Abstract
Fluorescence microscopy enables detailed observation of the effects of the antimicrobial peptide Cecropin A on the outer membrane (OM) and cytoplasmic membrane (CM) of single E. coli cells with sub-second time resolution. Fluorescence from periplasmic GFP decays and cell growth halts when the OM is permeabilized. Fluorescence from the DNA stain Sytox Green rises when the CM is permeabilized and the stain enters the cytoplasm. The initial membrane disruptions are localized and stable. Septating cells are attacked earlier than non-septating cells, and curved membrane surfaces are attacked in preference to cylindrical surfaces. Below a threshold bulk Cecropin A concentration, permeabilization is not observed over 30 minutes. Above this threshold, we observe a lag time of several minutes between Cecropin A addition and OM permeabilization and ~30 s between OM and CM permeabilization. The long lag times and the existence of a threshold concentration for permeabilization suggest a nucleation mechanism. However, the roughly linear dependence of mean lag time on bulk peptide concentration is not easily reconciled with a nucleation step involving simultaneous insertion of multiple peptides into the bilayer. Monte Carlo simulations suggest that within seconds the OM permeability becomes comparable to that of a pore of 100-nm diameter, or of numerous small pores distributed over a similarly large area.
Introduction
Antimicrobial peptides (AMPs) are ubiquitous host defense agents essential to the immune system of a variety of organisms, from microbes to insects, plants, and animals.1 These peptides may prove effective in combating multi-drug resistant pathogens. Literally thousands of AMPs have been discovered in nature, and dozens of synthetic variants have been developed and tested for antibacterial efficacy. They vary in sequence length (up to 50 residues), net charge (often highly cationic), secondary structure (α-helix, β-hairpin), and the fraction, sequence, and composition of hydrophobic and hydrophilic residues.2, 3 The selectivity of attack on bacterial cells vs eukaryotic cells presumably arises from electrostatic attraction of the cationic peptides to the anionic outer layer of both Gram negative and Gram positive bacteria. While it is known that AMPs at sufficiently high concentration disrupt bacterial membranes, the detailed mechanism by which they halt growth and kill bacterial cells is not certain, and may well vary from case to case. A clear relationship between peptide sequence and function has not yet emerged. A balance between hydrophobic and hydrophilic resides is important, but amphipathicity is evidently not essential.2, 4 Experiments that mix bulk bacterial cultures with AMPs have revealed information about the timing of antibacterial action as well as biochemical mechanisms.3 Suggested mechanisms of AMP activity against bacterial cells include interference with cell wall biosynthesis, loss of key periplasmic or cytoplasmic components after permeabilization of membranes, and triggering of signaling pathways that alter the bacterial metabolic state.2
There is a long history of biophysical studies of the interaction of AMPs with model lipid bilayers, including large unilamellar vesicles (LUVs) and giant unilamellar vesicles (GUVs).2 Kinetics studies have monitored release of LUV content2, 5, 6 and the thinning and bursting of GUVs vs time after addition of the AMP.7–11 In some cases there is evidence of transient membrane disruption (“graded” content release), while in other cases permeability persists until all content is released (“all or none” release).12 Structural studies have used x-ray and neutron diffraction,13 oriented circular dichroism,14 and NMR15 to study AMPs bound to multilayers, LUVs, or micelles. Many AMPs fold into amphipathic helices on binding to lipid bilayers.16 At low surface coverage, helical AMPs bind with the long axis parallel to the membrane surface; the resulting tension leads to membrane thinning. 9 At higher surface coverage, helices insert into the bilayer with the axis perpendicular to the membrane surface.
The nature of the membrane disruptions induced by antimicrobial peptides has been controversial.2 One standard concept is the formation of discrete pores, either “barrel-stave” or “toroidal”. In lipid multilayers, x-ray diffraction provides direct evidence of pore-like structures at high peptide surface density.13, 14 However, recent molecular dynamics simulations argue against a well-defined geometry comprising a fixed number of AMPs.17, 18 Membrane disruptions are likely to be much more disordered and fluxional than depicted by typical cartoons of toroidal pores.19 A seemingly different concept is the “carpet” model and its variants, all of which involve detergent-like solubilization or micellization of lipids by the AMP.20, 21 Carpets could be localized or global. Recent structural studies found a correlation between the activity of antimicrobial oligomers and their ability to form a three-dimensional, inverted hexagonal phase in mixtures with lipids.22, 23 This highly curved phase is perhaps reminiscent of the micelle-like structures proposed in the carpet mechanism. In our view, the distinctions among pores, chaotic pores, localized carpets, and localized “hexagonal phase precursor states” are blurry.
Cecropin A is a well-studied AMP isolated from the moth Hyalophora cecropia.24 Its 37-amino acid sequence contains seven Lys, one Arg, one Glu, and one Asp for a net charge of +7 at neutral pH. (The N-terminus contributes +1, while the C-terminus is amidated.) On binding to lipid bilayers, it adopts a helix-break-helix motif, with amphipathic helical segments formed by residues 5–21 and 24–37. At low surface concentrations, both helical segments lie parallel to the lipid bilayer. 25, 26 Cecropin A induces content release from lipid vesicles.27 Both L- and D- optical isomers of Cecropin A show comparable ability to kill bacterial cells.28 Evidently the killing mechanism is related to interactions with bacterial membranes, not to binding to specific enzymatic targets.
The detailed nature of the membrane disruptions induced by Cecropin A is unclear. Early studies showed that Cecropin A releases encapsulated dye from liposomes and inhibits E. coli growth within 10 min of incubation.29 Cecropin A forms pore-like ion-channels on planar lipid bilayers.30 In liposomes, Cecropin A disrupts ion gradients at low concentrations, while much higher concentrations are required to release encapsulated probes.31 On the other hand, in bacterial cells Cecropin A exerts its bactericidal effect, disrupts membrane potential and permeabilizes membranes at the same concentration.32 Both “pore-like” and “carpet-like” models have been proposed to explain different experimental results.21, 27 The strength of the connection between studies of model membranes and the mechanisms by which AMPs disrupt real bacterial cell membranes remains to be seen.
Fluorescence microscopy of AMP interactions with single, live bacterial cells provides a completely new level of real-time mechanistic detail that is not discernible from bulk measurements.33, 34 Here we present a study of the interaction of Cecropin A with single E. coli cells in real time with sub-second resolution. Fast movies at 2 frames/s show that the initial OM permeabilization event occurs preferentially in specific membrane regions that depend on whether or not the cell is septating. The event is local and persistent. We directly observe a lag time of several minutes between injection of Cecropin A and disruption of the outer membrane (OM) and a second lag time of ~30 s between entry of Cecropin A into the periplasm and disruption of the cytoplasmic membrane (CM). Comparisons with Monte Carlo simulations indicate that the initial degree of permeabilization is large and expands over several seconds. While superficially similar in length, charge, and structure, LL-37 and Cecropin A show different propensities for which membrane locations are attacked and for the degree of permeability induced.
Experimental Procedures
Materials
Cecropin A (Anaspec, catalog no. 24010, > 95% purity) and LL-37 (Anaspec, catalog no. 61302, 95% purity) were purchased as lyophilized powders and used without further purification. All peptide stock solutions were made in sterile, ultrapure (18 MH) water. A 5 mM solution (in DMSO) of Sytox Green was purchased from Molecular Probes (S7020). Cell cultures were grown in EZ Rich Defined Medium (EZRDM),35 which consists of MOPS buffer (M2130, Teknova), nucleic acids (M2103, Teknova), amino acids (M2104, Teknova), glucose (2 mg/mL), K2HPO4 (1.32 mM) and NaCl (76 mM).
Bacterial strains and cell cultures
The E. coli strain is K12 (MG1655). For experiments monitoring periplasmic GFP, TorA-GFP was expressed from the plasmid pJW1 as previously described.36 TorA-GFP consists of a short sequence (43 residues) from trimethylamine N-oxide (TMAO) reductase that signals the twin-arginine translocase (TAT) pathway.37 The TorA signal sequence is cleaved from GFP in the periplasm. The 12-hr MIC of Cecropin A is 0.5 µM.
Cell cultures were grown overnight at 30°C to stationary phase. Sub-cultures were grown to exponential phase (optical density ~ 0.5 at 600 nm) and injected into the flow chamber for imaging. We select a region that has at least 10 cells lying flat within the 50 µm diameter observation region prior to initiation of image acquisition and injection of Cecropin A.
Flow chamber
The flow chamber has been described earlier.30 It consists of two silicone gaskets (Warner Instruments, catalog no. 64-0335, thickness 375 µm per gasket) sandwiched between two poly-L-Lysine-coated glass coverslips (Fisher Scientific). The chamber volume is ~72 KL. The base plate is maintained at 30°C by a TC-344B dual channel temperature controller and CC-28 heating cables attached to RH-2 heater blocks (all from Warner Instruments). Solutions are injected into the flow chamber by hand using 1 mL NORM-JECT injection syringes. Typically, ~400 KL of peptide solution is injected over the course of ~5 s. By imaging solutions of fluorescent dyes, we have shown that this volume and flow rate fill the chamber uniformly. After injection of the peptide solution, the observation volume remains static throughout the course of the experiment.
