Abstract
With their utilization of light-driven allostery to control biochemical activities, photosensory proteins are of great interest as model systems and novel reagents for use by the basic science and engineering communities. One such protein, the light-activated EL222 transcription factor, from the marine bacterium Erythrobacter litoralis HTCC2594, is appealing for such studies, as it harnesses blue light to drive the reorientation of Light-Oxygen-Voltage (LOV) sensory and Helix-Turn-Helix (HTH) effector domains to allow photoactivation of gene transcription in natural and artificial systems. The protein conformational changes required for this process are not well understood, due in part to the relatively short lifetime of the EL222 photoexcited state (τ~29 s) which complicates its characterization with certain biophysical methods. Here we report how we have circumvented this limitation by creating an EL222 variant harboring V41I, L52I, A79Q and V121I point mutations (AQTrip) that stabilizes the photoactivated state. Using the wild-type and AQTrip EL222 proteins, we have probed EL222 activation using a combination of solution scattering, NMR and electromobility shift assays. Size exclusion chromatography and light scattering indicates that AQTrip oligomerizes in the absence of DNA, and selects for an EL222-dimer-DNA complex in the presence of DNA substrates. These results are confirmed in wild-type EL222 with a high-affinity DNA binding site that stabilizes the complex. NMR analyses of the EL222-DNA complex confirm a 2:1 stoichiometry in the presence of a previously characterized DNA substrate. Combined, these novel approaches have validated a key mechanistic step, whereby blue-light induces EL222 dimerization through LOV and HTH interfaces.
Keywords: Blue-light sensor, LOV, HTH, DNA binding, NMR, photoreceptor, optogenetics
Rapid adaptation to environmental changes requires that organisms coordinate biological responses using a combination of sensory and signal transduction machinery. One sensory component, the Light Oxygen Voltage (LOV) domains, enable diverse enzymatic and signaling proteins to detect changes in blue light intensity and redox state1, 2. Despite these varied output signals, LOV proteins use a common photochemistry, as has been well studied in isolated LOV domains1–5. However, because only few full-length LOV sensor/signal transduction proteins have been characterized with biophysical methods, we only partly understand the mechanisms of relaying alterations within the LOV core to downstream signaling components.
Some insight into these issues is provided by recognizing that LOV domains are a subset of the larger family of Period-ARNT-Singleminded (PAS) protein/protein interaction domains, many of which are ligand regulated themselves6, 7. These small domains (~110 residues) adopt a common α/β fold with a central β-scaffold flanked on one side by a series of short helices. Most ligand-regulated PAS domains typically function by binding environmentally-sensitive small molecule cofactors between the helical and β-sheet secondary structures, as seen for FMN or FAD chromophores within LOV domains1, 2, 6. While these are non-covalent flavoprotein complexes in the dark, illumination leads to the formation of a covalent adduct between the C4a position of the flavin ring and the sulfur of a nearby conserved cysteine1, 2, constituting the first step in environmental control of LOV proteins.
Subsequent signal transduction steps have been probed in an array of mechanistic studies conducted on isolated LOV domains, suggesting three general ways that structural changes triggered by adduct formation are propagated to effector domains or proteins1. These include light-driven 1) release of effector helices or domains, freeing them from inhibitory interactions with the LOV β-sheet5, 8–12, 2) reorganization of flanking protein elements to allow LOV:LOV dimerization3, 13, or 3) reorientation of a pre-existing LOV-LOV β-scaffold-mediated dimer4, 14, 15. In all three mechanisms, adduct-induced changes to the isoalloxazine ring configuration (including protonation of the N5 position) propagate via altered protein/flavin interactions to the LOV β-sheet1, 8.
To fully characterize the structural and functional changes involved in LOV signaling, it is necessary to examine the light-induced biophysical and biochemical features of full-length proteins containing both sensory and effector domains. To the best of our knowledge, only one natural protein meeting these criteria has provided high-resolution structural information needed for such studies: EL222, a bacterial light-regulated DNA binding protein10. EL222 consists simply of a N-terminal LOV domain followed by a C-terminal NarL/LuxR-type helix-turn-helix (HTH) DNA-binding domain, suggesting it functions as a light-dependent DNA binding protein. This was borne out by initial structural and functional characterization, showing that EL222 adopts an inactive, monomeric conformation in the dark, stabilized by inhibitory LOV-HTH contacts10. Upon illumination, EL222 undergoes standard LOV photochemistry and breaks these interdomain contacts, facilitating EL222 binding to its preferred 12 bp DNA sequence, which we identified with genomic ChIP-Seq and artificial SELEX methods16. Within the native Erythrobacter litoralis HTCC2594 host, we further observed light-dependent enhanced transcription of genes located near some EL222 binding sites in the genome, suggesting that EL222 serves as an environmentally-responsive transcription factor in vivo.
As with other LOV proteins, the mechanism of EL222 activation remains incompletely characterized. Based on two lines of reasoning, one facet is clear: EL222 likely oligomerizes as part of the activation process, converting from its monomeric inhibited state into a multimeric DNA-bound form. First, the activities of many proteins that contain LOV or HTH domains are regulated at the level of oligomeric complex formation and modification3, 4, 17–19. Notably, DNA binding by HTH-containing proteins occurs via such complexes17–19, the formation of which are often controlled by N-terminal regulatory domains. Within EL222, the dimerization surfaces typically used by both LOV (β-sheet) and HTH (4α helix) domains are occluded in the dark-state monomer, preventing their use in assembling higher-order complexes. Second, DNA binding sites for EL222 reveal an internal repeat, consistent with binding by multiple proteins16. Taken together, these precedents imply that the structural changes observed upon EL222 activation10 likely lead to oligomerization of the protein.
While this hypothesis is reasonable, its examination has been complicated by the short lifetime of the photogenerated cysteinyl-flavin adduct in EL222, hampering the application of most biophysical and biochemical methods normally used to directly observe oligomerization. Here we report how we have addressed these limitations, combining improved reagents and different techniques to obtain experimental evidence of EL222 oligomerization. We start by rationally-designing variants of EL222, which extend the adduct-state lifetime, facilitating solution light scattering and gel filtration studies. These rate-altering variants reveal light-induced formation of El222 dimers that can bind DNA. Complementary experiments with wildtype EL222 and a new high-affinity DNA sequence16 demonstrate that native EL222 also forms an EL2222/DNA complex. We close by using solution NMR methods, which allow direct measurement of protein/DNA complex formation with in situ illumination, to examine the ability of EL222 to bind various DNA substrates. Taken together, these independent approaches directly establish that oligomerization is a key step of EL222 activation.
