Abstract
Background and aims: This study was aimed at the development of a novel noninvasive treatment system, “pinpoint plantar long-wavelength infrared light irradiation (PP-LILI)”, which may be able to relieve mental stress and normalize blood glucose level via the reduction of stress hormones in type 2 (non-insulin-dependent) diabetes mellitus (DM) patients.
Materials (Subjects) and methods: Based on this hypothesis, the present study was undertaken to examine effects of PP-LILI on stress hormones (ACTH and cortisol), blood glucose, HbA1c, and insulin levels in 10 patients with type 2 DM. Each patient received PP-LILI of the foot for 15 minutes once weekly using a stress free apparatus (infrared wavelength, 9,000-12,000 nm/power 30 mW).
Results: In response to this therapy, ACTH (P<0.01) and cortisol (P<0.05) levels decreased significantly. Fasting blood glucose (P<0.05) and insulin (P<0.05) levels also decreased significantly along with a tendency for HbA1c to decrease.
Conclusions: The present data raise the possibility that PP-LILI can normalize blood glucose levels by reducing stress hormones such as cortisol, which aggravate DM, and by improving insulin sensitivity, thereby contributing to prevention and treatment of DM.
Keywords: Pinpoint plantar long-wavelength infrared light irradiation (PP-LILI), relieving stress, type 2 (non-insulin-dependent) diabetes mellitus (DM), blood glucose, stress hormones
Introduction
We recently published a report dealing with effects of Stress Free Therapy® (Registered Trademark No. 5495960) consisting of pinpoint plantar long-wavelength infrared light irradiation (PP-LILI) which provides peripheral deep-body temperature elevation and blood pressure stabilization while lowering serum adrenocorticotropic hormone (ACTH) and cortisol levels as well as the salivary amylase concentration. 1)
Cortisol is generally recognized as being involved in stress-induced blood glucose elevation by interfering with release of insulin, which lowers the blood glucose level, as well as with normal glucose metabolism. 2) Excessive release of cortisol under stressful conditions reportedly leads to diverse adverse reactions such as exaggerated fluctuations in blood glucose levels, hypertension, depressed immune function, depressed higher brain function (cognitive function), decreased bone mass density, and a decrease in muscle tissue mass. 3) Furthermore, patients with diabetes mellitus (DM) sustain stress due to dietary restrictions, exercise therapy, etc. in daily living, which may exacerbate DM.
Control of cortisol release, one of the major causes of stress, by non-pharmacotherapeutic means has not yet been achieved. As blood glucose elevation and cortisol secretion are closely related, this study was conducted to investigate clinical responses to PP-LILI Stress Free Therapy® in terms of the effects of this therapy on blood glucose, glycosylated hemoglobin (HbA1c), insulin, and stress hormones (ACTH and cortisol) in patients with type 2 DM.
Subjects and Methods
The study was conducted on 10 patients with type 2 DM (6 men and 4 women; mean age, 62.0 ± 7.7 years) whose glycemic control was not achieved despite taking oral anti-diabetic medications. Each patient received PP-LILI of the center of the area between the epiphyses of the second and third metatarsophalangeal joints of the foot for 15 minutes once weekly over an average 4-week period using a stress free apparatus (Controlled Medical Device Approval No. 224AFBZX00075000; probe diameter, 20 mm; infrared wavelength, 9,000-12,000 nm/power 30 mW) after a 15-minute rest in the supine position at each session (Fig. 1). The oral anti-diabetic medications were continued during the PP-LILI therapy period.
