Abstract
We previously demonstrated that γδ T cells played an important role in tumor immune surveillance by providing an early source of IFN-γ. The precise role of different subsets of γδ T cells in the antitumor immune response, however, is unknown. Vγ1 and Vγ4 γδ T cells are the principal subsets of peripheral lymphoid γδ T cells and they might play distinct roles in tumor immunity. In support of this, we observed that reconstitution of TCRδ−/− mice with Vγ4, but not Vγ1, γδ T cells restored the antitumor response. We also found that these effects were exerted by the activated (CD44high) portion of Vγ4 γδ T cells. We further determined that IFN-γ and perforin are critical elements in the Vγ4-mediated antitumor immune response. Indeed, CD44high Vγ4 γδ T cells produced significantly more IFN-γ and perforin on activation, and showed greater cytolytic activity than did CD44high Vγ1 γδ T cells, apparently due to the high level of eomesodermin (Eomes) in these activated Vγ4 γδ T cells. Consistently, transfection of dominant-negative Eomes in Vγ4 γδ T cells diminished the level of IFN-γ secretion, indicating a critical role of Eomes in the effector function of these γδ T cells. Our results thus reveal distinct functions of Vγ4 and Vγ1 γδ T cells in antitumor immune response, and identify a protective role of activated Vγ4 γδ T cells, with possible implications for tumor immune therapy.
Introduction
There are many unique features and functions of γδ T cells (1–3). Similar to αβ T cells, γδ T cells produce an array of cytokines and possess cytolytic functions. Our previous studies have demonstrated that γδ T cells predominantly produce IFN-γ on activation and the underlying controlling mechanisms are different from those of αβ T cells (4, 5). Moreover, we have shown that γδ T cells play an important role in tumor immune surveillance as an early source of IFN-γ (6). However, it remained unclear whether all or only some γδ T cells contribute to host protection.
Vγ1 and Vγ4 γδ T cells are the two dominant subsets of peripheral lymphoid γδ T cells. Recently, these subsets have been demonstrated to have different functions in regulating CD4 T cell Th1/Th2 differentiation; Vγ1 γδ T cells promote CD4 Th2 responses, wheraes Vγ4 γδ T cells promote CD4 Th1 cell responses in Coxsackievirus B3 infections and airway hyperactive response (7–12). These two subsets of γδ T cells have also shown to mediate divergent functions in macrophages and play a distinctive role in autoimmune diseases models as well as infectious immunity (13–15). Although these two subsets of γδ T cells have divergent functions; however, the involvement of TCR or other receptors has not been implicated. So far, the roles of Vγ1 versus Vγ4 γδ T cells in antitumor immune responses also remain to be investigated.
Unlike αβ T cells, most peripheral γδ T cells spontaneously activate, upregulating surface expression of the activation marker CD44 and experiencing rapid turnover (16, 17). They also expand quickly on pathogenic challenge in the first several days postinfection (18–20). In experimental models of infectious diseases, γδ T cell responses develop between 4 h and 96 h postinfection, bridging the gap between the innate immune (NK and macrophages) and adaptive immune responses (Ag-specific CD4 and CD8 T cell responses) (21, 22). Our previous studies have demonstrated that CD44high, but not CD44low, γδ T cells spontaneously express IFN-γ and T-bet, and rapidly produce IFN-γ on TCR activation (4, 5). Therefore, it seemed likely that CD44high γδ T cells play an important role in antitumor immune responses.
In this study, we demonstrate that Vγ4 γδ T cells are indeed protective in the immune response against the aggressive B16 melanoma, and that the CD44high fraction of this γδ T cell subset is critical. We further show that both IFN-γ and perforin are essential for CD44high Vγ4 γδ T cell-mediated tumor protection. On activation, CD44high Vγ4 γδ T cells produce higher levels of IFN-γ and perforin than do CD44high Vγ1 γδ T cells, at least in part due to the high expression level of the transcription factor eomesodermin (Eomes). Our study thus provides the first evidence for a critical role of Vγ4 γδ T cells in protective antitumor immune responses.
Materials and Methods
Mice
C57BL/6J (B6) mice were purchased from the National Cancer Institute. C57BL/6J-Tcrb tm1Mom (B6 TCRβ-deficient mice [TCRβ −/−]), C57BL/6J-Tcrdtm1Mom (B6 TCRδ −/−), C57BL/6-prf1tm1 (B6 Prf1-deficient [perforin−/−]) and C57BL/6-Ifngtm1Ts (B6 IFN-γ–deficient [IFN-γ−/−]) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Some of experimental mice were purchased from Chinese Medical Academy of Sciences (Beijing, China). All animals were maintained under specific pathogen-free conditions and used at 6–8 wk of age.
Reagents
Recombinant murine IL-2, IL-12 was purchased from R&D Systems (Minneapolis, MN). Anti-mouse IL-4, and Abs for phenotypic and cytokine analysis were purchased from BD Biosciences (San Jose CA).
Tumor Models
B16 F0 melanoma cells (provided by Dr. Mark Mamula, Yale School of Medicine, New Haven, CT) were injected s.c., and tumor growth was monitored and recorded daily for >3 wk, as described (6).
Expansion of CD44high gd T cells in vitro
CD44high γδ T cells were sorted from splenocytes of either B6 Wt (wild-type) or perforin−/− or IFN-γ−/− mice by FACS. Sorted cells were cultured with plate-coated Vγ4-specific Ab UC3 and Vγ1-specific Ab 2.11, respectively (10 μg/ml), and IL-2 (2 ng/ml) for 8 d. Expanded Vγ4 and Vγ1 γδ T cells were confirmed by FACS analysis.
Effector function of CD44high Vg4 and Vg1 gd T cells in antitumor immune response in vivo
Expanded CD44high Vγ1 or Vγ4 γδ T cells were prepared as described previously, mixed with B16 tumor cells in a ratio of 1:4, and coinjected into B6 TCR δ−/− mice s.c. The presence of tumors and their size was monitored and measured as described (6).
Intracellular cytokine staining
Cells were activated as described conditions. After 3 h of culture, brefeldin A was added to the culture for additional 3 h. The cells were then collected and fixed with 2% formaldehyde in PBS, permeabilized with 0.5% saponin, and stained with fluorescently labeled cytokine-specific Abs, as described (5).
