Abstract
Brucella strains produce abortion and infertility in their natural hosts and a zoonotic disease in humans known as undulant fever. These bacteria do not produce classical virulence factors, and their capacity to successfully survive and replicate within a variety of host cells underlines their pathogenicity. Extensive replication of the brucellae in placental trophoblasts is associated with reproductive tract pathology in natural hosts and prolonged persistence in macrophages leads to the chronic infections that are a hallmark of brucellosis in both natural hosts and humans. This review describes how Brucella strains have efficiently adapted to their intracellular lifestyle in the host.
Introduction
The Brucella spp. are Gram-negative bacteria that cause economically important diseases in food animals worldwide [44]. Brucella melitensis, B. abortus and B. suis strains cause abortion and infertility in their natural hosts - goats and sheep, cattle and swine, respectively. Humans can also acquire a severe, debilitating febrile illness known as brucellosis, or “undulant fever”, as the result of contact with infected animals or their products [133]. Naturally-occurring human brucellosis is strictly a zoonotic infection. In areas of the world where Brucella infections in food animals have been controlled by successful eradication programs, human infections are predominately an occupational hazard for animal handlers, veterinarians, slaughterhouse workers and others who work with potentially infected animals [130]. In contrast, human brucellosis remains a significant public health concern in areas of the world where Brucella infections are endemic in food animals. Indeed, brucellosis has been described as being the leading zoonosis worldwide [133].
Brucella ovis is a natural pathogen of sheep where it primarily causes epididymitis and infertility in rams and occasionally abortion in ewes [23]. B. canis infection leads to abortion and infertility in dogs [187]. Although B. ovis and B. canis are important veterinary pathogens, human infection with B. canis is rare [44], and human infection with B. ovis has not been reported.
Brucella pinnipedialis and B. ceti strains are being isolated from marine mammals with increasing frequency [48], but the role of these bacterial strains in disease in these hosts or how these strains are disseminated between marine mammals is presently unresolved [83]. Marine mammal strains of Brucella have also been isolated from human disease [171] indicating that these strains are potential zoonotic pathogens.
Brucella strains are highly infectious via the aerosol route [69]. Human brucellosis is also debilitating, long-lasting and difficult to treat with antibiotics, and there are no safe and effective vaccines available to prevent human infection [196]. This combination of characteristics has led to the inclusion of B. melitensis, B. suis and B. abortus strains on lists of etiologic agents considered to pose risks for use as bioweapons [134]. Accordingly, the possession and handling of Brucella strains in both clinical and research laboratories are subject to strict regulations in many countries.
The brucellae are members of the α–proteobacteria [126]. Other members of this group of bacteria include those in the genera Bartonella, Agrobacterium, Rhizobium, Sinorhizobium, and Mesorhizobium. All of these bacteria inhabit eukaryotic cells, and comparative genomic studies indicate that they evolved from a common ancestor [25]. There are remarkable parallels between the mechanisms and gene products employed by these bacteria to establish successful interactions with their plant and animal hosts [16,110]. Recognition of these parallels has greatly improved our understanding of the host-pathogen interactions that take place during Brucella infections.
Brucella strains are intracellular pathogens in vivo
Brucella strains live in close association with their mammalian hosts. They are not found free-living in the environment. Although many texts refer to these bacteria as being facultative intracellular parasites, it has been proposed that they are more appropriately termed “facultatively extracellular intracellular parasites” [127]. This is based on the fact that although the Brucella spp. are relatively easy to cultivate on artificial media, they maintain predominately an intracellular existence within their mammalian hosts. Within these hosts, the brucellae occupy both professional and non-professional phagocytes, and their interactions with these host cells dictate the outcomes of infection [101,125,156].
Interactions of Brucella strains with professional phagocytes
Macrophages
It is well documented that the capacity of Brucella strains to survive and replicate for prolonged periods within host macrophages underlies their ability to produce chronic, and sometimes life-long, infections [101,156]. This intracellular niche provides a safe haven for the brucellae in terms of protecting these bacteria from antibodies and complement during dissemination in the host. Localization of persistently infected macrophages in organs of the reticuloendothelial system such as the spleen and liver also provides foci for the maintenance of chronic infection [60,128]. An interesting feature of the interactions of Brucella strains with macrophages is that experimental evidence indicates that these bacteria have the ability to prevent apoptosis of the macrophages within which they reside [79]. This property conceivably allows the brucellae to extend the longevity of their safe haven.
Dendritic cells
The link between persistent infection of macrophages and the virulence of Brucella strains has been recognized for decades. Recent work, however, suggests that another type of professional phagocyte may also play a key role in the pathobiology of Brucella infections. Specifically, in contrast to several other intracellular pathogens, the brucellae survive and replicate in human and murine dendritic cells [21,159]. Strikingly, the intracellular replication of virulent Brucella strains interferes with the maturation of these host cells [22,159]. Considering the importance of dendritic cells in the development of host immune responses [12], it is easy to see how the capacity of the brucellae to inhibit the maturation of these antigen-processing cells allows these bacteria to circumvent host immune responses. As is the case with macrophages, it is also possible that dendritic cells serve as safe havens to prevent exposure of the brucellae to components of the immune response and act as vehicles for the dissemination of these bacteria in the host.
Interactions of Brucella strains with non-professional phagocytes
Placental trophoblasts
During pregnancy in natural hosts, Brucella strains can infect and replicate within placental trophoblasts [123,160]. These host cells are epithelial in nature, and although they are considered to be non-professional phagocytes, some placental trophoblasts acquire the capacity to engulf and degrade erythrocytes from the maternal circulation; hence they are known as erythrophagocytic trophoblasts [86]. This activity provides an important source of iron for the developing fetus. Large numbers of brucellae can be isolated from the placenta of infected ruminants (e.g. 1013 CFU/g of tissue in fetal cotyledons) [2], and this extensive intracellular proliferation of the brucellae can disrupt the integrity of the placenta leading to abortion or the birth of weak and infected offspring, two of the hallmark clinical presentations associated with Brucella infections in their natural hosts [5,6,23,60,187]. It seems likely that the physical and hormonal characteristics of the placenta that facilitate immune suppression and prevent maternal rejection of the developing fetus play an important role in allowing the brucellae to replicate to high numbers in the gravid reproductive tract of their natural hosts. The deposition of heavily infected placental tissues into the environment is important for transmission of Brucella infections between natural hosts [5,6,23,46,187]. In contrast to the situation in natural hosts, abortion is not a predominant clinical presentation associated with human brucellosis, but it does occur and is an issue of medical concern in regions where this disease is endemic [196].
Ruminant placental trophoblasts produce erythritol during the third trimester of pregnancy [160]. This sugar alcohol is a favored carbon and energy source for many Brucella strains [174], and it has been postulated that the presence of this compound contributes to the rapid and extensive replication of the brucellae in the ruminant reproductive tract [169]. This proposed link between erythritol utilization and virulence of Brucella strains in ruminants, however, has yet to be verified experimentally.
Epithelial cells
The brucellae gain entrance into the host at mucosal barriers, and thus the interactions of these bacteria with host epithelial cells at these locations represent an important point of initial contact between the pathogen and host. Brucella strains have been shown to invade a variety of epithelial cells in culture [64,166], but the efficiency with which these bacteria “invade” epithelial cells is low compared to bacterial pathogens that are considered to be truly “invasive” [128]. Consequently, the extent of the contribution that epithelial cell invasion makes to the initiation of Brucella infections unclear, and some investigators have proposed that M cells located at mucosal surfaces serve as the major site of entry of Brucella strains into the host [128]. It is important to note, however, that the human epithelial cell line HeLa and the African green monkey kidney fibroblastic cell line Vero have both been used widely and effectively as models for studying the interactions of Brucella strains with mammalian cells [52,128,139].
The brucellae proactively alter their intracellular trafficking in host cells
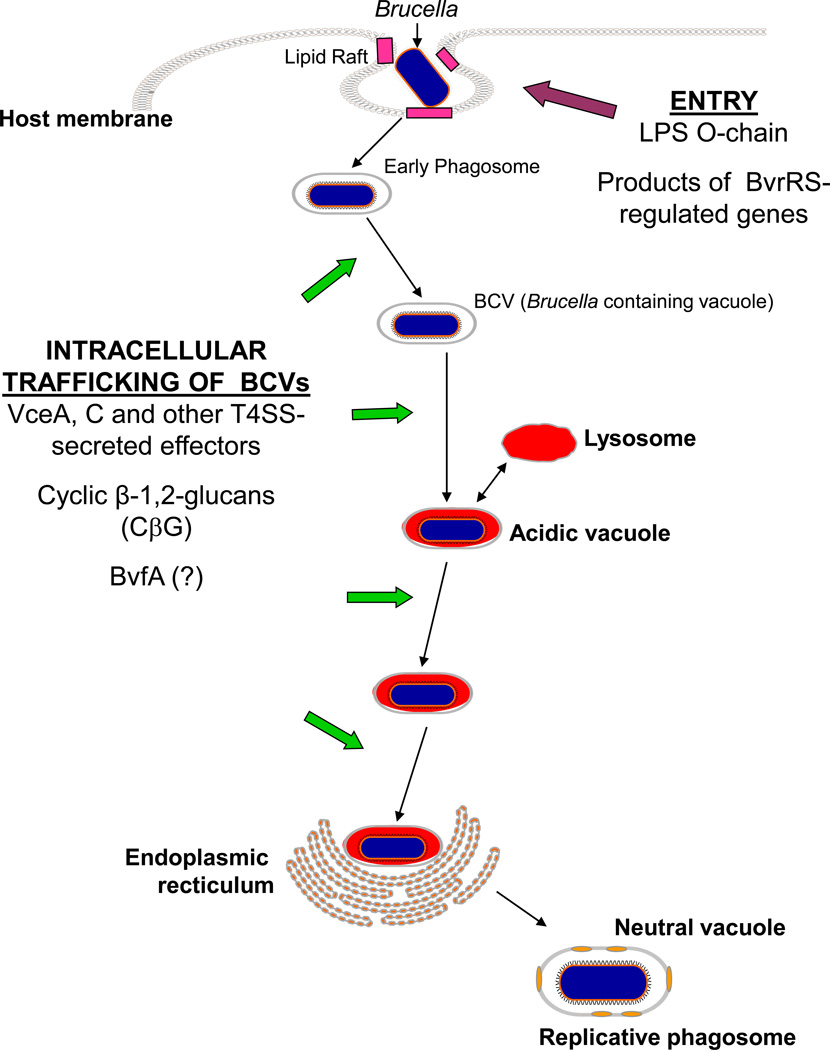
When unopsonized B. melitensis, B. abortus and B. suis strains are ingested by cultured macrophages and epithelial cells, the Brucella-containing vacuoles (BCVs) enter into an intracellular trafficking pathway that results in the development of specialized membrane-bound compartments [8,32,96,129,139,150] known as replicative phagosomes [96], replicative vacuoles [128] or brucellosomes [101] (Figure 1). Interactions between the O-chain of the smooth LPS of these Brucella strains and lipid rafts on the surface of macrophages have been shown to be important for mediating entry into host cells in a manner that leads to the development of the replicative phagosome [141]. During the initial stages of intracellular trafficking of the BCVs, these compartments undergo transient interactions with lysosomes [176] which results in their acidification [8,140]. These vacuoles then begin to interact extensively with the endoplasmic reticulum [32] and eventually their intracellular pH rises to a level that allows intracellular replication of the brucellae. During development of the replicative phagosome in epithelial cells, the BCVs acquire properties resembling autophagosomes [139], but this does not appear to be the case during the development of the BCVs in macrophages [32]. Studies employing the human monocytic cell line THP-1 and B. abortus strains opsonized with hyperimmune IgG have also shown that when the brucellae enter host macrophages in this manner, the resulting BCVs also undergo transient association with the lysosomal compartment and become acidified, but these BCVs do not interact extensively with the ER [20]. An obvious potential benefit of this altered intracellular trafficking is that limiting the fusion of the BCVs with lysosomes minimizes the exposure of these bacteria to the bactericidal proteins that reside in these intracellular compartments. A potential nutritional benefit to the brucellae of the fusion of the BCVs with the ER in host cells will be discussed in a later section.
Figure 1.
Gene products that influence their intracellular trafficking of Brucella strains in host cells.
The Type IV secretion system (T4SS) of Brucella strains encoded by the virB operon plays an essential role in the development of the replicative vacuole within which these bacteria reside in host cells [32,40,50,96] (Figure 1). The T4SS of Legionella pneumophila secretes “effector” proteins into host cells that alter the intracellular trafficking of this bacterium [167], and genetic and biochemical studies support a similar function for the Brucella T4SS. Specifically, the vacuoles containing B. melitensis, B. abortus and B. suis virB mutants fuse extensively with lysosomes but do not interact with the ER in cultured macrophages, dendritic cells and HeLa cells (32,40,50,159]. Recently, de Jong et al. (49) identified two putative effector proteins (designated VceA and VceC) secreted by the T4SS of B. abortus 2308. These investigators showed that VceC is also secreted by B. suis 1330. The biological functions of VceA and VceC have yet to be defined, but the importance of the Brucella T4SS for virulence is clearly evident from the attenuation that virB mutants exhibit compared to their parental strains in cultured macrophages [67,131,146], HeLa cells [67,97,131,168], human and murine dendritic cells [21,159] and experimentally infected mice [84,92,146,168] and goats [93,198]. It is also notable that two of the major environmental stresses encountered by the brucellae during their intracellular residence in host macrophages, exposure to acidic pH and nutritional deprivation (see below), serve as important stimuli for induction of expression of the virB operon in B. suis [24].
