Abstract
Objective
To assess whether African-Americans are at increased risk of developing peripartum cardiomyopathy.
Background
Peripartum cardiomyopathy is a heart disease of unknown cause that affects young women, often with devastating consequences. The frequency of peripartum cardiomyopathy varies markedly between African and non-African regions.
Methods
A case-control study was performed at a regional center that provides medical care to a racially heterogeneous population. For each case, 3 normal control patients were randomly selected that delivered babies within the same month.
Results
African-American women had a 15.7-fold higher relative risk of peripartum cardiomyopathy than non-African-Americans (odds ratio (OR) 15.7, 95% confidence interval (CI): 3.5–70.6). Other significant univariate risk factors were hypertension (OR 10.8, CI: 2.6–44.4), being unmarried (OR 4.2, 95% CI: 1.4–12.3), and > 2 previous pregnancies (OR 2.9, CI: 1.1–7.4). African-American ethnicity remained a significant risk factor for peripartum cardiomyopathy when other risk factors were considered in multivariable (OR 31.5, CI: 3.6–277.6) and stratified analyses (OR range 12.9–29.1, p<0.001). Although the frequency of peripartum cardiomyopathy (185 per 100,000 deliveries) at this center was higher than previous U.S. reports, it was comparable to the frequency in countries with more women of African descent (100–980 per 100,000). Analysis of other U.S. studies confirmed that the frequency of peripartum cardiomyopathy was significantly higher in African-American women.
Conclusions
African-American women have significantly higher odds of developing peripartum cardiomyopathy that could not be explained by several other factors. Further research will be necessary to determine the potential environmental and/or genetic factors associated with African descent that confer this risk.
Keywords: Heart failure, pregnancy, race
INTRODUCTION
Peripartum cardiomyopathy is a major cause of heart failure and cardiovascular mortality in women of child-bearing age. It occurs in women without pre-existing heart disease during the last month of pregnancy or within 5 months after giving birth.1 The prognosis for peripartum cardiomyopathy can be grim with mortality rates ranging from 15–56%.1, 2 These patients have a significant risk of thromboembolism, atrial fibrillation, persistent cardiomyopathy and severe or fatal recurrence with subsequent pregnancies.2, 3 Recent analysis suggests that the incidence of peripartum cardiomyopathy may be increasing.4
Peripartum cardiomyopathy was described more than 150 years ago but the risk factors or causes have not been firmly established.5, 6 An early review of peripartum cardiomyopathy noted that “viral infections, autoimmune mechanisms, hormonal changes, genetic disorders and toxemia” were possible etiologies.7 Yet despite a number of investigations, no specific cause or pathophysiologic mechanism has been found that distinguishes peripartum cardiomyopathy from other cardiomyopathies. For example, myocarditis has been identified in some patients with peripartum cardiomyopathy but myocarditis is also found with comparable frequency in patients with idiopathic dilated cardiomyopathy.8–11 There is evidence for activation of the immune system in patients with peripartum cardiomyopathy, but these findings are also found in other patients with heart failure.12, 13 Viral DNA has been identified in the hearts of patients with peripartum cardiomyopathy though the frequency was not different from controls and other studies have shown conflicting results.14, 15
There is a striking variation in the frequency of peripartum cardiomyopathy around the world. An analysis of U.S. hospital discharge data suggested a frequency of 31 women per 100,000 deliveries.4 Other published estimates in the US have ranged from 12–25 per 100,000.16,17 A comparable rate of 34 per 100,000 deliveries has been reported in Malaysia.18 In contrast, rates as high as 334 in Haiti, 100 in South Africa and 980 in Nigeria per 100,000 have been reported. 19–21 These countries differ markedly in factors that may affect the risk of peripartum cardiomyopathy such as nutritional status and types of infections.1, 2 Although these incidence data suggest that African women have a greater risk for peripartum cardiomyopathy, it is difficult to assess the relative risk of race and environmental factors in racially homogenous populations.12, 19, 20 Studies in the U.S. have drawn different conclusions about whether race or ethnicity influences the risk of peripartum cardiomyopathy. Recent hospital data have suggested that peripartum cardiomyopathy is more frequent among African Americans than other groups. 4, 17 However, a seminal paper by Demakis on peripartum cardiomyopathy concluded that there was no association with race, 10 and in other case-series, the overwhelming majority of patients have been Caucasian.3, 22
One explanation for the discrepancy between reports is that peripartum cardiomyopathy is uncommon; to identify predisposing factors for a rare condition requires observations from thousands of deliveries. For relatively rare conditions such as this, a case-control study provides an efficient mechanism for identifying risk factors. To determine if African-American race and other factors are associated with an increased risk of developing peripartum cardiomyopathy, we performed a case-control study at a regional medical center that provides care to a racially heterogeneous population.