Figure 1:
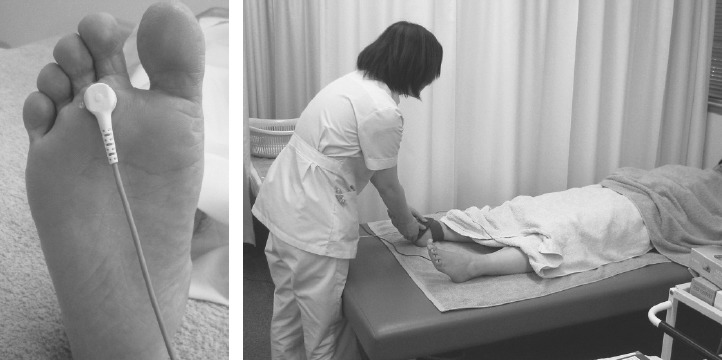
Pinpoint planter long-wavelength infrared light irradiation (PP-LILI) treatment. Halfway between the heads of the 2nd and 3rd metatarsals was irradiated.
For assays of blood glucose, HbA1c, insulin, and stress markers (ACTH and cortisol), 3 mL of blood were drawn through the cubital vein before and after PP-LILI, centrifuged (at 1200 g, 5 min), and analyzed for ACTH by chemiluminescent immunoassay (CLIA), for cortisol by electrochemiluminescence immunoassay (ECLIA), for glucose by the standard enzymatic method, for HbA1c by latex agglutination-turbidimetric immunoassay, and for insulin by CLIA. All blood sampling was carried out under identical conditions in view of the influence of such factors as assay time period and circadian rhythm.
This study was conducted after review and approval by the Ethics Committee of Ryotokuji University. Written informed consent was obtained from each patient after a full explanation of the nature and aims of the study.
Statistical evaluation of the data was performed using the sign test and paired t-test, with p-values of less than 0.05 considered to be significant.
Results
Changes in blood glucose and insulin levels
We examined blood glucose changes following PP-LILI and found that the blood glucose level decreased significantly from a pre-treatment value of 153.6±107.8 mg/dL to 96.2±11.8 mg/dL after treatment (P<0.05) (Fig. 2). The serum insulin level was 31.1±27.1 µU/mL before treatment and decreased significantly to 10.8±10.9 µU/mL after treatment (P<0.05) (Fig. 3).
Figure 2:
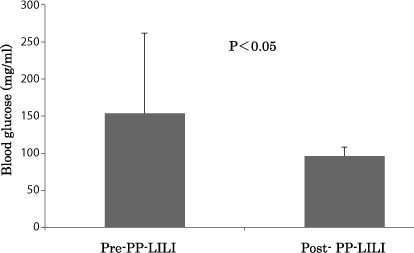
Improvement of the value of blood glucose after the PP-LILI.
Figure 3:
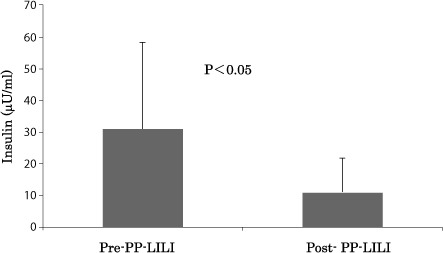
Improvement of the value of insulin after the PP-LILI.
Then we measured HbA1c (NGSP: National Glycohemoglobin Standardization Program). HbA1c levels decreased from a pre-treatment value of 6.9±2.6% to a post-treatment value of 5.8±0.4%, and HbA1c (JDS: Japan Diabetes Society) from a pre-treatment value of 6.6±2.6% to a post-treatment value of 5.6±0.4%; hence, both parameters showed a tendency to drop in parallel with the insulin level decrease (Figs. 4).