BvfA is a Brucella protein discovered in a genetic screen designed to identify substrates of the T4SS [106]. B. suis bvfA mutants are highly attenuated in cultured murine and human macrophages, HeLa cells and mice. Although the bvfA gene in B. suis 1330 exhibits a similar regulatory pattern in cultured macrophages as the virB genes, whether or not BvfA is a substrate for the T4SS has not been definitively resolved, and the function of this protein is unknown.
Like their phylogenetic relatives Agrobacterium tumefaciens and Sinorhizobium meliloti, Brucella strains produce periplasmic cyclic β-1,2-glucans (CβG) [87], and these glucose polymers are required for the successful interactions of all three of these bacteria with their eukaryotic hosts [26,57,144]. In the case of the brucellae, experimental evidence suggests that CβG disrupts the integrity of lipid rafts in the membrane of the BCVs during intracellular trafficking, preventing extensive interactions of these vacuoles with lysosomes [10] (Figure 1). It is unclear how these CβG molecules, which reside in the periplasm of Brucella strains, make their way to the membrane of the BCV. A plausible proposition that has been put forth is that CβG may be released from intact bacterial cells as components of outer membrane vesicles [10].
The capacity of the brucellae to both avoid and interfere with components of the host immune response contributes to their intracellular persistence
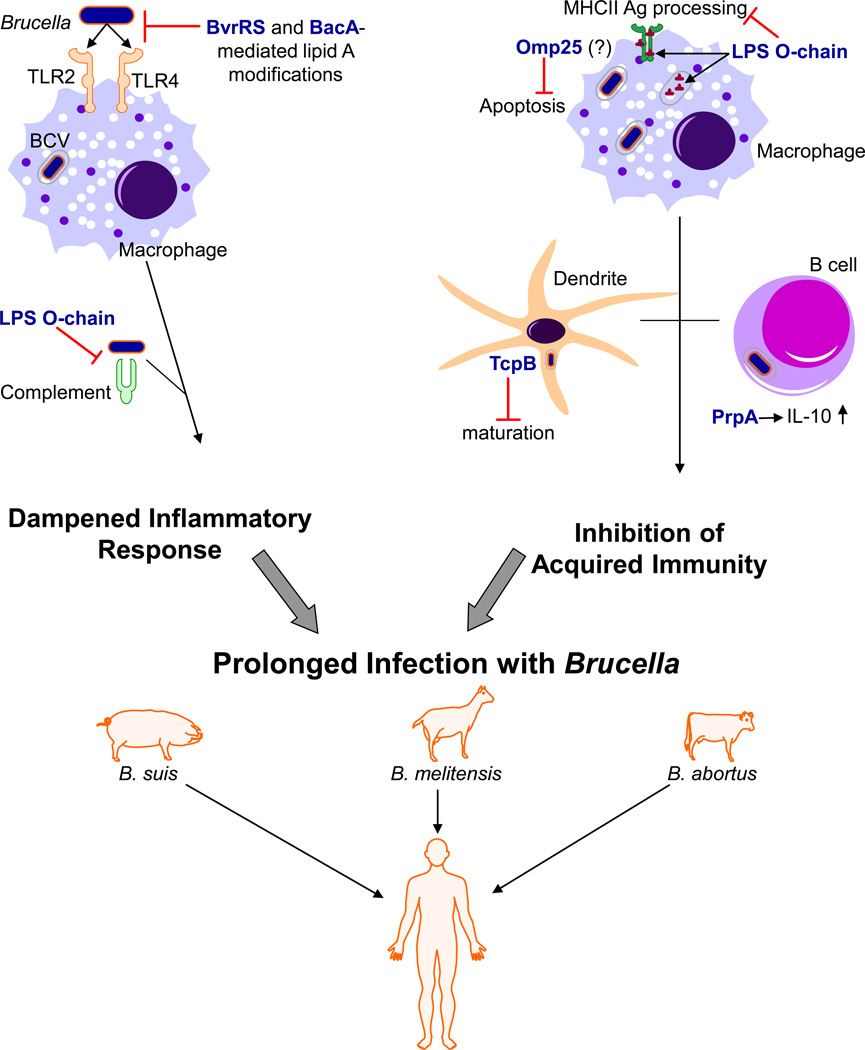
The Brucella LPS is a weak inducer of the host inflammatory response compared to LPS molecules from many other Gram-negative bacterial pathogens (Figure 2). Mice infected with virulent B. abortus 2308, for example, do not show signs of sepsis. This is in contrast to mice infected with a virulent strain of Salmonella typhimurium [14], which typically exhibit malaise, wasting and eventually death. Unlike mice infected with Salmonella, those infected with B. abortus do not exhibit an acute phase response, do not recruit neutrophils to the site of inoculation and do not strongly induce production of the proinflammatory cytokines IL-1β, IL-6 or TNF-α. Brucella cells are also relatively inefficient at activating complement. These experimental findings support previous work showing that the Brucella LPS has greatly reduced “endotoxin” activity compared to similar molecules from other Gram-negative pathogens [125,148]. They are also in agreement with the fact that although human brucellosis is a febrile illness, Brucella infections do not elicit the same sepsis response observed in patients with systemic infections caused by Gram-negative bacteria possessing a highly endotoxic LPS such as the enterics and the Pseudomonas spp. [14].
Figure 2.
Subversion of host immune defenses allows Brucella strains to establish and maintain chronic infections in their hosts. Brucella gene products linked to immune evasion are shown in blue font.
The endotoxin component of the Brucella LPS, the lipid A, has several biochemical features (e.g. diaminoglucose and long chain [C28] fatty acids) [88] that distinguish it from the “classic” lipid A found in the other Gram-negative bacteria that induces strong inflammatory responses in infected hosts [104]. Moreover, there is genetic evidence supporting the proposition that the Brucella lipid A plays a major role in the capacity of these bacteria to avoid the induction of a full-scale inflammatory response in the host (Figure 2). Specifically, a Brucella bacA mutant which has a lipid A that is deficient in its long chain (e.g. C28) fatty acid content [63] produces a stronger inflammatory response in experimentally infected mice than does its parental strain [136] and is attenuated. Brucella bvrRS mutants which also have lipid As with reduced long chain fatty acid content compared to their parent strains [117] are likewise highly attenuated in experimentally infected mice [172].
In addition to their ability to avoid induction of an optimal inflammatory response in the host, Brucella strains are also able to actively interfere with the host acquired immune response (Figure 2). The perosamine O-chain of the LPS of Brucella strains is poorly degraded by host macrophages [65] and forms complexes with components of the MHCII machinery of these phagocytes which interferes with their antigen processing capacity [66] (Figure 2).
Brucella strains also produce a protein designated TcpB [39,145] or Btp1 [159] that contains a Toll/interleukin-1 receptor (TIR) domain. TIR domain containing proteins serve as important components of the host cell signaling pathways that link the Toll-like receptors to NF-κB and are important for the induction of innate immunity [132]. When expressed in eukaryotic cells, the Brucella TcpB blocks TLR2- and TLR4-mediated induction of NF-κB expression [39,145,159, Sengupta et al, manuscript submitted] through its capacity to elicit the targeted degradation of the TLR signaling adapter MAL (also known as TIRAP) [Sengupta et al., manuscript submitted]. Although Brucella tcpB mutants are not attenuated in cultured murine macrophages, HeLa cells or immunocompetent mice (145,159, Sengupta et al., manuscript submitted], these strains do exhibit delayed virulence in the immunocompromised IRF-1−/− mouse model [145]. Studies employing a murine intestinal loop model indicate that TcpB plays a role in the capacity of Brucella strains to interfere with dendritic cell maturation and function [159] (Figure 2).
PrpA is another protein produced by Brucella strains that interferes with host immune response [173]. This protein is a proline racemase that acts as a T-cell independent B lymphocyte mitogen that stimulates the production of the anti-inflammatory cytokine IL-10 (Figure 2). B. abortus prpA mutants exhibit significant attenuation in experimentally infected mice at 12 weeks post infection and beyond. It has been proposed that PrpA induces a transient immune suppression that helps the brucellae maintain chronic infections.
Physiologic adaptation of the brucellae to their intracellular niche
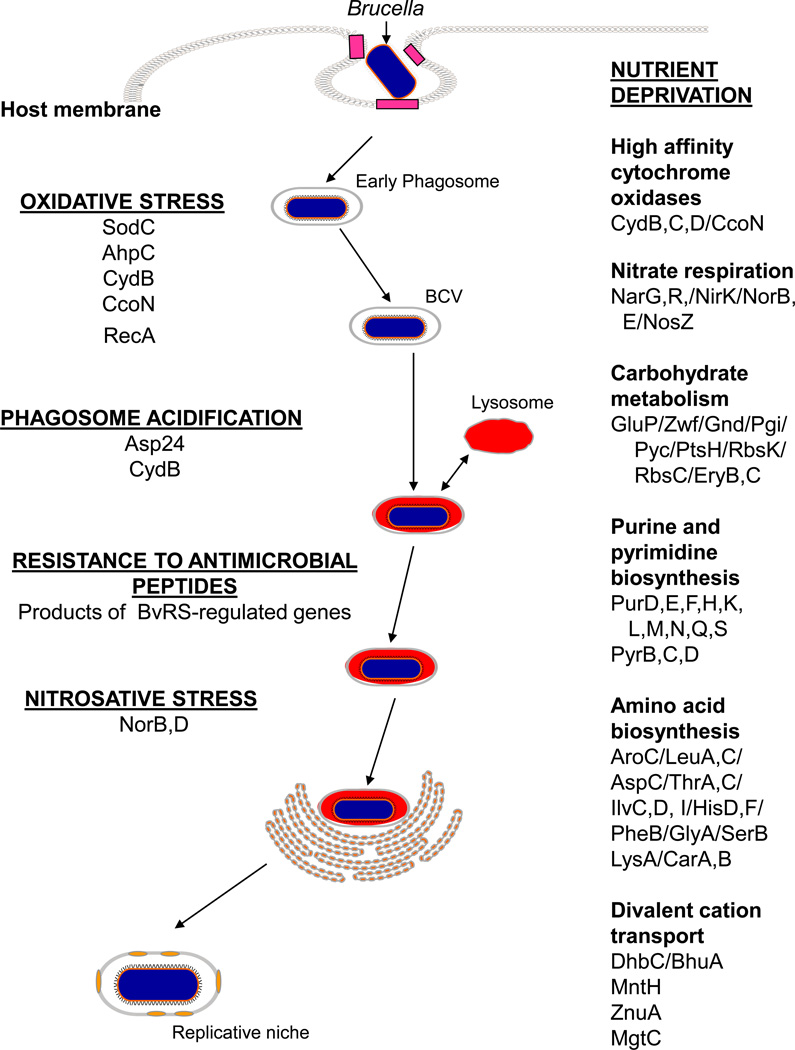
Even though Brucella strains are able to actively alter the intracellular trafficking of the host cell vacuoles within which they reside and avoid the induction of a full scale inflammatory response, these bacteria still encounter formidable environmental stresses during their interactions with macrophages. These stresses include exposure to reactive oxygen (ROS) and nitrogen species (RNS), exposure to acidic pH, nutritional deprivation and at least transient exposure to the lytic peptides contained in lysosomes (Figure 3). Correspondingly, the brucellae are well equipped from both a physiologic and metabolic standpoint to withstand these environmental stresses [101,156]. This trait undoubtedly plays an important role in the success with which Brucella strains maintain prolonged residence in these host phagocytes. The intracellular “stresses” encountered by the brucellae within non-professional phagocytes such as epithelial cells are less severe than those encountered in professional phagocytes [128].
Figure 3.
Gene products that play important roles in allowing Brucella strains to resist the environmental stresses they encounter during intracellular replication in the host.
Resistance to oxidative damage
Brucella strains generate ROS such as O2− and H2O2 endogenously as a consequence of their aerobic respiratory-type metabolism [149]. Exogenous production of these ROS has also been shown to be important for the brucellacidal activity of macrophages [90]. Because O2− is a charged molecule, it does not readily cross bacterial membranes. Consequently, bacteria have compartmentalized defenses against this ROS. Periplasmic superoxide dismutases such as the Cu/Zn SOD, for instance, are important for protecting bacteria from O2− of exogenous origin [115]. Cytoplasmic SOD such as the Mn SOD (SodA) or Fe SOD (SodB), on the other hand, protect bacterial cells from endogenous O2− generated by aerobic metabolism. Brucella strains produce both SodC and SodA [175]. Studies have shown that SodC plays an important role in protecting B. abortus 2308 from the respiratory burst of host macrophages [74] and is required for maintenance of chronic infection in the mouse model [74,181]. Genetic analysis of Brucella sodA mutants indicates that SodA plays a major role in protecting these bacteria from the endogenous O2− that is generated by aerobic metabolism (Baumgartner and Martin, unpublished). The importance of SodA to Brucella strains during their residence in the host is presently under investigation.
