Abstract
GAB2 is a scaffold protein with diverse upstream and downstream effectors. MAPK and PI3K signaling pathways are known effectors of GAB2. It is amplified and overexpressed in a variety of human tumors including melanoma. Here we show a previously undescribed role for GAB2 in NRAS-driven melanoma. Specifically, we found that GAB2 is co-expressed with mutant NRAS in melanoma cell lines and tumor samples and its expression correlated with metastatic potential. Co-expression of GAB2WT and NRASG12D in melanocytes and in melanoma cells increased anchorage independent growth by providing GAB2-expressing cells a survival advantage through upregulation of BCL-2 family of anti-apoptotic factors. Of note, collaboration of GAB2 with mutant NRAS enhanced tumorigenesis in vivo and led to an increased vessel density with strong CD34 and VEGFR2 activity. We found that GAB2 facilitiated an angiogenic switch by upregulating HIF-1α and VEGF levels. This angiogenic response was significantly suppressed with the MEK inhibitor PD325901. These data suggest that GAB2-mediated signaling cascades collaborate with NRAS-driven downstream activation for conferring an aggressive phenotype in melanoma. Second, we show that GAB2/NRAS signaling axis is non-linear and non-redundant in melanocytes and melanoma, and thus are acting independent of each other. Finally, we establish a link between GAB2 and angiogenesis in melanoma for the first time. In conclusion, our findings provide evidence that GAB2 is a novel regulator of tumor angiogenesis in NRAS-driven melanoma through regulation of HIF-1α and VEGF expressions mediated by RAS-RAF-MEK-ERK signaling.
Keywords: oncogene, melanoma, angiogenesis, scaffold, adaptor
Introduction
The main function of scaffold proteins is to bring other proteins together to form complexes. GAB2 is a scaffold protein that lacks enzymatic activity (1). Once it is activated it becomes phosphorylated on tyrosine residues providing binding sites for multiple proteins involved in signal transduction (2). It is a key mediator for the regulation of cellular processes such as proliferation, survival, migration, differentiation, and morphogenesis (3). There are three paralogues of GAB proteins in vertebrates, GAB1, GAB2, and GAB3 that are conserved throughout evolution. Receptor tyrosine kinases (EGFR, KIT, MET, FGFR, PDGFR, NGFR), cytokine receptors, Fc receptors, T and B cell antigen receptors, and G-coupled receptors act as upstream regulators of GAB2 (4–11). It can also be activated by various fusion oncoproteins such as BCR-ABL and has key functions in transformation of hematopoietic stem cells and leukemogenic signaling (12). GAB2 recruits proteins that serve as downstream effectors. MAPK and PI3K-AKT signaling represent two major effector pathways regulated by GAB2 that have been extensively studied. Binding of GAB2 to SHP2 activates ERK signaling, whereas its binding to the p85 subunit of PI3K leads to AKT activation, and subsequent biological responses (1). Additionally, PLCγ directly binds to GAB2. It has multiple consensus binding sites for the Crk adaptor proteins. 14-3-3 isoforms associate with GAB2 and attenuate its function. Thus, association with these molecules is critical for the function of GAB2 in mediating intracellular signaling from the receptors. There is growing body of evidence implicating GAB2 in oncogenic transformation, tumor progression and metastasis. The candidacy of GAB2 as a potential oncogenic event comes from studies in breast (13–15) and ovarian cancer (16), leukemia (12, 17) and melanoma (18, 19). 11q13.5-q14.1 that includes the GAB2 gene is amplified in ~10–15% of human malignancies (13). Functional studies implicate GAB2 as a driving event.
Melanoma results from transformation of melanocytes that are primarily located in the skin. There are more than 68,000 estimated new cases of invasive and 53,000 in situ melanoma cases in the United States annually resulting in over 8,700 deaths (20, 21). Early melanoma is typically cured by surgery whereas metastatic melanoma is an aggressive disease with a poor prognosis. NRAS and BRAF are bona fide oncogenes in melanoma critical for tumor initiation (22). In NRAS and BRAF-driven oncogenesis, progression to melanoma and metastasis requires silencing of tumor suppressor genes including PTEN, INK4A and/or ARF (23). There are three RAS (HRAS, KRAS, NRAS) and three RAF (ARAF, BRAF, CRAF) genes in humans (24). Gain-of-function mutations in NRAS (~25%) or BRAF (~40%) are found in melanoma in a mutually exclusive fashion. NRAS mutations involve codons 12, 13, and 61 (NRASG12/G13/Q61). More than 90% of BRAF mutations are valine to glutamic acid substitutions at codon 600 (BRAFV600E) (24). Oncogenic mutations in NRAS can activate both MAPK and PI3K-AKT pathways, whereas mutant BRAF activates the MAPK pathway. Co-expression of NRASQ61R and BRAFV600E results in a senescent phenotype in melanoma cells, thus explaining the epistatic relationship due to selection against double mutant cells (25). A role for RAS mutations in melanoma initiation has been confirmed through generation and analysis of transgenic animal models. Activated HRAS expression on an INK4A, ARF, or p53 mutant background promotes non-metastatic melanoma in mice whereas NRAS expression in INK4A/ARF deficiency promotes metastatic melanoma (26–28). BRAFV600E cannot transform human melanocytes due to induction of senescence, but transforms murine melanocytes that are ARF deficient (29). RAS mutants transform melanocytes more efficiently than BRAF possibly due to the PI3K-AKT component (29, 30). BRAFV600E in the presence of PTEN suppression results in PI3K-AKT activation, and leads to full transformation to melanoma in vivo (31). Although several genetic events cooperating with NRAS and BRAF in melanoma have been identified, full characterization of collaborating partners has not yet been established. Moreover, the role of scaffold proteins in NRAS- and BRAF-driven tumorigenesis is unknown.
GAB2 is expressed at significantly higher levels in metastatic melanomas as compared to benign melanocytic nevi and primary melanomas (18). Melanoma is a heterogeneous cancer consisting of different subgroups. GAB2 is amplified in the acral and mucosal melanoma subset with ~10% frequency (melanomas arising from melanocytes on palms, soles and mucous membranes), suggesting oncogenic potential (19). To date, the role of GAB2 in melanocyte transformation has not been examined. In cell culture systems, GAB2 promotes migration and invasion. Importantly, GAB2 accelerates tumorigenic potential and metastatic capability in vivo (18).
In this study, we examined the potential cooperative activity of GAB2 in NRAS-driven melanoma. We found that this collaboration increases anchorage independent growth by providing GAB2-expressing cells a survival advantage, enhances tumorigenesis in vivo, and facilitates an angiogenic switch by upregulating HIF-1α and VEGF in tumors with mutant NRAS. These findings support a role for GAB2 in NRAS-driven melanoma and provide, for the first time, a model to study the role of GAB2 in melanoma tumorigenesis.
Results
GAB2 is overexpressed in melanomas harboring NRAS or BRAF mutations, and its expression correlates with metastatic potential
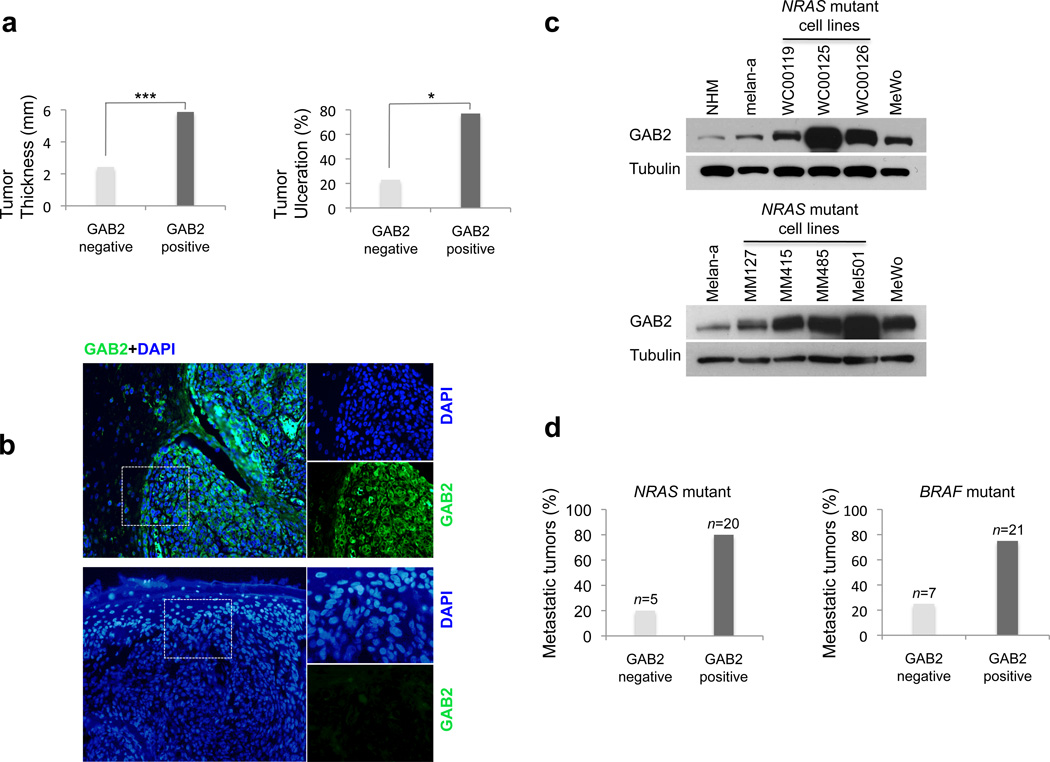
Primary tumor thickness and primary tumor ulceration are parameters that are accepted as independent predictors of survival (prognostic markers) for patients affected with melanoma by the American Joint Committee on Cancer (32, 33). To evaluate whether GAB2 correlates with prognostic markers for melanoma and to determine its impact on patient survival, we examined its protein expression in a cohort of 128 primary cutaneous melanoma samples. Ninety-nine of the 128 cases were informative for GAB2 protein expression by immunofluorescence assay. High GAB2 levels were found in 58 out of 99 cases (59%). Tumors displaying a positive phenotype were notably thicker (mean 5.87 mm, median 3.63 mm) as compared tumors displaying low or undetectable GAB2 levels (mean 2.43 mm, median 1.01 mm). GAB2 expression correlated significantly with tumor thickness (p<0.0001) and tumor ulceration (p=0.016) (Figures 1a and b, Supplemental Table 1). Notably, when the cohort was stratified by tumor thickness in three categories; thin (<1.0), intermediate thick (1.0–4.0) and thick (>4.0), the 2-year survival was significantly worse in patients with GAB2 expressing primary melanomas of intermediate thickness (p=0.045). Taken together, clinical correlative studies on human primary melanoma samples presented in this study show that GAB2 is a molecular correlate for melanoma prognostic markers underscoring its impact in melanoma progression and metastatic potential.