Figure 4:
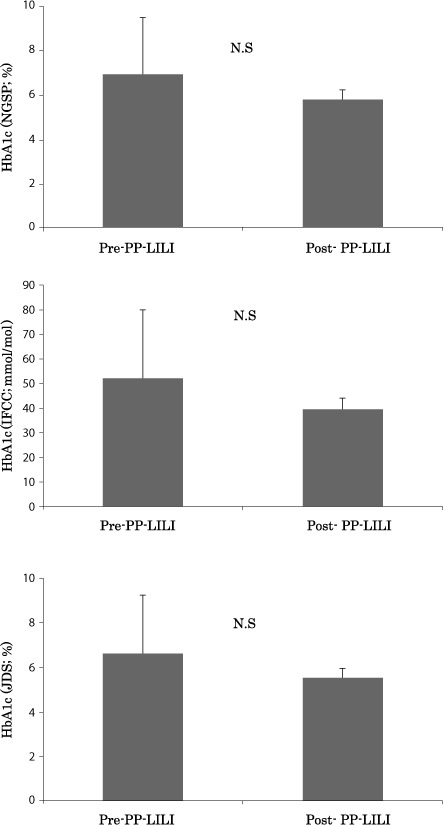
Changes of the value of HbA1c after the PP-LILI.
Stress hormone changes
The results on the HbA1c and blood glucose levels reveal that PP-LILI is effective to improve these values. Next, we have investigated the effects of PP-LILI on the changes in stress hormone levels. Cortisol secretion decreased significantly to 8.4±3.5 µg/dL after treatment, compared to 11.6±2.9 µg/dL before treatment (P<0.05) (Fig. 5). ACTH secretion was also reduced from a pre-treatment value of 29.6±21.9 pg/mL to 14.5±8.6 pg/mL after treatment (P<0.01) (Fig. 6).
Figure 5:
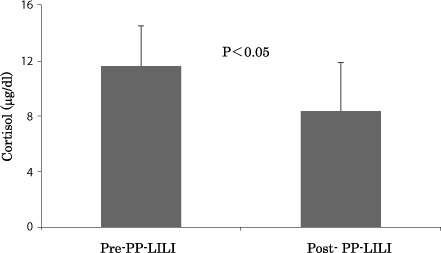
Improvement of the value of cortisol after the PP-LILI.
Figure 6:
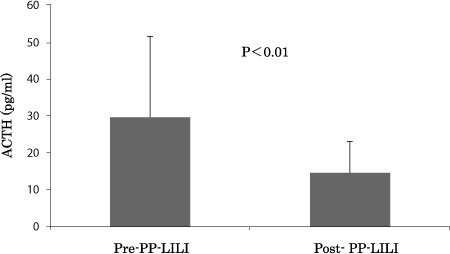
Improvement of the value of ACTH after the PP-LILI.
Discussion
We have reported, for the first time, the efficacy of PP-LILI for elevating peripheral deep-body temperature and stabilizing blood pressure as well as for significantly reducing secretions of such stress hormones as ACTH and cortisol, and that of amylase, and we have been applying PP-LILI as Stress Free Therapy® in our daily clinical practice setting. 1)
It is universally recognized that psychological stress stimulates the sympathetic nervous system via the brain and central nervous system to constrict blood vessels and lower deep body temperature, and also stimulates the hypothalamus (paraventricular nucleus) triggering release of corticotropin releasing hormone, that ACTH is thereby secreted from the anterior pituitary lobe and stimulates cortisol secretion from the adrenal cortex. 5,6)
The relationship of stress to DM has recently been receiving considerable attention. Type 2 DM is ascribed primarily to the following two factors: insufficient insulin secretion and tissue hyposensitivity to this hormone. These factors account for more than 90% of all DM cases in Japan. Furthermore, DM as a lifestyle-related disease is usually of this type. As regards the relationships between type 2 DM and hormones, secretions of cortisol and ACTH are known to be associated with complications. 7) Analysis of data from 170 type 2 DM cases revealed a positive correlation of cortisol secretion with the development of complications of DM. 8,9) Based on these reports, we explored the clinical feasibility of reducing blood glucose and stress hormone levels in patients with type 2 DM through the stress-relieving effects of PP-LILI. In response to this irradiation, blood glucose dropped significantly from a pre-treatment level of 153.6±107.8 mg/dL to a post-treatment level of 96.2±11.8 mg/dL (P<0.05). Although patients with a serum insulin level 15.0 µU/mL would obviously be insulin-resistant, the present series exhibited a significant decrease in the serum insulin level from a pre-treatment value of 31.1±27.1 µU/mL to 10.8±10.9 µU/mL after treatment (P<0.05), demonstrating that PP-LILI is highly likely to contribute to amelioration of insulin resistance.