Two major antioxidants with the capacity to degrade H2O2 have been described in Brucella strains, the periplasmic monofunctional catalase KatE [165] and the peroxiredoxin AhpC [155]. Genetic studies have shown that KatE detoxifies supraphysiologic levels of H2O2 [73,95,165] in these bacteria, while AhpC appears to be the major scavenger of the endogenous H2O2 that is generated by aerobic metabolism (Steele, manuscript in preparation). B. abortus ahpC and katE mutants exhibit wild-type virulence in experimentally infected mice [Steele, manuscript in preparation; 162] and a B. melitensis katE mutant produces abortion and fetal pathology in pregnant goats [73]. A B. abortus ahpC katE double mutant, in contrast, displays severe attenuation in both the C57BL6 and BALB/c mouse models (Steele, manuscript in preparation), and this attenuation is not diminished in C57BL6 knockout mice lacking a functional NADP oxidase or inducible nitric oxide synthase. These experimental findings indicate that, unlike SodC, neither AhpC and KatE plays a direct role in protecing Brucella strains from the oxidative or nitrosative bursts of host phagocytes. Rather, the combination of these two antioxidants appears to provides the brucellae with an efficient means of protecting themselves from potentially lethal levels of endogenous H2O2 that are generated as a consequence of their respiratory metabolism during residence in the host.
In addition to their ability to directly detoxify ROS, the brucellae also appear to have developed mechanisms for indirectly avoiding oxidative damage. Cytochrome bd ubiquinol oxidases and the cbb3-type cytochrome c oxidases have high affinity for O2, and the O2 “scavenging” capacity of these terminal cytochrome oxidases has been linked to the prevention of ROS toxicity in other bacteria [54,143]. Cytochrome bd ubiquinol oxidase and the cbb3-type cytochrome c oxidase are both required for wild-type virulence of Brucella strains in cell cultures and experimentally infected mice [59,91], and increased sensitivity to ROS has been experimentally linked to the attenuation of a B. abortus abortus cydB [59].
DNA is a target of ROS-mediated damage in all living cells, and experimental evidence indicates that DNA repair pathways such as base excision repair and recombination repair play an important role in protecting Brucella strains from ROS toxicity in vitro [85,158]. To date, however, recA mutants are the only Brucella strains with defects in DNA repair that have been shown to be attenuated in experimentally infected animals [182].
Resistance to nitrosative damage
Nitric oxide (NO) produced by the inducible nitric oxide synthase (iNOS) of murine macrophages has been shown to play a role in the capacity of these phagocytes to control the intracellular replication of the brucellae [78,90]. Brucella strains produce a nitric oxide reductase (Nor), and genetic studies suggest that in addition to its metabolic role in denitrification (see below), Nor may also play an important role in the detoxification of NO by Brucella strains during their replication in host macrophages [82,113]. Genetic studies have also uncovered a link between a D-alanyl-D-alanine carboxypeptidase (encoded by the dacF gene), expression of norB, and the resistance of B. abortus 544 to NO in vitro and in cultured macrophages [94], but the nature of this link is not readily apparent.
Peroxynitrite (ONOO−) is produced as the product of the reaction of O2− with NO. This ROS-RNI hybrid has potent microbicidal activity, and it is considered to be an important component of the antibacterial arsenal of host macrophages [62]. In addition to their ability to detoxify H2O2 and organic peroxides, the AhpC proteins from Salmonella, Mycobacterium and Helicobacter have also been shown to have peroxynitrite reductase activity in vitro [28]. Genetic studies suggest that AhpC protects Mycobacterium strains from exposure to ONOO− in vitro and is required for their virulence in cultured macrophages [121] and guinea pigs [192]. A B. abortus ahpC mutant exhibits increased sensitivity to ONOO− generated by the compound SIN-1 in in vitro assays (K. Steele, unpublished), but whether or not AhpC protects this strain from exposure to ONOO− in host macrophages remains to be determined experimentally.
Resistance to acidic pH
It is well documented that the intracellular compartment within which Brucella strains reside in cultured macrophages and epithelial cells is acidified during the early stages of its development [53,140]. In fact, if this acidification is blocked by the addition of bafilomycin or neutralized by the addition of NH4Cl during the early stages of development of the Brucella containing vacuole in these cells, the brucellae will not initiate intracellular replication in either cell type. As noted previously, this low pH apparently serves as an important environmental stimulus for the induction of the virB genes which encode the components of the Type IV secretion system [24].
Several gene products have been linked to acid resistance in Brucella strains. HdeA is a periplasmic chaperone that functions at low pH and plays an important role in acid resistance in E. coli [72] and Shigella flexneri [188]. A B. abortus hdeA mutant displays a decreased resistance to acidic pH (e.g. pH 4) compared to its parental strain [185], but this mutant is not attenuated in the mouse model. Asp24 is a putative “EF hands”-type Ca++-binding protein (T. Ficht, personal communication) originally identified in a screen for gene products produced by B. abortus 2308 in response to exposure to acid pH [112]. Notably, Brucella asp24 mutants are attenuated in mice [92] and goats [93] and have been proposed for use as vaccine candidates. Specifically how Asp24 protects Brucella strains from acid stress has not been described. Increased sensitivity to acid pH is a phenotype that has also been reported for Brucella cydB [59] and hfq mutants [152]. In the case of the latter strains, there is an as yet undefined regulatory link between the RNA chaperone Hfq (see below) and hdeA in B. abortus 2308 [185]. The basis for the acid sensitive phenotype of B. abortus cydB mutants is unclear.
Most Brucella strains produce a functional urease that protects these bacteria from extremely low pH (pH 2) under laboratory conditions when urea is present in the growth medium [13,163]. Results from experimental infections in mice suggest that urease may play an important role in protecting Brucella strains from the acidic conditions encountered during passage through the gastrointestinal tract after oral ingestion in the host. These same studies, however, indicate that urease does not play a role in protecting Brucella strains from the acidic conditions encountered within host cells [13,163].
Bacterial glutamate decarboxylases (GadA or GadB) and the associated γ–amino-butyric acid (GABA) exporters (GadC) represent important components of acid resistance because GadC is a proton symporter [31]. Brucella strains possess gadC, but the gadB genes appear to be pseudogenes, and mutational analysis indicates that neither GadB nor GadC contributes to the acid resistance of B. abortus 2308 in vitro or the virulence in this strain in mice [27].
Resistance to antimicrobial peptides
Brucella strains have an inherently higher level of resistance to many of the bactericidal cationic peptides found in mammalian hosts compared to other Gram-negative bacterial pathogens [120]. Experimental evidence indicates that this heightened resistance is linked to the acylation status of the lipid A moiety of the Brucella LPS [117]. Although this trait would be expected to be particularly beneficial to the brucellae in the extracellular environment in the host and during their interactions with neutrophils, this increased resistance to antimicrobial peptides may also provide these bacteria with protection from the lytic peptides contained in lysosomes during the transient interactions of the Brucella-containing vacuole with these organelles in host cells [176].
Resistance to nutrient deprivation
Studies employing both defined mutants as well as those generated by transposon mutagenesis have demonstrated that Brucella strains experience a significant degree of nutritional deprivation during their intracellular replication in host cells. One of the major limiting nutrients in this environment is elemental O2 that can be used as a terminal electron acceptor to fuel the respiratory metabolism of these bacteria. Brucella mutants that lack the cbb3-type cytochrome c oxidase (CcoNOQP), the cytochrome bd ubiquinol oxidase (CydCDAB) or components of the denitrification pathway (NarGHIJK/NirKV/NorBCDEFQ/NosDFLRXYZ) exhibit defective intracellular survival and replication in cultured macrophages [59,82,100,113] and HeLa cells [97] and are attenuated in experimentally infected mice [59,82,91]. The CcoNOQP and CydCDAB complexes allow these bacteria to respire efficiently at low O2 concentrations, while the components of the denitrification pathway allow the bacteria to use NO3− as an alternative terminal electron acceptor instead of O2. A recent comparative study of isogenic B. suis ccoN and cydB mutants in the mouse model [91] and an evaluation of B. melitensis cydAB mutants in pregnant goats [93] indicate that the cbb3-type cytochrome c oxidase may play a more important than the cytochrome bd ubiquinol oxidase in allowing Brucella strains to adjust to the environmental conditions encountered in the host, but this relationship merits further examination.
Brucella strains have a blocked Embden-Meyerhof pathway, and they rely on the pentose phosphate pathway and TCA cycle for the efficient use of carbohydrates as carbon and energy sources [61]. Several studies have shown that intact carbohydrate transport and metabolism pathways are essential for the successful intracellular replication of Brucella strains [67,84,97,100,103,108,194]. As noted in an earlier section, the capacity of B. abortus and B. melitensis strains to use erythritol as a preferred carbon source has been postulated to play an important role in their virulence in ruminant placental trophoblasts. Interestingly, experimental studies also suggest that some of the erythritol metabolism genes are required for the wild type virulence of B. suis 1330 in cultured human and murine macrophages and experimentally infected mice [29,100]. A derivative of B. abortus 2308 with Tn5 inserted into the eryB gene, in contrast, exhibits wild-type virulence in the mouse model [164]. The role that the eryB and eryC genes play in the intracellular survival and replication of B. suis 1330 in murine and human macrophages is unclear [29] since erythritol is not considered to be a major constituent of murine and human tissues.
Multiple investigators have shown that intact purine biosynthesis pathways are essential for efficient replication of Brucella strains in cultured murine [1,194] and human [56,100] macrophages, HeLa cells [97] as well as the wild-type virulence of these strains in mice [1,45] and goats [38]. The biosynthesis of pyrimidines and several classes of amino acids also appear to be required for the successful adaptation of the brucellae to their intracellular niche [67,68,97,100,108,109].
In addition to the macronutrients listed above, a number of micronutrients that are essential for the intracellular replication of Brucella strains have also been identified. One class of micronutrient that appears to be particularly important for these strains is the divalent cations, which serve as co-factors for a wide array of cellular proteins. With the notable exceptions of the Lactobacillus spp. [190] and Borrelia burgdorferi [142], all of the other bacteria that have been examined require iron, and the brucellae are no exception. The activity of two Brucella iron acquisition systems has been described in the literature, and a survey of several publicly available Brucella genome sequences suggests that several more exist [157]. B. abortus 2308 produces two siderophores, 2,3-dihydroxybenzoic acid (2,3-DHBA) [114] and brucebactin [77], in response to iron limitation in vitro. Genetic evidence indicates that brucebactin is derived from 2,3-DHBA, but the structure of brucebactin has not been determined. Although the genes required for the biosynthesis and transport of 2,3-DHBA and brucebactin are strongly expressed in B. abortus 2308 during intracellular replication in cultured murine macrophages [103], neither siderophore is required for the virulence of this strain in cultured murine or human macrophages or in experimentally infected mice [17,77,135, Bellaire, unpublished]. In contrast, B. abortus dhbC mutants (which can produce neither 2,3-DHBA nor brucebactin) are highly attenuated in pregnant cattle [18]. In vitro studies have established a link between siderophore production and the capacity of B. abortus 2308 to efficiently utilize erythritol as a carbon and energy source during growth under iron limiting conditions [19]. To what extent this link contributes to the attenuation of B. abortus dhbC mutants in ruminants, however, remains to be determined.
Brucella strains are also capable of transporting the intact heme molecule [4] and using it as an iron source [137]. A B. abortus mutant lacking the outer membrane heme transporter BhuA cannot maintain chronic spleen infection in experimentally infected mice [137], which suggests that heme represents a major iron source for the brucellae during their intracellular replication in host macrophages. The degradation of the hemoglobin contained in senescent erythrocytes by macrophages plays a central role in the recycling of iron in mammals [47]. Heme is a toxic compound, however, and unless it is directly used by these phagocytes, it is transported to their endoplasmic reticulum for degradation by heme oxygenase [179]. Thus, the possibility exists that one of the benefits to the brucellae of residence in an intracellular compartment that has extensive interaction with the endoplasmic reticulum is that it allows these bacteria access to a critical source of iron.
The efficient transport of Mg++, Zn++ and Mn++ has also been shown to be critical for the success of Brucella strains as intracellular pathogens [7,98,105,195]. Although the brucellae possess a single high affinity Mn++ transporter, MntH, transport of this divalent cation appears to be very important for these bacteria in the mammalian host as mntH mutants exhibit extreme attenuation in mice that lack Nramp1 [7]. This mammalian divalent cation transporter plays a critical role in the metal withholding response in host macrophages that is an important component of the host innate immune response [33], and bacterial Mn++ transport mutants often do not exhibit attenuation in mice unless these animals possess a functional Nramp1 [197]. The precise basis for the importance of Mn++ as a micronutrient for Brucella strains is unknown, but experimental evidence suggests that MntH-mediated Mn transport plays an important role in facilitating optimal production of the Mn SOD in B. abortus 2308. Mn++ transport may also be important for wild-type activity of Rsh, the mediator of the stringent response in this bacterium (see below) [7].
Production of flagella contributes to virulence via an as yet undefined mechanism
Although Brucella strains are uniformly described as being non-motile, genome sequence data suggests that they have the genetic capacity to produce flagella. Moreover, under certain growth conditions B. melitensis 16M produces a polar organelle that resembles a flagellum [70]. Intriguingly, B. melitensis flgI, fliF, fliC, flhA, motB and flgE mutants which do not produce this flagellum are not attenuated in cultured bovine macrophages or HeLa cells, but do exhibit attenuation in experimentally infected mice [70]. A transcriptional regulator, FtcR, that appears to lie downstream of VjbR in the regulatory pathway of the flagellar biosynthesis genes in B. melitensis 16M, has been identified [107], and isogenic ftcR mutants constructed in this strain are also attenuated in mice. The basis for the attenuation of Brucella mutants that are defective in flagellar biosynthesis has not been resolved, but one possibility is that the polar organelle produced by the Brucella “flagellar” genes is a secretion apparatus rather than one linked to motility. The components of bacterial Type III secretion systems share many similarities with those involved in the transport and assembly of flagella [75].
