samples (50 µg) were loaded onto a 10% SDS gel and ran using constant current (45 mA/2 gels) for 1 h. Then the proteins were transferred for 1.5 hours with a constant voltage of 100 V. The membrane was blocked using 5% nonfat skim milk for 1 h at room temperature and then washed with TBS-T (Tris Base Solution-0.1%Tween 20). The primary antibody (p-ERKs T202/Y204 diluted 1:1000 in 5% BSA-1X TBS-0.1%Tween 20) was incubated overnight at 4° C (Cell Signaling, Cat#9101, Danvers, MA). The membrane was then washed with TBS-T 3 × 10 min and incubated for 1 h at room temperature with a goat anti-rabbit horse radish peroxidase (HRP) secondary antibody diluted 1:5000 in 5% nonfat skim milk (Santa Cruz Biotechnologies SC-2004, Santa Cruz, CA). The membrane was washed 3 × 10 min with TBS-T and exposed in the dark room. Exposure time was 8 min to detect phosphorylated ERKs. After exposure, the membrane was washed with TBS-T and then stripped with 1× Millipore Re-Blot Plus Strong Solution (Cat #2504) at room temperature for 50 min. The stripping buffer was removed by washing 3 × 10 min with TBS-T. The membrane was then blocked using 5% nonfat skim milk, and washed using TBS-T to remove milk residue. A total ERKs antibody, diluted 1:1000 in 5% BSA-1X TBS-0.1%Tween 20, was added and the membrane incubated at 4° C overnight (Cell Signaling, Cat #9102, Danvers, MA). The blots were then washed with TBS-T 3 × 10min, and incubated with a goat anti-rabbit HRP secondary antibody diluted 1:5000 in 5% nonfat skim milk for 1 h at room temperature. Finally, the membranes were washed 3 ×10 min in TBS-T and exposed in the dark room. Exposure time was 1–4 min to detect ERKs.
Figure 3.
Frozen Erlotinib-Treated or Vehicle-Treated Mammary Tumor Samples. Tissues were disrupted and proteins were resolved by SDS-PAGE. Western blot analysis was performed using a primary antibody to detect phosphorylated ERKs. The membrane was stripped as described in Materials and Methods, and total ERKs were detected to verify equal protein loading.
Pharmacokinetic Studies
Serum samples were collected from 5 rats per group administered erlotinib either daily (6 mg/kg BW/day) or weekly (42 mg/kg BW). Serum was collected at time 0, 2, 4, 8, 24 and 48 hours after the last gavage dose of erlotinib. Analysis of erlotinib and an active demethylated metabolite (OSI 420) employing isocratic reverse phase /tandem mass spectrometry methods were performed as previously described (20,21). In brief, diluted plasma in buffer was extracted with methyl tertiary butyl ether. The dried product was re-suspended and separated on a Waters Symmetry C18 column using ammonium formate buffer and methanol as the mobile phase. The eluate were ionized by a heated nebulizer and the mass transitions monitored at 393.4/277.8, 379.3/277.9 and 407.4/292.1 m/z for erlotinib, OSI 420, and a standard, respectively. Non-compartmental pharmacokinetic parameters were calculated using linear-log trapezoid rule with WinNonlin version 5.2.
RESULTS
Prevention
In our initial studies, the preventive activity of various daily doses of erlotinib was examined (data not shown). It was found that a dose of 6 mg/Kg BW/day was highly effective; reducing both tumor incidence and multiplicity. In the present studies, we altered the dose by implementing intermittent dosing schedules (Figure 1A, B, C). In the first study, erlotinib (6 mg/kg BW) was administered using the following schedules: daily, one day on/one day off, or two days on/two days off. This resulted in reductions of tumor incidence and increases in latency for all three regimens vs controls (P<.01). However, the intermittent dosings were less effective than daily dosing: (P<.06, 1 day on/1 day off; P<.065, 2 days on/2 days off). We have determined tumor multiplicity at various time points (Supplementary Fig 1A) and found that daily, 1 day on/1 day off, and 2 days on/2 days off treatments reduced final tumor multiplicity by 90, 65 and 75%, respectively. All three regimens greatly reduced final tumor multiplicity compared to vehicle controls (P<.001) while daily dosing decreased final tumor multiplicity more than either of the intermittent dosing schedules (P<.05).