The long-term marker of blood glucose control HbA1c tended to decrease, though the decrement did not reach statistical significance, in response to the PP-LILI. Our present findings, i.e., favorable glycemic control with repeated or intermittent PP-LILI, make this a promising treatment strategy and we plan to assess this therapeutic effect in the near future. As for stress markers, there was a significant decrease in serum cortisol from a pre-treatment level of 11.6±2.9 µg/dL to a posttreatment level of 8.4±3.5 µg/dL (P<0.05). Serum ACTH also decreased significantly from a pre-treatment level of 29.6±21.9 pg/mL to a post-treatment level of 14.5±8.6 pg/mL (P<0.01). Many reports have documented that hypothalamic-pituitary-adrenal axis in stress signaling is related to DM. 7-9) Therefore, it would not be unreasonable to assume that PP-LILI, which has been proven to reduce serum levels of adrenal glucocorticoid hormones, contributes to the suppression of blood glucose elevation and thereby improves insulin resistance in patients with type 2 DM.
Attention has been focused in recent years on the major involvement of AMP-activated protein kinase (AMPK) in systemic metabolic regulation of carbohydrates and lipids. This enzyme expressed in the cells of various tissues, is activated in response to a decrease in ATP level and depresses ATP consumption, thereby regulating various metabolic pathways in a direction favoring increased ATP production. Several studies have explored the possibility that the gene encoding adiponectin is involved in the enhancement of insulin sensitivity via activation of hepatic AMPK, thereby possibly improving the relative insulin insensitivity in type 2 DM. 10,11) Adiponectin has recently been attracting attention as a beneficial substance of adipocytic origin. It exerts diverse physiological effects. Perhaps most significantly, it has been demonstrated that the insulin resistance-improving effect of adiponectin is attributable to activities including inhibition of enhanced fatty acid oxidation and of gluconeogenesis in the liver, facilitation of fatty acid oxidation and of glucose uptake in skeletal muscles, and inhibition of tumor necrosis factor-α (TNF-α) production in adipose tissues. It has also been shown that adiponectin levels are low in patients with DM. In the present study as well, we quantified the expression level the adiponectin gene using the microarray method before and after the PP-LILI therapy and these assays demonstrated a significant post-treatment increase in adiponectin receptor 2 (Adip R 2) expression (data not shown), along with a decrease in blood glucose to 101 mg/dL in a patient whose pre-treatment blood glucose level was 435mg/dL. Adip R 2 is known to facilitate fatty acid decomposition in the liver, and the preset results suggest that PP-LILI may act on adiponectin receptors, thereby accelerating fat combustion and improving insulin resistance.
A cohort study reported by Helmrich and colleagues 12) consisting of a 14-year follow-up survey of 5,990 University of Pennsylvania graduates revealed that, for each 500 kcal increase in energy expenditure due to physical activity, the risk of type 2 DM was decreased by 6%. This report stresses, particularly, the importance of improving vascular resistance and peripheral circulation for amelioration of DM insomuch as 40-60% of DM patients have concurrent hypertension which, together with the DM, promotes diabetic nephropathy, cerebrovascular disorders and coronary artery diseases. PP-LILI elevates peripheral deep-body temperature and stabilizes blood pressure via normalization of sympathetic nerve activity; therefore, PP-LILI may serve as an effective therapy for DM and hypertension even in patients who cannot receive exercise therapy due to motor dysfunction.
Conclusions
These obtained data raise the possibility that PP-LILI are useful for the normalization of blood glucose levels by reducing stress hormones such as cortisol, which aggravate DM, and by improving insulin sensitivity, thereby contributing to prevention and treatment of DM.