CTL assay
The cytotoxicity of Vγ1 and Vγ4 γδ T cells was determined by JAM assay as described previously (23). Briefly, sorted CD44high Vγ4 and Vγ1 γδ T cells from B6 mice were stimulated with the Vγ4-specific Ab UC3 and Vγ1 specific-Ab 2.11, respectively, and cultured with IL-2 for 8 d. YAC-1 cells (target cells, 1000 cells/well) were cultured for 6 h with [3H]thymidine (5 μCi/ml). Various amounts of activated cells (effector cells) were added to the target cells in triplicate in a 96-well round-bottom plate. Spontaneous 3H retention was determined by adding medium instead of effector cells. After 4 h of culture, cells were harvested onto filters, which were read by a scintillation counter to calculate the specific lysis using the following formula: ((spontaneous cpm − experimental cpm) × 100)/spontaneous cpm.
Co-culture of gd T cells and B16 melanoma
Expanded Vγ1 or Vγ4 γδ T cells (1.5 × 105 cells/well) were mixed with B16 cells (7500 cells/well) in a total volume of 200 μl in 96-well plate in triplicate for 24 h. Live cells were counted with trypan blue vital stain (Invitrogen, San Diego, CA) and the supernatants were collected for analysis of secreted IFN-γ. For Ab blocking experiments, expanded Vγ1 and Vγ4 cells (1 × 106 cells/ml) were then incubated with control Ab, anti-mouse γδ TCR (clone UC7) as TCR block or anti-mouse NKG2D (clone CX5) as NKG2D block at a concentration of 5 μg/ml on ice for 15 min. Cells were then washed and reconstituted in RPMI 1640 supplemented with 10% FBS for the coculture experiments. The specificity of blocking NKG2D was confirmed by its effect on secondary staining with allophycocyanin–anti-mouse NKG2D as described (24). Blocking Abs and isotype control Ab were added again to the coculture system (5 μg/ml).
ELISA
Mouse IFN-γ ELISA kit was purchased from BioLegend (San Diego, CA) and ELISA was performed according to the manufacturer’s protocol. Supernatants collected from the coculture system as described previously were assayed using a standard curve generated by recombinant IFN-γ.
Real-time PCR for gene transcription
Total RNA was extracted from cells using the RNeasy Mini kit (Qiagen, Valencia, CA) and reverse-transcribed using the Strata Script First Strand Synthesis System (Stratagene, La Jolla, CA). PCR was performed on an iCycler (Bio-Rad, Hercules, CA). Cycling conditions were 12 min at 95°C, followed by 40 repeats of 95°C for 15 s and 60°C for 60 s. Analysis was performed by sequence detection software that was supplied with the instrument. Each gene transcript was analyzed concurrently on the same plate with hypoxanthine-guanine phosphoribosyltransferase (HPRT) and transcripts were normalized to HPRT using primers as described. The primers were as follows: HPRT sense, 5′-CTGGTGAAAAG GACCTCTCG-3′; HPRT antisense, 5′-TGAAGTACTCATTATAGTCAAGGGCA-3′; HPRT probe, VIC-5′-TGTTGGATACAGGCCAGACTTTGTTGGAT-3′-TAMRA; T-bet sense, 5′-CAACAACCCCTTTGCCAAAG-3′; T-bet antisense, 5′-TCCCCCAAGCAGTTGACAGT-3′; T-bet probe, FAM-5′-CCGGGAGA ACTTTGAGTCCATGTACGC-3′-TAMRA; Eomes sense, 5′-CCTTCAC CTTCTCAGAGACACAGTT-3′; Eomes antisense, 5′-TCGATCTTTAG CTGGGTGATATCC-3′; Eomes probe, FAM-5′-TCGCTGTGACGGCCT ACCAAAACA-3′-BHQ.
Retroviral Transduction
Retroviral constructs of dominant negative (DN) T-bet, DN Eomes, and empty vector were the same as used before (25). Retroviral transduction was performed as previously described (26). Naive (CD44low) Vγ1 and Vγ4 γδ T cells were sorted from the splenocytes of Wt B6 mice and were infected with viral supernatants collected from the transfected Phoenix packaging cell line under the Th1 condition (5). Cells were cultured with fresh medium with IL-2 and restimulated on day 5 for intracellular cytokine staining.
Preparation of Cell Suspensions from Tumor Injection Site
C57BL/6 mice (n = 10, aged 6 wks) were injected with 2 × 105 B16 F0 melanoma cells. At day 4 postinoculation, small areas of shaved skin were resected, digested with trypsin-GNK solution (0.29% trypsin, 0.86% NaCl, 0.041% KCl, and 0.1% glucose) at 37°C for 2 h, and treated with collagenase/hyaluronidase digestion solution buffer (0.27% collagenase, 0.025% hyaluronidase, 1% DNase, 0.01% HEPES, and 0.01% sodium pyruvate in RPMI 1640) for 2 h at 37°C as described (6). The digested skin was filtered through a 40-μM cell strainer (Becton Dickinson, San Jose, CA), and a single-cell suspension was obtained that contained resident cells and infiltrating cells. Cells were stained with anti–Vγ1-FITC or anti–Vγ4-FITC, fixed with 2% formaldehyde, and stained for intracellular perforin as described previously.
Statistics
Statistical significance was evaluated by two-tailed unpaired Student t test or nonparameter analysis if SDs were significantly different between two groups using Instat version 2.03 software for Macintosh (GraphPad, San Diego, CA). The incidence of tumor development was compared and analyzed using the log-rank test, performed by GraphPad Prism version 3.0a for Macintosh (GraphPad). Throughout the text, figures, and legends, the following terminology is used to denote statistical significance: *p < 0.05; **p < 0.01.