EXPERIMENTAL PROCEDURES
Cloning and Protein Purification
EL222 variants harboring V41I, L52I, A79Q, L120K, V121I, S137Y mutations alone or in combination were constructed within the context of an N-terminal truncation construct (containing residues 14–222) using the Quikchange protocol (Agilent Technologies) and subcloned into the His6-Parallel expression vector20. Of note, EL222 is derived from the ELI_04755 gene, but different predictions of the translation start site lead to slightly different predicted protein products. In this and all of our prior work10, 21, we use residue numbers assuming that translation starts at the methionine that generates a protein which begins with a MGQDR… sequence; sequences in several databases (e.g. NCBI YP_457840.1) have a three-residue shift by assuming an earlier methionine is used (MLDMGQDR…). Resulting variants were sequenced in their entirety (UT Southwestern Sequencing Facility). WT EL222 and rate-altering variants were overexpressed in E. coli BL21(DE3) cells grown in either Luria Broth or M9 minimal media supplemented with 1 g/L 15NH4Cl. Cells were grown to an OD600 of 0.6–0.8 prior to induction with 100 µM isopropylthiogalactoside (IPTG) at 18°C. Proteins were expressed for 22 hr prior to harvesting.
EL222 proteins were purified by lysing cell pellets via sonication in 50 mM Tris (pH 8.0), 100 mM NaCl buffer at 4°C before subsequent purification with Ni-Sepharose affinity chromatography. Proteins were obtained using gradient elution from 5–500 mM imidazole, subsequently treated with His6-TEV protease22 overnight at 4°C to remove affinity tags and subjected to another round of affinity chromatography to remove the freed His6 tags and His6-TEV protease. Purification was completed with a final Superdex 75 size exclusion chromatography step, leaving samples in either 50 mM Tris (pH 8.0), 100 mM NaCl buffer (for kinetic and DNA binding studies) or 50 mM sodium phosphate (pH 6.0), 100 mM NaCl buffer (for NMR studies).
UV-visible Absorbance Spectroscopy and Kinetics
UV-visible absorbance spectroscopy and LOV dark-state reversion kinetics were conducted on a Varian Cary 50 spectrophotometer as previously reported21.
Electrophoretic mobility shift assay (EMSA)
Experiments were conducted as described in Nash et al.10, using the 45 bp oligomer 1 from that publication (referred to as AN-45 in Rivera-Cancel et al.16 and herein; sequence listed in Supporting Table S1) unless otherwise noted. Briefly, 32P end-labeled versions of double stranded DNA were incubated with increasing concentrations of EL222 WT or mutant protein under either dark- or light-state conditions. Light-state WT EL222 samples were generated under a white 150 W floodlight. EL222 variants were illuminated using either an analogous illumination protocol or a series of three camera flashes while the samples were incubating on ice. Importantly, both illumination protocols yielded identical results.
Initial assays of DNA binding were conducted with reactions containing a final concentration of 0.01 mg/mL BSA, 0.02 mg/mL poly dI/dC, and 1.45 nM 32P end-labeled AN-45 DNA (45 bp). Protein concentrations were varied between 0.1 and 20 µM, prepared in 50 mM Tris (pH 8.0), 100 mM NaCl, 2 mM MgCl2 and 12% glycerol. DNA and protein mixtures were incubated on ice for 30 min in the dark or with light. Samples were then run on a 10% polyacrylamide gel in TAE buffer for 2 hr at 100 V and 4°C.
EMSA experiments to demonstrate EL222 dimerization (Figure 2c) were conducted using 1.3 nM 32P end-labeled Clone-1 DNA16 (45 bp; Figure S1a for sequence) and an equimolar mixture of untagged and His6-Gβ15 tagged EL222 in a buffer containing (final concentration) 0.01 mg/mL BSA, 0.025 mg/mL poly dI:dC, 10 mM Tris (pH 8.0), 80 mM NaCl, 3 mM MgCl2 and 10% glycerol. Reactions were incubated under light conditions for 25 min on ice and then run on a native 5% acrylamide gel in TAE at 150 V for 2 hr at 4°C.
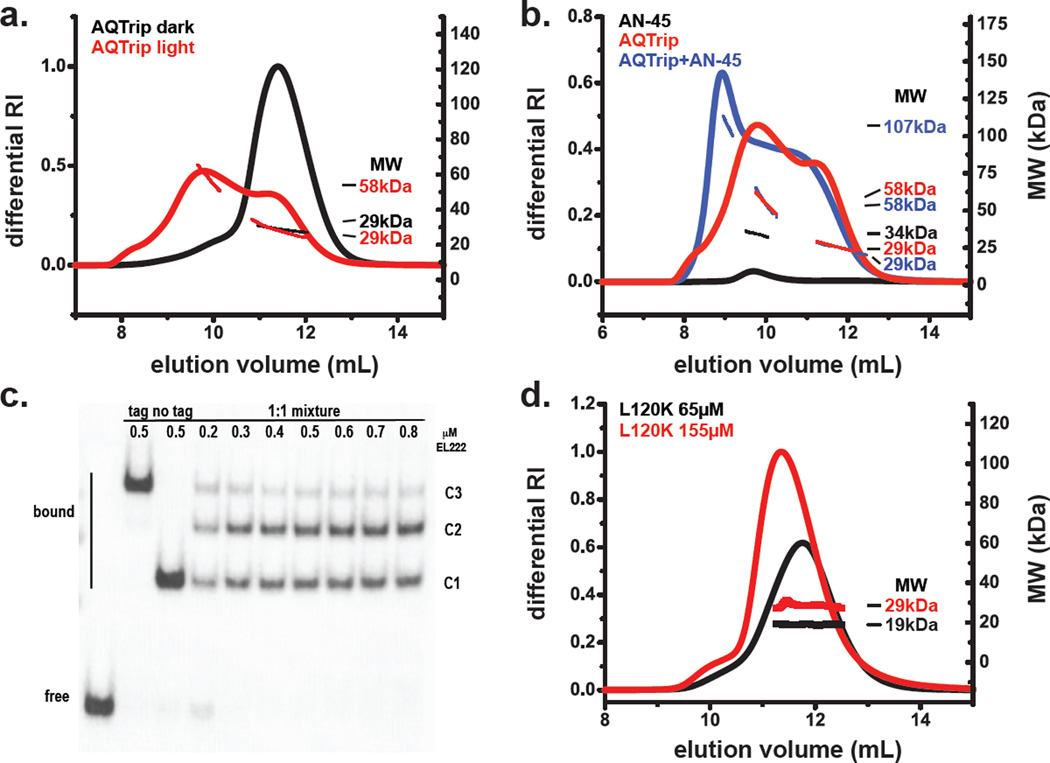
Figure 2. Dimerization of EL222 variants both free in solution and bound to DNA.