Figure 1. GAB2 is co-expressed with mutant NRAS or mutant BRAF in metastatic melanoma and its expression correlates with predictive markers for survival.
(a) Formalin-fixed paraffin-embedded tumor sections from a cohort of primary cutaneous melanomas (n=99) with annotated clinical and pathologic data were stained using an antibody against GAB2 and counterstained with DAPI. The correlation of GAB2 expression with melanoma prognostic markers such as tumor thickness and tumor ulceration was analyzed using Pearson’s Chi Square test. ***p<0.001, **p<0.01, *p<0.05.
(b) Immunofluorescence analysis of representative primary melanoma cases expressing high (top panels) and low levels, if any, (lower panels) of GAB2 protein is shown.
(c) Expression levels of GAB2 in normal human melanocytes, mouse melanocytes (melan-a), and a panel of metastatic melanoma cell lines were assayed by Western blotting. NRAS mutant metastatic cell lines include WC00119 (G12D), WC00125 (Q61L), WC00126 (Q61L), MM127 (G13R), MM415 (Q61L), MM485 (Q61R), and Mel501 (G12D). MeWo cell line is wild type for both NRAS and BRAF.
(d) GAB2 expression was examined in tumor sections from patients with metastatic melanomas harboring mutations in either NRAS (n=25) or BRAF (n=28) using immunofluorescence staining. The bars show percentage of cases with positive or negative GAB2 staining.
To determine the activation status of GAB2 in metastatic disease, we next examined its expression in a panel of metastatic melanoma cell lines. We also extended these translational studies by analyzing metastatic tumors from patients with melanoma harboring gain-of-function mutations in NRAS or BRAF. We have previously reported that GAB2 is overexpressed in a subset of BRAF mutant metastatic melanoma cell lines (18). Here we report that a subset of NRAS mutant cell lines including WC119, WC00125, WC00126, MM415, MM485, and Mel501 also have high levels of GAB2 expression relative to human melanocytes by western blotting (Figure 1c). MeWo cell line that is wild type for NRAS and BRAF but has GAB2 amplification and high GAB2 protein levels (18) was used for comparison. In metastatic melanoma tumor specimens, GAB2 was overexpressed in 20 out of 25 (80%) NRAS mutant, and 21 out of 28 (75%) BRAF mutant cases (Figure 1d and Supplemental Figure 1). These data show that GAB2 is co-expressed with the melanoma oncogenes NRAS or BRAF in metastatic disease suggesting potential cooperative function. Based on these observations, we sought to investigate its role in NRAS mutant melanomas.
GAB2 cooperates with oncogenic NRAS to promote anchorage independence
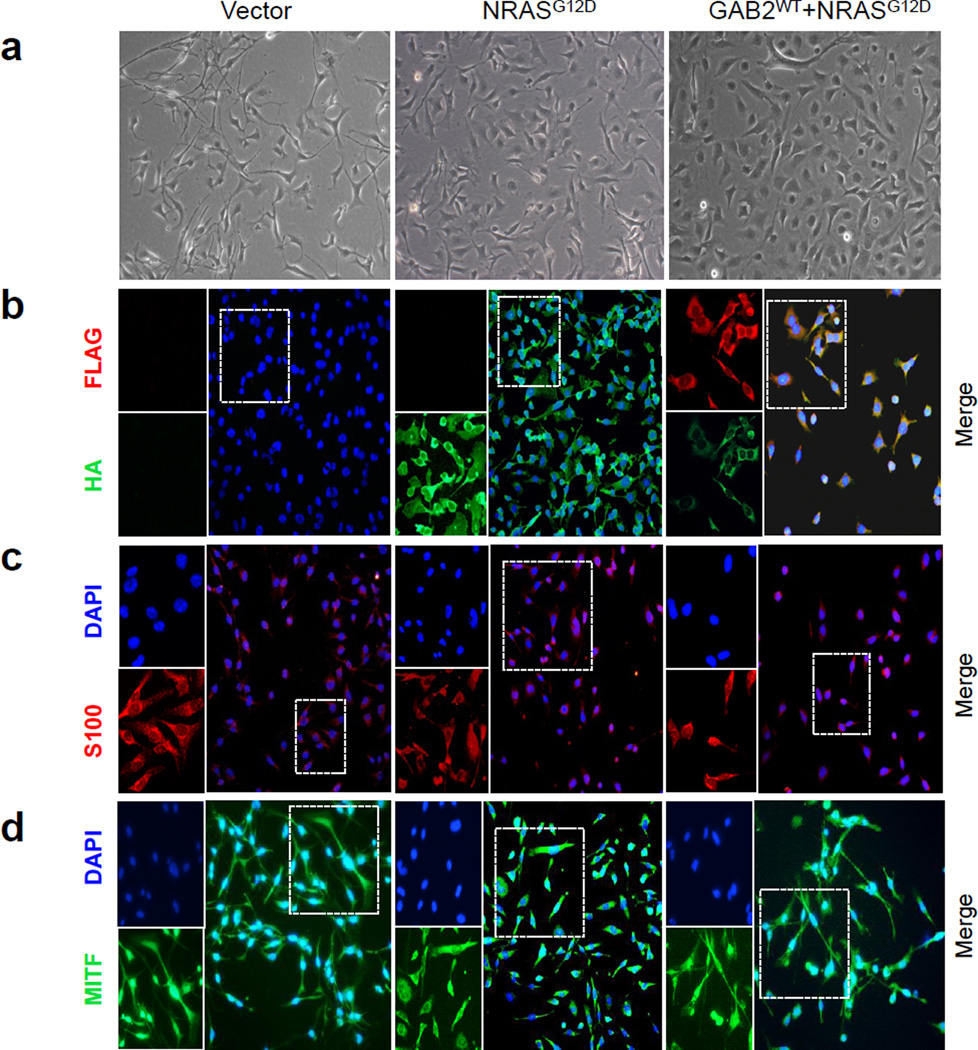
To investigate the consequences of activated GAB2 signaling in the context of oncogenic NRAS, we co-expressed GAB2WT with NRASG12D in mouse melanocytes (melan-a cells). Melan-a is a non-transformed mouse line that retains many of the characteristics of normal melanocytes that is CDKN2A-deficient (34–36). CDKN2A deficiency allows bypassing senescence in primary melanocytes and is frequently inactivated in human melanoma (37). We stably expressed vector, FLAG-epitope tagged GAB2WT and HA-epitope tagged NRASG12D alone or in combination in melan-a melanocytes. Selection was carried out in the absence of 12-O-tetradecanoyl phorbol 13-acetate (TPA). Melanocytes require TPA for growth and therefore survival in the absence of TPA is a hallmark for transformation. Vector- and GAB2WT-expressing melanocytes grown in medium lacking TPA ceased to proliferate whereas those expressing NRASG12D and GAB2WT+NRASG12D continued to grow and exhibited transformed morphology (Figure 2a). Expression levels of exogenous wild type GAB2 and oncogenic NRAS were determined using immunofluorescence staining and western blotting (Figures 2b and 3a). GAB1 levels remained unchanged in all of the three cell lines. Melanocytic origin of the cells was confirmed using melanocyte-specific markers, S100 and MITF, both of which were expressed in vector, NRASG12D and GAB2WT+NRASG12D cells (Figures 2c and 2d). To examine whether GAB2 overexpression induces genomic instability, we performed aCGH on the xenograft tumors injected with the transformed cells, and compared NRASG12D tumors with GAB2WT+NRASG12D. Whole genome DNA copy number profile did not exhibit differentially lost or gained regions between the groups, suggesting that GAB2 does not lead to genomic instability in transformed GAB2WT+NRASG12D mouse melanocytes (Supplemental Figure 2).