Results
Naturally activated (CD44high), but not naïve (CD44low), Vγ4 γδ T cells protect against tumor growth
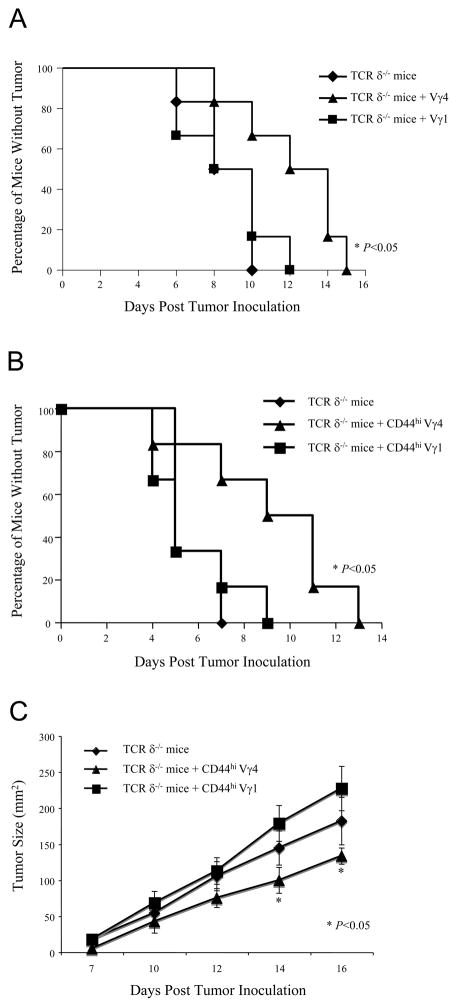
To define the role of the two subsets of γδ T cells in tumor immunity, sex- and age-matched B6 TCRδ −/− mice were transferred with small numbers of sorted Vγ1 or Vγ4 γδ T cells (1 × 105/mouse), followed by inoculation with B16 F0 cells (2 × 105/mouse, n = 10) on the second day. Tumor growth was monitored daily. Mice reconstituted with Vγ4 γδ T cells were better protected against tumor development than those that received Vγ1 γδ T cells (Fig. 1A, p < 0.05). These results suggested that Vγ4, but not Vγ1, γδ T cells contribute to the antitumor immune response.
Figure 1. Spontaneously activated (CD44high), but not naïve (CD44low), Vγ4 and Vγ1 γδ T cells play different roles in the antitumor immune response.
(A). B6 TCR δ−/− mice (n =10 per each group) were intravenously reconstituted with either Vγ4 or Vγ1 γδ T cells sorted from B6 β−/− mice (1×105 cells/mouse). On the following day, reconstituted mice were inoculated subcutaneously with B16 F0 tumor cells (2×105 cells/mouse), and tumor growth was observed and recorded daily. Data represent three independent experiments. (B). Sex- and age-matched B6 TCRδ−/− mice (n =10 per each group) were reconstituted with activated (CD44high) Vγ1 and Vγ4 γδ T cells sorted from B6 TCR β−/− mice (1×105 cells/mouse). On the following day, reconstituted mice were inoculated subcutaneously with B16 F0 tumor cells (2×105 cells/mouse). Recipient mice were monitored for the presence of tumors. Tumor size bigger than 4×4 mm2 was considered positive. Data represent 3 independent experiments. *, p < 0.05. (C). The tumor size was measured. Each data point represents the mean size of tumors seen in 10 identically treated animals. These results are typical of 3 independent experiments. *, p < 0.05.
Our previous study demonstrated that activated (CD44high) γδ T cells express IFN-γ and T-bet mRNA, and release IFN-γ protein on TCR triggering (4, 5). We hypothesized that CD44high and CD44low Vγ4 γδ T cells have different functions in the antitumor immune response. To test our hypothesis, sex- and age-matched B6 TCRδ−/− mice were reconstituted with either activated (CD44high) or naive (CD44low) Vγ1 and Vγ4 γδ T cells (1 × 105/mouse) i.v., followed with s.c. inoculation with B16 F0 cells (2 × 105/mouse, n = 10), and tumor growth was monitored and recorded daily. No significant difference was observed in tumor growth between mice reconstituted with naive Vγ4 and Vγ1 γδ T cells (data not shown). In contrast, mice that were reconstituted with activated Vγ4 γδ T cells were better protected against tumor growth than those reconstituted with activated Vγ1 γδ T cells (Fig. 1B, 1C, p < 0.05), suggesting that activated Vγ4 γδ T cells are the protective γδ T cell cohort in the antitumor immune response.
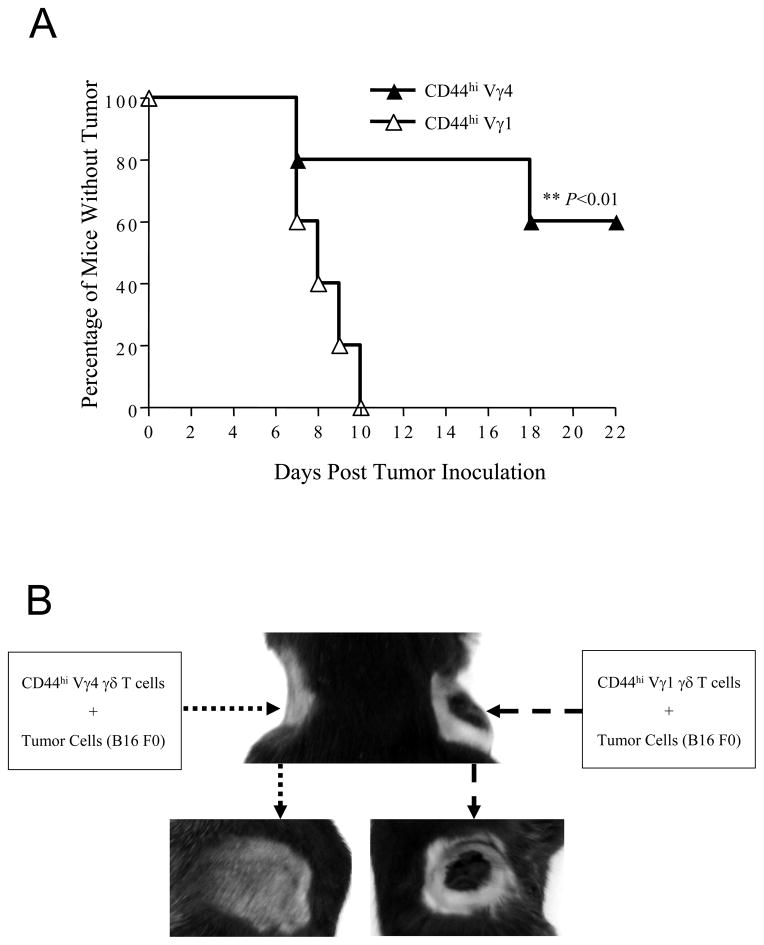
To further confirm the role of Vγ1 and Vγ4 γδ T cells in tumor resistance, naturally activated Vγ1 and Vγ4 cells were expanded in vitro for 8 d, mixed with B16 cells at a 1:4 ratio and inoculated s.c. into two flanks of B6 TCR δ−/− mice, and the presence as well as growth rate of tumors in both flanks of the transplant receipts were monitored daily. As predicted, the side with inoculation of the mixture of CD44high Vγ4 γδ T cells showed significantly less tumor growth than the opposite side receiving the mixture of CD44high Vγ1 γδ T cells (Fig. 2A, 2B, p < 0.01). This observation confirmed that activated CD44high Vγ4 γδ T cells are the preeminent antitumor effectors in the in vivo response against the B16 melanoma.