Phosphatidylcholine is a major component of the OM of Brucella strains
Unlike many bacteria, Brucella strains and other α–proteobacteria have outer membranes enriched in phosphatidylcholine (PC), a phospholipid that is typically associated with eukaryotic cell membranes [170]. The presence of PC in the outer membrane appears to be required for the successful interactions of Bradyrhizobium japonicum [124] and Agrobacterium tumefaciens [191] with their plant hosts, and likewise Brucella abortus mutants lacking PC in their outer membranes are attenuated in the mouse model [41,42]. The precise role that PC plays in the virulence of Brucella strains is undefined, but studies suggest that this phospholipid may be important for maintaining the integrity and permeability characteristics of the outer membrane and in particular may be involved in resistance to complement and other antimicrobial peptides [42]. Because PC is a major component of eukaryotic membranes and the degradation of PC by eukaryotic cells produces two important eukaryotic cell-signaling molecules (diacylglycerol and phosphatidic acid); it has also been postulated that the presence of this phospholipid in the Brucella outer membranes plays a role in immune evasion via molecular mimicry [41,42]. Furthermore, studies with Legionella pneumophila [43] raise the possibility that the presence of PC in the outer membrane is important for the proper assembly or function of the components of the T4SS or flagella on the surface of Brucella strains.
The mystery behind the virulence of naturally occurring rough strains of Brucella
It is well-established that the LPS O-chain is a major virulence determinant of B. abortus, B. melitensis and B. suis strains, and O-chain deficient mutants derived from these strains (so-called “rough” mutants) are uniformly attenuated in experimental hosts [3,37,58,76,122,153,183,193]. B. canis and B. ovis strains, in contrast, naturally lack the LPS O-chain, yet they produce disease in their natural hosts. Compared to the naturally-occurring smooth strains, little work has been done on the interactions of B. canis and B. ovis strains with host cells. What can be derived from these studies is that, in general, naturally-occurring rough Brucella strains appear to be taken up into host cells with greater efficiency than smooth strains [52,64,151], but these strains do not replicate as well in host cells as their smooth counterparts [52,64,119]. This may be due to the fact that trafficking studies indicate that BCVs containing B. canis and B. ovis strains fuse more extensively with lysosomes in host cells than BCVs containing smooth Brucella strains [141,151]. Laboratory derived rough B. abortus mutants exhibit cytotoxicity in cultured macrophages [138], and it has been postulated that the spontaneous occurrence of these cytotoxic mutants may facilitate cell-to-cell spread of naturally-occurring smooth strains in vivo. It is notable in this regard, however, that B. canis and B. ovis strains are not cytotoxic for cultured macrophages [138]. It is clear that a lot more needs to be learned about the interactions of B. canis and B. ovis with host cells and how these interactions influence the progression of canine and ovine brucellosis.
Programmatic changes in gene expression required for intracellular survival by the brucellae
BvrRS
A genetic screen for Brucella mutants with decreased resistance to the antimicrobial cationic peptide polymyxin B led to the discovery of the two-component regulator BvrRS in B. abortus [172]. The outer membrane properties of Brucella bvrRS mutants are considerably altered compared to their parental strains [117], and these mutants are highly attenuated and exhibit altered intracellular trafficking patterns in cultured macrophages and HeLa cells [172]. These strains also exhibit significant attenuation in experimentally infected mice [172]. BvrRS controls the expression of the genes encoding the outer membrane proteins Omp3a and Omp3b [80,102] as well as yet undefined genes whose products play a role in proper acylation of the lipid A component of the LPS (Figure 2) [117]. As noted earlier, these latter genes appear to play a major role in the high level of resistance that Brucella strains display to killing by cationic antimicrobial peptides. Altered expression of the omp3a and omp3b genes, in contrast, does not appear to be linked to this phenotypic trait or the attenuation of Brucella bvrRS mutants in mice [118]. Proteomic analysis suggests that numerous other Brucella genes are also subject to either direct or indirect regulation by BvrRS [102], but the individual contributions of these genes to virulence remains to be determined.
From a phylogenetic standpoint it is notable the Brucella BvrRS system is homologous to the Agrobacterium ChvIG and Sinorhizobium ChvI/ExoS two component regulators. These regulators control expression of genes whose products make the modifications of the cell envelope for the wild-type interactions of Agrobacterium [34] and Sinorhizobium [36] strains with their plant hosts. The environmental stimuli recognized by the Brucella BvrS have not been reported, but studies indicate that ChvG senses acidic pH in Agrobacterium tumefaciens [111]. If the same is true for the Brucella BvrS, it is easy to envision the potential benefit of such a regulatory link for the brucellae in successful adaptation to their intracellular niche.
VjbR and BlxR
Cell-to-cell communication via the process known as “quorum sensing” has been shown to be important for the successful adaptation of many bacteria to changing environmental conditions [189]. This process is also critical for the virulence of many bacterial pathogens [30] and the successful establishment of symbiotic relationships [161]. Brucella strains produce an acyl-homoserine lactone (AHL)-type signaling molecule (C12-HSL) [180]. LuxR-type transcriptional regulators respond to AHL in bacterial quorum sensing systems [189], and two of these transcriptional regulators have been identified in Brucella. VjbR [51] controls expression of the virB operon, flagellar biosynthesis genes and genes encoding several outer membrane proteins [184], and vjbR mutants exhibit attenuation in cultured macrophages, HeLa cells and experimentally infected mice [9,51]. Another LuxR-type regulator, designated BlxR [147], has also been shown to play a role in the regulation of the virB and flagellar biosynthesis genes, but comparative studies in mice indicate that the loss of VjbR has a much more dramatic effect on the virulence of B. melitensis 16M than does loss of BlxR. As the authors of this study point out, these findings suggest although VjbR and BlxR are both LuxR homologs, they do not perform functionally redundant roles in B. melitensis 16M [147].
Rsh
Bacteria can undergo a global change in gene expression known as the stringent response when they are faced with severe nutrient deprivation [89]. In response to nutrient deprivation, bacteria produce the alarmone guanosine 3’,5’-bispyrophosphate (ppGpp) via the activity of the ppGpp synthetases RelA or SpoT. This alarmone, in turn, binds to RNA polymerase and changes the efficiency with which it recognizes promoter sequences, leading to reduced expression of genes encoding components of the translational machinery and increased expression of amino acid biosynthetic genes and other genes required for adjusting the cells metabolism to a maintenance mode [116]. Brucella strains produce a single ppGpp synthetase (designated Rsh for RelA/SpoT homolog), and studies have shown that Brucella rsh mutants quickly lose viability when subjected to nutrient deprivation in vitro and are attenuated in cultured macrophages, HeLa cells and experimentally infected mice [55,99]. These experimental findings suggest that the stringent response plays a key role in the successful adaptation of the brucellae to the nutritional deprivation they encounter during intracellular residence in the host. Notably, nutritional deprivation appears to be an important environmental stimulus for induction of the genes encoding the virB genes [24], and the presence of Rsh is required optimal expression of these genes in B. melitensis 16M [55].
Hfq and sRNAs
Small regulatory RNAs (sRNAs) play an important role in regulating the expression of a wide variety of bacterial genes [177]. They perform this function predominantly by interacting with mRNAs and facilitating or interfering with the translation of these transcripts and/or accelerating or delaying their degradation by cellular RNases. Many of these sRNAs have limited complementarities with their mRNA targets and require the participation of the RNA chaperone Hfq for efficient interaction with these transcripts [186]. A B. abortus hfq mutant exhibits increased sensitivity to multiple environmental stresses compared to the parental 2308 strain and is attenuated in cultured murine and human macrophages and experimentally infected mice [20,152]. A B. melitensis hfq mutant is also attenuated in pregnant goats [154] and mice and non-human primates (M. J. Nikolich, personal communication). These experimental findings suggest that sRNAs play an important role in controlling the expression of genes required for successful adaptation of the brucellae to their intracellular niche in the host. Genetic and proteomic studies have linked Hfq to the wild-type expression of the sodC [71,74] and several other genes known to be required for the virulence in B. abortus 2308 [155, Gaines and Caswell, unpublished]. The nature of the regulatory links between Hfq and these genes and the identity of the sRNAs involved is presently under investigation.
NolR, MucR, and the LOV domain histidine kinase
The α–proteobacteria employ similar strategies to establish and maintain sustained interactions with their eukaryotic hosts, and as noted above for BvrRS, homologous regulatory systems appear to be responsible for proper expression of the bacterial genes required for these interactions. A couple of other examples of these shared regulatory networks have recently been discovered. NolR is a transcriptional regulator that controls the expression of nodulation genes in Sinorhizobium meliloti [35]. A targeted mutational analysis of genes predicted to encode transcriptional regulators in B. melitensis 16M has shown that a NolR homolog is required for wild-type expression of the virB genes and the virulence of this strain in cultured murine macrophages, HeLa cells and mice [81]. The transcriptional regulator MucR provides a regulatory link between exopolysaccharide synthesis and motility in S. meliloti [11], and insertion of the mariner transposon Himar1 into a mucR homolog in B. melitensis 16M severely attenuates this strain in macrophages and mice [194].
A histidine kinase carrying an LOV (light, oxygen, or voltage) domain has recently been identified in Brucella strains [178]. Biochemical studies indicate that this protein is responsive to light, and genetic studies have shown that B. abortus strains lacking this protein or carrying a mutated version of the protein that is not light-responsive are attenuated in the murine J774 macrophage-like cell line. Because of their close association with the host, it is unclear when exposure to blue light would be a relevant environmental stimulus. One proposition that has been put forth is that this exposure may take place when the brucellae are expelled into the environment in the infected placenta, and this exposure may stimulate the expression of Brucella genes important for initiating infection in a newly infected host [178].
Summary
The capacity of Brucella strains to successfully survive and replicate in host cells is critical to their virulence. The brucellae employ several strategies to establish and maintain persistent intracellular residence in host cells. These bacteria are able to avoid a full blown inflammatory response during the initial stages of infecting the host. Once the brucellae enter into host cells they proactively influence the intracellular trafficking of the membrane bound compartments within which they reside so that these vacuoles avoid becoming “phagolysosomes”. After the brucellae reach the vacuolar compartments within which they maintain their intracellular residence, they are well equipped from a physiologic standpoint to withstand the environmental stresses they encounter. The bacteria also appear to exploit some of the environmental stresses they encounter (e.g. acidic pH and nutrient deprivation) as stimuli for the induction of genes required for alteration of their intracellular trafficking. Finally, the intracellular brucellae alter the biological functions of the professional phagocytes within which they reside in such a manner that these cells lose their antigen-processing capacity and in the case of macrophages, become resistant to apoptosis.
Many of the cell components and strategies that the brucellae employ to successfully adapt to their intracellular lifestyle and produce chronic infections in the host appear to be same as those employed by other α–proteobacteria to establish and maintain prolonged infections of their plant and animal hosts [16]. Recent comparative studies with the closely related bacterium Ochrobactrum anthropi also provide insight into evolutionary pathways that the brucellae have followed to improve their adaptation to the specific challenges associated with their prolonged residence in mammalian cells [15]. Continued efforts to better understand how these remarkable intracellular pathogens have adapted to their intracellular niche will undoubtedly provide us with critical information that is needed for the rational design of better vaccines and chemotherapy for use against brucellosis in both natural hosts and in humans.
Acknowledgements
Research in the laboratory of RMR is supported by grants (AI48499 and AI63516) from the National Institutes of Allergy and Infectious Disease.