Figure 2. Melanocytes co-expressing GAB2 and NRASG12D do not require TPA for survival.
(a) Morphologic features of melan-a cells stably expressing empty vector alone, HA-tagged NRASG12D, or those co-expressing FLAG-tagged GAB2WT + HA-tagged NRASG12D grown in monolayer were examined on light microscopy. Cultures were grown in RPMI 1640 medium with 10% FBS in the absence of 12-O-tetradecanoyl phorbol 13-acetate (TPA) with the exception of vector expressing melanocytes which required TPA for growth.
(b–d) Cells grown on cover slips in monolayer culture were fixed and examined for FLAG (green) and HA (red) expression using double immunofluorescence staining. In addition, expression of melanocyte markers, S-100 (red) and MITF (green), was assessed by immunofluorescence analysis. Nuclei were counterstained with DAPI. FLAG, HA, S-100, and MITF are shown in the left and merged images on the right panels.
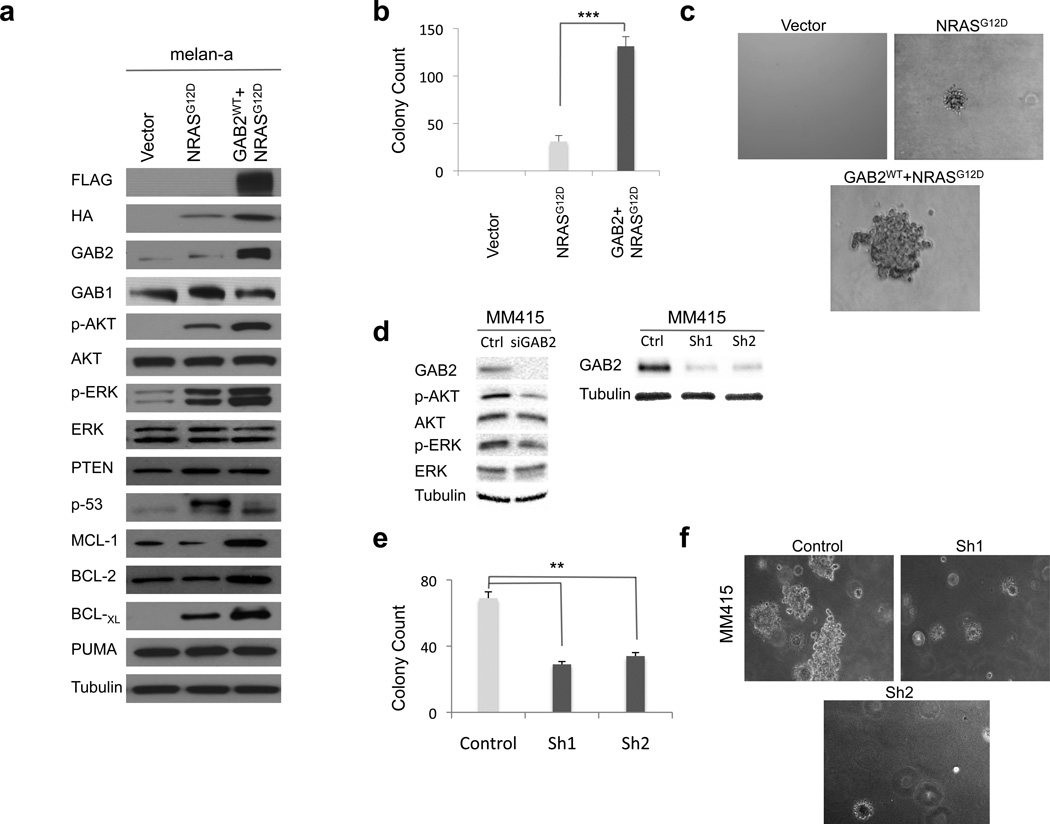
Since both GAB2 and NRAS can activate PI3K and MAPK signaling (2, 3), we examined downsteam effectors, AKT and ERK, respectively. As expected, NRASG12D expressing melanocytes showed increased phosphorylation of AKT and ERK as compared to vector expressing cells (Figure 3a). AKT and ERK levels were higher in cells expressing GAB2WT+NRASG12D compared to NRASG12D alone. Silencing of GAB2 in MM415 melanoma cells reduced AKT and ERK phosphorylation (Figure 3d). Although PI3K signaling was activated upon GAB2 overexpression in NRAS mutant melanocytes, its negative regulator PTEN levels remained unchanged. These results suggest that GAB2 potentiates both PI3K and MAPK signaling in melanocytes and melanoma cells.
Figure 3. Co-expression of GAB2WT with NRASG12D promotes anchorage independence in melanocytes.
(a) Melanocytes stably expressing empty vector, NRASG12D (HA-tagged), or GAB2WT+NRASG12D (FLAG- and HA-tagged, respectively) were cultured and protein lysates were assayed by Western blotting. PI3K and MAPK activation were examined by measuring phospho-AKT and phospho-ERK levels, respectively. Expression of PUMA, p53 and BCL-2 family of anti-apoptotic proteins, MCL-1, BCL-2 and BCL-XL, were also evaluated. Tubulin was used as loading control.
(b) and (c) Quantification and representative images of soft agar colony formation assay of melanocytes expressing vector, NRASG12D, and GAB2WT+NRASG12D are shown. Briefly, 1×105 cells were resuspended in 0.35% agarose and overlaid onto 0.7% agarose in growth medium containing 10% FBS in 6-well plates. Colonies were stained with thiazolyl blue tetrazolium bromide and counted at 4 weeks after plating. Experiments were performed in triplicates. Error bars indicate ± standard error. *p<0.05, **p<0.01, and ***p<0.001.
(d–f). MM415 is a mutant NRAS melanoma cell line with GAB2 overexpression. GAB2 was silenced with scrambled (control) or siRNA and GAB2-dependent signal transduction pathways were analyzed by western blotting. Stable knockdown of GAB2 was achieved by infecting cells with control, Sh1 and Sh2. Quantification and representative images of soft agar colony formation assay of melanomas expressing control and shGAB2 are shown. Colonies were counted two weeks after plating. Experiments were performed in triplicates. Error bars indicate ± standard error. *p<0.05, **p<0.01.