Figure 2. Activated (CD44high) Vγ4, but not Vγ1, γδ T cells contribute to anti-tumor immune responses.
(A). CD44high γδ T cells were sorted from B6 mouse spleens and lymph nodes and stimulated with Vγ4-specific antibody UC3 and Vγ1-specific antibody 2.11, respectively, and cultured with IL-2 for 8 days. B16 F0 tumor cells (2×105 cells/mouse) were mixed with these expanded Vγ4 or Vγ1 γδ T cells (0.5×105 cells/mouse) and subcutaneously injected into B6 TCR δ−/− mice (n =10 per each group). Tumor growth was observed and recorded daily. Tumor size bigger than 4×4 mm2 was considered positive. Data represent 3 independent experiments. *, p < 0.05. (B). Tumor growth in one representative mouse is shown.
IFN-γ and perforin are required for CD44high Vγ4 γδ T cell mediated tumor protection
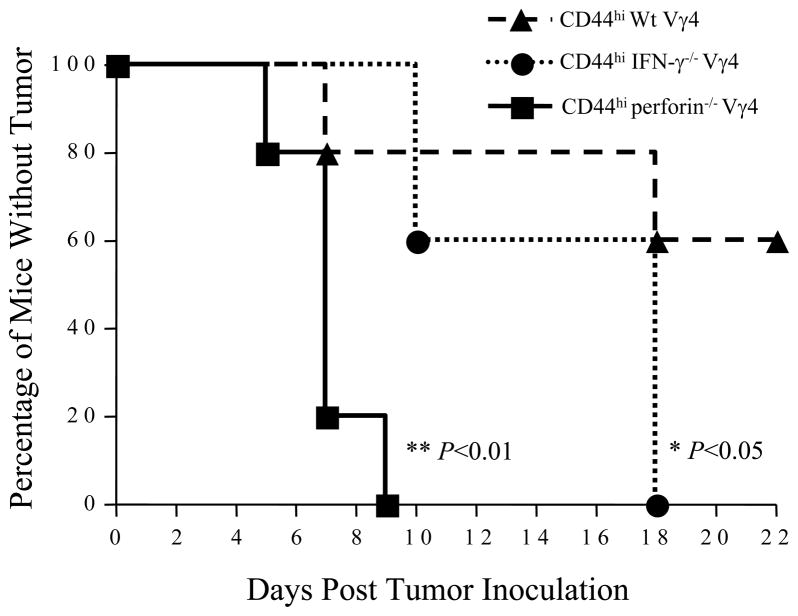
Next, we tried to determine critical elements in the protective function of CD44high γδ T cells. Expanded Wt, IFN-γ−/−, or perforin−/− CD44high Vγ4 γδ T cells were prepared, mixed with B16 cells at a ratio of 1:4, and inoculated into recipient B6 TCR δ −/− mice (n = 10 per each group) s.c. as described previously. Tumor growth was monitored and recorded daily. Compared with mice that were injected with Wt CD44high Vγ4 γδ T cells, those receiving either IFN-γ−/− or perforin−/− CD44high Vγ4 cells were more susceptible to tumor growth, demonstrating that both IFN-γ and perforin are required for the protective function of CD44high Vγ4 γδ T cells in this model (Fig. 3). Notably, IFN-γ−/− Vγ4 γδ T cells retained some protective function; whereas, perforin−/− Vγ4 γδ T cells were no longer protective (Fig. 3). This suggests that perforin is even more critical in Vγ4-mediated local tumor protection.
Figure 3. IFN-γ and perforin are required for Vγ4-mediated anti-tumor immune responses.
CD44high Vγ4 γδ T cells were sorted from either B6 wild-type mice, or perforin−/− or IFNγ−/− mice spleens and lymph nodes, and stimulated with UC3 antobody in the presence of IL-2 for 8 days. B16 F0 tumor cells (2×105 cells/mouse) were mixed with these expanded Vγ4 γδ T cells (0.5×105 cells/mouse), injected into B6 TCR δ−/− mice (n =10 per each group) subcutaneously. Tumor growth was observed and recorded daily, Tumor size greater than 4×4 mm2 was considered positive. Data represent 3 independent experiments. *, p < 0.05. **, p < 0.01.
Naturally Activated Vg4 gd T cells produce more IFN-g than Activated Vg1 gd T cells
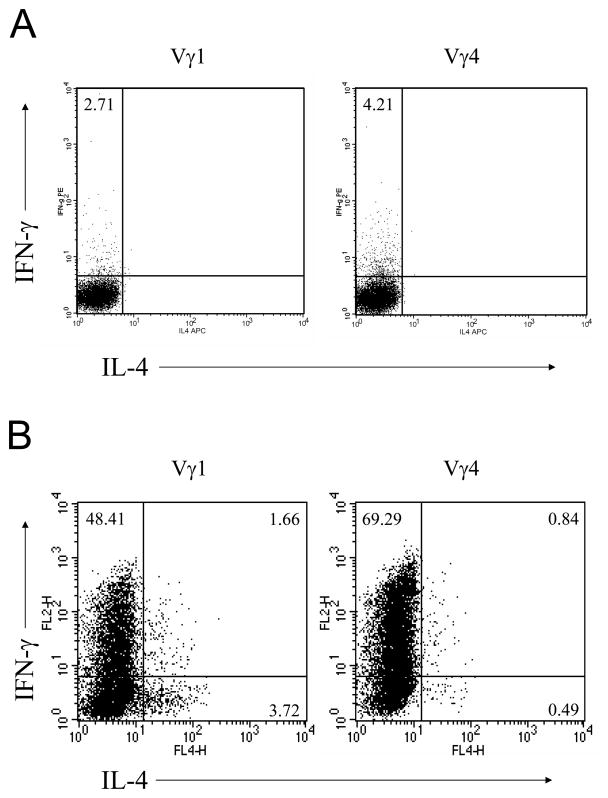
To assess the ability of spontaneously activated Vγ1 or Vγ4 γδ T cells to produce IFN-γ on TCR stimulation, CD44high Vγ1 and Vγ4 γδ T cells were sorted from splenocytes of naive B6 mice and stimulated immediately with anti-CD3 for 6 h prior to intracellular cytokine staining. Freshly isolated CD44high Vγ4 γδ T cells produced IFN-γ at higher cell frequencies than Vγ1 γδ T cells (Fig. 4A). Moreover, when CD44high Vγ1 and Vγ4 γδ T cells were expanded in vitro for 8 d and restimulated with anti-CD3 mAb for 6 h for intracellular staining, Vγ4 γδ T cells again produced IFN-γ at higher cell frequencies than activated Vγ1 γδ T cells (Fig. 4B). Conversely, activated Vγ1 γδ T cells produced IL-4 at higher cell frequency than activated Vγ4 γδ T cells (Fig. 4B).