RESEARCH DESIGN AND METHODS
The study protocol was approved by the MCG Institutional Review Board. The medical records of all patients admitted to the Medical College of Georgia (MCG) were screened to identify cases between July 2003 and July 2008. To meet the diagnosis for peripartum cardiomyopathy all cases had to satisfy the National Heart, Lung, and Blood Institute criteria:1 (1) an ejection fraction ≤ 45% by echocardiogram or radionuclide ventriculography; (2) clinical symptoms of heart failure; (3) development of cardiac failure in the last month of pregnancy or within 5 months of delivery; (4) absence of identifiable cause of cardiac failure and, (5) absence of recognizable heart disease prior to the last month of pregnancy. Of 35 potential cases identified, 7 were excluded because they did not meet the criteria for peripartum cardiomyopathy (2 had EF >45%, 1 was diagnosed >5 months post-partum, 1 had HIV, and 3 had another identifiable cause of heart failure). For each case, 3 normal control patients were randomly selected from women who had delivered babies at MCG within the same month as the cases. No control patients were excluded. Data was extracted from a standardized clinical admission form by using strictly defined criteria to minimize bias. Race or ethnic data was self-reported at the time of admission and was indicated for all the cases or controls. For the other variables analyzed in this study, at least one data item was missing in 18% of the sample.
Statistical analysis
The unadjusted odds ratios (OR) of peripartum cardiomyopathy were calculated for race (African-American vs. non-African-American) and the following risk factors: household income (estimated from 2000 census demographic data by zip codes, www.zip-code.com), number of pregnancies (live-births and miscarriages), and age at delivery. Other factors included the presence or absence of hypertension, diabetes mellitus, marital status at delivery, or the use of alcohol, drugs or cigarettes smoking during pregnancy. Statistical tests were two-tailed with α = 0.05 level of significance.
The risk of peripartum cardiomyopathy was modeled using multivariable forward-selection binary logistic regression analysis using the following binary variables: African-American ethnicity, hypertension, drug use, married at time of delivery, cigarette smoking during pregnancy, diabetes mellitus, and alcohol use during pregnancy. The remaining variables were dichotomized using a median split for use in the logistic regression analysis: number of pregnancies (> 2 vs. ≤ 2), household income (≤ $33,680 vs. > $33,680), and age at delivery (> 24 vs. ≤ 24). To further assess the possibility of effect modification on the variables chosen in the first regression analysis, an additional forward-selection logistic regression was performed using these factors and two-way interaction terms for of all of the factors. Finally, to minimize any effects of missing data, a stratified analysis was performed to analyze the effect of individual risk factors on the relationship between African American race and peripartum cardiomyopathy. This stratified analysis included a Breslow-Day test of homogeneity of odds ratios, a Mantel-Haenszel (M-H) estimate of a common odds ratio across strata and, a M-H test of odds ratio unity for each factor combined across strata.
RESULTS
To examine whether African-American descent was associated with a strong risk for development of peripartum cardiomyopathy, we analyzed the frequency of this disorder at our institution between July 1, 2003 and July 31, 2008. When categorized by race, the incidence was (give actual numbers e.g. 2 cases per XXX deliveries (or 24 cases per 100,000) deliveries for non-African-Americans and 26 cases per XXX deliveries 340 cases per 100,000 deliveries for African-Americans. Each case represented a unique individual. Analyzed in this fashion, the OR for women of African-American descent to develop peripartum cardiomyopathy was 14.4-fold higher than for non-African-American women (p<0.001). In our institution, peripartum cardiomyopathy did not occur in women of Hispanic or other descent although they represented a small proportion of the deliveries (6% Hispanic, and 5.2% other--Asian, Indian, or unidentified).