In Figure 1B, rats were treated with erlotinib (6 mg/kg BW) daily, intermittent erlotinib (6 mg/kg BW) two days on/two days off, or erlotinib weekly at 42 mg/kg BW (the equivalent daily dose over a one week period). All three treatment regimens reduced tumor incidence and increased tumor latency compared to controls (P<.01). The intermittent dosings were marginally less effective than daily dosing (P<.125), whereas the efficacy of daily or weekly dosing was comparable. As shown in Supplement Fig 1b, we found daily, 2 days on/2 days off, and weekly dosing reduced multiplicity 85, 69 and 89%, respectively; all highly significant relative to controls (P<.005). However, based on final multiplicity the dosing of 2 days on/2 days off was less effective than daily dosing (P<.05). Weekly dosing at either 42 or 21 mg/kg BW strongly inhibited tumor incidence (Figure 1C) and tumor multiplicity (Supplementary Fig 1C).
Pharmacokinetics
Table 1 shows the plasma concentrations of erlotinib and OSI 420 (an active metabolite). These data indicate that a dose of 42 mg/kg BW erlotinib in rats was similar to a human dose of 150 mg. As shown, the Cmax for erlotinib at 6 mg/Kg BW was <50% of human levels, while the Cmax for the 42mg dose was similar to that achieved in humans. This difference is even more apparent in the respective AUCs where the 6 mg/Kg BW dose is <20% of the human dose, while the 42 mg dose is similar to the human (Table 2). This large decrease in AUC for the rat is reflective of a half-life of 5–8 hours in the rat and approximately 24 hours in humans. It is generally thought that serum levels <25 ng/ml are suboptimal based on cell culture results showing efficacy at doses ≥100 nM in vitro. Thus, therapeutic levels were achieved for approximately 18 hours following a daily dose. However, therapeutic serum levels were achieved for less than 48 hours after the weekly dosing.
TABLE 1.
Serum Levels of Erlotinib and Its Major Metabolite OSI-420 in Female Sprague-Dawley Rats
| (a) Erlotinib, 6 mg/kg BW/day | ||
| Hours After Final Gavage | Erlotinib | OSI-420 |
| 2 | 708 ± 48* | 133 ± 17 |
| 4 | 427 ± 19 | 88 ± 13 |
| 8 | 277 ± 102 | 58 ± 19 |
| 24 | 25 ± 9 | 18 ± 5 |
| 48 | 0 | 10 ± 4 |
| (b) Erlotinib, 42 mg/kg BW, 1x/week | ||
| Hours After Final Gavage | Erlotinib | OSI-420 |
| 2 | 1352 ± 96 | 246 ± 21 |
| 4 | 1060 ± 184 | 226 ± 32 |
| 8 | 1590 ± 259 | 323 ± 42 |
| 24 | 470 ± 121 | 130 ±36 |
| 48 | 52 ± 11 | 13 ± 4 |
| (c) Controls | ||
| None detected | ||
Values are ng/ml of serum; mean ± SEM
TABLE 2.
Erlotinib Non-compartmental Pharmacokinetic Parameters in Female Sprague-Dawley Rats and Humans (steady state)
| Species | Dose mg/kg |
Tmax h |
Cmax ng/mL |
AUC0–24 ng h/mL |
T1/2 h |
|---|---|---|---|---|---|
| Rat | 6 | 2.00 | 708 | 4680 | 4.61 |
| 42 | 8.00 | 1590 | 28400 | 8.06 | |
| Human | 150 (mg) | 2.44 | 1740 | 26500 | 24.4 |
Therapy
The MNU cancer model was also used to examine the therapeutic efficacy of erlotinib by allowing palpable tumors to develop before initiating treatment (Figure 2). Ten rats with tumors were treated with erlotinib (6 mg/kg BW) on a daily basis, 16 rats were treated with erlotinib (42 mg/kg BW) on a weekly basis, and 10 rats received the vehicle. Daily or weekly treatment reduced average tumor size by 40 and 25%, respectively; while cancers in the vehicle-treated group increased in size by 250% over the 6 weeks period. With either daily or weekly dosing, at least 50% of the mammary tumors decreased by 60% in size. We consider a 60% decrease in size to be a highly significant regression. Thus, daily and weekly dosing were extremely effective.
Biomarker Study
A biomarker directly related to the mechanism of action of erlotinib was measured; specifically, the phosphorylation of the downstream effector molecule ERK. A striking decrease in phosphorylation of ERK in cancers from animals treated on a daily basis for 7 days with erlotinib was observed in a Western blot (Figure 3). Surprisingly, a strong decrease in phosphorylated ERK was similarly observed in animals given a single weekly dose of erlotinib and examined 6 days following the treatment.