Fluorescence imaging
The flow chamber, microscopy protocol, and the minimum inhibitory concentration (MIC) assay employed in this work have been described earlier.33 We image Cecropin A-induced permeabilization of the outer membrane to GFP and permeabilization of the cytoplasmic membrane to the DNA the stain Sytox Green as follows. The E. coli cells export GFP to the periplasm.36 At t = 0, growth medium containing Cecropin A and also 5 nM Sytox Green is injected into the flow chamber over a period of ~5 s. Sytox Green in buffer is non-fluorescent, but it fluoresces green on binding to chromosomal DNA. On excitation at 488 nm, each single cell exhibits a thin shell of GFP fluorescence. When the outer membrane becomes permeabilized to GFP, the thin shell of fluorescence gradually disappears. In the absence of Cecropin A, we detect no Sytox Green staining of DNA under our conditions. When both the outer membrane and cytoplasmic membrane become permeabilized to Sytox Green, we observe the onset of fluorescence from Sytox Green in a nucleoid staining pattern. To observe localized loss of periplasmic GFP and nucleoid staining by Sytox Green, images were acquired at a frame rate of 2 frames/s with an exposure time of 50 ms/frame.
Cells were typically imaged for 10 min at 2 µM Cecropin A (four times the MIC) and 20 min at 1 µM (twice the MIC). The cost of the peptide precludes studies under flow conditions that would stabilize the bulk concentration. The stated Cecropin A concentrations are only nominal. The actual bulk concentration is likely smaller than that of the injected solution due to unquantified losses at the walls of the flow chamber.
To monitor cell length vs time (a proxy for growth) in the presence of Cecropin A, the cells were alternately excited by 488 nm and white light at a rate of 1 frame every 3 seconds with an exposure time of 50 ms/frame. The interleaved fluorescence and white light images were separated during image processing. An elliptical mask was made in MATLAB that matched the cell boundaries. The major axis length of this mask was measured to estimate the cell length vs time for correlation with events in the same cell signaled by changes in fluorescence intensity vs time.
Data Analysis
Images were acquired using Andor Solis (version 4.8.30002.0) and data analyses were performed in ImageJ (version 1.47a), Microcal Origin (version 9.0) and MATLAB (version 7.11.0). To measure the total fluorescence intensity in a single cell, a region of interest (ROI) enclosing the cell was drawn in ImageJ. Background signal was measured by duplicating the same ROI on a region of the image that did not contain a cell. The fluorescence intensity of the ROI corresponding to the cell was measured and background was subtracted. This process was repeated for each frame of the entire 10–20 min movie. All cells that remain in focus long enough for antimicrobial effects to be observed are included in the analysis. Plots of axial intensity projections are obtained as described in Supporting Information.
Results
Attack on Septating Cells
Late in the cell cycle, as E. coli prepares to divide into two daughter cells, the previously straight cylindrical flank of the cell begins to septate at the cell midplane. The parent cell will eventually pinch off two daughter cells, and the septal region will become two new hemispherical endcaps. The early stages of septation are directly observable in phase contrast images and in periplasmic GFP images as a slight depression in the previously straight cell body. In this study, we classify cells as “septating” or “non-septating” based on visual inspection of the phase contrast and periplasmic GFP images; compare Figs. 1 and 2. All cells labeled as septating are surely in the process of septation, but some cells in the very early stages of septation will be categorized as non-septating.
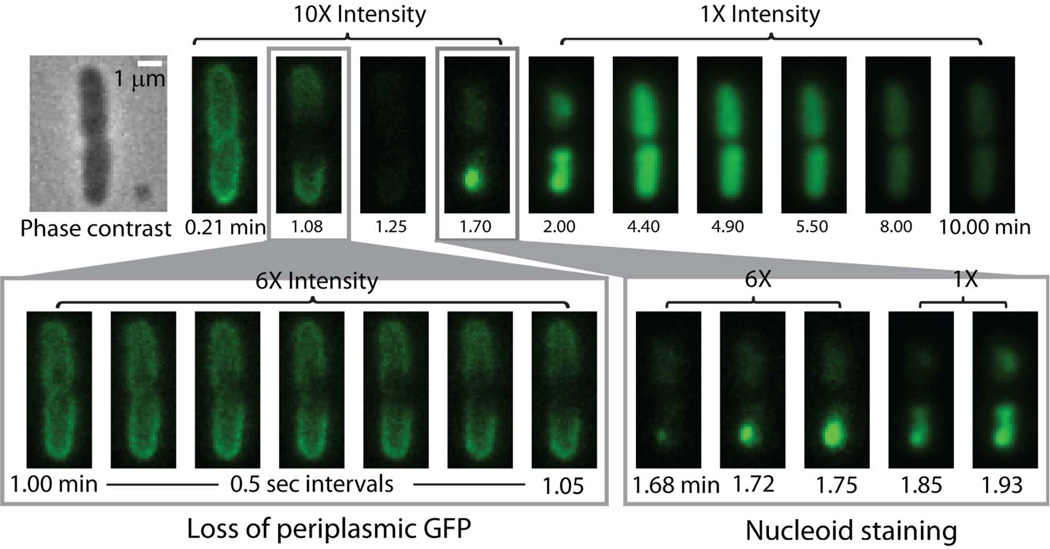
Figure 1.
Sequence of events for a septating cell at 2 µM nominal Cecropin A concentration. The overall sequence over 10 min is shown in the top row, while expanded views of GFP loss and Sytox Green entry are shown in the bottom row. Note left-right asymmetry in loss of GFP from septal region in first three frames after t = 1.00 min. Also note punctal entry of Sytox Green into the cytoplasm at t = 1.68 min.
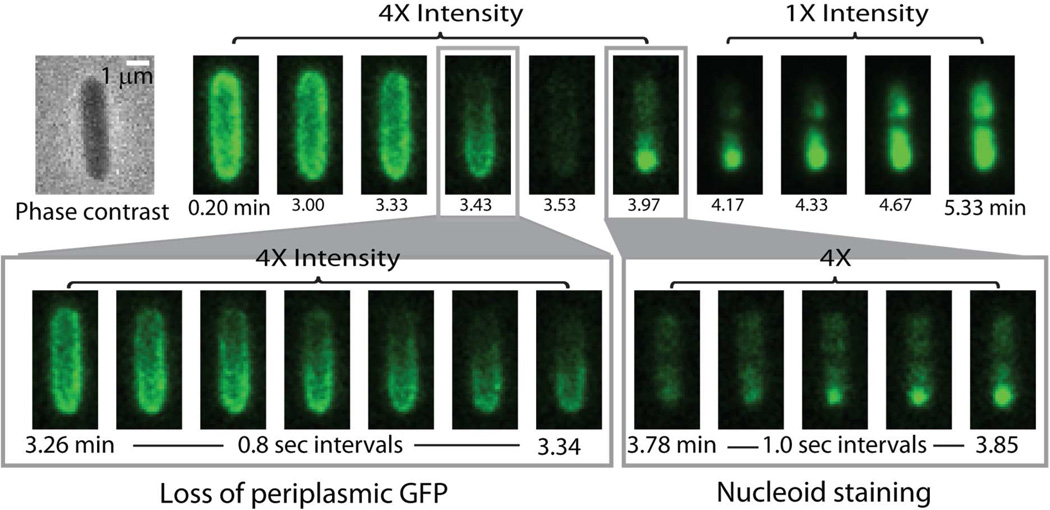
Figure 2.
Sequence of events for a non-septating cell at 2 µM nominal Cecropin A concentration. The overall sequence over 5 min is shown in the top row, while expanded views of GFP loss and Sytox Green entry are shown in the bottom row. Note loss of GFP from one endcap (2–4 frames after t = 3.26 min) and predominantly punctal initial staining by Sytox Green.
Cecropin A at nominal concentration of 2 µM, four times the MIC, attacks the membranes of septating cells earlier than non-septating cells, as described in detail below. The sequence of events in the antimicrobial action of Cecropin A at 2 µM on a septating cell is illustrated in Fig. 1. For this particular cell, there is a lag time of ~1 min after injection of Cecropin A during which no effects on GFP are observed. Cecropin A has permeabilized the OM at the nascent septum at t = 1.08 min after addition of the AMP, as shown by the dark septal region due to loss of periplasmic GFP fluorescence. Evidently the loss of periplasmic GFP to the surround is sufficiently rapid that diffusion cannot keep up.
The expanded time scale with images spaced by 0.5 s (Fig. 1) shows clearly that the initial loss of GFP causes a localized dark patch on one side of the septal region, clear evidence of radially asymmetric loss of GFP. Over 3–5 s, the dark patch spreads around the circumference of the septum, as shown by the images gradually becoming axially symmetric. Over 10–15 s, the dark region spreads axially outward towards the endcaps. The outward flux of GFP is evidently diffusion-limited. About 15 s after the onset of GFP loss (t = 1.25 min), the thin shell of GFP fluorescence has been lost entirely. All septating cells exhibit a central dark patch, but only a few septating cells exhibit the radially asymmetric dark patch. Some localized dark patches may occur near the top or bottom of the cell rather than on the side, and thus appear less asymmetric.
For the same cell, after an additional lag time of ~30 s Cecropin A permeabilizes the CM to Sytox Green, as evidenced by an increase in green fluorescence (t = 1.7 min). The expanded time scale shows that nucleoid staining by Sytox Green begins near one endcap as a bright dot, indicating localized entry into the cytoplasm. Evidently binding to DNA is sufficiently strong that the earliest Sytox Green molecules diffuse only a short distance within the nucleoid region before binding. Subsequent entry and binding of more Sytox Green causes the bright initial dot to spread. In this particular cell, the staining pattern suggests that a second localized disruption of the CM occurs in the other half of the cell beginning at t = 1.85 min. In many cases, only one bright dot is observed.
Attack on Non-septating Cells
When Cecropin A at nominal concentration of 2 µM attacks non-septating cells, permeabilization of the OM and loss of periplasmic GFP almost always begin near one endcap. The lag time is 2–8 min, much longer than for septating cells. As illustrated in Fig. 2, periplasmic GFP is lost from one end of the cell. For this particular cell, OM permeabilization occurs almost 4 min after injection of Cecropin A. The dark patch spreads across the entire cell periphery in ~15 s, as shown in the expanded view. In five examples, the non-septating cell under study had remained adhered to its sister cell prior to the Cecropin A attack, enabling us to distinguish the new pole from the old pole. The OM permeabilization always occurs at the new pole.