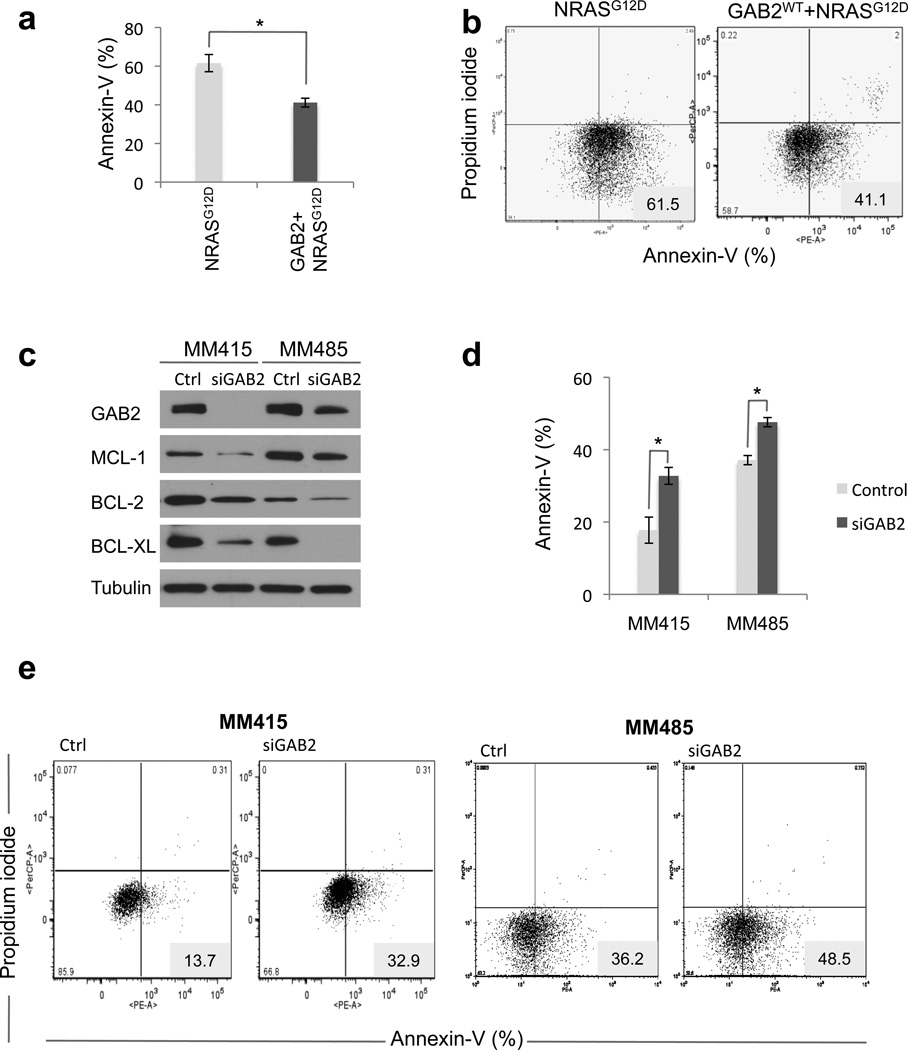
Since growth in anchorage independent conditions is essential for tumor cell transformation as well as for metastatic potential (38), we examined growth characteristics of these cells in soft agar. We found that GAB2WT+NRASG12D melanocytes resulted in larger and three times as many colonies in soft agar as NRASG12D cells (Figures 3b and c). Similarly, silencing of GAB2 in an NRAS mutant (MM415) melanoma cell line resulted in significant decrease of soft agar colonies (Figures 3d–f). Enhanced colony formation was associated with increased survival of GAB2WT+NRASG12D expressing melanocytes and melanoma cells as observed in three-dimensional (3D) collagen. Annexin-V staining and propidium iodide uptake (PI) of GAB2WT+NRASG12D expressing cells grown in 3D collagen showed significantly less apoptosis than NRASG12D cells (Figures 4a and b), suggesting that GAB2 provides a specific survival benefit under anchorage-independent conditions in oncogenic NRAS-driven tumorigenesis. In order to gain mechanistic insight, we next examined expression of BCL-2 family of anti-apoptotic proteins such as MCL-1, BCL-2 and BCL-XL. In GAB2WT+NRASG12D expressing melanocytes, expression of MCL-1, BCL-2 and BCL-XL were elevated when compared with NRASG12D and vector expressing cells. Expression of proapoptotic PUMA remained unchanged and p53 phosphorylation was decreased (Figure 3a). We next identified two NRAS mutant metastatic melanoma cell lines (MM415 and MM485) with increased levels of BCL-2 family of prosurvival proteins. Silencing of GAB2 in these cells resulted in significant decrease of MCL-1, BCL-2, and BCL-XL in 2D culture conditions (Figure 4c). We then transfected MM415 and MM485 cells with control or siGAB2 followed by plating the cells in 3D collagen for 48 hours in serum-free medium. We noted significant increase of apoptosis upon GAB2 silencing in these conditions (Figures 4d and e), suggesting that GAB2-mediated survival advantage in NRAS mutant tumorigenesis is in part mediated by upregulation of BCL-2 family of anti-apoptotic factors. Taken together, these data support our hypothesis that GAB2-mediated signaling cascades collaborate with NRAS-driven downstream activation for conferring an aggressive phenotype in melanoma. Importantly, it underscores that GAB2/NRAS signaling axis is non-linear and thus non-redundant in melanocytes and melanoma, suggesting that NRAS and GAB2 are acting independent of each other and their effects are additive to a certain degree.
Figure 4. GAB2 knockdown sensitizes NRAS mutant melanoma cells to apoptosis.
(a) and (b). Melanocytes stably expressing NRASG12D and GAB2WT+NRASG12D were plated onto 3D collagen gels for 24 hours in serum-free medium and analyzed for Annexin-V staining and PI uptake. Experiments were performed in triplicates. Error bars indicate ± standard error. *p<0.05.
(c) MM415 and MM485 are NRAS mutant whereas A2058 and G361 are BRAF mutant melanoma cell lines that overexpress GAB2. These melanoma cells were transfected with control or GAB2 siRNA. Forty-eight hours later cell lysates were analyzed for MCL-1, BCL-2, BCL-XL by Western blotting. Tubulin was used as loading control.
(d) and (e). Forty-eight hours following transfection with control and siGAB2, MM415, MM485, A2058, and G361 cells were replated onto 3D collagen for 48 hours in serum-free medium. Harvested cells were analyzed for Annexin-V staining and PI uptake. Bar graphs indicate percentage of Annexin-V positive cells. Representative traces from one of three experiments are shown. *p<0.05, **p<0.01, and ***p<0.001.
GAB2 promotes angiogenesis in NRAS mutant melanoma
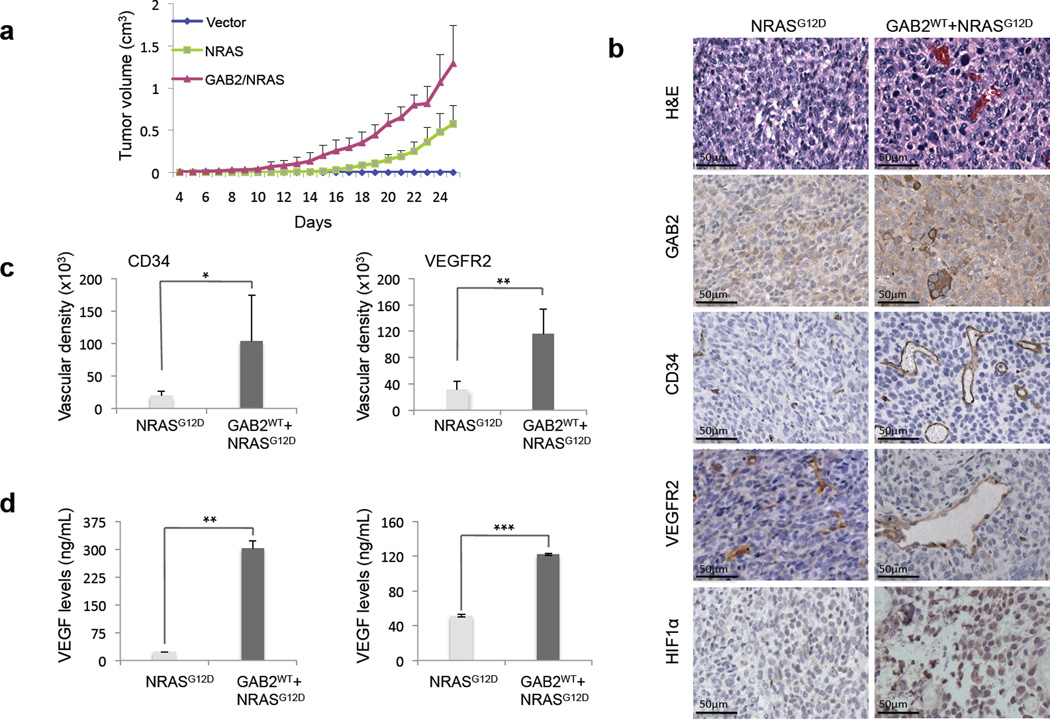
To determine whether GAB2 upregulation in the context of oncogenic NRAS can lead to increased tumorigenesis in vivo, we injected vector, NRASG12D, or GAB2WT+NRASG12D expressing cells into nude mice subcutaneously and analyzed tumor growth. Expression of exogenous wild type GAB2 (FLAG) and oncogenic NRAS (HA) in the tumor xenografts were confirmed (Supplemental Figure 3). GAB2WT+NRASG12D expressing cells formed significantly larger, soft, pink-red tumors compared to smaller, firm, brown-black tumors in NRASG12D group and no tumors in vector controls (Figure 5a). Histologic examination of GAB2WT+NRASG12D xenografts revealed marked pleomorphism and multinucleation of tumor cells together with increase in vascular structures (~4–6 fold difference) as compared to NRASG12D tumors (Figures 5b and c). In GAB2WT+NRASG12D tumors, vessel numbers were significantly increased as well as vessel length, lumina were markedly dilated, and vessels showed strong CD34 and VEGFR2 staining. These results suggest that GAB2-mediated signaling cascades not only lead to increased tumorigenesis but also significant angiogenic response in NRAS mutant melanocytes. Since the transcription factor HIF-1α regulates genes involved in tumor angiogenesis, we examined its expression by immunohistochemistry. We found that HIF-1α protein expression is markedly increased in xenograft tumors expressing GAB2WT+NRASG12D whereas minimal staining was noted in NRASG12D-induced tumors (Figure 5b). HIF-1α activity leads to angiogenesis through VEGF. VEGF directly stimulates endothelial cell proliferation, migration, and survival. We therefore examined VEGF levels by ELISA in NRASG12D and GAB2WT+NRASG12D-expressing xenografts and found that GAB2 expression led to significantly higher levels of VEGF (13 times higher) in these tumors (Figure 5d, left panel). In order to determine whether hypoxia was the driving force for VEGF expression, we measured VEGF levels in NRASG12D and GAB2WT+NRASG12D-expressing melanocytes cultured in 2D conditions and found that GAB2 led to higher levels of VEGF under these conditions as well (2.5 times higher) (Figure 5d, right panel). We next evaluated the behavior of these cells in a lung seeding assay which showed increased tumor cell invasion into the lung parenchyma and prominent vascular density in GAB2WT+NRASG12D expressing cells as compared with NRASG12D cells (Supplemental Figure 3). These data confirm the angiogenic response upon GAB2 upregulation in oncogenic NRAS-driven tumorigenesis.