(a) SEC-MALLS measurements of dark- and light-state AQTrip indicate the light-driven formation of a dimeric species. Under dark-state conditions AQTrip is largely monomeric (black) at the concentration used (140 µM). In contrast, at equivalent concentrations, AQTrip is a mixture of monomer and dimer in the light-state (red). (b) Mixtures of 120 µM light-treated AQTrip and 60 µM AN-45 DNA results in the formation of a more rapidly eluting species (blue) compared to light-state AQTrip alone (red) and DNA alone (black). The MW of the light-state DNA-bound EL222 complex is consistent with a 2:1 EL222: DNA ratio. (c) EMSA analysis of EL222 interactions with Clone-1 DNA16 (see Figure S1a for sequence), done in the presence of His6-Gβ1-tagged and untagged WT EL222, confirms a 2:1 stoichiometry of EL222:DNA interactions. The presence of a mixed species (C2) compared to tagged (C3) and untagged (C1) protein/DNA complexes indicates that EL222 dimers interact with this 45 bp oligo. The lack of additional bands suggests monomeric or larger oligomeric states do not bind appreciably at the concentrations tested. (d) L120K is monomeric (MW=19 kDa) under dark-state conditions at 65 µM (black). Increasing the concentration to 155 µM (red) results in a small shift in both elution time and MW, up to a ~29 kDa species, consistent with a rapidly exchanging low affinity dimer27, 30. For a, b, and d, each graph includes both UV absorbance measurements (top lines) and MALLS traces (bottom lines); MW measurements are provided from the midpoint of the MALLS traces shown.
Nuclear Magnetic Resonance (NMR) Titration Experiments
NMR-based titration experiments were conducted by collecting solution 15N/1H HSQC spectra23 on samples of 80 µM 15N-labeled EL222 in the presence of increasing concentrations of DNA oligos (described below) and 25 mM sodium phosphate (pH 6.0), 50 mM NaCl buffer. Data were collected in the presence and absence of DNA oligos ranging from 15–45 bp and concentrations ranging from 5–100 µM. Oligos were based on 5’ and 3’ truncations of AN-45, generating AN-15 through AN-35 (Figure S1a). All spectra were collected at 25°C on Varian 600 and 800 MHz spectrometers equipped with cryogenically-cooled 15N/13C/1H triple resonance probes. Light-state samples were collected as described previously10. All data was processed using NMRPipe24 and analyzed using NMRViewJ25.
Quantification of fractional EL222 occupancies was determined by ratios of peak intensities for 35 light-state peaks of EL222, previously assigned to specific residues in the protein10. For purposes of quantification, peak intensities in the absence of DNA (control) were assigned a concentration of 80 µM. The concentration of free protein at each DNA concentration was thus:
| (eq. 1) |
The resultant fraction bound was then:
| (eq. 2) |
The calculated fractional protein bound was averaged over all 35 peaks to reduce random error.
To evaluate binding affinities and stoichiometries in a length-independent manner, DNA concentrations were normalized to the relative concentration of 20 bp equivalents. Calculation of the concentrations of 20 bp equivalents were achieved by the following equation:
| (eq. 3) |
DNA titrations were then plotted with normalized [DNA] and actual [DNA] and fit with a one site-specific, cooperative binding model as provided in GraphPad Prism (GraphPad Software).
Reversibility of DNA binding was quantified by comparing the initial peak intensities of the dark-state locations of these 35 assigned residues before illumination and again after a dark-light-dark cycle. These data were compared to an analogous experiment conducted in the absence of DNA to separate the effects of the illumination protocol from DNA binding. Data for A79Q were collected at a protein concentration of 140 µM with 70 µM AN-35.
Size-Exclusion Chromatography-Multi Angle Laser Light-Scattering (SEC-MALLS)
Determination of the absolute MW of light-state and DNA-bound complexes were determined using SEC-MALLS experiments. All SEC-MALLS experiments were conducted on a mini-DAWN Treos static light scattering instrument (Wyatt) equipped with an in-line refractive index detector. All experiments were conducted on 400 µL samples with protein concentrations varying from 40–350 µM and DNA concentrations of up to 60 µM. Samples were injected onto a Superdex 75 (10/300) analytical gel filtration column to separate oligomeric species and protein aggregates. Light-state data was collected by first illuminating samples with three successive camera flashes immediately prior to injection. MW determinations were subsequently determined via in-line MALLS detection and calculated using Wyatt Astra software.
RESULTS
Utility of rate-altering EL222 mutants
Our prior studies of EL222 revealed that it has a relatively short-lived photochemically-generated adduct (τ=29 s at 25°C)21, particularly when compared to the times required for commonly-used methods for evaluating DNA binding and stoichiometry (e.g., EMSA, Size Exclusion Chromatography). The short lifetime of the EL222 active state thus introduces several practical complications into measurements of EL222:DNA interactions, from the need to utilize constant illumination to challenges with the detection of relatively short-lived species with different biophysical methods. To address these experimental issues, we used EL222 variants that contain point mutations, which stabilize the light-state adduct and used these to discern the stepwise process of EL222 photoactivation and DNA recognition.