References
- 1.Alcantara RB, Read RD, Valderas MW, Brown TD, Roop RM., II Intact purine biosynthesis pathways are required for wild-type virulence of Brucella abortus 2308 in the BALB/c mouse model. Infect. Immun. 2004;72:4911–4917. doi: 10.1128/IAI.72.8.4911-4917.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander B, Schnurrenberger PR, Brown RR. Numbers of Brucella abortus in the placenta, umbilicus and fetal fluid of two naturally infected cows. Vet. Rec. 1981;108:500. doi: 10.1136/vr.108.23.500. [DOI] [PubMed] [Google Scholar]
- 3.Allen CA, Adams LG, Ficht TA. Transposon-derived Brucella abortus rough mutants are attenuated and exhibit reduced intracellular survival. Infect. Immun. 1998;66:1008–1016. doi: 10.1128/iai.66.3.1008-1016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almirón M, Martínez M, Sanjuan N, Ugalde RA. Ferrochelatase is present in Brucella abortus and is critical for its intracellular survival and virulence. Infect. Immun. 2001;69:6225–6230. doi: 10.1128/IAI.69.10.6225-6230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alton GG. Brucella melitensis. In: Nielsen K, Duncan JR, editors. Animal brucellosis. Boca Raton, FL: CRC Press; 1990. pp. 383–409. [Google Scholar]
- 6.Alton GG. Brucella suis. In: Nielsen K, Duncan JR, editors. Animal brucellosis. Boca Raton, FL: CRC Press; 1990. pp. 411–422. [Google Scholar]
- 7.Anderson ES, Paulley JT, Gaines JM, Valderas MW, Martin DW, Menscher E, Brown TD, Burns CS, Roop RM., II The manganese transporter MntH is a critical virulence determinant for Brucella abortus 2308 in experimentally infected mice. Infect. Immun. 2009;77:3466–3474. doi: 10.1128/IAI.00444-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arenas GN, Staskevich AS, Aballay J, Mayorga LS. Intracellular trafficking of Brucella abortus in J774 macrophages. Infect. Immun. 2000;68:4255–4263. doi: 10.1128/iai.68.7.4255-4263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Rice-Ficht AC. Immunization with a single dose of a microencapsulated Brucella melitensis mutant enhances protection against wild-type challenge. Infect. Immun. 2008;76:2448–2455. doi: 10.1128/IAI.00767-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arrelano-Reynoso B, Lapaque N, Salcedo S, Briones G, Ciocchini AE, Ugalde R, Moreno E, Moriyón I, Gorvel JP. Cyclic β-1,2-glucan is a Brucella virulence factor required for intracellular survival. Nature Immunol. 2005;6:618–625. doi: 10.1038/ni1202. [DOI] [PubMed] [Google Scholar]
- 11.Bahlawane C, McIntosh M, Krol E, Becker A. Sinorhizobium meliloti regulator MucR couples exopolysaccharide synthesis and motility. Mol. Plant. Microbe Inter. 2008;21:1498–1509. doi: 10.1094/MPMI-21-11-1498. [DOI] [PubMed] [Google Scholar]
- 12.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 13.Bandara AB, Contreras A, Contreras-Rodriguez A, Martins AM, Dobrean V, Poff-Richow S, Rajasekaran P, Sriranganathan N, Schurig GG, Boyle SM. Brucella suis urease encoded by ure1 but not ure2 is necessary for intestinal infection of BALB/c mice. BMC Microbiol. 2007;7:57. doi: 10.1186/1471-2180-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barquero-Calvo E, Chaves-Olarte E, Weiss DS, Guzmán-Verri C, Chacón-Díaz C, Rucavado A, Moriyón I, Moreno E. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS ONE. 2007;7:e631. doi: 10.1371/journal.pone.0000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barquero-Calvo E, Conde-Alvarez R, Chacón-Díaz C, Quesada-Lobo L, Martirosyan A, Guzmán-Verri C, Iriarte M, Mancek-Keber M, Jerala R, Gorvel JP, Moriyón I, Moreno E, Chaves-Olarte E. The differential interaction of Brucella and Ochrobactrum with innate immunity reveals traits related to the evolution of stealthy pathogens. PLoS ONE. 2009;4:e5893. doi: 10.1371/journal.pone.0005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batut J, Andersson SGE, O’Callaghan D. The evolution of chronic infection strategies in the α–proteobacteria. Nature Rev. Microbiol. 2004;2:933–945. doi: 10.1038/nrmicro1044. [DOI] [PubMed] [Google Scholar]
- 17.Bellaire BH, Elzer PH, Baldwin CL, Roop RM., II The siderophore 2,3-dihydroxybenzoic acid is not required for virulence of Brucella abortus in BALB/c mice. Infect. Immun. 1999;67:2615–2618. doi: 10.1128/iai.67.5.2615-2618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bellaire BH, Elzer PH, Hagius S, Walker J, Baldwin CL, Roop RM., II Genetic organization and iron-responsive regulation of the Brucella abortus 2,3-dihydoxybenzoic acid biosynthesis operon, a cluster of genes required for wild-type virulence in pregnant cattle. Infect. Immun. 2003;71:1794–1803. doi: 10.1128/IAI.71.4.1794-1803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellaire BH, Elzer PH, Baldwin CL, Roop RM., II Production of the siderophore 2,3-dihydroxybenzoic acid is required for wild-type growth of Brucella abortus in the presence of erythritol under low-iron conditions in vitro. Infect. Immun. 2003;71:2927–2932. doi: 10.1128/IAI.71.5.2927-2932.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellaire BH, Roop RM, II, Cardelli JA. Opsonized virulent Brucella abortus replicates within nonacidic, endoplasmic reticulum-negative, LAMP-1-positive phagosomes in human monocytes. Infect. Immun. 2005;73:3702–3713. doi: 10.1128/IAI.73.6.3702-3713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billard E, Cazevieille C, Dornand J, Gross A. High susceptibility of human dendritic cells to invasion by the intracellular pathogens Brucella suis B. abortus and B. melitensis. Infect. Immun. 2005;73:8418–8424. doi: 10.1128/IAI.73.12.8418-8424.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billard E, Dornand J, Gross A. Brucella suis prevents human dendritic cell maturation and antigen presentation through regulation of tumor necrosis factor alpha secretion. Infect. Immun. 2007;75:4980–4989. doi: 10.1128/IAI.00637-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blasco JM. Epididymite contagieuse du belier ou infection à Brucella ovis. In: Lefevre PC, Blancou J, Chermette R, editors. Principales maladies infectieuses et parasitaires du bétail. Lavoiser: Paris; 2003. pp. 905–917. [Google Scholar]
- 24.Boschiroli ML, Ouahrani-Bettache S, Foulongne V, Michaux-Charachon S, Bourg G, Allardet-Servent A, Cazevieille C, Liautard JP, Ramuz M, O’Callaghan D. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. USA. 2002;99:1544–1549. doi: 10.1073/pnas.032514299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boussau B, Karlberg EO, Frank AC, Legault BA, Andersson SGE. Computational inference of scenarios for α–proteobacterial genome evolution. Proc. Natl. Acad. Sci. USA. 2004;101:9722–9727. doi: 10.1073/pnas.0400975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briones G, Iñón de Iannino N, Roset M, Vigliocco A, Paulo PS, Ugalde RA. Brucella abortus cyclic β-1,2-glucan mutants have reduced virulence in mice and are defective in intracellular replication in HeLa cells. Infect. Immun. 2001;69:4528–4535. doi: 10.1128/IAI.69.7.4528-4535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown TD. M.S. thesis. East Carolina University; 2007. The glutamate decarboxylase genes, gadBC, are not required for Brucella abortus reistance to low pH or virulence in the mouse model. [Google Scholar]
- 28.Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 29.Burkhardt S, Jiménez de Bagüés MP, Liautard JP, Köhler S. Analysis of the behavior of eryC mutants of Brucella suis attenuated in macrophages. Infect. Immun. 2005;73:6782–6790. doi: 10.1128/IAI.73.10.6782-6790.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castanie-Cornet MP, Penfound TA, Smith D, Elliott JF, Foster JW. Control of acid resistance in Escherichia coli. J. Bacteriol. 1999;181:3525–3535. doi: 10.1128/jb.181.11.3525-3535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Celli J, de Chastellier C, Franchini DM, Pizarro-Cerda J, Moreno E, Gorvel JP. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 2003;198:545–556. doi: 10.1084/jem.20030088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cellier MF, Courville P, Campion C. Nramp1 phagocytic intracellular metal withdrawal defense. Microbes Infect. 2007;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Charles TC, Nester EW. A chromosomally encoded two-component sensory transduction system is required for virulence of Agrobacterium tumefaciens. J. Bacteriol. 1993;175:6614–6625. doi: 10.1128/jb.175.20.6614-6625.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H, Gao K, Kondrosi E, Kondrosi A, Rolfe BG. Functional genomic analysis of global regulator NolR in Sinorhizobium meliloti. Mol. Plant Microbe Interact. 2005;18:1340–1352. doi: 10.1094/MPMI-18-1340. [DOI] [PubMed] [Google Scholar]
- 36.Cheng HP, Walker GC. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J. Bacteriol. 1998;180:20–26. doi: 10.1128/jb.180.1.20-26.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheville NF, Jensen AE, Halling SM, Tatum FM, Morfitt DC, Hennager SG, Frerichs WM, Schurig G. Bacterial survival, lymph node changes, and immunologic responses of cattle vaccinated with standard and mutant strains of Brucella abortus. Am. J. Vet. Res. 1992;53:1881–1888. [PubMed] [Google Scholar]
- 38.Cheville NF, Olsen SC, Jensen AE, Stevens MG, Florance AM, Houng HSH, Drazek ES, Warren RL, Hadfield TL, Hoover DL. Bacterial persistence and immunity in goats vaccinated with a purE deletion mutant or the parental 16M strain of Brucella melitensis. Infect. Immun. 1996;64:2431–2439. doi: 10.1128/iai.64.7.2431-2439.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cirl C, Wieser A, Yadav M, Duerr S, Schubert S, Fischer H, Stappert D, Wantia N, Rodriguez N, Wagner H, Svanborg C, Miethke T. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nature Med. 2008;14:399–406. doi: 10.1038/nm1734. [DOI] [PubMed] [Google Scholar]
- 40.Comerci DJ, Martinez-Lorenzo MJ, Sieira R, Gorvel JP, Ugalde RA. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 2001;3:159–168. doi: 10.1046/j.1462-5822.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 41.Comerci DJ, Altabe S, de Mendoza D, Ugalde RA. Brucella abortus synthesizes phosphatidylcholine from choline provided by the host. J. Bacteriol. 2006;188:1929–1934. doi: 10.1128/JB.188.5.1929-1934.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conde-Alvarez R, Grilló MJ, Salcedo SP, de Miguel MJ, Fugier E, Gorvel JP, Moriyón I, Iriarte M. Synthesis of phosphatidylcholine, a typical eukaryotic phospholipid, is necessary for full virulence of the intracellular bacterial parasite Brucella abortus. Cell. Microbiol. 2006;8:1322–1335. doi: 10.1111/j.1462-5822.2006.00712.x. [DOI] [PubMed] [Google Scholar]
- 43.Conover GM, Martinez-Morales F, Heidtman MI, Luo ZQ, Tang M, Chen C, Geiger O, Isberg RR. Phosphatidylcholine synthesis is required for optimal functions of Legionella pneumophila virulence determinants. Cell. Microbiol. 2008;10:514–528. doi: 10.1111/j.1462-5822.2007.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corbel MJ. Brucellosis: an overview. Emerg. Infect. Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crawford RM, van der Verg L, Yuan L, Hadfield TL, Warren RL, Drazek ES, Houng HSH, Hammack C, Sasala K, Polsinelli T, Thompson J, Hoover DL. Deletion of purE attenuates Brucella melitensis in mice. Infect. Immun. 1996;64:2188–2192. doi: 10.1128/iai.64.6.2188-2192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crawford RP, Huber JD, Adams BS. Epidemiology and surveillance. In: Nielsen K, Duncan JR, editors. Animal brucellosis. Boca Raton, FL: CRC Press; 1990. pp. 131–151. [Google Scholar]
- 47.Crichton RR, Wilmet S, Legssyer R, Ward RJ. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. J. Inorg. Biochem. 2002;91:9–18. doi: 10.1016/s0162-0134(02)00461-0. [DOI] [PubMed] [Google Scholar]
- 48.Dawson CE, Stubberfield EJ, Perrett LL, King AC, Whatmore AM, Bashiruddin JB, Stack JA, MacMillan AP. Phenotypic and molecular characterization of Brucella isolates from marine mammals. BMC Microbiol. 2008;8:224. doi: 10.1186/1471-2180-8-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Jong MF, Sun YH, den Hartigh AB, van Dijl JM, Tsolis RM. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol. Microbiol. 2008;70:1378–1396. doi: 10.1111/j.1365-2958.2008.06487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delrue RM, Martinez-Lorenzo M, Lestrate P, Danese I, Bielarz V, Mertens P, de Bolle X, Tibor A, Gorvel JP, Letesson JJ. Identification of Brucella spp. genes involved in intracellular trafficking. Cell. Microbiol. 2001;3:487–497. doi: 10.1046/j.1462-5822.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 51.Delrue RM, Deschamps C, Leonard S, Nijksens C, Danese I, Schaus JM, Bonnot S, Ferooz J, Tibor A, de Bolle X, Letesson JJ. A quorum-sensing regulator controls expression of both the type IV secretion system and the flagellar apparatus of Brucella melitensis. Cell. Microbiol. 2005;7:1151–1161. doi: 10.1111/j.1462-5822.2005.00543.x. [DOI] [PubMed] [Google Scholar]