Figure 4. Spontaneously activated (CD44high) Vγ4 γδ T cells produce higher levels of IFN-γ than activated Vγ 1 cells.
(A). CD44high Vγ4 and Vγ1 γδ T cells were sorted from B6 spleens and lymph nodes, stimulated with anti-CD3 for 6 hours, and GolgiPlug was added for the last 3 hours. Cells were then fixed with 2% formaldehyde and permeabilized with 0.5% saponin for intracellular IFN-γ and IL-4 staining. Results represent one of three repeated experiments. (B). CD44high γδ T cells were sorted from B6 spleens and lymph nodes, stimulated with UC3 and 2.11 respectively in the presence of IL-2 for 8 days. Expanded Vγ4 and Vγ1 γδ T cells were restimulated with anti-CD3 for 6 hours for intracellular cytokine staining as described above. Results represent one of three repeated experiments.
Naturally activated Vg4 gd T cells are more cytolytic and suppressive than Vg1 gd T cells due to increased IFN-g and perforin production
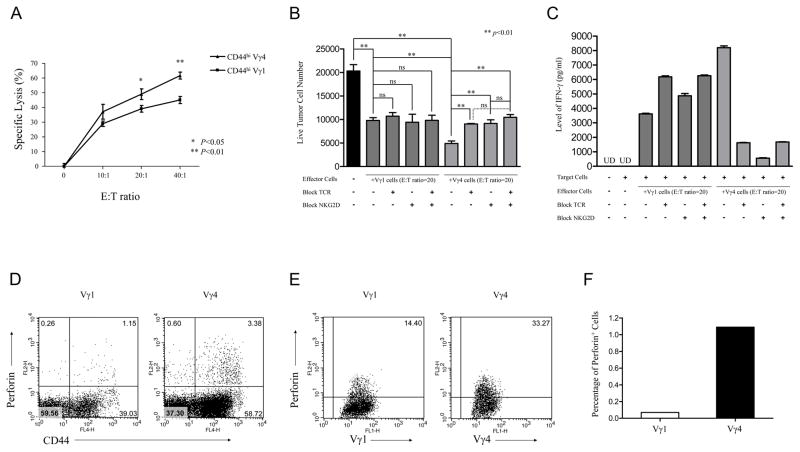
To assess the cytotoxicity of Vγ4 γδ T cells against tumor cells in vitro, sorted CD44high Vγ4 and Vγ1 cells were expanded and analyzed for cytolytic activity using the JAM assay, with YAC-1 tumor cells as targets. Vγ4 γδ T cells showed significantly increased CTL activity at E:T ratios of 20:1 up to 40:1 (p < 0.05), indicating that CD44high Vγ4 cells are more cytotoxic toward these tumor cells in vitro than CD44high Vγ1 cells (Fig. 5A). However, no significant cytolysis effect against B16 cells was observed by either Vγ4 or Vγ1 γδ T cells (data not shown). To determine whether these two γδ T cell subsets suppressed expansion of B16 cells in vitro, expanded Vγ1 or Vγ4 γδ T cells were cocultured with B16 melanoma cells (ratio of 20:1). Vγ4 γδ T cells exerted a much stronger inhibitory effect than that of Vγ1 γδ T cells (Fig. 5B). Interestingly, blocking either TCR or NKG2D partially blocked the suppressive effect of Vγ4 γδ T cells, but not Vγ1 γδ T cells (Fig. 5B), suggesting that both TCR and NKG2D are important, but not synergistic in Vγ4 γδ T cell-mediated B16 suppression. Moreover, to test the role of IFN-γ in γδ T cell-mediated tumor suppression, the level of IFN-γ in the supernatant of cocultures was determined by ELISA. The level of IFN-γ in Vγ4 T cell-B16 coculture medium was significantly higher than that of Vγ1 one, and the IFN-γ production by Vγ4 γδ T cells was significantly reduced by blockage of TCR or NKG2D (Fig. 5C).
Figure 5. Naturally Activated (CD44high) Vγ4 γδ T cells are more cytolytic and suppressive than CD44high Vγ1 γδ T cells.
(A). CD44high γδ T cells were sorted from B6 spleens, stimulated with UC3 and 2.11, respectively, and cultured with IL-2 for 8 days. Expanded Vγ1 and Vγ4 γδ T cells were transferred to 96-well round-bottom plates with 3H-pulsed YAC cells, and CTL activity was analyzed by JAM assay. *, p < 0.05. **, p < 0.01. (B–C). Vγ1 and Vγ4 γδ T cells were expanded with UC3 and 2.11 antibody respectively and TCR or NKG2D were blocked with UC7 or CX5 antibody, respectively. Then Vγ1 or Vγ4 γδ T cells were mixed with B16 cells at an E:T ratio of 20:1. Cells were co-cultured for 24 hours. (B). Live B16 cells were counted and recorded. ns, no significant difference. (C). IFN-γ level in the supernatants of the co-culture mediums were assessed by ELISA. UD, undetected. (D) Splenocytes were first stained with APC-anti-mouse CD44, and then stimulated with anti-CD3, anti-CD28 for 3 hours, and given GolgiPlug for the remaining 3 hours. Cells were then fixed and permeabilized for intracellular perforin staining. One representative dot plots was shown based on the gate of Vγ1+γδ TCR+ or Vγ4+γδ TCR+ cells. Results represent 1 of 3 repeated experiments. (E). Expanded Vγ1 or Vγ4 γδ T cells were restimulated with anti-CD3 for 3 hours, and given GolgiPlug for an additional 3 hours. Cells were then collected, fixed and permeabilized for perforin staining. One of three independent experiments is shown. (F). B6 wild-type mice were inoculated with B16 F0 melanoma cells (2×105 cells/mouse). At Day 4 after inoculation, cell suspensions were prepared from pools of 10 tissues collected from tumor injection sites, and cells were stained with anti-Vγ1 or anti-Vγ4 and intracellular perforin. One representative experiment is shown.