To assess the factors that may affect the relationship between African-American descent and peripartum cardiomyopathy we performed a case-control analysis of medical records. When compared to controls in this study (Table 1), cases had lower estimated household incomes (p<0.05). However, there were no significant differences between cases and controls in number of pregnancies or the mother’s age at delivery (Table 1).
Table 1.
Clinical Characteristics of Cases Compared to Controlsa.
| Variable | Cases (n = 28) | Controls (n = 84)k |
|---|---|---|
| Income ($×1000) | 29.3±10.7 | 33.8±9.5* |
| Gravidity | 2.8±1.5 | 2.3±1.5 |
| Age at Delivery (yrs) | 26.3±5.2 | 24.5±5.5 |
| Ejection Fraction (%) | 24.6±9.8 | - |
Data represent the mean ± SD
p < 0.05,
Case-control analysis of our data (Table 2) showed that African-American race increased the univariate odds of peripartum cardiomyopathy 15.7-fold (p<0.001). Women of African-American descent comprised 45.2% of our control subjects, but 92.9% of our peripartum cardiomyopathy cases. Other factors associated with a significantly increased univariate odds (p<0.05) of peripartum cardiomyopathy were a history of hypertension (OR 10.8), being unmarried at the time of delivery (OR 4.2), and 2 or more previous pregnancies (OR 2.9).
Table 2.
Unadjusted Odds Ratios for Peripartum Cardiomyopathy
| Variable | Cases n (%) | Controls n (%) | Odds Ratio | p-value | 95% CI |
|---|---|---|---|---|---|
| Race | |||||
| African-American | 26 (92.9) | 38 (45.2) | 15.7 | < 0.001* | 3.5, 70.6 |
| Other | 2 (7.1) | 46 (54.8) | |||
|
| |||||
| Hypertension | |||||
| Yes | 8 (28.6) | 3 (3.6) | 10.8 | 0.001* | 2.6, 44.4 |
| No | 20 (71.4) | 81 (96.4) | |||
|
| |||||
| Drug Use During Pregnancy | |||||
| Yes | 3 (11.1) | 2 (2.4) | 5.1 | 0.083 | 0.8, 32.4 |
| No | 24 (88.9) | 82 (97.6) | |||
|
| |||||
| Married | |||||
| No | 20 (80.0) | 38 (48.7) | 4.2 | 0.009* | 1.4, 12.3 |
| Yes | 5 (20.0) | 40 (51.3) | |||
|
| |||||
| Cigarettes During Pregnancy | |||||
| Yes | 8 (29.6) | 13 (15.5) | 2.3 | 0.262 | 0.8, 6.4 |
| No | 19 (70.4) | 71 (84.5) | |||
|
| |||||
| Diabetes Mellitus | |||||
| Yes | 2 (7.1) | 2 (2.4) | 3.2 | 0.262 | 0.4, 23.5 |
| No | 26 (92.9) | 82 (97.6) | |||
|
| |||||
| Number of Pregnanciesa | |||||
| > 2 | 14 (58.3) | 27 (32.5) | 2.9 | 0.025* | 1.1, 7.4 |
| ≤ 2 | 10 (41.7) | 56 (67.5) | |||
|
| |||||
| Household Incomea | |||||
| ≤ $33,680 | 17 (65.4) | 44 (56.4) | 1.5 | 0.422 | 0.6, 3.7 |
| > $33,680 | 9 (34.6) | 34 (43.6) | |||
|
| |||||
| Age at Deliverya | |||||
| >24 | 14 (53.8) | 35 (41.7) | 1.6 | 0.277 | 0.6, 4.0 |
| ≤ 24 | 12 (46.2) | 49 (58.3) | |||
|
| |||||
| Alcohol During Pregnancy | |||||
| Yes | 1 (3.8) | 5 (6.0) | 0.6 | 0.682 | 0.1, 5.7 |
| No | 25 (96.2) | 79 (94.0) | |||
Significant p < 0.05;
Based on median split.