The subsequent permeabilization of the CM to Sytox Green occurs 32 s after the OM is permeabilized to GFP. CM permeabilization is again fairly localized in space, as evidenced by an initial bright dot of fluorescence that gradually spreads across both nucleoid lobes. The location of CM permeabilization varies from cell to cell, as described next.
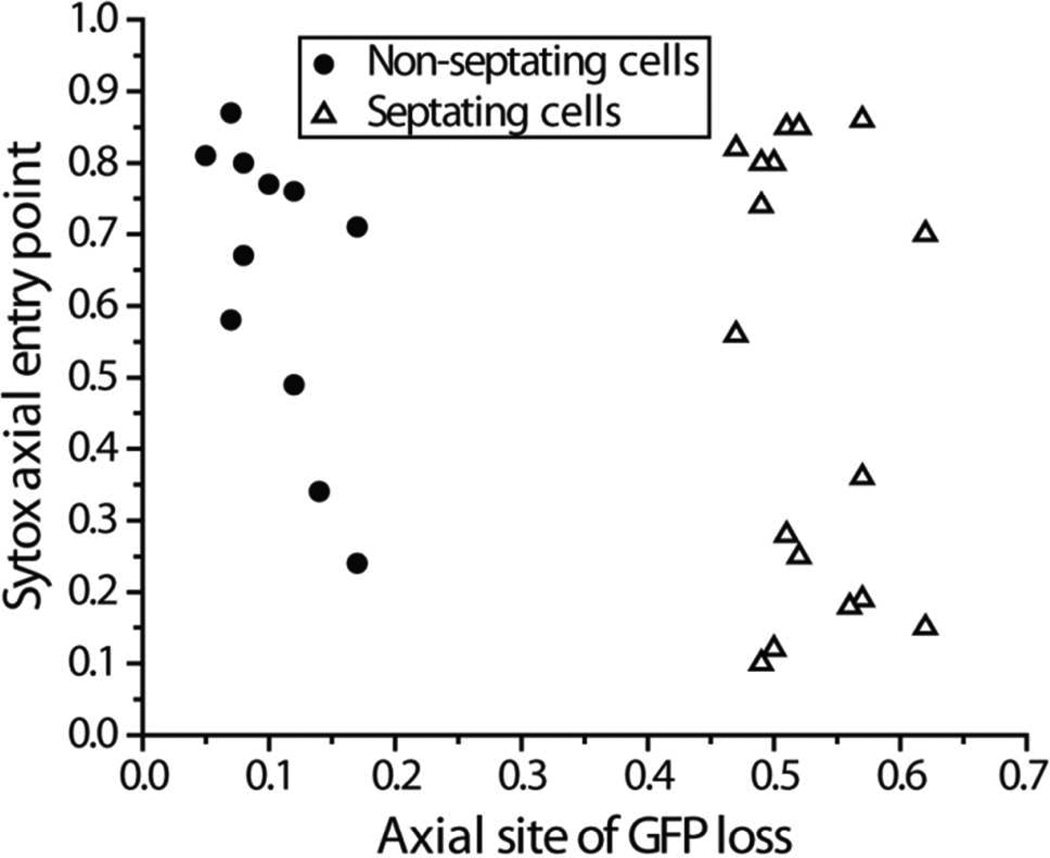
Distribution of Permeabilization Events Along the Cell Axis
In Fig. 3, the axial locations of each pair of OM and CM permeabilization events are plotted using a relative axial coordinate scale. Here xrel = 0 is defined as one tip of the cell and xrel = 1 is defined as the other tip. The septum lies near xrel = 0.5. In septating cells, xrel = 0 is chosen arbitrarily at one tip. In non-septating cells, xrel = 0 is defined as the tip near which GFP loss begins. Details regarding cell coordinates and determination of the values of xrel at which GFP leaves and Sytox Green enters are given in Supporting Information (Fig. S2).
Figure 3.
Correlation of axial locations of GFP loss vs Sytox Green entry, with axial location defined on a relative scale from 0 to 1 (tip-to-tip). For non-septating cells (circles), 0 is chosen at the end of the cell from which GFP leaves. Sytox Green tends to enter the cytoplasm at locations distant from the GFP exit point. For septating cells (triangles), choice of 0 is arbitrary. GFP leaves near the septum, while Sytox Green enters near one pole. See Supporting Information for details of measurements.
In visibly septating cells, GFP is always lost from the septal region, while Sytox Green tends to enter the cytoplasm near one endcap. This occurs in spite of the fact that the concentration of Cecropin A within the periplasm must initially be highest near the septum, where entry into the periplasm occurs. For non-septating cells, permeabilization of the OM to GFP occurs preferentially near one endcap (xrel ~ 0.1). The subsequent site for permeabilization of the CM to Sytox Green is broadly distributed along the cell axis, but tends to occur on the half of the cell distal to the OM permeabilization site. There is a preference for the opposite endcap.
Timing of Membrane Permeabilization Events and Cell Shrinkage Events vs Cecropin A concentration
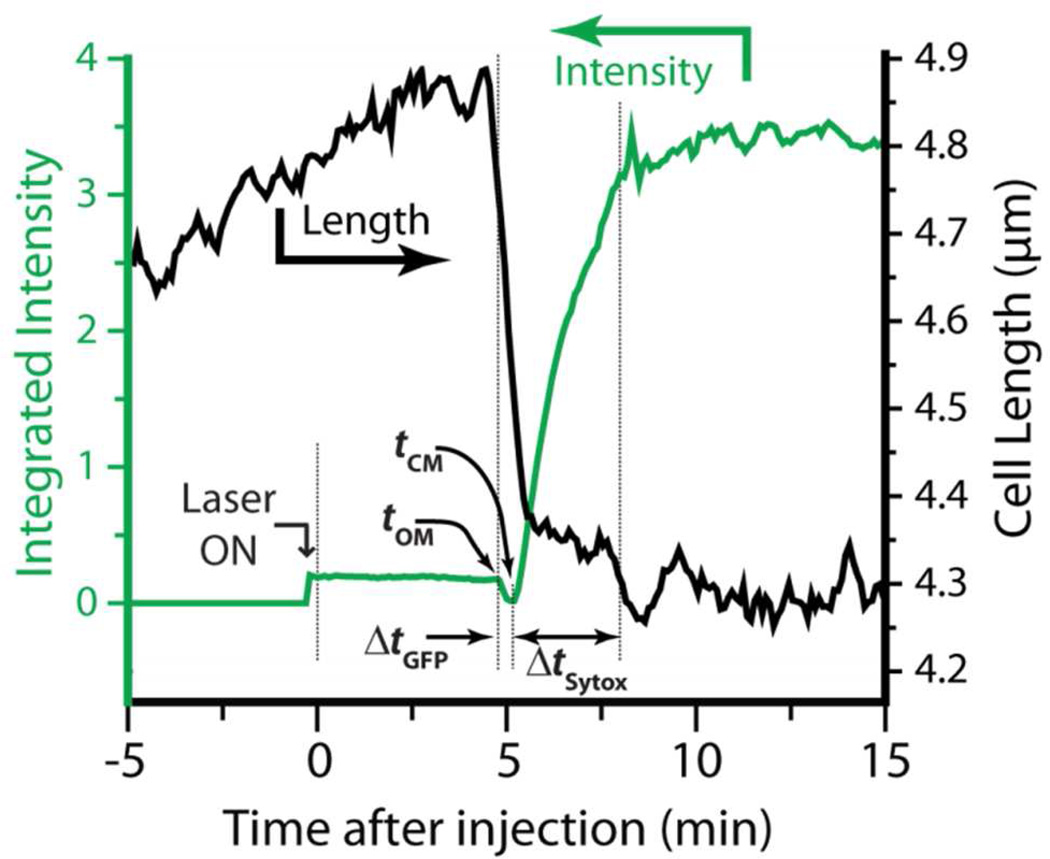
We have also measured the distribution of timings of specific membrane permeabilization events across cells and correlated these events with the halting of cell growth. An example of total green fluorescence intensity vs time for a single, non-septating cell, obtained at an acquisition rate of 1 frame every 6 s and corrected for background intensity, is shown in Fig. 4 (green trace). We define t = 0 as the time of injection of Cecropin A at nominal 2 µM concentration. As evidenced by the transverse intensity linescan (Figure S1), the green fluorescence intensity prior to addition of Cecropin A is due primarily to periplasmic GFP, with small contributions from cytoplasmic autofluorescence and from GFP that has not been exported to the periplasm. At tOM = 4.8 min, the outer membrane is permeabilized to GFP and the cell length shrinks abruptly. The cell loses all fluorescence intensity over the next 18 s. At tCM = 5.2 min, the CM is permeabilized to Sytox Green and the fluorescence abruptly rises, this time in a nucleoid-staining pattern. The Sytox Green signal plateaus when all binding sites are saturated and then slowly decreases due to photobleaching. Analogous measurements were carried out for Cecropin A at nominal concentrations of 1 µM and 4 µM (twice and eight times the MIC). Data from multiple experiments (four experiments at 1 µM, seven at 2 µM, and one at 4 µM, with each experiment involving 5–15 unique cells) was pooled to obtain cell-averaged results.
Figure 4.
Plot of green fluorescence intensity (green) and cell length (black) vs time for one non-septating cell at nominal Cecropin A concentration of 2 µM. Laser is turned on just prior to Cecropin A injection at t = 0. Lag times to onset of GFP loss from periplasm (tOM) and to Sytox Green entry into cytoplasm (tCM) are shown. Time intervals required for loss of GFP from periplasm (ΔtGFP) and entry of Sytox Green into cytoplasm (ΔtSytox) are also indicated.