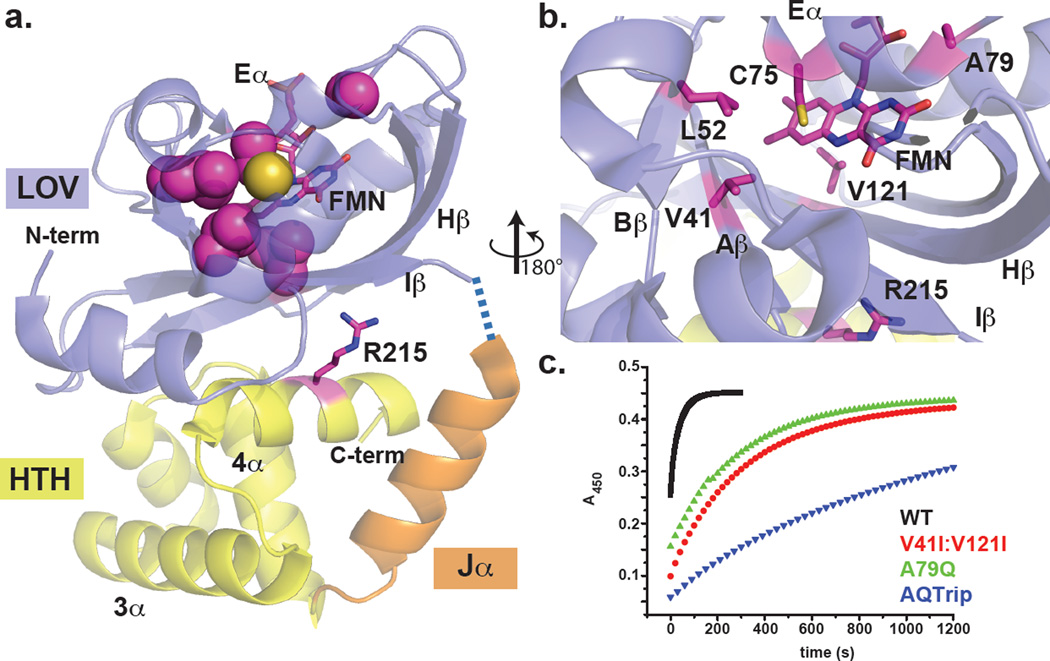
Comparable rate-altering variants have been characterized in several LOV proteins21, 26, 27; in particular, variants of the N. crassa Vivid (VVD) protein can modulate the active state lifetime over four orders of magnitude without affecting the in vitro function of the protein27. Building from this foundation, we compared the EL222 and VVD LOV domain sequences and identified three residues where VVD mutations alter the lifetime of the C4a adduct (EL222 residues V41, L52 and V121; Figure 1, Figure S2). Given that these mutations were found to modulate adduct-state stability in VVD, AsLOV2 and YtvA across several orders of magnitude26–29, we resolved to target the analogous residues in EL222 for mutagenesis. Interestingly, analysis of the sequence immediately surrounding the critical LOV cysteine residue revealed an additional substitution at the Gln residue usually found here (canonical GRNCRFLQ vs. GRNCRFLA in EL222); mutations to this site (A79Q)21 or nearby hydrophobic residues (V41I:V121I) each slow the adduct cleavage rate by an order of magnitude compared to wildtype protein (Figure 1c). Combining these mutations with a change at another nearby hydrophobic site (V41I:L52I:A79Q:V124I, referred to as AQTrip) revealed some additivity among these effects, culminating in a stabilized adduct that decayed with a time constant of ~2000 s (Figure 1c). While this ~70-fold attenuation of adduct cleavage observed in AQTrip is not as large as observed in some other LOV systems27, it is more than sufficient for biophysical studies of oligomerization and DNA recognition in EL222. Because the mechanisms of adduct-rate attenuation of these types of mutations in VVD, AsLOV2 and YtvA have been analyzed in detail elsewhere26–29, and also in EL22221, they will not be discussed here.
Figure 1. Active site variants of EL222 extend the lifetime of the light-state adduct.
(a) EL222 consists of two folded domains: an N-terminal LOV domain (blue), coupled by a connecting Jα helix (orange) to a C-terminal HTH DNA binding domain (yellow). The dark-state crystal structure10 shows that the LOV β-sheet directly contacts the 4α dimerization helix of the HTH domain; photochemically-induced adduct formation involving the FMN (magenta sticks) within the LOV domain causes conformational changes that perturb the LOV/HTH interaction, as demonstrated by large light-induced chemical shift changes in R215 (magenta sticks) following photoexcitation10. Variants identified to date which alter the lifetime of the photochemically-generated adduct are confined to residues whose side chains directly protrude into the FMN binding cavity (magenta spheres for carbon atoms of these sidechains; yellow sphere indicates location of C75 Sγ atom). (b) An expansion of the structure in panel a shows the residues localized to the active site that when mutated affect the lifetime of the light-state adduct. V41I and L52I alter interactions with both the adduct-forming C75 and the active site FMN, while V121I is positioned at the re-face of the flavin and regulates flavin reduction potentials in VVD27. A79 is located in a surface Eα-Fα loop and does directly contact the flavin chromophore; however, the A79Q variant alters H-bonds at the flavin N1 position and directly stabilizes the light-state adduct21. (c) Dark-state reversion rates were determined by fitting the absorbance at 450 nm after illumination. EL222 variants tune the lifetime of the adduct state relative to WT (black, τ~29 s at 25°C)21, with V41I:V121I (red) and A79Q (green) both increasing the lifetime of the adduct state ten-fold (τ~300 s). Combining these mutations with a further L52I variation increases the lifetime to a maximum of τ~2000 s in a V41I:L52I:A79Q:V121I (AQTrip, blue) variant.
Characterization of the oligomeric state of a slow-cycling EL222 variant
As noted above, LOV and HTH proteins are often regulated by controlling protein oligomeric state. To test if such a regulatory model holds for EL222, we used size exclusion chromatography coupled to multi-angle laser light scattering (SEC-MALLS) to probe the oligomeric state of light-state EL222 in the presence and absence of a double-stranded DNA substrate. As expected, SEC-MALLS data show that dark-state AQTrip elutes as a monomer (Figure 2a), with a 29 kDa MALLS-determined molecular weight, which is close to the 24 kDa theoretical mass. Following photo-excitation, AQTrip elutes as a mixture of three species: a monomeric 29 kDa form consistent with dark-state AQTrip, a 58 kDa dimeric complex, and a rapidly-eluting high molecular weight species (Figure 2a). Notably, the apparent MW of the oligomer is concentration dependent, indicative of a rapid monomer:dimer equilibrium (Figure S3) as observed for several other PAS-containing proteins3, 30. Increasing the protein concentration to 300 µM results in formation of a light-state tetramer (Figure S3). Thus, at high concentrations, stabilized forms of EL222 are capable of light-induced oligomerization in the absence of DNA.
To determine how the light-induced oligomerization is involved in DNA recognition, we conducted analogous SEC-MALLS experiments on light-state AQTrip in the presence of DNA (Figure 2b). Taking advantage of the previously-characterized AN-45 substrate for EL22210, 16, we used this double-stranded oligo in our SEC-MALLS experiments. Similar to experiments done in the absence of DNA, we observed three species in the AQTrip-DNA samples. The predominant species eluted more rapidly than either free DNA and light-state AQTrip oligomers with a MW of ~107 kDa, consistent with a 2:1 protein-to-DNA ratio (predicted MW of 92 kDa, Figure 2b). The two additional species eluted with 58 and 29 kDa masses consistent with free dimeric and monomeric EL222, respectively. Unfortunately, while sample constraints complicated the reproduction of these results at protein and DNA concentrations sufficient to observe putative tetrameric species (350 µM protein and greater than 100 µM DNA), it is readily apparent from these data that a DNA-bound protein tetramer is not the preferred configuration.
Characterization of wildtype and constitutively-active EL222 oligomerization
While comparable SEC-MALLS experiments with wildtype (WT) EL222 protein were complicated by the short lifetime of the light state, we were able to use EMSA experiments with samples containing mixtures of His6-Gβ1-tagged and untagged WT EL222 to demonstrate the presence of mixed dimeric states on DNA (Figure 2c). For these experiments, we used a different DNA substrate called Clone-1, a higher affinity 45 bp duplex (EC50~0.3 µM), which we identified with SELEX-based selection among a pool of artificial DNA sequences16. Similar to our SEC-MALLS experiments with AQTrip, EMSA analysis of WT protein with the Clone-1 DNA revealed analogous EL222-dimer/DNA interactions. Importantly, three DNA-bound complexes were observed: a rapidly migrating species consistent with an untagged protein dimer (labeled C1), a slower migrating species similar to a His6-Gβ1-tagged protein dimer (labeled C3) and an intermediate species representative of a His6-Gβ1-tagged:untagged protein complex (labeled C2) (Figure 2c). Given the lack of additional species, we conclude that EL222 preferentially interacts with DNA as a dimer and not as monomer or tetramer. Taken together, our SEC-MALLS and EMSA data demonstrate the light-induced formation of an EL222 dimer that is capable of binding to DNA.