DISCUSSION
EGFR was discovered approximately 30 years ago, and its associated receptor EGFR was clearly defined as a relevant target for tumor cell therapy more than 15 years ago (9). EGFR was shown to be integral to cell signaling and cell replication. It has also been shown to be over-expressed in a wide variety of tumors including lung, head and neck, urinary bladder, and basal cell subtype of breast cancer (10). EGFR inhibitors progressed into the clinics, and based on testing in advanced cancers have proven highly effective against a subset of non-small cell lung cancer (NSCLC); with particularly striking and extended responses in adenocarcinomas which have mutations in the EGFR gene (11). Lung adenocarcinomas with EGFR mutations are more prevalent in non-smokers and persons of Asian descent.
Previous clinical breast cancer studies using EGFR inhibitors are particularly interesting and partially conflicting. In advanced breast cancer patients which had undergone prior therapies, EGFR inhibitors used as mono-therapies yielded minimal activity (16,17). These studies used mixed populations of patients who had been heavily pre-treated. In advanced breast cancer, EGFR inhibitors alone or EGFR inhibitors administered with an aromatase inhibitor have proven effective in the subgroup of ER+, tamoxifen-resistant breast cancers (14,15). These tumors tended to over-express various growth factors. Interestingly, there are at least two reports implying the efficacy of EGFR inhibitors in earlier stages of ER+ breast cancer. One was a true neoadjuvant study in which gefitinib was administered for 12–16 weeks (12). Roughly 40% of ER+ tumors were shown to have EGFR expression based on immunohistochemistry. These tumors were treated with gefitinib alone for a period of up to 16 weeks, and approximately 40% showed a complete response. A subsequent study comparing anastrozole alone vs anastrozole plus gefitinib revealed no significant improvement in early stage breast cancer. However, in this case anastrozole alone was profoundly effective (23). The second study was a biomarker study in a pre-surgical setting (13). This biomarker study showed striking decreases in Ki-67 labeling in ER+ breast cancer as contrasted with Neu positive or triple-negative breast cancer (TNBC). Interestingly, in the gefitinib study in tamoxifen resistant breast cancer mentioned above (15), significant decreases in breast cancer proliferation were associated with the efficacy of gefitinib clinically. Large decreases in proliferation have been shown to be associated with the therapeutic and preventive efficacy of hormonal agents (SERMs and aromatase inhibitors both in humans and rodents) (24,25). The positive, albeit mixed, results in ER+ breast cancer might appear to be particularly unexpected since the general consensus has been that among subtypes of breast cancer ER+ tumors generally have the lowest expression of EGFR (13). In fact, EGFR expression has been considered a biomarker of TNBC; although that subtype of cancers does not routinely respond to therapy with these agents (26).
Our laboratories had previously shown that MNU-induced rat mammary cancers were highly sensitive to the EGFR inhibitor gefitinib (18). These tumors are ER+ and appear similar to highly differentiated ER+ human cancers; as determined by gene arrays (3). The tumors respond to the same treatments that alter human ER+ cancers (SERMs, aromatase inhibitors, ovariectomy). In our earlier studies, it was found that gefitinib caused a dose dependent decrease in tumor multiplicity, and that the highest dose (10 mg/kg BW), which is somewhat lower than the human equivalent dose based on standard scaling factors, was therapeutic in the model. Not unexpectedly, we found that gefitinib decreased levels of phosphorylated EGFR and the downstream phosphorylated proteins AKT and ERK (18).