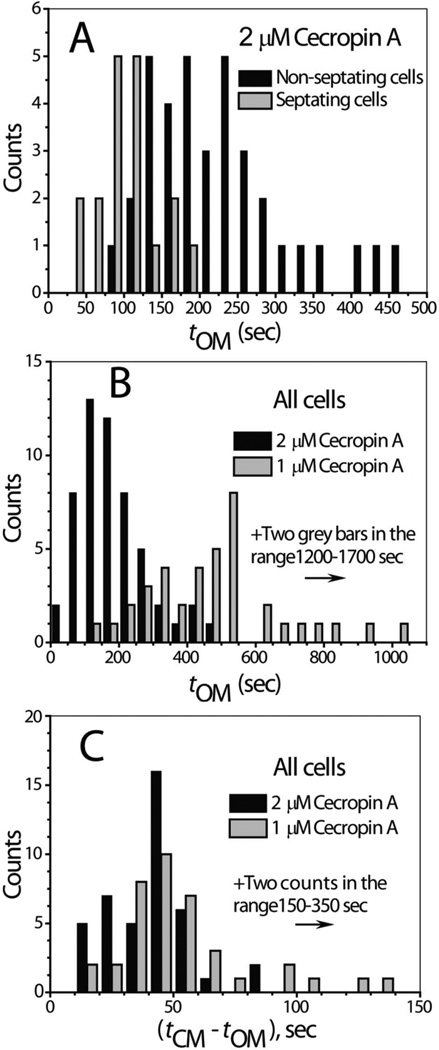
In Fig. 5A, we compare histograms of tOM for septating vs non-septating cells at 2 µM Cecropin A. The mean value < tOM> is 100 ± 39 s (± one standard deviation) for septating cells, compared with 225 ± 94 s for non-septating cells. Both distributions are broad, with standard deviations about half the mean. This ensemble data clearly indicates that the membranes of septating cells are permeabilized earlier than those of non-septating cells, as in the examples of Figs. 1 and 2.
Figure 5.
Histograms of timing events for onset of permeabilization of outer and cytoplasmic membranes. A: Comparison of distribution of tOM for septating vs non-septating cells at nominal 2 µM Cecropin A. B: Comparison of distribution of tOM for all cells at nominal 1 µM vs 2 µM Cecropin A. C: Comparison of distribution of (tCM – tOM) for all cells at nominal 1 µM vs 2 µM Cecropin A. Bin width is 25 s in Panel A, 50 s in Panel B, and 10 s in Panel C.
The bulk Cecropin A concentration significantly affects the distribution of tOM. In Fig. 5B we compare 1 µM and 2 µM in histograms including all cells, both septating and non-septating. At 1 µM, the distribution has a long tail. As the nominal concentration increases from 1 µM to 2 µM to 4 µM, the mean value < tOM> for all cells combined decreases from 540 ± 300 s to 183 ± 99 s to 108 ± 51 s. In Fig. 5C, we show a histogram of the lag time between OM permeabilization and CM permeabilization, (tCM – tOM), at 1 µM and 2 µM bulk Cecropin A. The two histograms are similar, except for several outliers at long delay times for the 1 µM data. The breadth of the distribution of tOM is quite similar to that of tCM (Figs. S3, S4, S5), while the breadth of the distribution of (tCM – tOM) is much smaller. Most of the variability in tCM is due to variability in tOM.
Table 1 summarizes < tOM>, < tCM>, and < tCM – tOM> for septating, non-septating, and all cells combined at all three Cecropin A concentrations. Additional histograms partitioning the data into septating and non-septating cells are included as Figs. S3 and S4.
Table 1.
Summary of mean timing data for OM and CM permeabilization of E. coli by Cecropin A.a
| Nominal Bulk Cecropin A Concentration | |||
|---|---|---|---|
| 1 µM | 2 µM | 4 µM | |
|
Ntot (All cells) |
43 | 54 | 7 |
| <tOM> | 540 ± 300 | 183 ± 99 | 108 ± 51 |
| <tCM> | 601 ± 310 | 222 ± 97 | 129 ± 49 |
| <tCM – tOM> | 64 ± 53 | 39 ± 17 | 21 ± 9 |
| <ΔtGFP> | 22 ± 21 (N = 36) | 15 ± 10 (N = 52) | 15 ± 6 |
| <ΔtSytox> | 98 ± 47 | 92 ± 44 | 117 ± 47 |
|
Nsept (septating) |
13 | 18 | 3 |
| <tOM> | 350 ± 120 | 100 ± 39 | 62 ± 9 |
| <tCM> | 410 ± 140 | 147 ± 45 | 84 ± 6 |
| <tCM – tOM> | 63 ± 39 | 47 ± 14 | 22 ± 9 |
| <ΔtGFP> | 23 ± 12 (N = 12) | 17 ± 8 | 18 ± 6 |
| <ΔtSytox> | 85 ± 31 | 71 ± 23 | 78 ± 12 |
|
Nnon-sept (non-septating) |
30 | 36 | 4 |
| <tOM> | 620 ± 320 | 225 ± 94 | 143 ± 38 |
| <tCM> | 680 ± 330 | 259 ± 95 | 164 ± 34 |
| <tCM – tOM> | 65 ± 59 | 34 ± 17 | 21 ± 9 |
| <ΔtGFP> | 22 ± 25 (N = 24) | 14 ± 11 (N = 34) | 15 ± 6 |
| <ΔtSytox> | 104 ± 52 (N = 29) | 103 ± 49 | 117 ± 47 |
All times in seconds. The ± values are one standard deviation of single measurements. N values give the number of cells in calculation of each mean.
Finally, we measured cell length vs time from the interspersed phase contrast images, enabling us to correlate the apparent halting of cell growth with the membrane permeabilization events. An example for a septating cell at 2 µM Cecropin A is included in Fig. 4 (black trace). Prior to injection of Cecropin A, cell length gradually increases with time. Cell growth halts abruptly at t = 4.7 min, some 6 s before the onset of GFP loss, and the cell gradually shrinks in length by 12% over the next 40 s. This is the general pattern for both septating and non-septating cells at all three concentrations studied. After shrinkage, cell length levels off and does not recover. Significantly, permeabilization of the CM to Sytox Green (and presumably loss of the transmembrane potential) consistently occurs about 30 s later than the start of cell shrinkage. We suggest that the abrupt shrinkage is an osmotic effect that occurs at the same time as entry of Cecropin A into the periplasm, but the detailed mechanism is unclear. Similar abrupt shrinkage events were observed for LL-37 on E. coli and on B. subtilis in earlier work.33, 34
There is mild evidence that Cecropin A at 2 µM sometimes decreases the cell growth rate shortly after injection and well before the OM permeabilization event. In Fig. S6, the plot of length vs time is curving upward prior to injection of Cecropin A but the curvature decreases immediately after injection. This decrease in the rate of growth is evident in 4 of 6 cases examined closely. Perhaps a stress signal transmitted through both membranes has slowed growth before Cecropin A permeabilizes the OM.38 Alternatively, some Cecropin A molecules may translocate across the OM and affect cell wall growth prior to forming GFP-sized disruptions. A similar slowing of growth of B. subtilis was observed on injection of sub-lethal concentrations of LL-37.34
Time Scale of GFP Release and of Sytox Green Entry
We can infer something of the nature and magnitude of the membrane disruption from the time course of the release of GFP from the periplasm (ΔtGFP, Fig. 4) and the entry of Sytox Green into the cytoplasm (ΔtSytox). Because each curve may have a different shape, we define ΔtGFP as the time required for the GFP signal to decay from 90% to 10% of its initial value and ΔtSytox as the time required for the Sytox Green signal to rise from 10% to 90% of its final value. Histograms of the distribution of these values are given in Figs. S3, S4, and S5. Means and standard deviations are provided in Table 1 for the three Cecropin A concentrations studied. The values of ΔtGFP will constrain Monte Carlo simulations of GFP loss, as described below.