Figure 5. GAB2 enhances tumorigenesis and induces angiogenesis in vivo.
(a). 1×106 melan-a melanocytes stably expressing vector, NRASG12D, GAB2WT+NRASG12D were injected into nude mice subcutaneously (n=8 mice per group). Primary tumor growth was examined by measuring tumor volume every other day.
(b). At four weeks, xenograft tumors were analyzed histologically using hematoxylin and eosin staining as well as for GAB2, CD34, VEGFR2, and HIF1α, using immunohistochemistry. Representative micrographs of NRASG12D and NRASG12D+GAB2WT expressing tumors are shown.
(c). CD34 or VEGFR2-staining vasculature was quantified by measuring the vessel areas (20× per field, 4 fields per section) using the Image J software. Error bars indicate ± standard error. *p<0.05, **p<0.01.
(d). Proteins were extracted from xenografts (left panel) or cells cultured in 2D conditions (right panel) expressing either NRASG12D or GAB2WT+NRASG12D, and VEGF levels were measured by ELISA. Experiments were performed in duplicates. Error bars indicate ± standard error. **p<0.01, ***p,0.001.
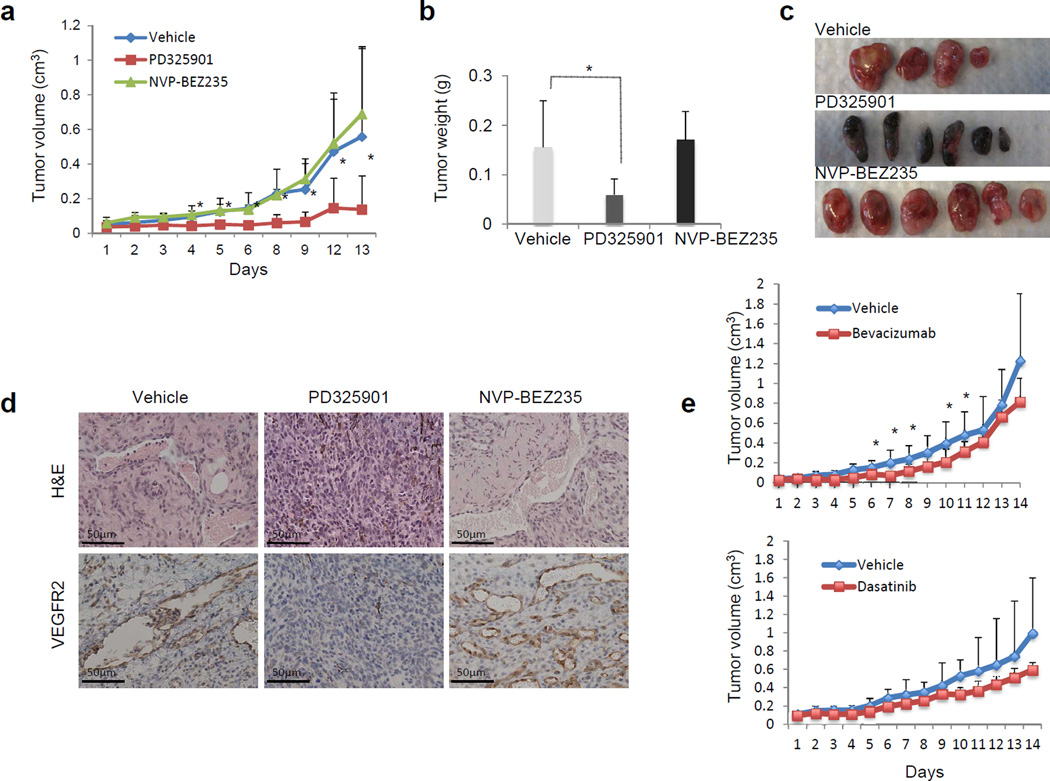
To obtain further mechanistic insight and to examine the therapeutic relevance of our findings, we compared MEK inhibitor (PD325901) with a dual PI3K/mTOR inhibitor (NVP-BEZ235) for their ability to decrease tumor burden in GAB2WT+NRASG12D-expressing tumors. We established xenografts by subcutaneous injection of melanocytes expressing GAB2WT+NRASG12D and mice were treated with vehicle, 20 mg/kg/day of PD325901 once a day, or 20 mg/kg/day of NVP-BEZ235 once a day by oral gavage for two weeks. PD325901 treatment inhibited primary tumor growth significantly relative to the vehicle control (p<0.01), whereas NVP-BEZ235 had no effect (Figures 6a–c). The tumors in vehicle and NVP-BEZ235 treated groups were amelanotic with increased vasculature whereas those treated with PD325901 showed increase in pigmentation (Figure 6c). Early lethality of mice in the combined group (PD325901+NVP-BEZ235) in this model made it difficult to detect any effect of combined MEK and PI3K pathway inhibition. Taken together, these findings indicate that GAB2 mediates angiogenic response and increases tumor growth via induction of MEK but not PI3K signaling in GAB2/NRAS-driven tumorigenesis.
Figure 6. Therapeutic efficacy of MEK, PI3K, VEGF and SRC inhibition in NRAS and GAB2-driven tumorigenesis.
(a–d). 1×106 melan-a melanocytes stably expressing GAB2WT+NRASG12D were injected into nude mice subcutaneously. Treatment began when tumors reached a volume of 0.1cm3. Mice were treated with vehicle (n=4), PD325901 (MEK inhibitor, n=6) or NVP-BEZ235 (PI3K/mTOR inhibitor, n=6) every day via oral gavage for two weeks. Tumor volume and weight were analyzed. Xenografts were dissected after treatment and analyzed histologically with H&E staining and VEGFR2 immunohistochemistry.
(e). Mice with xenografts expressing GAB2WT+NRASG12D were treated with vehicle (n=8) or Bevacizumab (n=8) twice a week via intraperitoneal injection for two weeks. Tumor volumes were analyzed (upper panel). Similarly, mice were treated with vehicle (n=8) or Dasatinib (n=8) everyday via oral gavage for two weeks and tumor volumes were measured (lower panel).
Since VEGF levels were significantly elevated in GAB2WT+NRASG12D-expressing tumors, we treated mice with vehicle or 5mg/kg/day of Bevacizumab (VEGF inhibitor) twice a week via intraperitoneal injection for two weeks. Mice treated with Bevacizumab had mild to moderate reduction in tumor growth as compared to vehicle treated group (Figure 6e, upper panel). Similarly, SRC inhibitor Dasatinib (15mg/kg/day) resulted in mild to moderate reduction in tumor growth as well that did not reach statistical significance (Figure 6e, lower panel).