In the present experiments, our earlier studies were expanded using the EGFR inhibitor erlotinib. We initially showed that erlotinib induced a dose dependent decrease in tumor incidence and multiplicity. The highest dose of erlotinib (6 mg/kg BW/day) administered would equate to a human dose of approximately 75 mg; or ½ the standard human dose of 150 mg. This is based on standard FDA scaling factors where the rat dose in mg/kg is divided by 6 to get the human dose, and then multiplied by kg to get the mg equivalent. The effects of altering the dosing schedule to one day on/one day off or dosing two days/two days off was performed in the first study. Both the daily dosing and intermittent dosing reduced tumor incidence and increased tumor latency (Fig 1A), while simultaneously reducing final multiplicity (65–75% by intermitant dosing and 90% by daily dosing) (Supplementary Fig 1A). As indicated, intermittent dosings were less effective than daily dosing when comparing tumor incidence and tumor latency (1 day on/1 day off vs daily, P< .06; 2 days on/2 days off vs daily, P< .065) or employing final tumor multiplicity (P<.05; intermittent vs daily dosing). We had previously observed less efficacy with gefitinib when comparing intermittent vs daily dosing (18). Based in part on these results, we substantially altered the dosing protocol; employing weekly dosing with erlotinib as compared with daily dosing or two days on/two days off schedule. The studies (Figs 1B and 1C, and Supplementary Figs 1B and 1C) demonstrated that weekly dosing was highly effective since even the lower weekly dose (21 mg/kg BW), which is one-half the total dose administered by daily gavage of 6 mg/kg BW, decreased tumor multiplicity approximately 85%. Given the relatively short half-life of erlotinib in rats (Tables 1 and 2) and the fact that apparently sub-therapeutic serum levels were reached in approximately 48 hours, the preventive efficacy was surprising. When standard PK parameters were examined (Table 2), it was observed that the effective daily dose in a rat is substantially lower than that in humans; yielding a Cmax that is approximately 60% lower than in the human and an AUC which is 5× less. These results reflect the shorter half-life of daily erlotinib in the rat vs the human (5–6 hours vs 16–24 hours). The weekly dosing regimen was also effective in a therapeutic setting (Figure 2). It should be noted, however, that the cancers in this model are not as genetically advanced as a typical human breast cancer.
In an attempt to understand the downstream effects of EGFR inhibition in this model, we examined levels of phosphorylated ERK in tumors of rats treated daily or weekly with erlotinib. We had previously shown daily dosing with gefitinib inhibited phosphorylation of EGFR, AKT, and ERK as determined by immunohistochemistry (18). In the present study, it was observed that a decrease occurred in ERK phosphorylation (as determined by Western blotting) even one week following a large bolus dose of erlotinib. We similarly observed the efficacy of both dosing regimens in the prevention and therapy of tumors in rats. This biomarker result was unexpected given the finding that effective serum levels of erlotinib were probably maintained for less than 48 hours, even following a single weekly dosing with erlotinib. The specific mechanism responsible remains to be determined.
There are a number of important questions derived from the results in these studies. First, could the results have been due to chance? The reproducibility of the effect in two separate experiments suggests otherwise. Second, is this effect generally applicable to all EGFR inhibitors? Similar results have been seen with two additional EGFR inhibitors in the same model system (27). Third, will the altered dosing regimen be applicable to a wide range of cancers in different organs? We have tested weekly Erlotinib treatment in both a transgenic model of ER negative breast cancer and a carcinogen-induced urinary bladder model; in both models, strong efficacy was observed (Lubet and Grubbs, unpublished data). Fourth, will this greatly altered dosing regimen be applicable to a wider range of kinase inhibitors? We have observed efficacy of weekly dosing with an allosteric AKT inhibitor; although this may reflect in part the pharmacokinetics of this specific agent.
The most important question, however, is whether the altered dosing regimen will alter the toxicity profile favorably enough to enable the testing (and ultimate use if shown to be effective) of erlotinib in a prevention setting. Milton and colleagues (28) examined the effects of weekly dosing of erlotinib in a phase I/II study performed in patients with previously treated NSCLC. They showed with doses up to 2000 mg/week (more than thirteen-fold greater than the FDA-approved single daily dose of 150 mg) that the toxicity profile was generally modest with no severe (grade 3) rash or diarrhea at any dose level, and moderate (grade 2) rash in 17% or 42% of patients treated at the 1200/1600 mg/week or 2000 mg/week dose levels, respectively. Similarly, a study of nine patients with brain metastases who were treated with a median dose of 1500 mg weekly also showed a low incidence of rash, with mild to moderate (grade 1–2) rash in only two patients (29); significant efficacy was observed at this dose. As with all preventive interventions, the balance between benefit, risk, and tolerability will need to be established before this class of drugs can be used in this setting.
The results presented show for the first time that a greatly altered dosing regimen (consisting of once weekly high doses of erlotinib) is effective in the prevention and treatment of carcinogen-induced mammary cancers. Given the human data showing diminished toxicity with weekly dosing (28,29), these data provide a scientific rationale for testing such regimens in human cancer prevention trials in appropriately high risk individuals.
Supplementary Material
ACKNOWLEDGEMENTS
Grant Support: National Cancer Institute; Contract Number HHSN261200433001C
Footnotes
Conflict of Interest: None
Contributor Information
Ronald A. Lubet, Email: lubetr@mail.nih.gov.