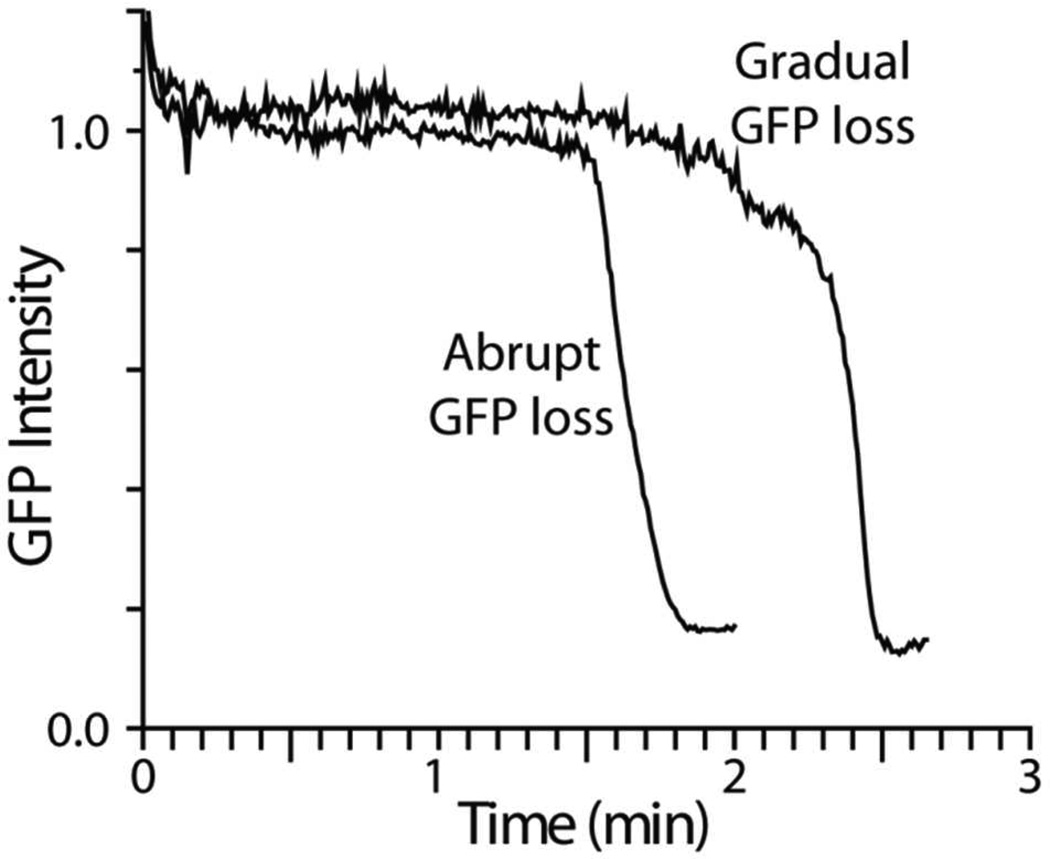
The rate of release of GFP shows distinct behavior for different cells, as shown in Fig. 6 for two septating cells at 2 µM Cecropin A. In most cells, the GFP loss is quite abrupt, while in a few cells the loss is initially gradual. The more gradual release events seem somewhat more likely for septating cells (4 out of 13 examples) than for non-septating cells (2 out of 15). In cells that exhibit the more gradual GFP loss, the dark patch occurs first on one half of the cell, but it is more delocalized and less sharply delineated at early times. The slower the GFP loss, the more effectively diffusion competes with efflux. The Monte Carlo simulations described next provide quantitative insights into the magnitude of membrane permeability and how it changes in time after the initial disruption event.
Figure 6.
Expanded view of loss of periplasmic GFP for two cells, both septating, at nominal Cecropin A concentration of 2 µM. Most cells exhibit the abrupt loss behavior; see text.
Simulations of GFP Loss from the Periplasmic Space
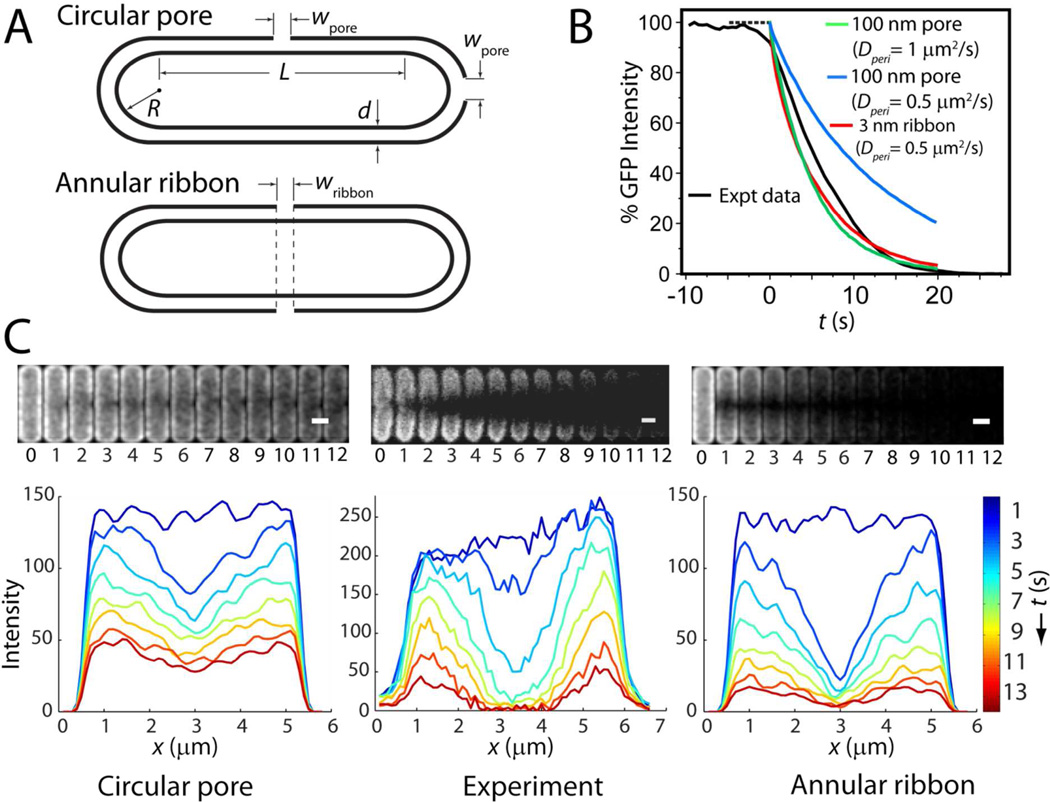
The spatial and temporal course of GFP release from the periplasm is governed by the diffusion coefficient of GFP and the size, location, and permeability of the OM disruption. We have carried out Monte Carlo (random walk) simulations of diffusion to an absorbing patch on the outer membrane in a model geometry chosen to mimic the periplasm: a thin space between two nested spherocylinders (Fig. 7A). The GFP population is modeled as 20,000 point particles, initially randomly distributed in the periplasmic space. The permeable region of the outer membrane is modeled as a perfectly absorbing patch.39 A particle is removed from the simulation whenever it crosses the absorbing surface patch. No “re-crossings” of the surface patch are permitted, meaning that the model yields the fastest possible leakage of GFP through a hole in the OM. In comparison, both a pore of finite thickness and a carpet patch would transmit GFP less efficiently. Parameters of the simulation include the GFP diffusion coefficient Dperi, the periplasm thickness d, and the geometry and location of the absorbing patch. Details are given in Supporting Information.
Figure 7.
Monte Carlo simulations of GFP loss from periplasm. A: Model geometry for circular pore (both septal and endcap) and for annular ribbon. B: Comparison of GFP loss from one septating cell (Expt data) with different models as shown. C: Two-dimensional experimental images and axial profiles from the same cell as in B (center) compared with models. At left is 100 nm pore with Dperi = 0.5 µm2-s−1; at right is 3 nm annular ribbon with the same Dperi = 0.5 µm2-s−1. The model at left fits the data reasonably well at short times, but does not decay sufficiently over 10 s. The model at right decays too fast at short times but fits the data reasonably well at longer times.
For septating cells, we experimented with circular absorbing patches of varying diameter placed at the septum and with an absorbing annular ribbon that circumscribes the entire cylinder (Fig. 7A). The diameter of the circular patch (wpore) and the width of the ribbon (wribbon) were varied along with the value of Dperi (Figs. S8 and S9). For non-septating cells, we use a circular patch centered at one cell tip (Fig. 7A). In each simulation, the size and shape of the absorbing patch is held constant in time. To simulate fluorescence images, the particle positions are first blurred by the diffraction limit. Simulated images in space and time can then be projected onto two dimensions (as in Fig. 7C, mimicking the microscopy experiments of Figs. 1 and 2) or integrated over the short cell axis to produce simulated axial intensity profiles (as in Fig. 7C). They can also be integrated over space to yield the time course of total GFP leakage and ΔtGFP (as in Figs. 4 and 7B and in Table 1). For both septating and non-septating cells, our simulations indicate that the permeable region must already be large within ~2 s and must grow even larger over the subsequent 5–10 s.
In Fig. 7C we compare the simulation results with the data from the same septating cell shown in Fig. 1. The simulations match experiment best when Dperi is chosen in the narrow range 0.5–1.0 µm2-s−1. For smaller diffusion coefficients ΔtGFP becomes too long, and for larger diffusion coefficients the observed spatial hole never becomes deep enough due to fast equilibration of the GFP distribution. Remarkably, the simulations show that in order to absorb GFP on the experimental time scale of ΔtGFP ~ 10–20 s (Fig. 7B), a single circular patch must have a very large diameter, ~100–200 nm. An absorbing ribbon at the septal region also matches the time scale well with Dperi = 0.5 µm2-s−1, independent of the value of wribbon (Fig. S8). Models tuned to match ΔtGFP decay more rapidly than experiment at t = 0 but less rapidly than experiment at later times. The model thus suggests that the OM permeability is increasing in time over the first several seconds, even in cases of nominally abrupt GFP loss curves (Fig. 6).
In addition, neither a static circular patch nor a static annular ribbon mimics the time-dependent spatial distribution well (Fig. 7C). The circular patch mimics the radial asymmetry at early times, but the asymmetry persists too long and the depth of the axial hole remains too shallow. The static ribbon cannot explain the radial asymmetry, but it captures the longer-term depth of the axial hole much better than the circular patch. We have not carried out simulations with time-evolving permeable patches. However, for the septating cell in Fig. 1 it seems likely that the observations could be well matched by a large, localized initial patch that evolved over the first few seconds into an annular ribbon.
For non-septating cells, the experimental results show <ΔtGFP> ~ 10–20 s. Again with Dperi = 0.5–1.0 µm2-s−1 and a circular patch at the tip of the cell of diameter as large as 100–200 nm, the simulation can match the experimental GFP loss times and approximate the time-dependent GFP spatial distribution (Fig. S10). We discuss possible interpretations of such large permeable patches below.
Simulations of Sytox Green entry into the cytoplasm would depend on the permeability of both OM and CM to Sytox Green as well as the effective binding strength of Sytox Green to DNA and its diffusion coefficient within the cytoplasm. Such a large parameter space is not well constrained by the present data. The fact that ΔtSytox is consistently larger than ΔtGFP does not necessarily indicate that the disruptions of the cytoplasmic membrane are less permeable than those of the outer membrane. The nucleoid binding capacity is large; it may absorb a very large number of Sytox Green molecules on the way to the maximum of the green fluorescence signal.