Although we cannot identify the protein/protein interface(s) used to assemble the EL222 dimer complex using SEC-MALLS alone, precedence from other LOV and HTH structures does suggest candidates for these interfaces, allowing us to predict mutations in EL222 that disrupt the observed dark-state LOV/HTH packing and produce constitutively active, DNA-binding variants which can provide some insight into structures involved in light induced dimerization10. Two such variants, L120K and S137Y, both alter residues on the LOV β-sheet adjacent to the HTH 4α helix10 (Figure S4a). The L120K variant, which we have shown to bind AN-45 in the dark with comparable affinity to light-state WT protein10, appears to be in a rapid exchange between monomeric and dimeric states as shown by the concentration dependence of its SEC elution profile and apparent MW (Figure 2D). EMSA analysis indicates that S137Y also binds AN-45 in the dark with comparable affinity to illuminated WT protein (Figure S4b) and appears to be in a monomer:dimer equilibrium based on SEC-MALLS results (Figure S4c). These data suggest that both mutations activate EL222 by releasing the inhibitory LOV/HTH interactions observed in the dark-state crystal structure, but their introduction of charge and/or steric constraints do not preclude the (albeit transient) formation of EL222 dimers. Such behavior is consistent with dimerization being driven by HTH/HTH interactions with potential side contributions from LOV/LOV dimerization.
DNA recognition by WT EL222 and rate-altering variants
While SEC-MALLS and EMSA analysis of WT and rate-altering variants indicate that dimer formation is required for DNA-binding, these experiments did not address the potential impact of the rate-altering variants on affinity of these interactions. We initially evaluated DNA-binding with EMSA analyses in a manner analogous to those conducted on WT EL222 and A79Q variant10, 21. Light-state EMSAs revealed that all of the variants inspected (A79Q, V41I:A79Q, AQTrip) bound AN-45 with equivalent (V41I:A79Q) or higher affinity (A79Q, AQTrip) than that established for WT EL222 (EC50~8 µM) (Figure S5). In addition, two variants (V41I:A79Q and A79Q) formed supershifted bands that had only been seen with WT EL222 at high protein concentrations (>20 µM) and the constitutively-active L120K variant10. Thus, mutations within the flavin-binding pocket have the potential for generating allosteric changes in the surrounding LOV domain that may impart increased affinity for DNA.
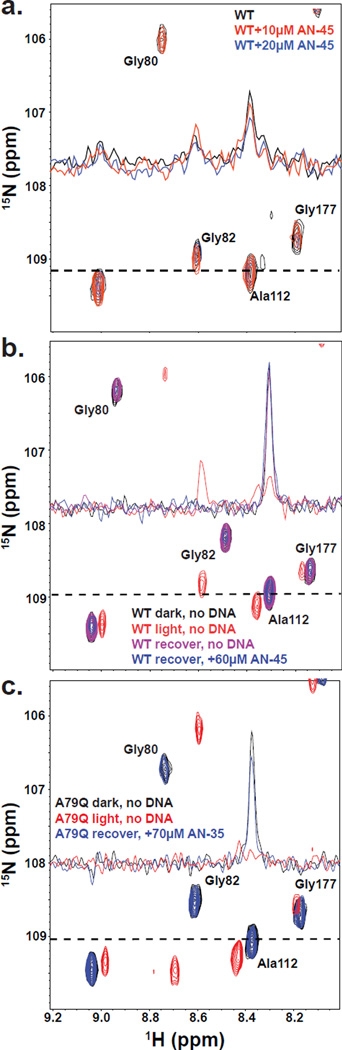
To complement these EMSA measurements with a solution method where illumination could be more readily controlled in real time, we developed an alternative NMR-based DNA binding assay. Initial 15N/1H HSQC spectra of uniformly 15N labeled WT EL222 in the dark showed minimal, concentration-independent effects on peak shifts or intensity without or with AN-45 duplex DNA, confirming the lack of DNA binding (at concentrations up to 80 µM) for dark state WT EL222 (data not shown). In contrast, titration of AN-45 DNA into illuminated EL222 samples exhibited substantial DNA-dependent loss of peak intensity (Figure 3a). Such changes are consistent with EL222 binding to AN-45 under these conditions, with line broadening caused by the slowed rotational diffusion of the complex, intermediate chemical exchange broadening, or both. Within these samples, greater than 90% of the signal intensity was restored after returning to dark-state conditions post-illumination, confirming reversibility of DNA binding by EL222 (Figure 3b,c). Quantifying peak intensities following dark-light-dark cycles, in the presence and absence of either AN-45 or a shorter 35 bp AN-35 fragment (Figure S1), we established that the minimal loss in signal intensity was not a consequence of DNA (Figure 3b, inset 1D traces), nor was it affected by the presence of the A79Q mutation (Figure 3c).
Figure 3. NMR-based DNA binding studies show reversible binding of EL222 to DNA.
(a) EL222 binding to DNA can be monitored by titrating increasing amounts of AN-45 DNA into 15N-labeled EL222 and analyzing peak intensities in 15N/1H HSQC light-state spectra. Dashed lines represent the location of the cross-section used for the 1D trace shown inset. Addition of 10 µM AN-45 (red) and 20 µM AN-45 (blue) to 80 µM WT EL222 decreases the intensity of all peaks relative to light-state WT EL222 protein alone (black) in a concentration-dependent manner. Loss of peak intensity is consistent with EL222 binding to AN-45 DNA. (b) Initial WT EL222 dark-state peak intensities (black) are comparable to peak intensities following a dark-light-dark cycle in the presence (blue) and absence of DNA (magenta). A light-state spectrum of EL222 (red) in the absence of DNA is shown for comparison. Up to 30% loss in peak intensity is observed in some experiments even in the absence of DNA; however, for all experiments, loss in peak intensity is similar between DNA and DNA-free samples. Concentration independent loss in peak intensity between dark- state spectra with and without DNA indicates that only minimal interaction occurs between EL222 and DNA in the dark. (c) DNA-binding is also reversible with A79Q (140 µM) when AN-35 DNA (70 µM) is present (blue), as evident from the absence of peaks observed with light-state A79Q alone (red). Peaks for dark-state A79Q (black) were similar to peaks observed with recovered A79Q in the presence of AN-35 DNA.