We next determined whether perforin production also differed between Vγ4 and Vγ1 γδ T cells. B6 splenocytes or expanded Vγ1, Vγ4 γδ T cells were stimulated with anti-CD3 for 6 h, and examined after intracellular staining. Both ex vivo isolated and expanded Vγ4 γδ T cells produced more perforin than did Vγ1 γδ T cells (Fig. 5D, 5E), consistent with the differential cytolytic activity of these two subsets of γδ T cells.
Finally, to test whether the infiltrating Vγ1 and Vγ4 γδ T cells produce perforin at different levels in situ, B6 mice were injected with B16 melanoma cells. Subsequently, tissues from the tumor injection sites were digested, and infiltrating cells were stained directly with anti–TCR-Vγ mAbs and intracellular perforin. We found that among tumor infiltrating γδ T cells, Vγ4 γδ T cells expressed perforin at higher cell frequencies than did Vγ1 γδ T cells (Fig. 5F), consistent with their protective role in this tumor model.
Eomes is critical for IFN-g production in Vg4 gd T cells
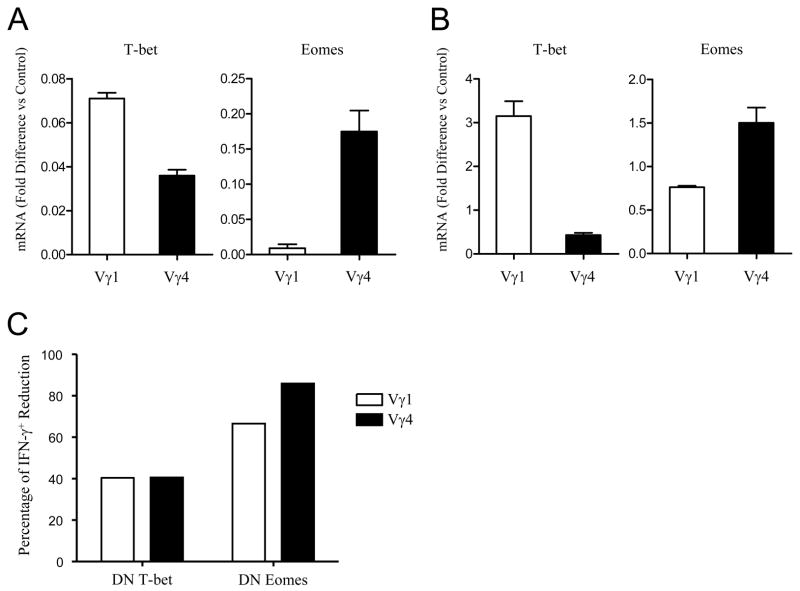
Both T-bet and Eomes are transcription factors that contribute to IFN-γ production in γδ T cells (26). We hypothesized that differential level of IFN-γ production between Vγ4 and Vγ1 γδ T cells might be due to the expression level of these two transcription factors. To test this hypothesis, CD44high Vγ1 and CD44high Vγ4 γδ T cells were sorted from B6 splenocytes and transcription of T-bet and Eomes in these different subsets of γδ T cells was examined by real-time PCR analysis. The abundance of T-bet transcripts in CD44high Vγ1 γδ T cells was slightly higher than that in CD44high Vγ4 γδ T cells (Fig. 6A, left panel). In sharp contrast, Eomes transcription in CD44high Vγ4 γδ T cells was ~20-fold higher than that in CD44high Vγ1 γδ T cells (Fig. 6A, right panel). Similar expression profiles of T-bet and Eomes were observed in the in vitro expanded γδ T cells (Fig. 6B), suggesting that Eomes plays a key role in determining the distinct function of Vγ4 γδ T cells.
Figure 6. Eomes is critical for Vγ4 γδ T cell IFN-γ production.
(A) CD44high Vγ4 and Vγ1 γδ T cells were sorted from B6 mice for real-time PCR analysis. T-bet and Eomes transcripts were normalized against HPRT. One example of 3 repeated experiments is shown. (B). Vγ1 or Vγ4 cells were expanded as described above and the transcription of T-bet and Eomes was determined by real-time PCR. (C). Naïve (CD44low) Vγ4 and Vγ1 γδ T cells were sorted from wild-type mice and activated with anti-CD3 and anti-CD28 in the presence of IL-12 and anti-IL-4. Cells were then infected with either control, DN T-bet or DN Eomes retrovirus-GFP as described in Materials and Methods. After 48hrs, cells were cultured in the presence of IL-2 for additional 3 days, restimulated with anti-CD3 and anti-CD28 for 6h for intracellular cytokine staining. One representative experiment for the percentage of IFN-γ+ reduction ((% of IFN-γ+ cells from control retrovirus infected - % of IFN-γ+ cells from DN retrovirus infected)/ % of IFN-γ+ cells from control retrovirus infected) is shown.
To further test this hypothesis, naive Vγ1 and Vγ4 (CD44low) γδ T cells from Wt mice were primed in Th1 condition and transduced with DN T-bet, DN-Eomes, or control retrovirus. Five days later, cells were restimulated with anti-CD3 for 6 h for intracellular cytokine staining as described previously. It has to be mentioned that most of these cultured Vγ1 and Vγ4 γδ T cells were CD44high (data not shown). Interestingly, transduction of DN T-bet reduced frequencies of IFN-γ producing Vγ4 and Vγ1 γδ T cells partially, and to a similar degree, consistent with the similar levels of T-bet expression in the two types of γδ T cells. Transduction of DN-Eomes reduced frequencies of IFN-γ producing γδ T cells even more drastically, especially among Vγ4 γδ T cells where IFN-γ producing cells disappeared almost entirely (Fig. 6C). This result emphasizes the critical role of the transcription factor Eomes in the functional differentiation of tumoricidal Vγ4 γδ T cells.
Discussion
γδ T cells have many unique features and functions. Our earlier studies established that splenic γδ T cells predominantly produce IFN-γ on activation and play a critical role in tumor immune surveillance through providing an early source of IFN-γ (4–6). However, the role of TCR-defined different subsets of γδ T cells in this capacity has not been identified. In the current study, we provide evidence that spontaneously activated CD44high Vγ4 γδ T cells play a critical role in tumor immunity through production of perforin and IFN-γ, which in turn depends on Eomes expression.