GAB2 enhances HIF-1α protein expression and VEGF transcription in NRAS mutant melanoma
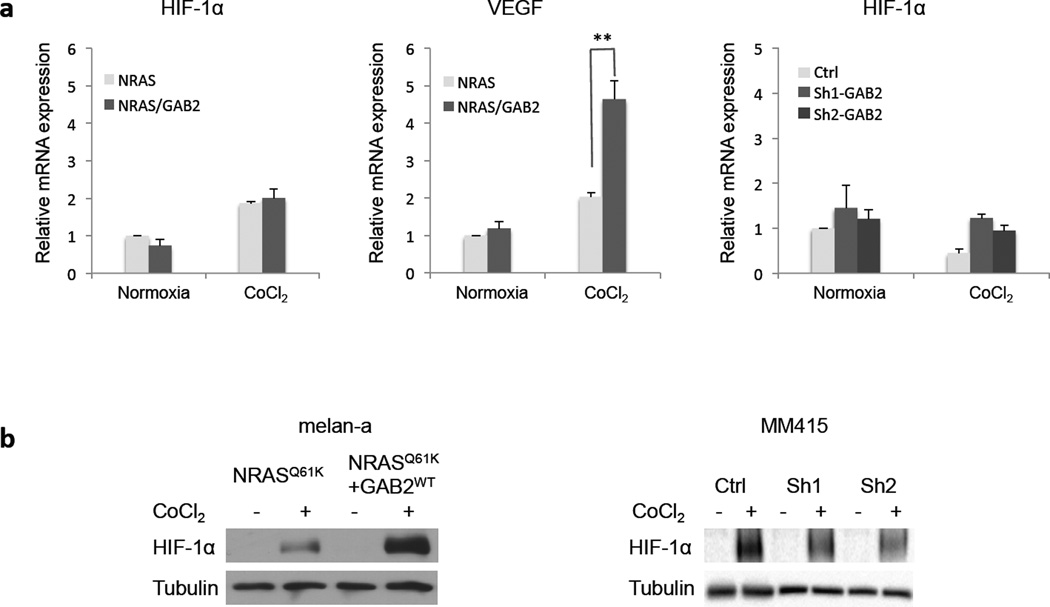
To determine how GAB2 regulates HIF-1α and its target VEGF expression, we examined messenger RNA (mRNA) levels of melanocytes expressing NRASG12D and GAB2WT+NRASG12D using qRT-PCR. Hypoxia condition was established by CoCl2 treatment of the cells. As expected, CoCl2 treatment led to an increase of HIF-1α and VEGF mRNA levels in both cell lines (Figure 7a). Although there was no elevation of HIF-1α mRNA levels when GAB2WT+NRASG12D cells were compared to NRASG12D cells, its protein expression was significantly increased upon CoCl2 treatment as determined by western blotting (Figure 7b). Silencing of GAB2 in MM415 melanoma cells did not affect HIF1-α mRNA levels but led to decrease in protein expression in CoCl2 treated cells (Figure 7a and b). Taken together, these data suggest that GAB2 modulates HIF-1α at the post-transcriptional level in NRAS mutant melanocytes.
Figure 7. Effects of GAB2 on HIF-1α transcriptional activity and protein expression.
(a) Melanocytes expressing NRASG12D and GAB2WT+NRASG12D or MM415 melanoma cells with stable knockdown of GAB2 (Sh1 and Sh2) were treated with DMSO or CoCl2 (100 µM) for 24 hours. Total RNA from these cells was analyzed for mRNA expression by qRT-PCR. qRT-PCR experiments were performed in triplicate. Error bars indicate ± standard error **p<0.01.
(b) Cell lysates from these cells were examined for HIF-1α protein expression by western blotting. Tubulin was used as loading control.
Discussion
Approximately 30% of human tumors harbor somatic gain-of-function mutations in one of the RAS genes. The mutations impair the GTPase activity of RAS, trapping it in the active GTP-bound state and promoting tumorigenesis. In melanoma, NRAS is mutated in 20–25% of cases whereas KRAS and HRAS are mutated with 2% and 1% frequency, respectively (39). Despite significant effort over the past years there is no effective treatment for RAS-driven tumors that is routinely used in clinical practice and thus they remain as one of the most difficult cancers to treat. Many RAS effector pathways are comprised of kinase cascades, providing multiple nodes for potential therapeutic intervention, such as the RAF-MEK-ERK (MAPK) and PI3K signaling pathways (40). Several small molecule compounds that potently and selectively inhibit the activity of MEK have been tested in clinical trials. However, the success of early trials was limited in part due to dose-limiting toxicity of the compounds thus necessitating the development of agents with improved pharmaceutical properties. Although RAS mutant tumors depend on the activation of the PI3K pathway, preliminary data suggest that RAS mutant tumors are insensitive to single agent PI3K inhibitors but show promising results when combined with MEK inhibitors (40). In our study we provide evidence that MEK but not PI3K inhibition alone is effective in GAB2/NRAS-driven tumorigenesis.
Our results establish that GAB2 signaling plays important roles in promoting VEGF expression, angiogenesis and tumor progression. Angiogenesis, the formation of new blood vessels from pre-existing vasculature, is a highly regulated process that is essential for tumor progression and metastasis. In some cases tumor cells can line the vessels by a process known as vascular mimicry (41) or putative cancer stem-like cells can generate tumor endothelium (42). Angiogenesis involves survival, proliferation and migration of endothelial cells, and tube formation (43). Multiple growth factors and cytokines contribute to endothelial cell activation in tumor-associated angiogenesis, one of which is vascular endothelial growth factor (VEGF) (44). At the surface of endothelial cells, VEGFR2 (Flt1) is the major mediator of VEGF-dependent signaling and cellular activities (45). Oncogenes such as RAS and BRAF are potent stimulators of VEGF expression (46). Immortalized endothelial cells expressing mutant RAS form angiosarcomas in mice (47). VEGF and VEGFRs are expressed and/or upregulated in most solid tumors, making this pathway a feasible target for therapy. VEGF inhibition has been consistently shown to slow tumor cell growth and angiogenesis (48). Bevacizumab, a humanized monoclonal antibody against VEGF, is approved as single agent or in combination in the treatment of several malignancies such as renal cell carcinoma, glioblastoma, colorectal carcinoma, and nonsquamous cell lung cancer (49). In this study, we show that Bevacizumab leads to a mild to moderate delay in tumor growth in GAB2/NRAS-driven tumorigenesis suggesting a potential use in this setting. However, it is noteworthy mentioning that MEK inhibition led to a more drastic effect in delaying tumor growth and decreasing vasculature than VEGF inhibition alone.
There are three paralogues of GAB proteins in vertebrates; GAB1, GAB2, and GAB3. GAB1 shares many biochemical properties with GAB2, has similar upstream and downstream effectors, and similar biological effects in various cellular systems. GAB1 and GAB2 are tyrosine phosphorylated upon VEGF stimulation of endothelial cells (50, 51). Recent data suggest that these two proteins display non-redundant functions in endothelial cells. GAB1 associates with VEGFR2 and promotes activation of the PLCγ, SRC, ERK, and AKT pathways that are essential for the angiogenic responses. GAB1 is a mediator of VEGF-induced endothelial cell migration and is required for in vitro capillary formation (52). Similar to GAB1, GAB2 contributes to VEGF-mediated cell migration. GAB1 but not GAB2 mediates endothelial cell survival induced by VEGF (51). Additionally, GAB1 expression within endothelial cells is required for developmental vasculogenesis. Endothelium specific GAB1 knock out mice display defects in postnatal ischemia and VEGF-induced angiogenesis (53). Pro- and anti-angiogenic factors enabling tumor cells to elicit endothelial cell responses are well characterized, however the role of scaffold protein signaling in this process is poorly defined. While it needs to be further determined whether GAB2-mediated angiogenesis is dependent on its physical interaction with SHP2, p85 subunit of PI3K, and/or PLCγ, thus downstream effects of ERK, AKT, and/or PKC activation, respectively, our findings support the conclusion that GAB2 signaling controls the assembly of protein complexes within the tumor cells that are essential for an angiogenic response. Moreover, it suggests that GAB2 may impact the development of vascular tumors or anomalies of the vascular structures.
The biologic significance of scaffold adaptor proteins is not fully understood. Since adaptor proteins have diverse upstream and downstream effectors, it is likely that the biological activities are dependent on the specific receptor’s ability to signal to specific adaptor protein in a cell-context manner. In this study, we found that GAB2 in collaboration with mutant NRAS increases anchorage independent growth by providing GAB2-expressing cells a survival advantage, enhances tumorigenesis in vivo, and facilitates an angiogenic switch by upregulating HIF-1α and VEGF in melanocytes and melanomas with mutant NRAS. Moreover, these findings suggest that MEK or VEGF inhibition may represent therapeutic approaches for NRAS mutant metastatic melanomas expressing high levels of GAB2. Future work will focus on modeling NRAS/GAB2 in mouse models and determine the use of combination regimens in this setting.
Methods
Cell lines, Plasmids and Transfection Protocols
Melan-a cells were obtained from D. Bennett (St. George’s, University of London) and maintained in RPMI 1640 containing 10% fetal bovine serum, 200 nm 12-Otetradecanoylphorbol- 13-acetate (TPA)(Sigma) and 300 pm cholera toxin (Sigma) at 37°C and 10% CO2. Primary human melanocytes were isolated from the epidermis of neonatal foreskin as previously described and maintained in melanocyte basal medium, MBM-4 (Lonza) (54). NRAS mutant metastatic cell lines, MM127, MM415, and MM485, were purchased from Cell Bank of Australia; Mel 501 from ATCC; and WC00119 (WM3670), WC00125 (WM3854), and WC00126 (WM3060) from Coriell Institute for Medical Research. MeWo cell line that is wild type for both NRAS and BRAF was obtained from ATCC. BRAF mutant metastatic melanoma cell lines, A2058 and G361, were purchased from ATCC and cultured according to provider’s instructions.
For stable expression, Phoenix cells were transfected with pBABE-puro (Addgene), pBABE-puro-GAB2WT-FLAG, or pBABE-zeo-HA-NRASG12D using Lipofectamine 2000 (Invitrogen). Selection was performed in either puromycin (2 µg/mL) or zeocin (500 µg/mL) in the absence of TPA. To generate GAB2WT+NRASG12D cells, melanocytes stably expressing GAB2 were infected with NRASG12D and selected in puromycin and zeocin in the absence of TPA.