Forward-selection multiple logistic regression was used to examine how race and other risk factors, in combination, affected the odds of developing peripartum cardiomyopathy. In this analysis (Table 3) African-American ethnicity remained a strong risk factor (OR 31.5, p<0.005) which emerged first in the analysis. Cigarette smoking during pregnancy was also identified as a risk factor (OR 6.0, p<0.02). No other variables were added to the model since they were not significant (p > 0.05) explanatory factors. To determine if any other factors modified the effect of African-American ethnicity or smoking on peripartum cardiomyopathy, another forward-selection regression was performed using all risk factor, two-way interaction terms. None of the two-way interactions were significant suggesting that no other factors modified the risk by these two variables. Since the multivariate adjusted OR for African-American ethnicity (31.5) was twice the estimated unadjusted OR (15.7) smoking may be a potential negative confounder of the relationship of race to the development of peripartum cardiomyopathy.
Table 3.
Final Statistics of the Forward-Selection Logistic Regression Analysis
| Variable/Risk Factor | p-value | Odds Ratio | 95% CI for OR | |
|---|---|---|---|---|
| Lower | Upper | |||
| Race (African-American vs. Other) | 0.002* | 31.5 | 3.6 | 277.6 |
| Smoke Cigarettes During Pregnancy (Yes vs. No) | 0.017* | 6.0 | 1.4 | 26.3 |
p < 0.05.
To further assess whether other variables confounded the strong relationship between African-American descent and peripartum cardiomyopathy, we performed a series of stratified analyses, controlling for one confounder at a time, using the Mantel-Haenszel (M-H) statistic. African-American descent remained a significant risk factor (OR 12.7–29.1) for the development of peripartum cardiomyopathy despite stratification for all variables (Table 4). Indeed the OR of African-American ethnicity was similar when stratified across all risk factors, as indicated by homogeneity tests. Thus, none of the other risk factors explained the strong risk of African-American descent for the development of peripartum cardiomyopathy.
Table 4.
Stratified Odds Ratio of Peripartum Cardiomyopathy for African-Americans
| Stratifying Variable | ORb | 95% CI | p-value |
|---|---|---|---|
| Household Incomea (≤ $33,680 vs. > $33,680) | 21.9 | 3.2, 147.5 | < 0.001* |
| Married (No vs. Yes) | 14.6 | 2.4, 87.2 | 0.001* |
| Hypertension (Yes vs. No) | 12.7 | 2.9, 55.3 | < 0.001* |
| Diabetes Mellitus (Yes vs. No) | 15.3 | 3.4, 68.6 | < 0.001* |
| Alcohol During Pregnancy (Yes vs. No) | 29.1 | 3.8, 221.3 | < 0.001* |
| Drug Use During Pregnancy (Yes vs. No) | 14.2 | 3.2, 63.7 | < 0.001* |
| Cigarette Smoking During Pregnancy (Yes vs. No) | 13.5 | 3.3, 54.6 | < 0.001* |
| Number of Pregnanciesa (>2 vs. ≤ 2) | 12.9 | 2.8, 59.2 | < 0.001* |
| Age at Deliverya (> 24 vs. ≤ 24) | 14.0 | 3.1, 63.2 | < 0.001* |
Based on median split;
Mantel-Haenszel common OR for African American race;
p < 0.05
DISCUSSION
In this case-control study, African-American race was the most important predictor of developing peripartum cardiomyopathy, with an odds ratio of 15.7 versus non-African-American patients. In multivariate regression and in stratified analysis, African-American descent remained a potent, significant risk factor (OR 31.5) for peripartum cardiomyopathy, even when the potential effects of other risk factors were considered.