Comparison Study of LL-37
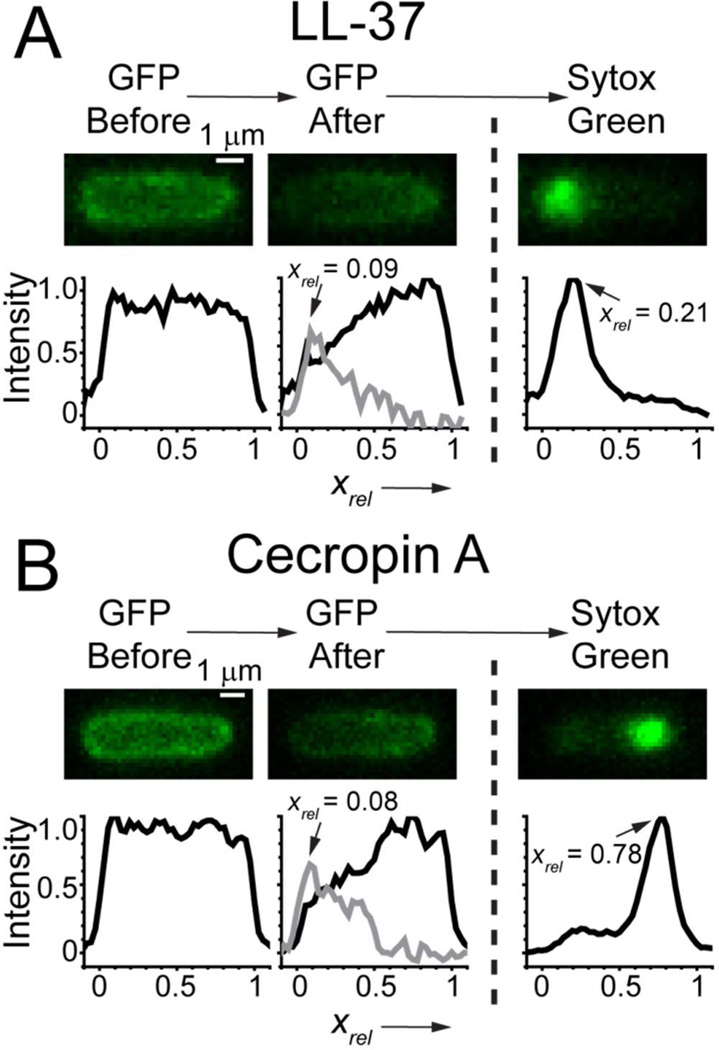
The length and charge of LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-NH2) and Cecropin A (KWKLFKKIEKVGQNIRDGIIKAGPAVAVVGQATQIAK-NH2) are similar,40 so it is of interest to compare their effects on E. coli in some detail. Our previous study of LL-37 focused primarily on Rhodamine-labeled LL-37. Using the fast image acquisition scheme introduced here, we have studied membrane permeabilization events for unlabeled LL-37 for comparison with Cecropin A. The 12-hour MIC for LL-37 is 1 µM. The timing results for permeabilization events and for the loss of GFP and the rise of Sytox Green signal are summarized in Table S1. As for Cecropin A, the LL-37-induced disruption of both OM and CM is localized, and there is a substantial lag time before each step. However, there are both qualitative and quantitative differences between LL-37 and Cecropin A. Like Cecropin A, LL-37 attacks septating cells earlier and disrupts the OM at the septal region, leading to complete loss of GFP. However, LL-37 then also disrupts the CM at the septal region (Fig. S11). In contrast, Cecropin A tends to attack the CM at one endcap. For non-septating cells, LL-37 locally disrupts the OM at one endcap (like Cecropin A) and then locally disrupts the CM at the same endcap. In contrast, for Cecropin A the location of the second event seems polarized towards the opposite end of the cell (Fig. 3). These differences in mode of attack are illustrated in Fig. 8 for a typical non-septating case and in Fig. S11 for a typical sepatating example.
Figure 8.
Comparison of the typical attack mode of (A) LL-37 and (B) Cecropin A on nonseptating cells. Both attack the OM at one endcap; LL-37 attacks the CM at the same endcap but Cecropin A attacks the CM at the opposite endcap. For (A) and (B), the two images and axial linescans at left were obtained just before and just after OM permeabilization to GFP. Subtraction of the two linescans makes the grey difference plot, whose maximum determines xrel for the OM permeabilization event. The image and linescan at right shows the initial localized Sytox Green signal. We determine xrel for CM permeabilization from the peak position of the axial linescan. See also Supporting Information (Fig. S2).
Comparisons of timing events between LL-37 and Cecropin A should be made with caution. The most appropriate comparison would be carried out at equal adsorbed surface concentration of the two peptides. However, in this work with unlabeled peptides, we can only compare the action of the two peptides at equivalent bulk concentrations relative to the MIC. Bulk peptide concentrations are only nominal due to unquantifiable losses of AMP at the surfaces of the chamber. Nevertheless, LL-37 at nominal bulk concentration of 4 µM (four times the MIC) takes much longer to disrupt the OM than Cecropin A at nominal bulk concentration of 2 µM (again four times the MIC). For all cells combined, <tOM> = 670 ± 540 s for LL-37 vs 180 ± 100 s for Cecropin A. The same holds for the lag time between disruption of the OM to GFP and disruption of the CM to Sytox Green (<tCM – tOM> = 320 ± 280 s for LL-37 vs 39 ± 17 s for Cecropin A). Surprisingly, although LL-37 preferentially attacks septating cells first, the time lag <tCM – tOM> is fourfold longer for septating cells vs non-septating cells.
Alternatively, we can compare LL-37 at 8 µM with Cecropin A at 2 µM, so that the lag times <tOM> become comparable for the two AMPs (220 ± 170 s vs 180 ± 100 s). Averaged over all cells, the second lag time <tCM – tOM> remains substantially longer for LL-37 (130 ± 150 s vs 39 ± 17 s), and again the difference is dominated by the long lag times for LL-37 to disrupt the CM of septating cells. Perhaps LL-37 binds to elements within the periplasm more strongly than Cecropin A, and is thus less available for attack on the CM.
As evidenced by ΔtGFP, the degree of OM permeability induced by LL-37 is smaller than that produced by Cecropin A. Again comparing 8 µM LL-37 with 2 µM Cecropin A (to make <tOM> comparable), <ΔtGFP> over all cells is 60 ± 64 s for LL-37 vs 15 ± 10 s for Cecropin A, a factor of four slower. Comparing 4 µM LL-37 with 1 µM Cecropin A, GFP loss is a factor of six slower for LL-37. In contrast, <ΔtSytox> is comparable for LL-37 and Cecropin A.
In summary, at comparable concentration relative to the MIC, LL-37 permeabilizes the OM and CM much slower than Cecropin A. In salty solution like growth medium, LL-37 folds into helical bundles and forms oligomers, 41 while Cecropin A exists primarily as monomers. 26 The slow kinetics of LL-37 mediated attack on E. coli could be related to the stability of oligomeric bundles within the lipopolysaccharide layer.
Discussion
Localized, Persistent Membrane Disruption Events
For the action of Cecropin A on both the outer and cytoplasmic membranes of E. coli, we find clear evidence of localized membrane disruption events that persist and are stable in time. In some studies of content release from LUVs, membrane disruptions evidently heal over time (graded release kinetics).2, 27 This may be due to the ability of the LUV to equilibrate the AMP concentration and relieve differential surface pressure across the two leaflets before all content is released. However, experiments on single GUVs have not revealed membrane disruptions that heal over time.7–11 Such a healing mechanism is presumably not available for the OM of E. coli. Cecropin A molecules that translocate into the periplasm may bind to periplasmic components such as the peptidoglycan layer or to the outer leaflet of the cytoplasmic membrane, thus maintaining differential AMP concentration between the outer and inner leaflets of the OM.
Localized permeabilization of the OM of E. coli by Cecropin A and LL-37 is inferred from the initial radial asymmetry of the dark patch that gradually symmetrizes and spreads axially as well (Fig. 1). Comparison of the time evolution of the shape of the axial intensity distribution with Monte Carlo simulations indicates that the permeability of the membrane disruption gradually increases over several seconds (Fig. 7B, C). Complete leakage of the periplasmic GFP to the surrounding medium and subsequent entry of Sytox Green across the OM demonstrates that the OM disruption remains for at least several minutes.
For the cytoplasmic membrane, localized disruption is inferred from the small initial spot of Sytox Green fluorescence, which then slowly spreads across the nucleoids. The CM remains permeable to Sytox Green for at least ~2 min, the time scale of Sytox Green staining of the nucleoid, and probably for much longer. The earliest discernible images of Sytox Green are not diffraction limited. For Cecropin A and LL-37 on E. coli, we estimate the initial full width at half-maximum intensity (FWHM) to be 330–440 nm, significantly larger than the diffraction limit of 200 nm FWHM at the emission wavelength of 540 nm. The same was true for LL-37 on B. subtilis.34 The breadth of the initial Sytox Green spots may be due in part to the breadth of the initial membrane disruption, consistent with the inferences from the Monte Carlo simulations. However, the structure of the cytoplasm also plays a role. Under these growth conditions, E. coli shows strong segregation of the two nucleoid lobes from three interspersed ribosome-rich regions (cell center and two endcaps).42 When Sytox Green enters the cytoplasm at either the septal region or an endcap, it must first diffuse through a ribosome-rich region before encountering dense DNA. Some spreading during this transit will inevitably occur.