It has been shown in many experimental systems that TCR-defined subsets of γδ T cells have distinctive functions. This is also true for Vγ1 and Vγ4 γδ T cells, the two main subsets in peripheral lymphoid tissues. Vγ4 γδ T cells tend to produce more IFN-γ and enhance CD4+ Th1 responses, whereas, Vγ1 γδ T cells seem to produce more IL-4 and promote airway hyperactivity and type 2 inflammation (9–11). However, the role of these two subsets of γδ T cells in tumor immunity has not yet been defined. Based on our previous studies that γδ T cells provide an early source of IFN-γ in tumor surveillance (6), we hypothesized that the Th1-like Vγ4 γδ T cells might be more critical in comparison with Vγ1 γδ T cells in immune response against tumor growth. We first demonstrated in this study that reconstitution of mice deficient in all γδ T cells with CD44high Vγ4 γδ T cells, but not Vγ1 γδ T cells rendered them better protected against tumor growth (Fig. 1). The importance of our results not only establishes a critical role of Vγ4 γδ T cells in tumor immunity, but also highlights a physiological function of spontaneously activated Vγ4 γδ T cells in vivo. It has been demonstrated from our previous studies that CD44high γδ T cells express IFN-γ and T-bet mRNA (4), but the physiological function of this subpopulation was unclear. Although it is uncertain how γδ T cells obtain the spontaneous activation phenotype in vivo, our work thus provides clear evidence that these activated Vγ4 γδ T cells join the defenses and play an important role in tumor immunity.
What are the molecules derived from activated Vγ4 γδ T cells that mediate the protective effect in tumor immunity? It is well established that certain key components of the host immune response, such as NK, NKT cells, IFN-γ, and perforin, are essential for immune surveillance (27–31). In support of this, both IFN-γ−/− or perforin−/− mice are highly susceptible to tumor growth. Likewise, humans with IFN-γ signaling mutations are also more susceptible to mycobacterial infections (32, 33). Consistently, we defined in this study IFN-γ and perforin as the critical factors produced by activated Vγ4 γδ T cells in tumor immune surveillance. Indeed, in comparison with Vγ1 γδ T cells, Vγ4 γδ T cells were more likely to produce IFN-γ and perforin on activation ex vivo and after culture in vitro (Figs. 4, 5), correlated with significantly enhanced cytolytic and the in vitro tumor suppressive functions (Fig. 5A, 5B). Interestingly, both TCR and NKG2D were involved in Vγ4-mediated tumor suppression, probably through affecting IFN-γ secretion by this subset of γδ T cells (Fig. 5B, 5C). Although both expanded Vγ1 and Vγ4 γδ T cells upregulated the expression of NKG2D (data not shown), it only involved in Vγ4-mediated antitumor effect. Our results thus provide additional evidence that TCR-defined subsets of γδ T cells have divergent functional and recognition properties. Reconstitution of TCR δ−/− mice with IFN-γ−/− or perforin−/− CD44high Vγ4 γδ T cells showed that these γδ T cells were functionally impaired and no longer protective in vivo (Fig. 3), further supporting a critical role of IFN-γ and perforin in mediating the protective effect of Vγ4 γδ T cells in tumor immunity. In contrast, although Granzyme B was found to play an important role in tumor immunity as well (34), we did not observe differences in its expression level between these two subsets of γδ T cells (data not shown). Additional studies will be needed to address the role of Granzyme B in γδ T cell-mediated tumor immunity.
What are the mechanisms that might determine the functional differences between TCR-defined γδ T cells? It has been shown from our early studies that both T-bet and Eomes contribute to the IFN-γ production by γδ T cells (26). A striking finding in this study was that the expression level of Eomes in CD44high Vγ4 γδ T cells was so much higher than those in CD44high Vγ1 γδ T cells (~20-fold), in contrast to the expression of T-bet (Fig. 6A). Expanded Vγ4 γδ T cells also have a higher Eomes level (Fig. 6B). Moreover, forced reduction of Eomes by retroviral infection significantly reduced IFN-γ expression in Vγ4 γδ T cells (Fig. 6C), emphasizing the critical role of Eomes in the protective effector function of Vγ4 γδ T cells. Indeed, Eomes knockdown also strongly abrogated Vγ1 γδ T cell IFN-γ production (Fig. 6C), most probably due to its effect on T-bet, which was highly expressed in expanded Vγ1 γδ T cells. Consistently, it has been previous reported that the DN Eomes can inhibit T-box factors, including T-bet (25). However, it is unclear what causes the differential expression level of Eomes in Vγ4 versus Vγ1 γδ T cells. One possible mechanism would be that different TCR (Vγ4 versus Vγ1) signals differently on TCR-ligation (35). Additional studies to define the signaling via the TCR in these two subsets of γδ T cells are therefore needed.
What is the significance of defining the function of TCR-defined γδ T cell subsets in tumor immunity? γδ T cells might be better objects for tumor immunotherapy when compared with conventional T cells. They can recognize tumor-associated Ags directly, even when the function of traditional Ag-presenting dentritic cells is impaired or suppressed in tumor microenvironment (3, 36). They also have direct cytolytic functions against tumor cells, which, in sharp contrast to CD8+ T cells, are not affected by reduced expression of MHC class I molecules in tumor tissues (37). Furthermore, the γδ T cells themselves can also serve as APCs, and thus support responses of Ag-specific αβ T cells (38). Because different TCR-defined subsets of γδ T cells have divergent functions in many disease models, including models of tumor immunity, and are highly effective in small cell numbers, intervention with mAbs against the γδ TCR or via transfer of purified γδ T cells may provide a novel therapeutic approach against tumors, without significantly affecting general immune competence.
Given the limited number of γδ T cells ex vivo, we used in vitro expanded γδ T cells in some of our studies. It has to be emphasized that although we cannot rule out quantitative changes in functional efficiency, expanded γδ T cells showed very similar functions as those of ex vivo γδ T cells, such as IFN-γ production (Fig. 4A, 4B), perforin expression (Fig. 5D, 5E), and T-bet/Eomes profiles (Fig. 6A, 6B).
In summary, we have presented novel evidence that CD44high Vγ4 γδ T cells play a critical role in preventing tumor formation and tumor growth, and that these cells in particular express high level of Eomes, resulting in their enhanced ability to produce more IFN-γ and perforin as well as an increased cytolytic function. These findings not only shed light on the molecular mechanisms of tumor immune surveillance, but also might lead to the development of new strategies for tumor immunotherapy.
Acknowledgments
We thank Xinglong Zhou for help with flow cytometry.