For transient knockdown of GAB2, melanoma cell lines MM415 and MM485 were transfected with scrambled siRNA or GAB2 specific siRNA (ON-TARGETplus SMARTpool GAB2, Dharmacon) using Lipofectamine (Invitrogen). For stable knockdown of GAB2, 293 cells were transfected with pLKO.1 or shGAB2 pLKO.1 #1 and #2 (Open Biosystems) using FuGENE 6 according to the manufacturer’s protocol (Promega). Viruses were harvested, filtered, and used for infecting MM415 melanoma cells together with polybrene. Selection was performed in puromycin.
Western blotting
Western blotting was performed as previously described (18). The following commercial antibodies were used in this study: GAB2, GAB1, phospho-AKT (Ser-473), total AKT, phospho-ERK, total ERK, FLAG, BCL-2, BCL-XL, and p53 (Cell Signaling); HA (Roche); MITF (Thermo Fisher Scientific); MCL-1 and actin (Santa Cruz Biotechnology).
Immunohistochemistry and Immunofluorescence Analyses
For immunofluorescence staining in cell lines, cells were first plated on coverslips in 60-mm culture dishes. Forty-eight hours later, they were washed twice with PBS followed by incubation with 100% methanol for 15 minutes and 100% acetone for 2 minutes at -20°C. Once the fixation process was completed cells were washed three times with PBS, incubated with 1% Triton X-100 in PBS for 5 minutes, and blocked with 10% donkey serum (Invitrogen) in PBS for 30 minutes at room temperature before the treatment with 50 µL of the diluted primary antibodies for 2 hours at room temperature. The coverslips were then double stained by incubation with 50 µL of diluted (1:100) Alexa Fluor 594 anti-mouse and Alexa Fluor 488 anti-rabbit secondary antibodies (Invitrogen) for 1 hour. Coverslips were washed three times with PBS and mounted with Vectashield mounting medium containing DAPI (Vector). The following primary antibodies were used: FLAG (Cell Signaling), HA (Roche), S-100 (DAKO), and MITF (Thermo Fisher Scientific). For immunohistochemistry and immunofluorescence in tissues, FLAG (Cell Signaling), HA (Roche), GAB2 and VEGFR2 (Cell Signaling), CD34 (ABcam), and HIF-1α (R&D Systems) were used.
Assays for Anchorage Independence
For soft agar colony formation assay, 1×105 cells were resuspended in 0.35% agarose and overlaid onto 0.7% agarose in growth medium containing 10% FBS with TPA in 6-well plates. Fresh medium was added twice a week. Colonies were stained with thiazolyl blue tetrazolium bromide (Sigma) and counted at 2 and 4 weeks after plating.
For apoptosis assay in 3D collagen, collagen solution was first prepared by mixing rat tail collagen (BD Biosciences), PBS, dH2O and 1N NaOH followed by resuspension of 5×105 cells in collagen solution and overlaid onto a layer of polymerized collagen. Forty-eight hours later collagen gels were dissolved in 1mg/mL collagenase (Sigma), cells were washed with PBS and resuspended in 500 µL of binding buffer at a concentration of 1×106 cells/mL. Cells were then incubated with Annexin V-FITC and PI (Beckman Coulter), diluted in binding buffer and analyzed by flow cytometry.
VEGF immunoassay
VEGF levels were measured using the mouse VEGF immunoassay kit (R&D systems) according to manufacturer’s instructions. In the final step, 100 µL of the stop solution was added to each well and the optical density was determined within 30 minutes using a microplate reader set to 450 nm with a wavelength correction of 540 nm.
Real-time RT-PCR
Total RNA was isolated using RNeasy kit (Qiagen). cDNA was synthesized using RNA to cDNA EcoDry Premix (Oligo dT) (Clontech). SYBR Premix Ex Taq II (Tli RNaseH Plus) (Takara) was used for quantitative RT-PCR. Amplification and real-time analysis was performed with BioRad CFX96. Transcript levels were normalized to 18S subunit of rRNA levels. The relative mRNA levels were calculated according to the comparative CT (Δ ΔCT) method as described by the manufacturer.
Array Comparative Genomic Hybridization (a-CGH)
We performed aCGH analysis the mouse xenografts expressing NRASG12D or GAB2WT+NRASG12D using the SurePrint G3 1×1M whole genome mouse microarrays (Agilent Technologies). Segmentation was performed of the log2 ratios of CGH data using Circular Binary Segmentation, followed by smoothing smaller fluctuations (<400,000bp).
In Vivo Studies
Five-week-old female NCR/Nude mice were purchased from Taconic, Inc. Tumor growth was evaluated after subcutaneous injection of 1×106 melan-a melanocytes expressing vector, NRASG12D, or GAB2WT+NRASG12D into the flanks of mice (n=8 in each group). Mice were sacrificed when tumor surface area reached 1.5 cm2, tumors were harvested and examined histologically. For therapeutic inhibition experiments, xenografts were established in a similar manner, mice were treated with vehicle or inhibitor (PD325901, NVP-BEZ235, Bevacizumab, or Dasatinib) for two weeks and tumor volumes were evaluated. For experimental metastasis (lung seeding) assay, 5×106 melan-a melanocytes expressing vector, NRASG12D, or GAB2WT+NRASG12D were injected into mice via the tail veins (n=8 in each group). Mice were sacrificed 3 weeks after inoculation, lungs were harvested and examined histologically.
Statistical Analysis
Pearson’s chi-square test was used for clinical correlative studies. Student’s t test was used for soft agar colony formation assay, apoptosis assay in 3D collagen, VEGF immunoassay, in vivo xenograft assays and vascular density analysis.
Supplementary Material
Acknowledgements
This work has been supported by grants from the NIH/NCI RO1 CA138678 to JTC, the Dow Foundation to JTC and the Neuberger Dorothy Rodbell Cohen Foundation to JTC. We appreciate the technical assistance of Ruzhen Chen, Tasneem Fatima, Yan Lu, Rong Du, and Tingting Wu. We are grateful to the following Shared Resources of the Herbert Irving Comprehensive Cancer Center, Pathology Shared Resource, Flow Cytometry Shared Resource, and Confocal & Microscopy Shared Resource as well as to the Department of Dermatology’s Skin Diseases Research Center Cell Culture Core and Division of Dermatopathology.