Our data do not enable us to classify the observed membrane disruptions as pores or carpets. Neither our observations nor the Monte Carlo models can distinguish a well-defined pore14 from a chaotic pore19 from a localized “carpet patch”21 from a leaky hexagonal phase analogue.22 The simulations of GFP efflux from the periplasmic space demonstrate that within a few seconds, the outer membrane acquires permeability comparable to that of a single pore of ~100 nm diameter. A toroidal pore can in principle expand to any size; in fact, micron-sized, AMP-induced pores were recently observed by AFM in a planar lipid bilayer.43 However, we expect that rapid formation of such a large pore in the OM would lead to blebbing of the cytoplasm, which is not observed. The GFP loss data are equally consistent with the abrupt formation of many smaller pores in a ~100 nm diameter region of space. As is well known in the theory of diffusion to absorbing patches,39 a sparse set of small absorbers will capture molecules almost as rapidly as a completely absorbing patch covering the same area. In a pore-like model, the increasing OM permeability over several seconds inferred from comparisons with the Monte Carlo simulations would be due to a rapid increase in the number of pores and perhaps also in the area they cover. The permeable region may evolve from a localized patch to a ribbon surrounding the septal region, as suggested in the examples in Figs. 1 and 7B, C. The GFP release data are also consistent with formation of a localized, highly permeable “carpet patch” of at least 100 nm diameter that subsequently spreads.
Formation of the initial membrane disruption takes minutes, but the subsequent enhancement of the permeability occurs over only a few seconds. This suggests that successful nucleation of the initial localized permeabilization site lowers the free energy barrier to formation of a larger permeabilization region, a kind of “cooperativity of permeabilization”.
Preferential Attack at Curved Membrane Surfaces
Both Cecropin A and LL-37 attack septating cells earlier than non-septating cells. For septating cells, both AMPs always permeabilize the OM in the septal region. This is the region of active biosynthesis of curved cell wall, including the lipopolysaccharide layer, underlying membrane, and the peptidoglycan layer. For non-septating cells, both Cecropin A and LL-37 permeabilize the OM at one endcap, where no cell wall synthesis is occurring. Intriguingly, in the five cells observed for sufficient time, Cecropin A permeabilizes the new pole (the site of the most recent septation) of non-septating cells in preference to the old pole. The septal region of septating cells and the new pole of non-septating cells both contain elements of the divisome machinery. Labeling studies have shown that in newborn cells, the poles and division septum are enriched in anionic phospholipids such as cardiolipin.44 The septal region is also the site of initiation of DNA replication by DnaA.45 It is possible that a cardiolipin-rich domain at the septum recruits the DNA replication machinery, and subsequent tly, the early cell division proteins such as FtsZ, FtsA and ZipA.46 Following cell division, cardiolipin might persist at the new pole before eventually migrating to the next division site. Cardiolipin is known to be present on the outer leaflet of the OM. Our data suggest that the special composition of the curved outer membrane at a septum or a new pole somehow facilitates permeabilization by Cecropin A. In a pore model of membrane disruption, the positively curved cardiolipin might alleviate strain at the curved edge of a toroidal pore.47 It might also be useful in nucleating a highly curved hexagonal-like phase.
The axial distribution of OM and CM permeabilization events is quite different for LL-37 and Cecropin A (Figs. 8 and S11). Evidently peptide sequence strongly affects the point of attack. For septating cells, LL-37 typically permeabilizes the CM at the septal region, the same location as the OM permeabilization. For non-septating cells, LL-37 permeabilizes both the OM and CM near the same end cap. In contrast, Cecropin A permeabilizes the CM far from the initial entry point into the periplasm (Fig. 3, 8, S11). The explanation is not obvious. While the length and net charge of the two peptides are similar, the distribution of charges along the sequence is quite different. In LL-37, positive and negative charges are widely distributed, while in Cecropin A the helix nearest to the N-terminus (residues 8–21) contains most of the charges and the other helix (residues 25–37) is mainly hydrophobic.
Kinetics of Membrane Disruption
This study of unlabeled Cecropin A lacks key ingredients necessary to build a quantitative kinetics model of membrane disruption, so the discussion that follows is necessarily speculative. Without flowing Cecropin A through the observation chamber, the bulk concentration remains ill-defined. More importantly, we lack information about the time-dependent concentration of Cecropin A at the cell surface, within the lipopolysaccharide (LPS)/outer membrane layer. Finally, we do not know the diffusion coefficient of Cecropin A within the LPS layer.
Nevertheless, the present results allow us to infer several qualitative features of the membrane disruption mechanism. Disruption of the OM by Cecropin A is a rare event that occurs only after a mean lag time of 2–9 min. A possibly analogous lag time has been observed in kinetics measurements of the disruption of GUVs by various AMPs.10, 11, 16 Above a threshold bulk Cecropin A concentration, the mean lag time <tOM> to OM permeabilization decreases with increasing bulk concentration, but only roughly linearly (Table 1). The mean lag time <(tOM – tCM)> between OM permeabilization and CM permeabilization is also weakly dependent on bulk Cecropin A concentration and shows no threshold. This suggests that any Cecropin A concentration sufficiently high to permeabilize the OM quickly creates a periplasmic Cecropin A concentration that lies well above the threshold for permeabilization of the CM.
The long lag time to OM permeabilization might arise from slow uptake of AMPs by the LPS layer. However, our earlier study of Rhodamine-labeled LL-3733 and unpublished data on Rhodamine-labeled Cecropin A indicate that for both septating and non-septating cells the AMP equilibrates between the lipopolysaccharide layer (LPS) and the bulk in less than 1 min. Disruption of the OM typically occurs only after a much longer lag time. Evidently the lag time to OM permeabilization is not primarily due to slow accumulation of Cecropin A within the LPS layer. Alternatively, slow diffusion within the LPS layer might severely limit the ability of Cecropin A to reach the membrane and disrupt it. Future studies using fluorescently labeled Cecropin A will provide quantitative data on the rate of equilibration of the bulk and surface-bound populations and also the diffusion constant of Cecropin A within the LPS layer.
The long lag times to OM and CM permeabilization combined with the existence of a threshold concentration for OM permeabilization suggest that membrane disruption involves a nucleation step that is rare and difficult due to a large free energy barrier. Suppose nucleation of a pore-like structure involved cooperative, simultaneous insertion of multiple Cecropin A monomers into the bilayer. Then classical nucleation theory predicts that the mean lag time to formation of a multi-peptide disruption from monomers scales as [M]n, where [M] is the surface concentration of monomer and n is the number of monomers in the critical nucleus configuration.48 Yet the observed mean lag time to OM permeabilization decreases only roughly linearly with bulk Cecropin A concentration, not as some higher power as suggested by nucleation theory. A remarkably similar combination of observations was made for Bax-mediated pore formation in isolated mitochondrial outer membranes.49 The lag time to membrane disruption was highly variable, but the mean rate of pore formation depended only linearly on the Bax concentration.
It is possible that for Cecropin A, the threshold surface concentration for pore formation in the OM is not much smaller than the saturation limit for binding of peptide to the LPS layer. If so, then the equilibrium surface concentration would change only slowly with bulk concentration in the regime that induces pores. The critical number n could still be substantial and nucleation theory could still hold. Future measurement of lag time distributions vs surface Cecropin A concentration using labeled peptide are an important next step. If a linear dependence of mean lag time on surface concentration is observed, it may prove useful to consider “lipocentric” models of pore formation, in which the peptide serves as a kind of catalyst that induces tension within the bilayer and then stabilizes the resulting transient pores.50
Supplementary Material
Acknowledgments
A helpful discussion with Prof. Qiang Cui is gratefully acknowledged.