- 52.Detilleux PG, Deyoe BL, Cheville NF. Entry and intracellular localization of Brucella spp. in Vero cells: fluorescence and electron microscopy. Vet. Pathol. 1990;27:317–328. doi: 10.1177/030098589002700503. [DOI] [PubMed] [Google Scholar]
- 53.Detilleux PG, Deyoe BL, Cheville NF. Effect of endocytic and metabolic inhibitors on the internalization and intracellular growth of Brucella abortus in Vero cells. Am. J. Vet. Res. 1991;52:1658–1664. [PubMed] [Google Scholar]
- 54.D’mello R, Hill S, Poole RK. The cytochrome bd quinol oxidase in Escherichia coli has an extremely high oxygen affinity and two oxygen-binding haems: implications for regulation of activity in vivo by oxygen inhibition. Microbiology. 1996;142:755–763. doi: 10.1099/00221287-142-4-755. [DOI] [PubMed] [Google Scholar]
- 55.Dozot M, Boigegrain RA, Delrue RM, Hallez R, Ouahrani-Bettache S, Danese I, Letesson JJ, de Bolle X, Köhler S. The stringent response mediator Rsh is required for Brucella melitensis and Brucella suis virulence, and for expression of the type IV secretion system virB. Cell. Microbiol. 2006;8:1791–1802. doi: 10.1111/j.1462-5822.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- 56.Drazek ES, Houng HSH, Crawford RM, Hadfield TL, Hoover DL, Warren RL. Deletion of purE attenuates Brucella melitensis 16M for growth in human monocyte-derived macrophages. Infect. Immun. 1995;63:3297–3301. doi: 10.1128/iai.63.9.3297-3301.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dylan T, Ielpi L, Stanfield S, Kashyap L, Douglas C, Yanofsky M, Nester EW, Helinski DR, Ditta G. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc. Natl. Acad. Sci. USA. 1986;83:4403–4407. doi: 10.1073/pnas.83.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Elzer PH, Enright FM, McQuiston JR, Boyle SM, Schurig GG. Evaluation of a rough mutant of Brucella melitensis in pregnant goats. Res. Vet. Sci. 1998;64:259–260. doi: 10.1016/s0034-5288(98)90135-7. [DOI] [PubMed] [Google Scholar]
- 59.Endley S, McMurray D, Ficht TA. Interruption of the cydB locus in Brucella abortus attenuates intracellular survival and virulence in the mouse model of infection. J. Bacteriol. 2001;183:2454–2462. doi: 10.1128/JB.183.8.2454-2462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Enright FM. The pathogenesis and pathobiology of Brucella infection in domestic animals. In: Nielsen K, Duncan JR, editors. Animal brucellosis. Boca Raton, FL: CRC Press; 1990. pp. 301–320. [Google Scholar]
- 61.Essenberg RC, Seshadri R, Nelson K, Paulsen I. Sugar metabolism by brucellae. Vet. Microbiol. 2002;90:249–261. doi: 10.1016/s0378-1135(02)00212-2. [DOI] [PubMed] [Google Scholar]
- 62.Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nature Rev. Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 63.Ferguson GP, Datta A, Baumgartner J, Roop RM, II, Carlson RW, Walker GC. Similarity to peroxisomal-membrane protein family reveals that Sinorhizobium and Brucella BacA affect lipid-A fatty acids. Proc. Natl. Acad. Sci. USA. 2004;101:5012–5017. doi: 10.1073/pnas.0307137101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferrero MC, Fossati CA, Baldi PC. Smooth Brucella strain invade and replicate in human lung epithelial cells without inducing cell death. Microbes Infect. 2009;11:476–483. doi: 10.1016/j.micinf.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 65.Forestier C, Moreno E, Pizarro-Cerda J, Gorvel JP. Lysosomal accumulation and recycling of lipopolysaccharide to the cell surface of murine macrophages, an in vitro and in vivo study. J. Immunol. 1999;162:6784–6791. [PubMed] [Google Scholar]
- 66.Forestier C, Deleuil F, Lapaque N, Moreno E, Gorvel JP. Brucella abortus lipopolysaccharide in murine peritoneal macrophages acts as a down-regulator of T cell activation. J. Immunol. 2000;165:5202–5210. doi: 10.4049/jimmunol.165.9.5202. [DOI] [PubMed] [Google Scholar]
- 67.Foulongne V, Bourg G, Cazevieille C, Michaux-Charachon S, O’Callaghan D. Identification of Brucella suis genes affecting intracellular survival in an in vitro human macrophage infection model by signature-tagged transposon mutagenesis. Infect. Immun. 2000;68:1297–1303. doi: 10.1128/iai.68.3.1297-1303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foulongne V, Walravens K, Bourg G, Boschiroli ML, Godfroid J, Ramuz M, O’Callaghan D. Aromatic compound-dependent Brucella suis is attenuated in both cultured cells and mouse models. Infect. Immun. 2001;69:547–550. doi: 10.1128/IAI.69.1.547-550.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Franz DR, Jahrling PB, Friedlander AM, McClain DJ, Hoover DL, Bryne WR, Pavlin JA, Christopher GW, Eitzen EM. Clinical recognition and management of patients exposed to biological warfare agents. JAMA. 1997;278:399–411. doi: 10.1001/jama.278.5.399. [DOI] [PubMed] [Google Scholar]
- 70.Fretin D, Fauconnier A, Köhler S, Halling S, Leonard S, Nisjkens C, Ferooz J, Lestrate P, Delrue RM, Danese I, Vandenhaute J, Tibor A, de Bolle X, Letesson JJ. The sheathed flagellum of Brucella melitensis is involved in persistence in a murine model of infection. Cell. Microbiol. 2005;7:687–698. doi: 10.1111/j.1462-5822.2005.00502.x. [DOI] [PubMed] [Google Scholar]
- 71.Gaines J, Tjaden B, Carroll B, Baumgartner J, Anderson E, Roop R. The small RNA regulatory twist on expression of sodC in Brucella abortus 2308. Abstr. 109th Gen. Meet. Amer. Soc. Microbiol., Abstr. 2009:B-042. [Google Scholar]
- 72.Gajiwala KS, Burley SK. HdeA, a periplasmic protein that supports acid resistance in pathogenic enteric bacteria. J. Mol. Biol. 2000;295:605–612. doi: 10.1006/jmbi.1999.3347. [DOI] [PubMed] [Google Scholar]
- 73.Gee JM, Kovach ME, Grippe VK, Hagius S, Walker JV, Elzer PH, Roop RM., II Role of catalase in the virulence of Brucella melitensis in pregnant goats. Vet. Microbiol. 2004;102:111–115. doi: 10.1016/j.vetmic.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Gee JM, Valderas MW, Kovach ME, Grippe VK, Robertson GT, Ng WL, Richardson JM, Winkler ME, Roop RM., II The Brucella abortus Cu,Zn superoxide dismutase is required for optimal resistance to oxidative killing by murine macrophages and wild-type virulence in experimentally infected mice. Infect. Immun. 2005;73:2873–2880. doi: 10.1128/IAI.73.5.2873-2880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghosh P. Process of protein transport by the Type III secretion system. Microbiol. Mol. Biol. Rev. 2004;68:771–795. doi: 10.1128/MMBR.68.4.771-795.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Godfroid F, Taminiau B, Danese I, Denoel P, Tibor A, Weynants V, Cloeckaert A, Godfroid J, Letesson JJ. Identification of the perosamine synthetase gene of Brucella melitensis 16M and involvement of lipopolysaccharide O side chain in Brucella survival in mice and in macrophages. Infect. Immun. 1998;66:5485–5493. doi: 10.1128/iai.66.11.5485-5493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.González-Carreró MI, Sangari FJ, Agüero J, García-Lobo JM. Brucella abortus 2308 produces brucebactin, a highly efficient catecholic siderophore. Microbiology. 2002;148:353–360. doi: 10.1099/00221287-148-2-353. [DOI] [PubMed] [Google Scholar]
- 78.Gross A, Spiesser S, Terraza A, Rouot B, Caron E, Dornand J. Expression and bactericidal activity of nitric oxide synthase in Brucella suis-infected murine macrophages. Infect. Immun. 1998;66:1309–1316. doi: 10.1128/iai.66.4.1309-1316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gross A, Terraza A, Ouahrani-Bettache S, Liautard JP, Dornand J. In vitro Brucella suis infection prevent the programmed cell death of human monocytic cells. Infect. Immun. 2000;68:342–351. doi: 10.1128/iai.68.1.342-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guzmán-Verri C, Manterola L, Sola-Landa A, Parra A, Cloeckaert A, Garin J, Gorvel JP, Moriyón I, Moreno E, López-Goñi I. The two-component system BvrR/BvrS essential for Brucella abortus virulence regulates the expression of outer membrane proteins with counterparts in members of the Rhizobiaceae. Proc. Nat. Acad. Sci. USA. 2002;99:12375–12380. doi: 10.1073/pnas.192439399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haine V, Sinon A, Van Steen F, Rousseau S, Dozot M, Lestrate P, Lambert C, Letesson JJ, de Bolle X. Systematic targeted mutagenesis of Brucella melitensis 16M reveals a major role for GntR regulators in the control of virulence. Infect. Immun. 2005;73:5578–5586. doi: 10.1128/IAI.73.9.5578-5586.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haine V, Dozot M, Dornand J, Letesson JJ, de Bolle X. NnrA is required for full virulence and regulates several Brucella melitensis denitrification genes. J. Bacteriol. 2006;188:1615–1619. doi: 10.1128/JB.188.4.1615-1619.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hernandez-Mora G, Gonzalez-Barrientos R, Morales JA, Chaves-Olarte E, Guzman-Verri C, Baquero-Calvo E, De-Miguel MJ, Marin CM, Blasco JM, Moreno E. Neurobrucellosis in stranded dolphins, Costa Rica. Emer. Infect. Dis. 2008;14:1430–1433. doi: 10.3201/eid1409.071056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hong PC, Tsolis RM, Ficht TA. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 2000;68:4102–4107. doi: 10.1128/iai.68.7.4102-4107.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hornback ML, Roop RM., II The Brucella abortus xthA-1 gene product participates in base excision repair and resistance to oxidative killing but is not required for wild-type virulence in the mouse model. J. Bacteriol. 2006;188:1295–1300. doi: 10.1128/JB.188.4.1295-1300.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Igwebuike UM. Trophoblast cells of ruminant planentas – a minireview. Anim. Reprod. Sci. 2006;93:185–198. doi: 10.1016/j.anireprosci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Iñón de Iannino N, Briones G, Tomalsky M, Ugalde RA. Molecular cloning and characterization of cgs, the Brucella abortus cyclic β(1–2) glucan synthetase gene: genetic complementation of Rhizobium meliloti ndvB and Agrobacterium tumefaciens chvB mutants. J. Bacteriol. 1998;180:4392–4400. doi: 10.1128/jb.180.17.4392-4400.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iriarte M, González D, Delrue RM, Monreal D, Conde R, López-Goñi I, Letesson JJ, Moriyón I. Brucella lipopolysaccharide: structure, biosynthesis and genetics. In: López-Goñi I, Moriyón I, editors. Brucella: molecular and cellular biology. Norfolk, U. K.: Horizon Bioscience; 2004. pp. 159–191. [Google Scholar]
- 89.Jain V, Kumar M, Chatterji D. ppGpp: stringent response and survival. J. Microbiol. 2006;44:1–10. [PubMed] [Google Scholar]
- 90.Jiang X, Leonard B, Benson R, Baldwin CL. Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell. Immunol. 1993;151:309–319. doi: 10.1006/cimm.1993.1241. [DOI] [PubMed] [Google Scholar]
- 91.Jiménez de Bagüés MP, Loisel-Meyer S, Liautard JP, Jubier-Maurin V. Different roles of the two high-oxygen-affinity terminal oxidases of Brucella suis: cytochrome c oxidase, but not ubiquinol oxidase, is required for persistence in mice. Infect. Immun. 2007;75:531–535. doi: 10.1128/IAI.01185-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kahl-McDonough MM, Ficht TA. Evaluation of protection afforded by Brucella abortus and Brucella melitensis unmarked deletion mutants exhibiting different rates of clearance in BALB/c mice. Infect. Immun. 2006;74:4048–4057. doi: 10.1128/IAI.01787-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kahl-McDonough MM, Elzer PH, Hagius SD, Walker JV, Perry QL, Seabury CM, den Hartigh AB, Tsolis RM, Adams LG, Davis DS, Ficht TA. Evaluation of novel Brucella melitensis unmarked deletion mutants for safety and efficacy in the goat model of brucellosis. Vaccine. 2006;24:5169–5177. doi: 10.1016/j.vaccine.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Kikuchi H, Kim S, Watanabe K, Watarai M. Brucella abortus D-alanyl-D-alanine carboxypeptidase contributes to its intracellular replication and resistance against nitric oxide. FEMS Microbiol. Lett. 2006;259:120–125. doi: 10.1111/j.1574-6968.2006.00253.x. [DOI] [PubMed] [Google Scholar]
- 95.Kim JA, Sha Z, Mayfield JE. Regulation of Brucella abortus catalase. Infect. Immun. 2000;68:3681–3866. doi: 10.1128/iai.68.7.3861-3866.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim S, Watarai M, Makino S, Shirahata T. Membrane sorting during swimming internalization of Brucella is required for phagosome trafficking decisions. Microb. Pathogen. 2002;33:225–237. doi: 10.1006/mpat.2002.0531. [DOI] [PubMed] [Google Scholar]
- 97.Kim S, Watarai M, Kondo Y, Erdenebaatar J, Makino S, Shirahata T. Isolation and characterization of mini-Tn5Km2 insertion mutants of Brucella abortus deficient in internalization and intracellular growth in HeLa cells. Infect. Immun. 2003;71:3020–3027. doi: 10.1128/IAI.71.6.3020-3027.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim S, Watanabe K, Shirahata T, Watarai M. Zinc uptake system (znuA locus) of Brucella abortus is essential for intracellular survival and virulence in mice. J. Vet. Med. Sci. 2004;66:1059–1063. doi: 10.1292/jvms.66.1059. [DOI] [PubMed] [Google Scholar]