Eva Szabo, Email: szaboe@mail.nih.gov.
Kenneth K. Iwata, Email: kenneth.iwata@us.astellas.com.
Stanley C. Gill, Email: stan.gill@us.astellas.com.
Chris Tucker, Email: chris.tucker@us.astellas.com.
Ann Bode, Email: ambode@hi.umn.edu.
Vernon E. Steele, Email: steelev@mail.nih.gov.
M. Margaret Juliana, Email: mmj@uab.edu.
Holly L. Nicastro, Email: nicastrohl@mail.nih.gov.
Clinton J. Grubbs, Email: clinton.grubbs@ccc.uab.edu.
REFERENCES
- 1.Huggins C. Two principles in endocrine therapy of cancers: hormone deprival and hormone interference. Cancer Res. 1965;25:1163–1167. [PubMed] [Google Scholar]
- 2.Grubbs CJ, Peckham JC, McDonnough KD. Effect of ovarian hormones on the induction of 1-methyl-1-nitrosourea induced mammary cancer. Carcinogenesis. 1983;4:495–497. doi: 10.1093/carcin/4.4.495. [DOI] [PubMed] [Google Scholar]
- 3.Chan MM, Lu X, Merchant FM, Inglehart JD, Miron PL. Gene expression profiling of NMU-induced rat mammary tumors: cross species comparison with human breast cancer. Carcinogenesis. 2005;26:1343–1353. doi: 10.1093/carcin/bgi100. [DOI] [PubMed] [Google Scholar]
- 4.Gottardis MM, Jordan C. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res. 1987;47:4020–4024. [PubMed] [Google Scholar]
- 5.Lubet RA, Steele VE, Casebolt TL, Eto I, Kelloff GJ, Grubbs CJ. Chemopreventive effects of the aromatase inhibitors vorozole (R-83842) and 4-hydroxyandrostenedione in the methylnitrosourea (MNU)-induced mammary tumor model in Sprague-Dawley rats. Carcinogenesis. 1994;15:2775–2780. doi: 10.1093/carcin/15.12.2775. [DOI] [PubMed] [Google Scholar]
- 6.Gottardis MM, Bischoff ED, Shirley MA, Wagoner MA, Lamph WW, Heyman RA. Chemoprevention of mammary carcinoma by LGD 1069 (Targretin): an RXR selective ligand. Cancer Res. 1996;56:5566–5570. [PubMed] [Google Scholar]
- 7.Lubet RA, Christov K, You M, Yao R, Steele VE, End DW, et al. Effects of the farnesyl transferase inhibitor R115777 (Zarnestra) on mammary carcinogenesis: prevention, therapy, and role of HaRas mutations. Mol Cancer Ther. 2006;5:1073–1078. doi: 10.1158/1535-7163.MCT-05-0398. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S. The epidermal growth factor (EGF) Cancer. 1983;51:1787–1791. doi: 10.1002/1097-0142(19830515)51:10<1787::aid-cncr2820511004>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 10.Arteaga CL. Overview of epidermal growth factor receptor biology and its role as therapeutic target in human neoplasia. Semin Oncol. 2002;29:3–9. doi: 10.1053/sonc.2002.35642. [DOI] [PubMed] [Google Scholar]
- 11.Hida L, Ogawa S, Park JC, Park JY, Shimazu J, Horio Y, et al. Gefitinib for the treatment of non small cell lung cancer. Expert Rev Anticancer Ther. 2009;9:17–35. doi: 10.1586/14737140.9.1.17. [DOI] [PubMed] [Google Scholar]
- 12.Polychronis A, Sinnett HD, Hadjiminas D, Singhal H, Mansi JL, Shivapatham D, et al. Preoperative gefitinib versus gefitinib and anastrozole in postmeanopausal patients with oestrogen receptor positive and epidermal growth factor receptor positive primary breast cancer: a double blind placebo controlled phase II randomized trial. Lancet Oncol. 2005;6:383–391. doi: 10.1016/S1470-2045(05)70176-5. [DOI] [PubMed] [Google Scholar]
- 13.Guix M, Granja-Nde M, Meszoely I, Adkins TB, Wieman BM, Frierson KE, et al. Short preoperative treatment with Erlotinib inhibits tumor cell proliferation in hormone receptor-positive breast cancers. J Clin Oncol. 2008;26:897–906. doi: 10.1200/JCO.2007.13.5939. [DOI] [PubMed] [Google Scholar]
- 14.Cristofanilli M, Valero V, Mangalik A, Royce M, Rabinowitz I, Arena FP, et al. Phase II, Randomized trial to compare anastrozole combined with gefitinib or placebo in post-menopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16:1904–1914. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 15.Gutteridge E, Agrawal A, Nicholson R, Leung K, Robertson J, Gee J. The effects of gefitinib in tamoxifen-resistant and hormone-insensitive breast cancer: a phase II study. Int J Cancer. 2010;126:1806–1816. doi: 10.1002/ijc.24884. [DOI] [PubMed] [Google Scholar]