Further quantitation of the intensities of 35 assigned light-state WT EL222 peaks10 as a function of AN-45 concentration provided additional confirmation of the 2:1 EL222:DNA stoichiometry observed in EMSA and SEC-MALLS analyses. Specifically, we observed progressive loss of peak intensity with the titration of AN-45 into 80 µM WT EL222, with a complete loss of peak intensity occurring at approximately 40 µM AN-45 (Figure 4). Saturation at this concentration is consistent with two EL222-binding sites per DNA molecule (Figure 4a), as expected from our experimental data and the presence of two half-sites within the consensus EL222-binding site found by ChIP-Seq and SELEX16 (Figure S1). In addition, fitting the titration data to an equation for potentially-cooperative, specific binding to a single site yields an apparent KD~11 µM for AN-45, similar to the EC50 established by EMSA (~8 µM) despite significant differences in the experimental conditions and approaches used to obtain these values.
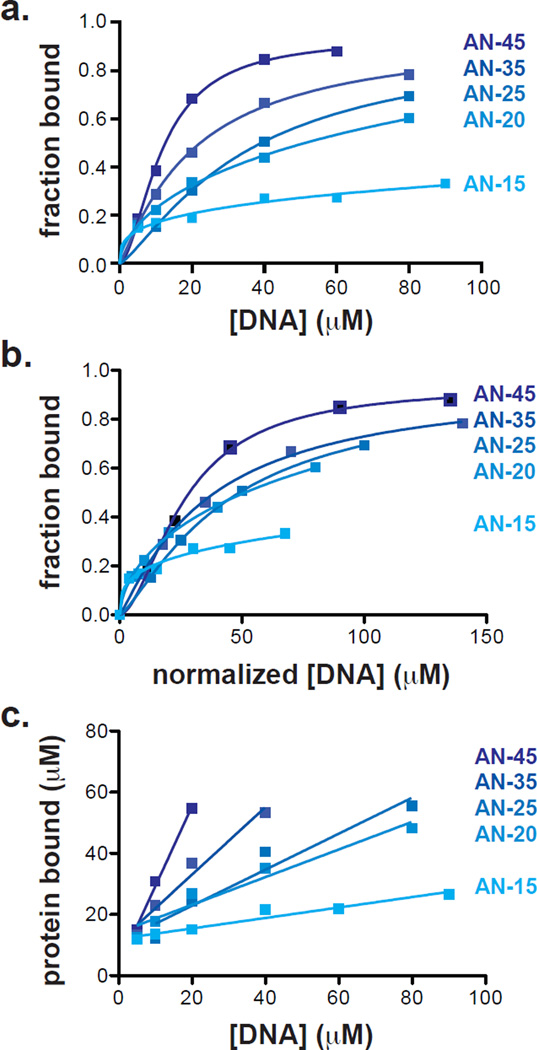
Figure 4. Quantification of affinity and stoichiometry of DNA binding in EL222 from NMR-based studies.
(a) Peak intensities obtained from NMR-based titration experiments were plotted as a function of DNA concentration. Five substrates all derived from the original 45 bp AN-45 DNA were tested for binding to WT EL222. Truncation of the ends by 5 bp each to create a 35 bp oligo (AN-35, Figure S1a) has a minor negative effect on DNA binding. Further truncations to a 25-mer (AN-25) or a 20-mer (AN-20) have larger effects, while both retain some binding to EL222. Binding is essentially lost in a 15-mer (AN-15). All curves were fit with a single site-specific binding curve, including a Hill coefficient for cooperativity. (b) Normalization of the DNA concentration in (a) to the number of 20 bp equivalents suggest that AN-45, 35, 25 and 20 have similar binding efficiencies. (c) Comparisons of EL222 protein bound as a function of DNA concentration indicates that smaller oligos do not bind with the same stoichiometry. On average, two protein molecules bind to the complete 45 bp AN-45 oligo; however, only 1 molecule binds efficiently to AN-25 and AN-20. AN-35 exhibits intermediate efficiency between these cases, consistent with its inclusion of a DNA sequence resembling a consensus EL222 binding site at the 3’ terminus of AN-45.
With these initial validations of the NMR-based approach for examining EL222/DNA complexes, we used this method to examine the effect of truncating the AN-45 oligo on EL222 binding affinity. Titrations of 15–45 bp fragments of AN-45 DNA into EL222 (80 µM) revealed substantial differences in saturation and apparent binding affinities among these fragments (Figure 4a, Figure S1a). The effects of truncation of the 5’ and 3’ ends of AN-45 on apparent affinity and saturation are most evident with the AN-15 substrate, which showed minimal, non-saturated DNA binding above the dark-state threshold (Figure 4a). Intermediate truncations affected both the affinity and minimal saturating concentration of EL222 in a rational manner. The effects on affinity are best seen in plots of protein bound as a function of DNA concentration normalized to the total concentration of available base pairs in all oligos (Figure 4b), allowing a discrimination among sequence specific or non-specific binding. When normalized, AN-45 shows a modest increase in binding affinity compared to AN-20/-30/-35, whose titration profiles were superimposable. AN-15 showed no effective binding. Analysis of the AN-45 sequence reveals some homology to the consensus EL222-binding site16 at the 3’ terminus (Figure S1), whereas this site is either flanked by a sole basepair (AN-35) or abolished altogether (AN-20/-25) in the truncated variants. In AN-15, the lack of effective binding likely reflects DNA too short to allow recognition by the EL222 dimer. Plots of total protein bound as a function of DNA (Figure 4c) complement these analyses and provide additional insight into AN-45 recognition by EL222. Analysis of the slopes of protein concentration vs. DNA concentration indicates that only AN-45 is saturated with two EL222 molecules per DNA, consistent with our prior SEC-MALLS and EMSA data. AN-35 is intermediate between a single copy and a dimeric unit; shorter DNAs bound only a single EL222 molecule on average. Taken together, we interpret these data to reflect the effects of truncating the 3’ terminal sequence on decreasing both the affinity and preferred stoichiometry of the EL222-DNA interaction.
DISCUSSION
As stated at the outset, the understanding of mechanistic basis of environmental sensing / signaling within protein receptors requires knowledge of stimuli-triggered structural, dynamic and functional changes. Within the LOV class of photoreceptors, such studies have been slowed in part by the lack of full-length proteins – containing both LOV and effectors – that are suitable for high-resolution biophysical and biochemical characterization. While two engineered “optogenetic” tools have recently provided some understanding as to how LOV domains might be adapted to control small GTPases and histidine kinases15, 31, there is a clear need to complement this work by examining natural proteins. Accordingly, we have focused on the EL222 LOV-HTH protein10, which can provide insight into natural regulatory processes as well as broaden our understanding of how LOV domains regulate different biochemical activities.