Abbreviations used in this paper
- DN
dominant negative
- Eomes
eomesodermin
- HPRT
hypoxanthine-guanine phosphoribosyltransferase
- n.s
no significant difference
- UD
undetected
- Wt
wild-type
Footnotes
This work was supported by grants from the National Basic Research Program of China (2007CB914801) and a National Outstanding Young Scientist Award of NSFC (30725015).
Disclosures
Authors have no financial conflict of interest.
References
- 1.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 2.Carding SR, Egan PJ. Gammadelta T cells: functional plasticity and heterogeneity. Nat Rev Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, Wands JM, Johnston M, Born WK. gammadelta T-cell receptors: functional correlations. Immunol Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 4.Yin Z, Chen C, Szabo SJ, Glimcher LH, Ray A, Craft J. T-Bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-gamma by gammadelta T cells. J Immunol. 2002;168:1566–1571. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- 5.Yin Z, Zhang DH, Welte T, Bahtiyar G, Jung S, Liu L, Fu XY, Ray A, Craft J. Dominance of IL-12 over IL-4 in gamma delta T cell differentiation leads to default production of IFN-gamma: failure to down-regulate IL-12 receptor beta 2-chain expression. J Immunol. 2000;164:3056–3064. doi: 10.4049/jimmunol.164.6.3056. [DOI] [PubMed] [Google Scholar]
- 6.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber SA, Born W, O’Brien R. Dual functions of murine gammadelta cells in inflammation and autoimmunity in coxsackievirus B3-induced myocarditis: role of Vgamma1+ and Vgamma4+ cells. Microbes Infect. 2005;7:537–543. doi: 10.1016/j.micinf.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Huber S. T cells in coxsackievirus-induced myocarditis. Viral Immunol. 2004;17:152–164. doi: 10.1089/0882824041310667. [DOI] [PubMed] [Google Scholar]
- 9.Huber SA, Graveline D, Newell MK, Born WK, O’Brien RL. V gamma 1+ T cells suppress and V gamma 4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 10.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O’Brien RL, Gelfand EW, Born WK. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–2902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 11.Hahn YS, Taube C, Jin N, Takeda K, Park JW, Wands JM, Aydintug MK, Roark CL, Lahn M, O’Brien RL, Gelfand EW, Born WK. V gamma 4+ gamma delta T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J Immunol. 2003;171:3170–3178. doi: 10.4049/jimmunol.171.6.3170. [DOI] [PubMed] [Google Scholar]
- 12.Huber S, Sartini D. T cells expressing the Vgamma1 T-cell receptor enhance virus-neutralizing antibody response during coxsackievirus B3 infection of BALB/c mice: differences in male and female mice. Viral Immunol. 2005;18:730–739. doi: 10.1089/vim.2005.18.730. [DOI] [PubMed] [Google Scholar]
- 13.Dalton JE, Howell G, Pearson J, Scott P, Carding SR. Fas-Fas ligand interactions are essential for the binding to and killing of activated macrophages by gamma delta T cells. J Immunol. 2004;173:3660–3667. doi: 10.4049/jimmunol.173.6.3660. [DOI] [PubMed] [Google Scholar]
- 14.Tramonti D, Andrew EM, Rhodes K, Newton DJ, Carding SR. Evidence for the opposing roles of different gamma delta T cell subsets in macrophage homeostasis. Eur J Immunol. 2006;36:1729–1738. doi: 10.1002/eji.200635959. [DOI] [PubMed] [Google Scholar]
- 15.Dodd J, Riffault S, Kodituwakku JS, Hayday AC, Openshaw PJ. Pulmonary V gamma 4+ gamma delta T cells have proinflammatory and antiviral effects in viral lung disease. J Immunol. 2009;182:1174–1181. doi: 10.4049/jimmunol.182.2.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tough DF, Sprent J. Lifespan of gamma/delta T cells. J Exp Med. 1998;187:357–365. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasper LH, Matsuura T, Fonseka S, Arruda J, Channon JY, Khan IA. Induction of gammadelta T cells during acute murine infection with Toxoplasma gondii. J Immunol. 1996;157:5521–5527. [PubMed] [Google Scholar]
- 19.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 20.Moore TA, Moore BB, Newstead MW, Standiford TJ. Gamma delta-T cells are critical for survival and early proinflammatory cytokine gene expression during murine Klebsiella pneumonia. J Immunol. 2000;165:2643–2650. doi: 10.4049/jimmunol.165.5.2643. [DOI] [PubMed] [Google Scholar]
- 21.Ponomarev ED, Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol. 2005;174:4678–4687. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- 22.Ponomarev ED, Novikova M, Yassai M, Szczepanik M, Gorski J, Dittel BN. Gamma delta T cell regulation of IFN-gamma production by central nervous system-infiltrating encephalitogenic T cells: correlation with recovery from experimental autoimmune encephalomyelitis. J Immunol. 2004;173:1587–1595. doi: 10.4049/jimmunol.173.3.1587. [DOI] [PubMed] [Google Scholar]
- 23.Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991;145:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 24.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 25.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, He W, Kim ST, Tao J, Gao Y, Chi H, Intlekofer AM, Harvey B, Reiner SL, Yin Z, Flavell RA, Craft J. Epigenetic and transcriptional programs lead to default IFN-gamma production by gammadelta T cells. J Immunol. 2007;178:2730–2736. doi: 10.4049/jimmunol.178.5.2730. [DOI] [PubMed] [Google Scholar]
- 27.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 28.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 31.Street SE, Trapani JA, MacGregor D, Smyth MJ. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med. 2002;196:129–134. doi: 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-gamma-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 33.Jouanguy E, Lamhamedi-Cherradi S, Lammas D, Dorman SE, Fondaneche MC, Dupuis S, Doffinger R, Altare F, Girdlestone J, Emile JF, Ducoulombier H, Edgar D, Clarke J, Oxelius VA, Brai M, Novelli V, Heyne K, Fischer A, Holland SM, Kumararatne DS, Schreiber RD, Casanova JL. A human IFNGR1 small deletion hotspot associated with dominant susceptibility to mycobacterial infection. Nature Genet. 1999;21:370–378. doi: 10.1038/7701. [DOI] [PubMed] [Google Scholar]
- 34.Tao J, Gao Y, Li MO, He W, Chen L, Harvev B, Davis RJ, Flavell RA, Yin Z. JNK2 negatively regulates CD8+ T cell effector function and anti-tumor immune response. Eur J Immunol. 2007;37:818–829. doi: 10.1002/eji.200636726. [DOI] [PubMed] [Google Scholar]
- 35.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 37.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 38.Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–268. doi: 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]