- 16.Baselga J, Albanell J, Ruiz A, Lluch A, Gascón P, Guillém V, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23:5323–5333. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Jonat W, Fasching P, du Bois A, Kleeberg U, Lűck HJ, et al. A multicentre phase II study on gefitinib in taxane- and anthracycline-pretreated metastatic breast cancer. Breast Cancer Res Treat. 2005;89:165–172. doi: 10.1007/s10549-004-1720-2. [DOI] [PubMed] [Google Scholar]
- 18.Lubet RA, Szabo E, Christov K, Bode AM, Ericson ME, Steele VE, et al. Effects of gefitinib (Iressa) on mammary cancers: preventive studies with varied dosages, combinations with vorozole or targretin, and biomarker changes. Mol Cancer Ther. 2008;7:972–979. doi: 10.1158/1535-7163.MCT-07-2141. [DOI] [PubMed] [Google Scholar]
- 19.Guttman-Yassky E, Mita A, De Jonge M, Matthews L, McCarthy S, Iwata KK, et al. Characterization of the cutaneous pathology in non-small cell lung cancer (NSLC) patients treated with EGFR tyrosine kinase inhibitor Erlotinib. Eur J Cancer. 2010;46:2010–2019. doi: 10.1016/j.ejca.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M, Wolf JL, Rusk J, Beard SE, Clark GM, Witt K, et al. Effects of smoking on the pharmacokinetics of Erlotinib. Clin Cancer Res. 2006;12:2166–2171. doi: 10.1158/1078-0432.CCR-05-2235. [DOI] [PubMed] [Google Scholar]
- 21.Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, et al. Phase I and Pharmacologic Study of OSI-744, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J. Clin Oncol. 2001;19:3267–3279. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, LaFortune TA, Krishnamurthy S, Esteva FJ, Cristofanilli M, Liu P, et al. Epidermal growth factor receptor tyrosine kinase inhibitor reverses mesenchymal to epithelial phenotype and inhibits metastasis in inflammatory breast cancer. Clin Cancer Res. 2009;15:6639–6648. doi: 10.1158/1078-0432.CCR-09-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith IE, Walsh G, Skene A, Llombart A, Mayordome JI, Detre S, et al. A phase II placebo-controlled trial of neoadjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol. 2007;25:3816–3822. doi: 10.1200/JCO.2006.09.6578. [DOI] [PubMed] [Google Scholar]
- 24.Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in breast cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christov K, Grubbs CJ, Shilkaitis A, Juliana MM, Lubet RA. Short-term modulation of cell proliferation and apoptosis and preventive/therapeutic efficcy of various agents in a mammary cancer model. Clin Cancer Res. 2007;13:5488–5496. doi: 10.1158/1078-0432.CCR-07-0404. [DOI] [PubMed] [Google Scholar]
- 26.Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, Ma CX, et al. TBCRC 001: Randomized Phase II study in cetuximab in combination with carboplatin in Stage IV triple-negative breast cancer. J Clin Oncol. 2012 Jun 4; doi: 10.1200/JCO.2010.34.5579. E Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubet R, Bode A, Szabo E, Grubbs CJ. Alternative dosing regimens with the EGFR inhibitors (Gefitinib and Lapatinib) in mammary cancer models: prevention and therapeutic efficcy. San Antonio Breast Cancer Symposium. 2011;71:380s. [Google Scholar]
- 28.Milton DT, Azzoli CG, Heelan RT, Venkatraman E, Gomez JE, Kris MG, et al. A phase I/II study of weekly high-dose Erlotinib in previously treated patients with non small cell lung cancer. Cancer. 2006;107:1034–1041. doi: 10.1002/cncr.22088. [DOI] [PubMed] [Google Scholar]
- 29.Grommes G, Oxnard R, Kris MG, Miller VA, Pao W, Holodny AI, et al. “Pulsatile” high-dose weekly Erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.