Grant Support: This work has been supported by grants from the NIH/NCI RO1 CA138678 to JTC, the Dow Foundation to JTC and the Neuberger Dorothy Rodbell Cohen Foundation to JTC.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Gu H, Pratt JC, Burakoff SJ, Neel BG. Cloning of p97/Gab2, the major SHP2-binding protein in hematopoietic cells, reveals a novel pathway for cytokine-induced gene activation. Mol Cell. 1998;2:729–740. doi: 10.1016/s1097-2765(00)80288-9. [DOI] [PubMed] [Google Scholar]
- 2.Gu H, Neel BG. The"Gab" in signal transduction. Trends Cell Biol. 2003;13:122–130. doi: 10.1016/s0962-8924(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 3.Wohrle FU, Daly RJ, Brummer T. Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun Signal. 2009;7:22. doi: 10.1186/1478-811X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishida K, Hirano T. The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors. Cancer Sci. 2003;94:1029–1033. doi: 10.1111/j.1349-7006.2003.tb01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett HL, Brummer T, Jeanes A, Yap AS, Daly RJ. Gab2 and Src co-operate in human mammary epithelial cells to promote growth factor independence and disruption of acinar morphogenesis. Oncogene. 2008;27:2693–2704. doi: 10.1038/sj.onc.1210928. [DOI] [PubMed] [Google Scholar]
- 6.Sun J, Pedersen M, Ronnstrand L. Gab2 is involved in differential phosphoinositide 3-kinase signaling by two splice forms of c-Kit. J Biol Chem. 2008;283:27444–27451. doi: 10.1074/jbc.M709703200. [DOI] [PubMed] [Google Scholar]
- 7.Lock LS, Maroun CR, Naujokas MA, Park M. Distinct recruitment and function of Gab1 and Gab2 in Met receptor-mediated epithelial morphogenesis. Mol Biol Cell. 2002;13:2132–2146. doi: 10.1091/mbc.02-02-0031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao Y, Lee AW. A novel role for Gab2 in bFGF-mediated cell survival during retinoic acid-induced neuronal differentiation. J Cell Biol. 2005;170:305–316. doi: 10.1083/jcb.200505061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meng S, Chen Z, Munoz-Antonia T, Wu J. Participation of both Gab1 and Gab2 in the activation of the ERK/MAPK pathway by epidermal growth factor. Biochem J. 2005;391:143–151. doi: 10.1042/BJ20050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kameda H, Ishigami H, Suzuki M, Abe T, Takeuchi T. Imatinib mesylate inhibits proliferation of rheumatoid synovial fibroblast-like cells and phosphorylation of Gab adapter proteins activated by platelet-derived growth factor. Clin Exp Immunol. 2006;144:335–341. doi: 10.1111/j.1365-2249.2006.03067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Alessio A, Cerchia L, Amelio I, Incoronato M, Condorelli G, de Franciscis V. Shp2 in PC12 cells: NGF versus EGF signalling. Cell Signal. 2007;19:1193–1200. doi: 10.1016/j.cellsig.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Sattler M, Mohi MG, Pride YB, Quinnan LR, Malouf NA, Podar K, et al. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell. 2002;1:479–492. doi: 10.1016/s1535-6108(02)00074-0. [DOI] [PubMed] [Google Scholar]
- 13.Bentires-Alj M, Gil SG, Chan R, Wang ZC, Wang Y, Imanaka N, et al. A role for the scaffolding adapter GAB2 in breast cancer. Nat Med. 2006;12:114–121. doi: 10.1038/nm1341. [DOI] [PubMed] [Google Scholar]
- 14.Bocanegra M, Bergamaschi A, Kim YH, Miller MA, Rajput AB, Kao J, et al. Focal amplification and oncogene dependency of GAB2 in breast cancer. Oncogene. 2010;29:774–779. doi: 10.1038/onc.2009.364. [DOI] [PubMed] [Google Scholar]
- 15.Ke Y, Wu D, Princen F, Nguyen T, Pang Y, Lesperance J, et al. Role of Gab2 in mammary tumorigenesis and metastasis. Oncogene. 2007;26:4951–4960. doi: 10.1038/sj.onc.1210315. [DOI] [PubMed] [Google Scholar]
- 16.Brown LA, Kalloger SE, Miller MA, Shih Ie M, McKinney SE, Santos JL, et al. Amplification of 11q13 in ovarian carcinoma. Genes Chromosomes Cancer. 2008;47:481–489. doi: 10.1002/gcc.20549. [DOI] [PubMed] [Google Scholar]
- 17.Zatkova A, Schoch C, Speleman F, Poppe B, Mannhalter C, Fonatsch C, et al. GAB2 is a novel target of 11q amplification in AML/MDS. Genes Chromosomes Cancer. 2006;45:798–807. doi: 10.1002/gcc.20344. [DOI] [PubMed] [Google Scholar]
- 18.Horst B, Gruvberger-Saal SK, Hopkins BD, Bordone L, Yang Y, Chernoff KA, et al. Gab2-mediated signaling promotes melanoma metastasis. Am J Pathol. 2009;174:1524–1533. doi: 10.2353/ajpath.2009.080543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernoff K, Bordone L, Horst B, Simon K, Twaddell WS, Lee K, et al. Gab2 amplifications refine molecular classification of melanoma. Clin Cancer Res. 2009;15:4288–4291. doi: 10.1158/1078-0432.CCR-09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 21.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 22.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh P, Chin L. Genetics and genomics of melanoma. Expert Rev Dermatol. 2009;4:131. doi: 10.1586/edm.09.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 25.Petti C, Molla A, Vegetti C, Ferrone S, Anichini A, Sensi M. Coexpression of NRASQ61R and BRAFV600E in human melanoma cells activates senescence and increases susceptibility to cell-mediated cytotoxicity. Cancer Res. 2006;66:6503–6511. doi: 10.1158/0008-5472.CAN-05-4671. [DOI] [PubMed] [Google Scholar]
- 26.Bardeesy N, Bastian BC, Hezel A, Pinkel D, DePinho RA, Chin L. Dual inactivation of RB and p53 pathways in RAS-induced melanomas. Mol Cell Biol. 2001;21:2144–2153. doi: 10.1128/MCB.21.6.2144-2153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, et al. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackermann J, Frutschi M, Kaloulis K, McKee T, Trumpp A, Beermann F. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 2005;65:4005–4011. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- 29.Wellbrock C, Ogilvie L, Hedley D, Karasarides M, Martin J, Niculescu-Duvaz D, et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 2004;64:2338–2342. doi: 10.1158/0008-5472.can-03-3433. [DOI] [PubMed] [Google Scholar]
- 30.Chudnovsky Y, Adams AE, Robbins PB, Lin Q, Khavari PA. Use of human tissue to assess the oncogenic activity of melanoma-associated mutations. Nat Genet. 2005;37:745–749. doi: 10.1038/ng1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickson PV, Gershenwald JE. Staging and prognosis of cutaneous melanoma. Surg Oncol Clin N Am. 2011;20:1–17. doi: 10.1016/j.soc.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gershenwald JE, Soong SJ, Balch CM. 2010 TNM staging system for cutaneous melanoma… and beyond. Ann Surg Oncol. 2010;17:1475–1477. doi: 10.1245/s10434-010-0986-3. [DOI] [PubMed] [Google Scholar]
- 34.Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- 35.Monsel G, Ortonne N, Bagot M, Bensussan A, Dumaz N. c-Kit mutants require hypoxia-inducible factor 1alpha to transform melanocytes. Oncogene. 2010;29:227–236. doi: 10.1038/onc.2009.320. [DOI] [PubMed] [Google Scholar]
- 36.Mishra PJ, Ha L, Rieker J, Sviderskaya EV, Bennett DC, Oberst MD, et al. Dissection of RAS downstream pathways in melanomagenesis: a role for Ral in transformation. Oncogene. 2010;29:2449–2456. doi: 10.1038/onc.2009.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ha L, Ichikawa T, Anver M, Dickins R, Lowe S, Sharpless NE, et al. ARF functions as a melanoma tumor suppressor by inducing p53-independent senescence. Proc Natl Acad Sci U S A. 2007;104:10968–10973. doi: 10.1073/pnas.0611638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsatsanis C, Spandidos DA. Oncogenic kinase signaling in human neoplasms. Ann N Y Acad Sci. 2004;1028:168–175. doi: 10.1196/annals.1322.019. [DOI] [PubMed] [Google Scholar]
- 39.Milagre C, Dhomen N, Geyer FC, Hayward R, Lambros M, Reis-Filho JS, et al. A mouse model of melanoma driven by oncogenic KRAS. Cancer Res. 2010;70:5549–5557. doi: 10.1158/0008-5472.CAN-09-4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gysin S, Salt M, Young A, McCormick F. Therapeutic strategies for targeting ras proteins. Genes Cancer. 2011;2:359–372. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 42.Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 43.Dufraine J, Funahashi Y, Kitajewski J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene. 2008;27:5132–5137. doi: 10.1038/onc.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerhardt H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis. 2008;4:241–246. doi: 10.4161/org.4.4.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kranenburg O, Gebbink MF, Voest EE. Stimulation of angiogenesis by Ras proteins. Biochim Biophys Acta. 2004;1654:23–37. doi: 10.1016/j.bbcan.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, et al. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci U S A. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 49.Wozniak A. Challenges in the current antiangiogenic treatment paradigm for patients with non-small cell lung cancer. Crit Rev Oncol Hematol. 2011 doi: 10.1016/j.critrevonc.2011.05.003. epub ahead of print Jun 27. [DOI] [PubMed] [Google Scholar]
- 50.Dance M, Montagner A, Yart A, Masri B, Audigier Y, Perret B, et al. The adaptor protein Gab1 couples the stimulation of vascular endothelial growth factor receptor-2 to the activation of phosphoinositide 3-kinase. J Biol Chem. 2006;281:23285–23295. doi: 10.1074/jbc.M600987200. [DOI] [PubMed] [Google Scholar]
- 51.Caron C, Spring K, Laramee M, Chabot C, Cloutier M, Gu H, et al. Non-redundant roles of the Gab1 and Gab2 scaffolding adapters in VEGF-mediated signalling, migration, and survival of endothelial cells. Cell Signal. 2009;21:943–953. doi: 10.1016/j.cellsig.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 52.Laramee M, Chabot C, Cloutier M, Stenne R, Holgado-Madruga M, Wong AJ, et al. The scaffolding adapter Gab1 mediates vascular endothelial growth factor signaling and is required for endothelial cell migration and capillary formation. J Biol Chem. 2007;282:7758–7769. doi: 10.1074/jbc.M611327200. [DOI] [PubMed] [Google Scholar]
- 53.Lu Y, Xiong Y, Huo Y, Han J, Yang X, Zhang R, et al. Grb-2-associated binder 1 (Gab1) regulates postnatal ischemic and VEGF-induced angiogenesis through the protein kinase A-endothelial NOS pathway. Proc Natl Acad Sci U S A. 2011;108:2957–2962. doi: 10.1073/pnas.1009395108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berking C, Takemoto R, Satyamoorthy K, Shirakawa T, Eskandarpour M, Hansson J, et al. Induction of melanoma phenotypes in human skin by growth factors and ultraviolet B. Cancer Res. 2004;64:807–811. doi: 10.1158/0008-5472.can-03-3438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.