Coupling precedence from other LOV and HTH proteins with initial biophysical characterization10, it was relatively straightforward to develop a regulatory model for EL222: Dark-state interactions between the LOV β-sheet and HTH 4α helix inhibit the DNA-binding capability of this protein, in part by holding it in an inactive monomeric conformation. A combination of biophysical and biochemical data establish that EL222 activation proceeds through light-induced disruption of the LOV β-sheet/HTH 4α helix interactions, with the ability of several β-sheet mutations to constitutively activate EL222 DNA binding further supporting this concept10, 21. However, a critical aspect of the model – dimerization of activated EL222 – remained unproven to date, in part due to complications with the rapid decay kinetics of the photoexcited state hampering direct physical observation of this state.
Here, we address this shortcoming by using variants of EL222 with long-lived active states in EMSA, SEC-MALLS and NMR experiments. Data derived from these experiments revealed that EL222 binds to DNA as a dimer and uses a consensus EL222 binding site similar to one we have previously reported16. We emphasize that prior technical challenges were addressed through three advances: 1) development of EL222 variants with longer-lived photoexcited states21, enabling SEC-MALLS (Figure 2); 2) identification of an artificially-selected DNA oligomer with ~20-fold higher affinity than our original candidate-based AN-4516, enabling EMSA-based observation of mixed dimers bound to DNA (Figure 2c; comparable studies on AN-45 show a smear of mixed dimers rather than distinct bands, consistent with rapid exchange [data not shown]); 3) utilization of solution NMR methods to examine protein/DNA complexes in equilibrium with the ability to easily photoactivate samples and without requiring separation of free protein from protein/DNA complexes.
Taken together, these results provide a model for the DNA activation process in EL222, where EL222 activation bears similarity to other characterized HTH proteins in the NarL/LuxR class of tetrahelical HTH DNA binding proteins. While the regulatory processes of NarL and LuxR themselves are more complex due to their involvement of a separate sensor-histidine kinase, other cases clearly document how an activatable, inhibitory domain (e.g., LOV, phosphoacceptor) docks to the HTH domain in a configuration not suitable for DNA recognition10, 32. Upon phosphorylation or light activation the inhibitory surface is released, allowing for dimerization and DNA binding32. Interestingly, different proteins in the HTH class utilize different elements for dimerization: some primarily use the HTH 4α helix (e.g. NarL32), while others utilize N-terminal domains as mediators of dimerization (e.g. LuxR, TraR33–35).
Within EL222, both types of potential dimerizing-domains are present, leaving open the possibility that either (or both) are involved in dimer formation. The LOV/PAS β-scaffold is a known mediator of protein:protein interactions and is the primary dimerization interface in several hetero- and homodimeric LOV proteins3, 4. Our constitutively-active point mutants in the EL222 LOV domain (L120K, S137Y) both exhibit some dark-state dimerization, albeit with apparently different exchange kinetics. This implies some role for the LOV domain in assembling these dimers, but with some degree of flexibility to accommodate these point mutations. Similar effects have been observed in β-sheet point mutants in other PAS dimeric systems (e.g. B. subtilis KinA PAS-A30). In addition to these effects on free proteins, we also suggest that the DNA scaffold will likely facilitate oligomerization as well.
Together, our data indicate that photoactivation of EL222 follows a three-step process. First, photochemical excitation results in C4a-flavin adduct formation and subsequent alterations in local structure. Propagation of conformational changes within the LOV β-sheet then alters LOV-HTH interactions in the vicinity of S137 and L120. Finally, the elongated monomeric form can then assemble with some combination of LOV-LOV and HTH-HTH mediated dimers that directly recognize DNA. Return to dark-state conditions results in rapid DNA release with kinetics determined by the LOV photocycle. A detailed understanding of the molecular mechanisms of the light-activated DNA binding protein described in this study should help to provide further insight into signal transduction and also facilitate the adaptation of EL222 into a ‘natural’, minimally-engineered photoswitchable system for use in various applications.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Giomar Rivera-Cancel and Abigail Nash for helpful discussions on EL222/DNA interactions, and all members of the Gardner Laboratory for useful comments.
Funding sources: We gratefully acknowledge funding from the NIH (R01 GM081875 to K.H.G.; F32 GM090671 supporting B.D.Z.) and the Robert A. Welch Foundation (I-1424 to K.H.G.) in support of this research.
ABBREVIATIONS
- LOV
Light Oxygen Voltage domain
- SEC-MALLS
Size Exclusion Chromatography – Multi Angle Laser Light Scattering
- NMR
Nuclear Magnetic Resonance
- EMSA
Electrophoretic Mobility Shift Assay
- HTH
helix-turn-helix
- FMN
Flavin Mononucleotide
- PAS
Period ARNT Single minded domain
- bp
basepair
- oligo
oligonucleotide
- AQTrip
EL222 variant with V41I:L52I:A79Q:V124I point mutations
Footnotes
SUPPORTING INFORMATION
Includes five Supporting Figures showing all DNA sequences used in this study (Figure S1), a sequence alignment of EL222 and VVD LOV domains (Figure S2), SEC-MALLS profiles of light-state AQTrip (Figure S3), SEC-MALLS and EMSA analysis of a constitutively-active S137Y variant (Figure S4), and EMSA analyses of rate-altered EL222 variants (Figure S5). This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Zoltowski BD, Gardner KH. Tripping the Light Fantastic: Blue-Light Photoreceptors as Examples of Environmentally Modulated Protein-Protein Interactions. Biochemistry. 2011;50:4–16. doi: 10.1021/bi101665s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crosson S, Rajagopal S, Moffat K. The LOV domain family: photoresponsive signaling modules coupled to diverse output domains. Biochemistry. 2003;42:2–10. doi: 10.1021/bi026978l. [DOI] [PubMed] [Google Scholar]
- 3.Zoltowski BD, Crane BR. Light activation of the LOV protein vivid generates a rapidly exchanging dimer. Biochemistry. 2008;47:7012–7019. doi: 10.1021/bi8007017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moglich A, Moffat K. Structural basis for light-dependent signaling in the dimeric LOV domain of the photosensor YtvA. J. Mol. Biol. 2007;373:112–126. doi: 10.1016/j.jmb.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harper SM, Neil LC, Gardner KH. Structural basis of a phototropin light switch. Science. 2003;301:1541–1544. doi: 10.1126/science.1086810. [DOI] [PubMed] [Google Scholar]