- 99.Kim S, Watanabe K, Suzuki H, Watarai M. Roles of Brucella abortus SpoT in morphological differentiation and intramacrophagic replication. Microbiology. 2005;151:1607–1617. doi: 10.1099/mic.0.27782-0. [DOI] [PubMed] [Google Scholar]
- 100.Köhler S, Foulongne V, Ouahrani-Bettache S, Bourg G, Teyssier J, Ramuz M, Liautard JP. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc. Natl. Acad. Sci. USA. 2002;99:15711–15716. doi: 10.1073/pnas.232454299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Köhler S, Michaux-Charachon S, Porte F, Ramuz M, Liautard JP. What is the nature of the replicative niche of a stealthy bug named Brucella? Trends Microbiol. 2003;11:215–219. doi: 10.1016/s0966-842x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 102.Lamontagne J, Butler H, Chaves-Olarte E, Hunter J, Schirm M, Paquet C, Tian M, Kearney P, Hamaidi L, Chelsky D, Moriyón I, Moreno E, Paramithiotis E. Extensive cell envelope modulation is associated with virulence in Brucella abortus. J. Proteome Res. 2007;6:1519–1529. doi: 10.1021/pr060636a. [DOI] [PubMed] [Google Scholar]
- 103.Lamontagne J, Forest A, Marazzo E, Denis F, Butler H, Michaud JF, Boucher L, Pedro I, Villeneuve A, Sitnikov D, Trudel K, Nassif N, Boudjelti D, Tomaki F, Chaves-Olarte E, Guzmán-Verri C, Brunet S, Côté-Martin A, Hunter J, Moreno E, Paramithiotis E. Intracellular adaptation of Brucella abortus. J. Proteome Res. 2009;8:1594–1609. doi: 10.1021/pr800978p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lapaque N, Takeuchi S, Corrales F, Akira A, Moriyón I, Howard JC, Gorvel JP. Differential inductions of TNF- and IGTP, IIGP by structurally diverse classic and non-classic lipopolysaccharides. Cell. Microbiol. 2006;8:401–413. doi: 10.1111/j.1462-5822.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 105.Lavigne JP, O’Callaghan D, Blanc-Potard AB. Requirement of MgtC for Brucella suis intramacrophage growth: a potential mechanism shared by Salmonella enterica and Mycobacterium tuberculosis for adaptation to a low-Mg2+ environment. Infect. Immun. 2005;73:3160–3163. doi: 10.1128/IAI.73.5.3160-3163.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lavigne JP, Patey G, Sangari FJ, Bourg G, Ramuz M, O’Callaghan D, Michaux-Charachon S. Identification of a new virulence factor, BvfA, in Brucella suis. Infect. Immun. 2005;73:5524–5529. doi: 10.1128/IAI.73.9.5524-5529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Léonard S, Ferooz J, Haine V, Danese I, Fretin D, Tibor A, de Walque S, de Bolle X, Letesson JJ. FtcR is a new master regulator of the flagellar system of Brucella melitensis 16M with homologs in Rhizobiaceae. J. Bacteriol. 2007;189:131–141. doi: 10.1128/JB.00712-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lestrate P, Delrue RM, Danese I, Didembourg C, Taminiau B, Mertens P, de Bolle X, Tibor A, Tang CM, Letesson JJ. Identification and characterization of in vivo attenuated mutants of Brucella melitensis. Mol. Microbiol. 2000;38:543–551. doi: 10.1046/j.1365-2958.2000.02150.x. [DOI] [PubMed] [Google Scholar]
- 109.Lestrate P, Dricot A, Delrue RM, Lambert C, Martinelli V, de Bolle X, Letesson JJ, Tibor A. Attenuated signature-tagged mutagenesis mutants of Brucella melitensis identified during the acute phage of infection in mice. Infect. Immun. 2003;71:7053–7060. doi: 10.1128/IAI.71.12.7053-7060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.LeVier K, Phillips RW, Grippe VK, Roop RM, II, Walker GC. Similar requirements of a plant symbiont and a mammalian pathogen for prolonged intracellular survival. Science. 2000;287:2492–2493. doi: 10.1126/science.287.5462.2492. [DOI] [PubMed] [Google Scholar]
- 111.Li L, Jia Y, Hou Q, Charles TC, Nester EW, Pan SQ. A global pH sensor: Agrobacterium sensor protein ChvG regulates acid-inducible genes on its two chromosomes and Ti plasmid. Proc. Natl. Acad. Sci. USA. 2002;99:12369–12374. doi: 10.1073/pnas.192439499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lin J, Ficht TA. Protein synthesis in Brucella abortus induced during macrophage infection. Infect. Immun. 1995;63:1409–1414. doi: 10.1128/iai.63.4.1409-1414.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Loisel-Meyer S, Jiménez de Bagüés MP, Bassères E, Dornand J, Köhler S, Liautard JP, Jubier-Maurin V. Requirement of norD for Brucella suis virulence in a murine model of in vitro and in vivo infection. Infect. Immun. 2006;74:1973–1976. doi: 10.1128/IAI.74.3.1973-1976.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.López-Goñi I, Moriyón I, Neilands JB. Identification of 2,3-dihydroxybenzoic acid as a Brucella abortus siderophore. Infect. Immun. 1992;60:4496–4503. doi: 10.1128/iai.60.11.4496-4503.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lynch M, Kuramitsu H. Expression and role of superoxide dismutases (SOD) in pathogenic bacteria. Microbes Infect. 2000;2:1245–1255. doi: 10.1016/s1286-4579(00)01278-8. [DOI] [PubMed] [Google Scholar]
- 116.Magnusson LU, Farewell A, Nyström T. ppGpp: a global regulator in Escherichia coli. Trends Microbiol. 2005;13:236–242. doi: 10.1016/j.tim.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 117.Manterola L, Moriyón I, Moreno E, Sola-Landa A, Weiss DS, Koch MHJ, Howe J, Brandenburg K, López-Goñi I. The lipopolysaccharide of Brucella abortus BvrS/BvrR mutants contains lipid modifications and has higher affinity for bactericidal cationic peptides. J. Bacteriol. 2005;187:5631–5639. doi: 10.1128/JB.187.16.5631-5639.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Manterola L, Guzmán-Verri C, Chaves-Olarte E, Barquero-Calvo E, de Miguel MJ, Moriyón I, Grilló MJ, López-Goñi I, Moreno E. BvrR/BvrS-controlled outer membrane proteins Omp3a and Omp3b are not essential for Brucella abortus virulence. Infect. Immun. 2007;75:4867–4874. doi: 10.1128/IAI.00439-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martín-Martín AI, Caro-Hernández P, Orduña A, Vizcaíno N, Fernández-Lago L. Importance of the Omp25/Omp31 family in the internalization and intracellular replication of virulent B. ovis in murine macrophages and HeLa cells. Microbes Infect. 2008;10:706–710. doi: 10.1016/j.micinf.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 120.Martínez de Tejada G, Pizarro-Cerdá J, Moreno E, Moriyón I. The outer membranes of Brucella spp. are resistant to bactericidal cationic peptides. Infect. Immun. 1995;63:3054–3061. doi: 10.1128/iai.63.8.3054-3061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Master SS, Springer B, Sander P, Boettger EC, Deretic V, Timmins GS. Oxidative stress response genes in Mycobacterium tuberculosis: role of ahpC in resistance to peroxynitrite and stage-specific survival in macrophages. Microbiology. 2002;148:3139–3144. doi: 10.1099/00221287-148-10-3139. [DOI] [PubMed] [Google Scholar]
- 122.McQuiston JR, Vemulapalli R, Inzana TJ, Schurig GG, Sriranganathan N, Fritzinger D, Hadfield TL, Warren RL, Snellings N, Hoover D, Halling SM, Boyle SM. Genetic characterization of a Tn5-disrupted glycosyltransferase gene homolog in Brucella abortus and its effect on lipopolysaccharide composition and virulence. Infect. Immun. 1999;67:3830–3835. doi: 10.1128/iai.67.8.3830-3835.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Meador VP, Deyoe BL. Intracellular localization of Brucella abortus in bovine placenta. Vet. Pathol. 1989;26:513–515. doi: 10.1177/030098588902600609. [DOI] [PubMed] [Google Scholar]
- 124.Minder AC, de Rudder KEE, Narberhaus F, Fischer HM, Hennecke H, Geiger O. Phosphatidylcholine levels in Bradyrhizobium japonicum membranes are critical for an efficient symbiosis with the soybean host plant. Mol. Microbiol. 2001;39:1186–1198. [PubMed] [Google Scholar]
- 125.Moreno E, Berman DT, Boettcher LA. Biological activities of Brucella abortus lipopolysaccharides. Infect. Immun. 1981;31:362–370. doi: 10.1128/iai.31.1.362-370.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Moreno E, Stackenbrandt E, Dorsch M, Wolters J, Busch M, Mayer H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the Class Proteobacteria. J. Bacteriol. 1990;172:3569–3576. doi: 10.1128/jb.172.7.3569-3576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moreno E, Moriyón I. Brucella melitensis: a nasty bug with hidden credentials for virulence. Proc. Natl. Acad. Sci. USA. 2002;99:1–3. doi: 10.1073/pnas.022622699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Moreno E, Gorvel JP. Invasion, intracellular trafficking and replication of Brucella organisms in professional and non-professional phagocytes. In: López-Goñi I, Moriyón I, editors. Brucella: molecular and cellular biology, Horizon Bioscience. Norfolk, UK: 2004. pp. 287–312. [Google Scholar]
- 129.Naroeni A, Jouy N, Ouahrani-Bettache S, Liautard JP, Porte F. Brucella suis-impaired specific recognition of phagosomes by lysosomes due to phagosomal membrane modifications. Infect. Immun. 2001;69:486–493. doi: 10.1128/IAI.69.1.486-493.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nicoletti PL. Relationship between animal and human disease. In: Young EJ, Corbel MJ, editors. Brucellosis: clinical and laboratory aspects. Boca Raton, FL: CRC Press; 1989. pp. 41–51. [Google Scholar]
- 131.O’Callaghan D, Cazevieille C, Allardet-Servent A, Boschiroli ML, Bourg G, Foulongne V, Frutos P, Kulakov Y, Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 132.O’Neill LAJ, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nature Rev. Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 133.Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect. Dis. 2006;6:91–99. doi: 10.1016/S1473-3099(06)70382-6. [DOI] [PubMed] [Google Scholar]
- 134.Pappas G, Panagopoulou P, Christou L, Akritidis N. Brucella as a biological weapon. Cell. Mol. Life Sci. 2006;63:2229–2236. doi: 10.1007/s00018-006-6311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Parent MA, Bellaire BH, Murphy EA, Roop RM, II, Elzer PH, Baldwin CL. Brucella abortus siderophore 2,3-dihydroxybenzoic acid (DHBA) facilitates intracellular survival of the bacteria. Microb. Pathogen. 2002;32:239–248. doi: 10.1006/mpat.2002.0500. [DOI] [PubMed] [Google Scholar]
- 136.Parent MA, Goenka R, Murphy E, LeVier K, Carreiro N, Golding B, Ferguson G, Roop RM, II, Walker GC, Baldwin CL. Brucella bacA mutant induces greater pro-inflammatory cytokines than the wild-type parent. Microbes Infect. 2007;9:55–62. doi: 10.1016/j.micinf.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 137.Paulley JT, Anderson ES, Roop RM., II Brucella abortus requires the heme transporter BhuA for maintenance of chronic infection in BALB/c mice. Infect. Immun. 2007;75:5248–5254. doi: 10.1128/IAI.00460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pei J, Ficht TA. Brucella abortus rough mutants are cytopathic for macrophages in culture. Infect. Immun. 2004;72:440–450. doi: 10.1128/IAI.72.1.440-450.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pizarro-Cerda J, Meresse S, Parton RG, van der Goot G, Sola-Landa A, López-Goñi I, Moreno E, Gorvel JP. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 1998;66:5711–5724. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Porte F, Liautard JP, Köhler S. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 1999;67:4041–4047. doi: 10.1128/iai.67.8.4041-4047.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Porte F, Naroeni A, Ouahrani-Bettache S, Liautard JP. Role of the Brucella suis lipopolysaccharide O antigen in phagosomal genesis and in inhibition of phagosome-lysosome fusion in murine macrophages. Infect. Immun. 2003;71:1481–1490. doi: 10.1128/IAI.71.3.1481-1490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- 143.Preisig O, Zufferey R, Thöny-Meyer L, Appleby CA, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J. Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Puvanesarajah V, Schell FM, Stacey G, Douglas CJ, Nester EW. Role for 2-linked β-D-glucan in the virulence of Agrobacterium tumefaciens. J. Bacteriol. 1985;164:102–106. doi: 10.1128/jb.164.1.102-106.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Radhakrishnan GK, Yu Q, Harms JS, Splitter GA. Brucella TIR domain-containing protein mimics properties of the Toll-like receptor adaptor protein TIRAP. J. Biol. Chem. 2009;284:9892–9898. doi: 10.1074/jbc.M805458200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rajashekara G, Glover DA, Banai M, O’Callaghan D, Splitter GA. Attenuated bioluminescent Brucella melitensis mutants GR109 (virB4), GR024 (galE), and GR026 (BEMI1090-BMEI1091) confer protection in mice. Infect. Immun. 2006;74:2925–2936. doi: 10.1128/IAI.74.5.2925-2936.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rambow-Larsen AA, Rajashekara G, Petersen E, Splitter G. Putative quorum-sensing regulator BlxR of Brucella melitensis regulates virulence factors including the Type IV secretion system and flagella. J. Bacteriol. 2008;190:3274–3282. doi: 10.1128/JB.01915-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rasool O, Freer E, Moreno E, Jarstrand C. Effect of Brucella abortus lipopolysaccharide on oxidative metabolism and lysozyme release by human neutrophils. Infect. Immun. 1992;60:1699–1702. doi: 10.1128/iai.60.4.1699-1702.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rest RF, Robertson DC. Characterization of the electron transport system in Brucella abortus. J. Bacteriol. 1975;122:139–144. doi: 10.1128/jb.122.1.139-144.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rittig M, Alvarez-Martinez MT, Porte F, Liautard JP, Rouot B. Intracellular survival of Brucella spp. in human monocytes involves conventional uptake but special phagosomes. Infect. Immun. 2001;69:3995–4006. doi: 10.1128/IAI.69.6.3995-4006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rittig MG, Kaufmann A, Robins A, Shaw B, Sprenger H, Gemsa D, Foulongne V, Rouot B, Dornand J. Smooth and rough lipopolysaccharide phenotypes of Brucella induce different intracellular trafficking and cytokine/chemokine release in human monocytes. J. Leukoc. Biol. 2003;74:1045–1055. doi: 10.1189/jlb.0103015. [DOI] [PubMed] [Google Scholar]