Acknowledgements
This research was conducted jointly by the medical education Ryotokuji College, Faculty of Health Sciences Ryotokuji University, and Medical Corporation Ryotokuji Group. We thank all the individuals involved in this study. Stress hormone quantification was performed in collaboration with Showa Medical Science.
Author Disclosure Statement
No competing financial interest exists.
Author Contributions
K.R. wrote manuscript, researched data. K.I. wrote manuscript, researched data. K.K. researched data. Y.N. reviewed/edited manuscript. N.H. contributed to discussion, reviewed/edited manuscript. K.I. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. We certify that this study involving human subjects (the accepted trial registry number: no. 2304) is in accordance with the Helsinky declaration of 1975 as revised in 2000 and that it has been approved by the relevant institutional Ethical Committee. We also certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
References
- 1: Ryotokuji K, Ishimaru K, Kihara K, Namiki Y, Hozumi N. (2013). Effect of Pinpoint Plantar Longwavelength Infrared Light Irradiation on Subcutaneous Temperature and Stress Markers. Laser Therapy 22: 93-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2: Loredana M, Francesco C. (2012). The effects of acute and chronic stress on diabetes control. Sci. Signal 23: 247-257 [DOI] [PubMed] [Google Scholar]
- 3: Roy MS, Roy A, Brown S. (1998). Increased urinary-free cortisol outputs in diabetic patients. J Diabetes Complications 12: 24-27 [DOI] [PubMed] [Google Scholar]
- 4: Andrews RC, Herlihy O, Livingstone DE, Andrew R, Walker BR. (2002). Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. J Clin Endocrinol Metab 87: 5587-5593 [DOI] [PubMed] [Google Scholar]
- 5: Smitz S, Giagoultsis T, Dewé W. (2000). Albert A Comparison of rectal and infrared ear temperatures in older hospital inpatients. J Am Geriatr Soc 48: 63-66 [DOI] [PubMed] [Google Scholar]
- 6: Huang M, Chen W. (2010). Theoretical simulation of the dual-heat-flux method in deep body temperature measurements. Eng Med Biol Soc 561-564 [DOI] [PubMed] [Google Scholar]
- 7: Luchsinger JA, Gustafson DR. (2009). Adiposity, type 2 diabetes, and Alzheimer's disease. J Alzheimers Dis 16: 693-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8: Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, Epaminonda P, Masserini B, Beck-Peccoz P, Orsi E, Ambrosi B. (2007). Cortisol Secretion in Patients with Type 2 Diabetes Relationship with chronic complications. Diabetes Care 30: 83-88 [DOI] [PubMed] [Google Scholar]
- 9: Richardson AP, Tayek JA. (2002). Type 2 diabetic patients may have a mild form of an injury response: a clinical research center study. Am J Physiol Endocrinol Metab 282: 1286-1290 [DOI] [PubMed] [Google Scholar]
- 10: Hara K, Boutin P, Mori Y, Tobe K, Dina C, Yasuda K, Yamauchi T, Otabe S, Okada T, Eto K, Kadowaki H, Hagura R, Akanuma Y, Yazaki Y, Nagai R, Taniyama M, Matsubara K, Yoda M, Nakano Y, Tomita M, Kimura S, Ito C, Froguel P, Kadowaki T. (2002). Genetic variation in the gene encoding adiponectin is associated with an increased risk of type 2 diabetes in the Japanese population. Diabetes 51: 536-540 [DOI] [PubMed] [Google Scholar]
- 11: Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Nagasawa A, Kobayashi H, Hiraoka H, Komai N, Kaibe M, Rakugi H, Ogihara T, Matsuzawa Y. (2003). Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 42: 231-234 [DOI] [PubMed] [Google Scholar]
- 12: Helmrich SP, Ragl DR, Leung RW, Paffenbarger RS. (1991). Physical activity and reduced occurrence of noninsulin-dependent diabetes mellitus. N Engl J Med 325, 147-152 [DOI] [PubMed] [Google Scholar]