We examined other reports to calculate whether African-American race was also associated with an increased risk of peripartum cardiomyopathy. An early, seminal study by Demakis et al, 16 at Cook County hospital failed to detect an increased risk for African-American women but their calculation was flawed because it was based on cases and birth data from two different historical epochs (1947–1967 and 1952–1965). However, examination of two large, more recent US studies with data on race (Table 5) 4, 17 showed that African-American women had significantly higher odds ratios (3.6 and 4.7) for developing peripartum cardiomyopathy. Both reports were based on discharge diagnoses, obtained from the Kaiser Permanente system or the National Hospital Discharge Study. 4, 17 The reliance of these studies solely on discharge data and specific ICD-9 codes could, as the authors acknowledge, affect the detection of some patients who had peripartum cardiomyopathy, due to errors in coding.17 Yet despite the differences in study design and methodology, there is an important consistency in our findings: the odds-ratio for peripartum cardiomyopathy in both the Brar 17 (OR 3.6) and Mielniczuk 4 (OR 4.7) fall within the 95% confidence interval estimated in our study (3.5, 70.6).
Table 5.
Relative risk of African-American descent for peripartum cardiomyopathy in this and other studies
| Source | Period | N (cases) | OR | 95% CI |
|---|---|---|---|---|
| This study† | 2003–2008 | 28 | 15.7** | 3.5 – 70.6 |
| Kaiser-CA17† | 1996–2005 | 60 | 3.6** | 2.0 – 6.2 |
| U.S. Hospitals4† | 1990–2002 | 10898 | 4.7** | 4.5 –4.9 |
OR calculated for African-Americans vs. non-African-Americans based on data available in each report;
p<0.001
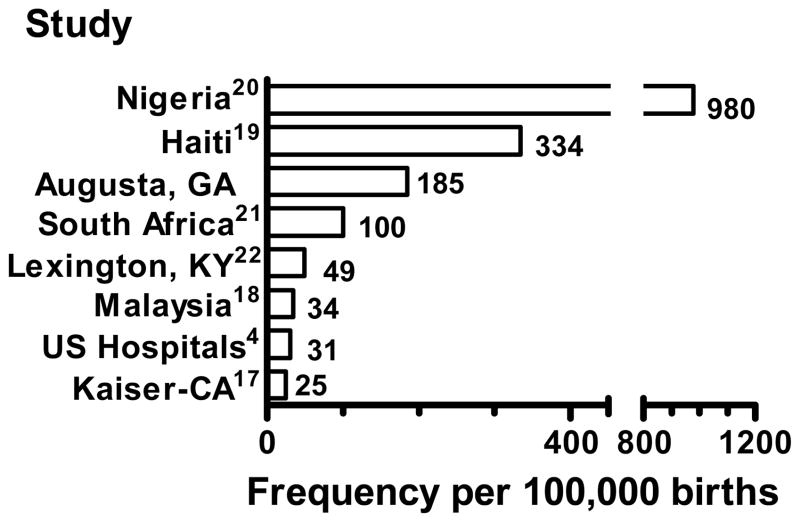
Worldwide, the frequency of peripartum cardiomyopathy is highest where a large proportion of the women were of African descent such as Nigeria, Haiti, South Africa and this study (Fig. 1).19–21 The lowest frequency of peripartum cardiomyopathy has been reported in studies where women of African descent were less common, such as the U.S. hospital discharge study, the Kaiser-Southern California report and a series in Malaysia. 4, 17, 18, 22 The highest reported incidence is in Nigeria, at 980 per 100,000 deliveries.20 This very high incidence may be related to a local custom of ingesting salt in the postpartum period which increased the detection of peripartum cardiomyopathy by increasing clinical heart failure symptoms.20 Desai found an incidence of 100 cases per 100,000 deliveries in South Africa.21 Fett has reported an incidence in a predominately rural population in Haiti of 334 cases per 100,000 deliveries.19 The incidence of peripartum cardiomyopathy in our population as a whole was 185 cases per 100,000 deliveries which is substantially higher than previously reported in the United States (Fig. 1). However, it is striking that the incidence among African-Americans in our study was 340 cases per 100,000, a number quite similar to the 334 cases per 100,000 deliveries reported in Haiti.19
Figure 1.
The frequency of peripartum cardiomyopathy in this (Augusta, GA) and other studies per 100,000 deliveries.