Funding
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM094510. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- AMP
antimicrobial peptide
- OM
outer membrane
- CM
cytoplasmic membrane
- GUV
giant unilamellar vesicle
- LUV
large unilamellar vesicle
- MIC
minimum inhibitory concentration
- GFP
green fluorescent protein
- ROI
region of interest
Footnotes
Supporting Information
Supporting Information includes: (1) Explanation of how axial and transverse intensity profiles are created. (2) Definition of the relative axial coordinate system xrel and explanation of how we estimate the axial positions at which the OM is permeabilized to GFP and the CM is permeabilized to Sytox Green. (3) Additional histograms of the timing and duration of permeabilization events at nominal 1 µM and 2 µM bulk Cecropin A concentration. (4) Figure S6 showing the subtle slowing of growth rate immediately after Cecropin A addition. (5) Further details of how the Monte Carlo simulations are carried out, plus figures showing one model trajectory and how the model outcomes depend on input parameters. (6) Comparison of the attack of Cecropin A and LL-37 on a septating cell. (7) Table S1, describing timing and duration of permeabilization events for LL-37 attacking E. coli. (8) A movie showing the attack of Cecropin A on one septating cell and one non-septating cell. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Wang Z, Wang G. APD: the Antimicrobial Peptide Database. Nucleic Acids Res. 2004;32:D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wimley WC, Hristova K. Antimicrobial peptides: successes, challenges and unanswered questions. The Journal of membrane biology. 2011;239:27–34. doi: 10.1007/s00232-011-9343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature reviews. Microbiology. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 4.Mowery BP, Lindner AH, Weisblum B, Stahl SS, Gellman SH. Structure-activity relationships among random nylon-3 copolymers that mimic antibacterial host-defense peptides. Journal of the American Chemical Society. 2009;131:9735–9745. doi: 10.1021/ja901613g. [DOI] [PubMed] [Google Scholar]
- 5.Clark KS, Svetlovics J, McKeown AN, Huskins L, Almeida PF. What determines the activity of antimicrobial and cytolytic peptides in model membranes. Biochemistry. 2011;50:7919–7932. doi: 10.1021/bi200873u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almeida PF, Pokorny A. Mechanisms of antimicrobial, cytolytic, and cellpenetrating peptides: from kinetics to thermodynamics. Biochemistry. 2009;48:8083–8093. doi: 10.1021/bi900914g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung WC, Chen FY, Lee CC, Sun Y, Lee MT, Huang HW. Membrane-thinning effect of curcumin. Biophys J. 2008;94:4331–4338. doi: 10.1529/biophysj.107.126888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang HW. Molecular mechanism of antimicrobial peptides: the origin of cooperativity. Biochim Biophys Acta. 2006;1758:1292–1302. doi: 10.1016/j.bbamem.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Huang HW, Chen FY, Lee MT. Molecular mechanism of Peptide-induced pores in membranes. Physical review letters. 2004;92:198304. doi: 10.1103/PhysRevLett.92.198304. [DOI] [PubMed] [Google Scholar]
- 10.Tamba Y, Ohba S, Kubota M, Yoshioka H, Yoshioka H, Yamazaki M. Single GUV method reveals interaction of tea catechin (-)-epigallocatechin gallate with lipid membranes. Biophys J. 2007;92:3178–3194. doi: 10.1529/biophysj.106.097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tamba Y, Yamazaki M. Single giant unilamellar vesicle method reveals effect of antimicrobial peptide magainin 2 on membrane permeability. Biochemistry. 2005;44:15823–15833. doi: 10.1021/bi051684w. [DOI] [PubMed] [Google Scholar]
- 12.Wimley WC. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS chemical biology. 2010;5:905–917. doi: 10.1021/cb1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian S, Wang W, Yang L, Huang HW. Structure of the alamethicin pore reconstructed by x-ray diffraction analysis. Biophys J. 2008;94:3512–3522. doi: 10.1529/biophysj.107.126474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee CC, Sun Y, Qian S, Huang HW. Transmembrane pores formed by human antimicrobial peptide LL-37. Biophys J. 2011;100:1688–1696. doi: 10.1016/j.bpj.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee E, Jeong KW, Lee J, Shin A, Kim JK, Lee J, Lee DG, Kim Y. Structure-activity relationships of cecropin-like peptides and their interactions with phospholipid membrane. BMB reports. 2013;46:282–287. doi: 10.5483/BMBRep.2013.46.5.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MT, Hung WC, Chen FY, Huang HW. Mechanism and kinetics of pore formation in membranes by water-soluble amphipathic peptides. Proc Natl Acad Sci U S A. 2008;105:5087–5092. doi: 10.1073/pnas.0710625105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlamadinger DE, Wang Y, McCammon JA, Kim JE. Spectroscopic and computational study of melittin, cecropin A, the hybrid peptide CM15. The journal of physical chemistry. B. 2012;116:10600–10608. doi: 10.1021/jp304021t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leontiadou H, Mark AE, Marrink SJ. Antimicrobial peptides in action. Journal of the American Chemical Society. 2006;128:12156–12161. doi: 10.1021/ja062927q. [DOI] [PubMed] [Google Scholar]
- 19.Axelsen PH. A chaotic pore model of polypeptide antibiotic action. Biophys J. 2008;94:1549–1550. doi: 10.1529/biophysj.107.124792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 21.Shai Y. Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta. 1999;1462:55–70. doi: 10.1016/s0005-2736(99)00200-x. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt NW, Tai KP, Kamdar K, Mishra A, Lai GH, Zhao K, Ouellette AJ, Wong GC. Arginine in alpha-defensins: differential effects on bactericidal activity correspond to geometry of membrane curvature generation and peptide-lipid phase behavior. The Journal of biological chemistry. 2012;287:21866–21872. doi: 10.1074/jbc.M112.358721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang L, Gordon VD, Mishra A, Som A, Purdy KR, Davis MA, Tew GN, Wong GC. Synthetic antimicrobial oligomers induce a composition-dependent topological transition in membranes. Journal of the American Chemical Society. 2007;129:12141–12147. doi: 10.1021/ja072310o. [DOI] [PubMed] [Google Scholar]
- 24.Steiner H, Hultmark D, Engstrom A, Bennich H, Boman HG. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292:246–248. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 25.Marassi FM, Opella SJ, Juvvadi P, Merrifield RB. Orientation of cecropin A helices in phospholipid bilayers determined by solid-state NMR spectroscopy. Biophys J. 1999;77:3152–3155. doi: 10.1016/S0006-3495(99)77145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silvestro L, Axelsen PH. Membrane-induced folding of cecropin A. Biophys J. 2000;79:1465–1477. doi: 10.1016/S0006-3495(00)76398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gregory SM, Cavenaugh A, Journigan V, Pokorny A, Almeida PF. A quantitative model for the all-or-none permeabilization of phospholipid vesicles by the antimicrobial peptide cecropin A. Biophys J. 2008;94:1667–1680. doi: 10.1529/biophysj.107.118760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wade D, Boman A, Wahlin B, Drain CM, Andreu D, Boman HG, Merrifield RB. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A. 1990;87:4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steiner H, Andreu D, Merrifield RB. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim Biophys Acta. 1988;939:260–266. doi: 10.1016/0005-2736(88)90069-7. [DOI] [PubMed] [Google Scholar]
- 30.Christensen B, Fink J, Merrifield RB, Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci U S A. 1988;85:5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvestro L, Gupta K, Weiser JN, Axelsen PH. The concentrationdependent membrane activity of cecropin A. Biochemistry. 1997;36:11452–11460. doi: 10.1021/bi9630826. [DOI] [PubMed] [Google Scholar]
- 32.Silvestro L, Weiser JN, Axelsen PH. Antibacterial and antimembrane activities of cecropin A in Escherichia coli. Antimicrob Agents Chemother. 2000;44:602–607. doi: 10.1128/aac.44.3.602-607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sochacki KA, Barns KJ, Bucki R, Weisshaar JC. Real-time attack on single Escherichia coli cells by the human antimicrobial peptide LL-37. Proc Natl Acad Sci U S A. 2011;108:E77–E81. doi: 10.1073/pnas.1101130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barns KJ, Weisshaar JC. Real-time attack of LL-37 on single Bacillus subtilis cells. Biochim Biophys Acta. 2013;1828:1511–1520. doi: 10.1016/j.bbamem.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neidhardt FC, Bloch PL, Smith DF. Culture medium for enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sochacki KA, Shkel IA, Record MT, Weisshaar JC. Protein diffusion in the periplasm of E. coli under osmotic stress. Biophys J. 2011;100:22–31. doi: 10.1016/j.bpj.2010.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sargent F. The twin-arginine transport system: moving folded proteins across membranes. Biochemical Society transactions. 2007;35:835–847. doi: 10.1042/BST0350835. [DOI] [PubMed] [Google Scholar]
- 38.Hong RW, Shchepetov M, Weiser JN, Axelsen PH. Transcriptional profile of the Escherichia coli response to the antimicrobial insect peptide cecropin A. Antimicrob Agents Chemother. 2003;47:1–6. doi: 10.1128/AAC.47.1.1-6.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berg HC. Random Walks in Biology. Princeton University Books; 1993. [Google Scholar]
- 40.Porcelli F, Verardi R, Shi L, Henzler-Wildman KA, Ramamoorthy A, Veglia G. NMR structure of the cathelicidin-derived human antimicrobial peptide LL-37 in dodecylphosphocholine micelles. Biochemistry. 2008;47:5565–5572. doi: 10.1021/bi702036s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oren Z, Lerman JC, Gudmundsson GH, Agerberth B, Shai Y. Structure and organization of the human antimicrobial peptide LL-37 in phospholipid membranes: relevance to the molecular basis for its non-cell-selective activity. Biochem J. 1999;341(Pt 3):501–513. [PMC free article] [PubMed] [Google Scholar]
- 42.Bakshi S, Siryaporn A, Goulian M, Weisshaar JC. Superresolution imaging of ribosomes and RNA polymerase in live Escherichia coli cells. Mol Microbiol. 2012;85:21–38. doi: 10.1111/j.1365-2958.2012.08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rakowska PD, Jiang H, Ray S, Pyne A, Lamarre B, Carr M, Judge PJ, Ravi J, UI MG, Koksch B, Martyna GJ, Hoogenboom BW, Watts A, Crain J, Grovenor CR, Ryadnov MG. Nanoscale imaging reveals laterally expanding antimicrobial pores in lipid bilayers. Proc Natl Acad Sci U S A. 2013;110:8918–8923. doi: 10.1073/pnas.1222824110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mileykovskaya E, Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta. 2009;1788:2084–2091. doi: 10.1016/j.bbamem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pogliano K, Pogliano J, Becker E. Chromosome segregation in Eubacteria. Curr Opin Microbiol. 2003;6:586–593. doi: 10.1016/j.mib.2003.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutkenhaus J, Pichoff S, Du S. Bacterial cytokinesis: From Z ring to divisome. Cytoskeleton. 2012;69:778–790. doi: 10.1002/cm.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MT, Hung WC, Chen FY, Huang HW. Many-body effect of antimicrobial peptides: on the correlation between lipid's spontaneous curvature and pore formation. Biophys J. 2005;89:4006–4016. doi: 10.1529/biophysj.105.068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Powers ET, Powers DL. The kinetics of nucleated polymerizations at high concentrations: amyloid fibril formation near and above the "supercritical concentration". Biophys J. 2006;91:122–132. doi: 10.1529/biophysj.105.073767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kushnareva Y, Andreyev AY, Kuwana T, Newmeyer DD. Bax activation initiates the assembly of a multimeric catalyst that facilitates Bax pore formation in mitochondrial outer membranes. PLoS Biol. 2012;10:e1001394. doi: 10.1371/journal.pbio.1001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuertes G, Gimenez D, Esteban-Martin S, Sanchez-Munoz OL, Salgado J. A lipocentric view of peptide-induced pores. Eur Biophys J. 2011;40:399–415. doi: 10.1007/s00249-011-0693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.