- 152.Robertson GT, Roop RM., II The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol. Microbiol. 1999;34:690–700. doi: 10.1046/j.1365-2958.1999.01629.x. [DOI] [PubMed] [Google Scholar]
- 153.Roop RM, II, Jeffers G, Bagchi T, Walker J, Enright FM, Schurig GG. Experimental infection of goat fetuses in utero with a stable, rough mutant of Brucella abortus. Res. Vet. Sci. 1991;51:123–127. doi: 10.1016/0034-5288(91)90001-5. [DOI] [PubMed] [Google Scholar]
- 154.Roop RM, II, Robertson GT, Grippe VK, Kovach ME, LeVier K, Hagius S, Walker JV, Booth N, Fulton T, Elzer PH, Walker GC. Virulence of Brucella melitensis hfq, katE and bacA mutants in pregnant goats. Proc. Brucellosis 2000 Meeting, Abstr. 2000;93:89. [Google Scholar]
- 155.Roop RM, II, Gee JM, Robertson GT, Richardson JM, Ng WL, Winkler ME. Brucella stationary-phase gene expression and virulence. Annu. Rev. Microbiol. 2003;57:57–76. doi: 10.1146/annurev.micro.57.030502.090803. [DOI] [PubMed] [Google Scholar]
- 156.Roop RM, II, Bellaire BH, Valderas MW, Cardelli JA. Adaptation of the brucellae to their intracellular niche. Mol. Microbiol. 2004;52:621–630. doi: 10.1111/j.1365-2958.2004.04017.x. [DOI] [PubMed] [Google Scholar]
- 157.Roop RM, II, Bellaire BH, Anderson E, Paulley JT. Iron metabolism in Brucella. In: López-Goñi I, Moriyón I, editors. Brucella: molecular and cellular biology, Horizon Bioscience. Norfolk, UK: 2004. pp. 243–262. [Google Scholar]
- 158.Roux CM. Doctoral dissertation. University of Louisiana at Lafayette; 2003. Characterization of DNA repair networks of Brucella abortus: analysis of their role in pathogenesis. [Google Scholar]
- 159.Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O, Alexapoulou L, Comerci DJ, Ugalde RA, Pierre P, Gorvel JP. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathogen. 2008;4:e21. doi: 10.1371/journal.ppat.0040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Samartino LE, Enright FM. Pathogenesis of abortion of bovine brucellosis. Comp. Immunol. Microbiol. Infect. Dis. 1993;16:95–101. doi: 10.1016/0147-9571(93)90001-l. [DOI] [PubMed] [Google Scholar]
- 161.Sanchez-Contrerase M, Bauer WD, Gao M, Robinson JB, Downie JA. Quorum-sensing regulation in rhizobia and its role in symbiotic interactions with legumes. Phil. Trans. R. Soc. B. 2007;362:1149–1163. doi: 10.1098/rstb.2007.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sangari FJ, Agüero J. Molecular basis of Brucella pathogenicity: an update. Microbiologia. 1996;12:207–218. [PubMed] [Google Scholar]
- 163.Sangari FJ, Seoane A, Rodríguez MC, Agüero J, García Lobo JM. Characterization of the urease operon of Brucella abortus and assessment of its role in virulence of the bacterium. Infect. Immun. 2007;75:774–780. doi: 10.1128/IAI.01244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sangari FJ, Grilló MJ, Jiménez de Bagües MP, González-Carreró MI, García-Lobo JM, Blasco JM, Agüero J. The defect in the metabolism of erythritol of the Brucella abortus B19 vaccine strain is unrelated with its attenuated virulence in mice. Vaccine. 1998;16:1640–1645. doi: 10.1016/s0264-410x(98)00063-2. [DOI] [PubMed] [Google Scholar]
- 165.Sha Z, Stabel TJ, Mayfield JE. Brucella abortus catalase is a periplasmic protein lacking a standard signal sequence. J. Bacteriol. 1994;176:7375–7377. doi: 10.1128/jb.176.23.7375-7377.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shepard CC. Nonacid-fast bacteria and HeLa cells: their uptake and subsequent intracellular growth. J. Bacteriol. 1959;77:701–714. doi: 10.1128/jb.77.6.701-714.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shin S, Roy CR. Host cell processes that influence the intracellular survival of Legionella pneumophila. Cell. Microbiol. 2008;10:1209–1220. doi: 10.1111/j.1462-5822.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- 168.Sieira R, Comerci DJ, Sánchez DO, Ugalde RA. A homologue of an operon required for DNA transfer in Agrobacterium is required for Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 2000;182:4849–4855. doi: 10.1128/jb.182.17.4849-4855.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smith H, Williams AE, Pearce JH, Keppie J, Harris-Smith PW, Fitzgeorge RB, Witt K. Foetal erythritol: a cause of the localization of Brucella abortus in bovine contagious abortion. Nature. 1962;193:47–49. doi: 10.1038/193047a0. [DOI] [PubMed] [Google Scholar]
- 170.Sohlenkamp C, Lopez-Lara IM, Geiger O. Biosynthesis of phosphatidylcholine in bacteria. Prog. Lipid Res. 2003;42:115–162. doi: 10.1016/s0163-7827(02)00050-4. [DOI] [PubMed] [Google Scholar]
- 171.Sohn AH, Probert WS, Glaser CA, Gupta N, Bollen AW, Wong JD, Grace EM, McDonald WC. Human neurobrucellosis with intracebral granuloma caused by a marine mammal Brucella spp. Emerg. Infect. Dis. 2003;9:485–488. doi: 10.3201/eid0904.020576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sola-Landa A, Pizarro-Cerdá J, Grilló MJ, Moriyón E, Blasco JM, Gorvel JP, López-Goñi I. A two-component regulatory system playing a critical role in plant pathogens and endosymbionts is present in Brucella abortus and controls cell invasion and virulence. Mol. Microbiol. 1998;29:125–138. doi: 10.1046/j.1365-2958.1998.00913.x. [DOI] [PubMed] [Google Scholar]
- 173.Spera JM, Ugalde JE, Mucci J, Comerci DJ, Ugalde RA. A B lymphocyte mitogen is a Brucella abortus virulence factor required for persistent infection. Proc. Natl. Acad. Sci. USA. 2006;103:16514–16519. doi: 10.1073/pnas.0603362103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sperry JF, Robertson DC. Erythritol catabolism by Brucella abortus. J. Bacteriol. 1975;121:619–630. doi: 10.1128/jb.121.2.619-630.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sriranganathan N, Boyle SM, Schurig G, Misra H. Superoxide dismutases of virulent and avirulent strains of Brucella abortus. Vet. Microbiol. 1991;26:359–366. doi: 10.1016/0378-1135(91)90029-f. [DOI] [PubMed] [Google Scholar]
- 176.Starr T, Ng TW, Wehrly TD, Knodler LA, Celli J. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic. 2008;9:678–694. doi: 10.1111/j.1600-0854.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 177.Storz G, Opdyke JA, Zhang A. Controlling mRNA stability and translation with small, noncoding RNAs. Curr. Opin. Microbiol. 2004;7:140–144. doi: 10.1016/j.mib.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 178.Swartz TE, Tseng TS, Frederickson MA, Paris G, Comerci DJ, Rajashekara G, Kim JG, Mudgett MB, Splitter GA, Ugalde RA, Goldbaum FA, Briggs WR, Bogomolni RA. Blue-light-activated histidine kinases: two-component sensors in bacteria. Science. 2007;317:1090–1093. doi: 10.1126/science.1144306. [DOI] [PubMed] [Google Scholar]
- 179.Taketani S. Acquisition, mobilization and utilization of cellular iron and heme: endless findings and growing evidence of tight regulation. Tohoku J. Exp. Med. 2005;205:297–318. doi: 10.1620/tjem.205.297. [DOI] [PubMed] [Google Scholar]
- 180.Taminiau B, Daykin M, Swift S, Boschiroli ML, Tibor A, Lestrate P, de Bolle X, O’Callaghan D, Williams P, Letesson JJ. Identification of a quorum-sensing signal molecule in the facultative intracellular pathogen Brucella melitensis Infect. Immun. 2002;70:3004–3011. doi: 10.1128/IAI.70.6.3004-3011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tatum FM, Detilleux PG, Sacks JM, Halling SM. Construction of a Cu/Zn superoxide dismutase deletion mutant of Brucella abortus: analysis of survival in vitro in epithelial and phagocytic cells and in vivo in mice. Infect. Immun. 1992;60:2863–2869. doi: 10.1128/iai.60.7.2863-2869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tatum FM, Morfitt DC, Halling SM. Construction of a Brucella abortus RecA mutant and its survival in mice. Microb. Pathogen. 1993;14:177–185. doi: 10.1006/mpat.1993.1018. [DOI] [PubMed] [Google Scholar]
- 183.Ugalde JE, Czibener C, Feldman MF, Ugalde RA. Identification and characterization of the Brucella abortus phosphoglucomutase gene: role of lipopolysaccharide in virulence and intracellular multiplication. Infect. Immun. 2000;68:5716–5723. doi: 10.1128/iai.68.10.5716-5723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Uzureau S, Godefroid M, Deschamps C, Lemaire J, de Bolle X, Letesson JJ. Mutations of the quorum sensing-dependent regulator VjbR lead to drastic surface modifications in Brucella melitensis. J. Bacteriol. 2007;189:6035–6047. doi: 10.1128/JB.00265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Valderas M, Alcantara RB, Baumgartner JE, Bellaire BH, Robertson GT, Ng WL, Richardson JM, Winkler ME, Roop RM., II Role of HdeA in acid resistance and virulence in Brucella abortus 2308. Vet. Microbiol. 2005;107:307–312. doi: 10.1016/j.vetmic.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 186.Valentin-Hansen P, Eriksen M, Udesen C. The bacterial Sm-like protein: a key player in RNA transactions. Mol. Microbiol. 2004;51:1525–1533. doi: 10.1111/j.1365-2958.2003.03935.x. [DOI] [PubMed] [Google Scholar]
- 187.Wanke MM. Canine brucellosis. Anim. Reprod. Sci. 2004;82–83:195–207. doi: 10.1016/j.anireprosci.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 188.Waterman SR, Small PLC. Identification of σS-dependent genes associated with the stationary-phase acid-resistance phenotype of Shigella flexneri. Mol. Microbiol. 1996;21:925–940. doi: 10.1046/j.1365-2958.1996.00058.x. [DOI] [PubMed] [Google Scholar]
- 189.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell. Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 190.Weinberg ED. The Lactobacillus anomaly: total iron abstinence. Perspect. Biol. Med. 1997;40:578–583. doi: 10.1353/pbm.1997.0072. [DOI] [PubMed] [Google Scholar]
- 191.Wessel M, Klüener S, Gödeke J, Fritz C, Hacker S, Narberhaus F. Virulence of Agrobacterium tumefaciens requires phosphatidylcholine in the bacterial membrane. Mol. Microbiol. 2006;62:906–915. doi: 10.1111/j.1365-2958.2006.05425.x. [DOI] [PubMed] [Google Scholar]
- 192.Wilson T, de Lisle GW, Marcinkeviciene JA, Blanchard JS, Collins DM. Antisense RNA to ahpC an oxidative stress defence gene involved in isoniazid resistance, indicates that AhpC of Mycobacterium bovis has virulence properties. Microbiology. 1998;144:2687–2695. doi: 10.1099/00221287-144-10-2687. [DOI] [PubMed] [Google Scholar]
- 193.Winter AJ, Schurig GG, Boyle SM, Sriranganathan N, Bevins JS, Enright FM, Elzer PH, Kopec JD. Protection of BALB/c mice against homologous and heterologous species of Brucella by rough strain vaccines derived from Brucella melitensis and Brucella suis biovar 4. Am. J. Vet. Res. 1996;57:677–683. [PubMed] [Google Scholar]
- 194.Wu Q, Pei J, Turse C, Ficht TA. Mariner mutagenesis of Brucella melitensis reveals genes with previously uncharacterized roles in virulence and survival. BMC Microbiol. 2006;6:102. doi: 10.1186/1471-2180-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang X, Becker T, Walters N, Pascual DW. Deletion of znuA virulence factor attenuates Brucella abortus and confers protection against wild-type challenge. Infect. Immun. 2006;74:3874–3879. doi: 10.1128/IAI.01957-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Young EJ. Brucella species. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia, PA: Elsevier Churchill Livingstone; 2005. pp. 2669–2685. [Google Scholar]
- 197.Zarahik ML, Cullen VL, Fung AM, Libby SJ, Choy SLK, Coburn B, Kehres DG, Maguire ME, Fang FC, Finlay BB. The Salmonella enterica serovar Typhimurium divalent cation transport systems MntH and SitABCD are essential for virulence in an Nramp1G169 murine typhoid model. Infect. Immun. 2004;72:5522–5525. doi: 10.1128/IAI.72.9.5522-5525.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zygmunt MS, Hagius SD, Walker JV, Elzer PH. Identification of Brucella melitensis 16M genes required for bacterial survival in the caprine host. Microbes Infect. 2006;8:2849–2854. doi: 10.1016/j.micinf.2006.09.002. [DOI] [PubMed] [Google Scholar]