- 6.Taylor BL, Zhulin IB. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol. Mol. Biol. Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry JT, Crosson S. Ligand Binding PAS Domains in a Genomic, Cellular, and Structural Context. Annu. Rev. Microbiol. 2011;65:261–286. doi: 10.1146/annurev-micro-121809-151631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halavaty AS, Moffat K. N- and C-terminal flanking regions modulate light-induced signal transduction in the LOV2 domain of the blue light sensor phototropin 1 from Avena sativa. Biochemistry. 2007;46:14001–14009. doi: 10.1021/bi701543e. [DOI] [PubMed] [Google Scholar]
- 9.Alexandre MT, Domratcheva T, Bonetti C, van Wilderen LJ, van Grondelle R, Groot ML, Hellingwerf KJ, Kennis JT. Primary reactions of the LOV2 domain of phototropin studied with ultrafast mid-infrared spectroscopy and quantum chemistry. Biophys. J. 2009;97:227–237. doi: 10.1016/j.bpj.2009.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nash AI, McNulty R, Shillito ME, Swartz TE, Bogomolni RA, Luecke H, Gardner KH. Structural basis of photosensitivity in a bacterial light-oxygen-voltage/helix-turn-helix (LOV-HTH) DNA-binding protein. Proc Natl Acad Sci U S A. 2011;108:9449–9454. doi: 10.1073/pnas.1100262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lungu OI, Hallett RA, Choi EJ, Aiken MJ, Hahn KM, Kuhlman B. Designing photoswitchable peptides using the AsLOV2 domain. Chem. Biol. 2012;19:507–517. doi: 10.1016/j.chembiol.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freddolino PL, Gardner KH, Schulten K. Signaling mechanisms of LOV domains: new insights from molecular dynamics studies. Photochem Photobiol Sci. 2013;12:1158–1170. doi: 10.1039/c3pp25400c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoltowski BD, Schwerdtfeger C, Widom J, Loros JJ, Bilwes AM, Dunlap JC, Crane BR. Conformational switching in the fungal light sensor Vivid. Science. 2007;316:1054–1057. doi: 10.1126/science.1137128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moglich A, Ayers RA, Moffat K. Design and signaling mechanism of light-regulated histidine kinases. J. Mol. Biol. 2009;385:1433–1444. doi: 10.1016/j.jmb.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diensthuber RP, Bommer M, Gleichmann T, Moglich A. Full-length structure of a sensor histidine kinase pinpoints coaxial coiled coils as signal transducers and modulators. Structure. 2013;21:1127–1136. doi: 10.1016/j.str.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Rivera-Cancel G, Motta-Mena LB, Gardner KH. Identification of natural and artificial DNA substrates for light-activated LOV-HTH transcription factor EL222. Biochemistry. 2012;51:10024–10034. doi: 10.1021/bi301306t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maris AE, Sawaya MR, Kaczor-Grzeskowiak M, Jarvis MR, Bearson SM, Kopka ML, Schroder I, Gunsalus RP, Dickerson RE. Dimerization allows DNA target site recognition by the NarL response regulator. Nat. Struct. Biol. 2002;9:771–778. doi: 10.1038/nsb845. [DOI] [PubMed] [Google Scholar]
- 18.Jeon Y, Lee YS, Han JS, Kim JB, Hwang DS. Multimerization of phosphorylated and non-phosphorylated ArcA is necessary for the response regulator function of the Arc two-component signal transduction system. J. Biol. Chem. 2001;276:40873–40879. doi: 10.1074/jbc.M104855200. [DOI] [PubMed] [Google Scholar]
- 19.Gautam US, Chauhan S, Tyagi JS. Determinants outside the DevR C-terminal domain are essential for cooperativity and robust activation of dormancy genes in Mycobacterium tuberculosis. PLoS One. 2011;6:e16500. doi: 10.1371/journal.pone.0016500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheffield P, Garrard S, Derewenda Z. Overcoming expression and purification problems of RhoGDI using a family of "parallel" expression vectors. Protein Expression Purif. 1999;15:34–39. doi: 10.1006/prep.1998.1003. [DOI] [PubMed] [Google Scholar]
- 21.Zoltowski BD, Nash AI, Gardner KH. Variations in protein-flavin hydrogen bonding in a light, oxygen, voltage domain produce non-Arrhenius kinetics of adduct decay. Biochemistry. 2011;50:8771–8779. doi: 10.1021/bi200976a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blommel PG, Fox BG. A combined approach to improving large-scale production of tobacco etch virus protease. Protein Expr Purif. 2007;55:53–68. doi: 10.1016/j.pep.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kay LE, Keifer P, Saarinen T. Pure absorption gradient enhanced heteronuclear single quantum correlation spectroscopy with improved sensitivity. J. Am. Chem. Soc. 1992;114:10663–10665. [Google Scholar]
- 24.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BA, Blevins RA. NMRView: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR. 1994;4:595–740. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 26.Raffelberg S, Mansurova M, Gartner W, Losi A. Modulation of the photocycle of a LOV domain photoreceptor by the hydrogen-bonding network. J. Am. Chem. Soc. 2011;133:5346–5356. doi: 10.1021/ja1097379. [DOI] [PubMed] [Google Scholar]
- 27.Zoltowski BD, Vaccaro B, Crane BR. Mechanism-based tuning of a LOV domain photoreceptor. Nat. Chem. Biol. 2009;5:827–834. doi: 10.1038/nchembio.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christie JM, Corchnoy SB, Swartz TE, Hokenson M, Han IS, Briggs WR, Bogomolni RA. Steric interactions stabilize the signaling state of the LOV2 domain of phototropin 1. Biochemistry. 2007;46:9310–9319. doi: 10.1021/bi700852w. [DOI] [PubMed] [Google Scholar]
- 29.Song SH, Freddolino PL, Nash AI, Carroll EC, Schulten K, Gardner KH, Larsen DS. Modulating LOV domain photodynamics with a residue alteration outside the chromophore binding site. Biochemistry. 2011;50:2411–2423. doi: 10.1021/bi200198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J, Tomchick DR, Brautigam CA, Machius M, Kort R, Hellingwerf KJ, Gardner KH. Changes at the KinA PAS-A dimerization interface influence histidine kinase function. Biochemistry. 2008;47:4051–4064. doi: 10.1021/bi7021156. [DOI] [PubMed] [Google Scholar]
- 31.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baikalov I, Schroder I, Kaczor-Grzeskowiak M, Grzeskowiak K, Gunsalus RP, Dickerson RE. Structure of the Escherichia coli response regulator NarL. Biochemistry. 1996;35:11053–11061. doi: 10.1021/bi960919o. [DOI] [PubMed] [Google Scholar]
- 33.Qin Y, Luo ZQ, Smyth AJ, Gao P, Beck von Bodman S, Farrand SK. Quorum-sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. EMBO J. 2000;19:5212–5221. doi: 10.1093/emboj/19.19.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto UM, Winans SC. Dimerization of the quorum-sensing transcription factor TraR enhances resistance to cytoplasmic proteolysis. Mol. Microbiol. 2009;73:32–42. doi: 10.1111/j.1365-2958.2009.06730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu J, Winans SC. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc Natl Acad Sci U S A. 2001;98:1507–1512. doi: 10.1073/pnas.98.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.