In addition to African-American descent, other risk factors were identified for the development of peripartum cardiomyopathy. Among these other significant univariate risk factors was a history of hypertension (OR 10.8), marital status (OR 4.2), and having more than 2 pregnancies (OR 2.9). Similar to case series from Haiti19 and South Africa21, we did not confirm the hypothesis that maternal age increases the risk of developing peripartum cardiomyopathy. Although hypertension is common in peripartum cardiomyopathy (2–43% of patients), this is the first study to demonstrate that hypertension confers a significant increase in risk 4, 20–22. Certainly uncontrolled, severe hypertension can lead to cardiac dysfunction. Still, none of our patients had known hypertensive heart disease or evidence of left ventricular hypertrophy on echocardiogram. Moreover, in the multivariable logistic regression analysis, race and smoking were the only significant predictors; hypertension was not.
Case-control studies are among the most powerful methods for identifying risk factors associated with uncommon diseases or condition. However, our hospital-based study may not have detected the occurrence of peripartum cardiomyopathy in women who were mildly affected and did not require hospital admission. Still, significant under-detection seems implausible, as the incidence of peripartum cardiomyopathy per 100,000 deliveries in this study was higher than other U.S. reports, although it was within the range of other studies in which the majority of women were of African descent.
To be certain that cases were not missed, we separately reviewed 3 years of hospital records for women of child-bearing age admitted with heart failure and found no new cases. In analyzing our data we were careful to be certain that the link between African-American descent and cardiomyopathy was not due to biased detection. Biased detection seems doubtful because: 1) race was self-reported and available for all cases and controls; 2) cardiomyopathy was independently confirmed by echocardiography and, 3) African-American descent had not previously been established as a risk factor for this condition. In addition, we used both the Mantel-Haenszel and multiple logistic regression techniques to determine whether the increased risk of peripartum cardiomyopathy in African-American women could be attributed to other variables.
These analyses confirmed that the risk of developing peripartum cardiomyopathy was consistently elevated when the effects of all other variables were considered.
In summary, these data and an analysis of previous reports provide strong, consistent evidence that the risk of peripartum cardiomyopathy is increased in women of African descent. It is important to consider whether the increased risk is due to genetic or environmental factors, or both. In future studies, more precise definitions of race and descent may identify better those at greatest risk for peripartum cardiomyopathy. As Yancy has pointed out “race, intermarriage, and reproduction are decidedly variable in North America” yet certain hypertensive conditions such as end-stage renal disease, left ventricular hypertrophy, etc. are more common in African-American patients 24. Consistent with that notion, our study found that hypertension was a significant univariate risk factor for the development of peripartum cardiomyopathy. Women with cardiomyopathy had a lower estimated family income than controls. Other factors associated with an increased univariate risk of peripartum cardiomyopathy included: being unmarried at the time of delivery, and 2 or more previous pregnancies. Although not significant in the univariate analysis, cigarette smoking during pregnancy was a significant risk factor in the multivariate logistic regression analysis. However, in the stratified analysis none of these factors could explain solely the increased risk for this disorder in African-American women. Still, our power to detect multivariate interactions that may affect the association we found between African-American descent and peripartum cardiomyopathy may have been limited by the relatively small size of our sample. Thus we are unable to determine in this study whether genetic factors of race, or other complex environmental, social, economic, or other factors that are linked to race, account for the increased risk of peripartum cardiomyopathy. Further research is needed to better identify women at increased risk of cardiomyopathy and to develop strategies for prevention.
Acknowledgments
The authors gratefully acknowledge the critical comments provided by Dr. Daniel E. Singer, Massachusetts General Hospital, Boston.
Funding source: This work was supported in part by NIH Grant HL058496 (to G.L.R.).
Footnotes
Disclosures: The authors have no conflicts of interest.
References
- 1.Pearson GD, Veille JC, Rahimtoola S, et al. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. Jama. 2000;283(9):1183–8. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 2.Sliwa K, Fett J, Elkayam U. Peripartum cardiomyopathy. Lancet. 2006;368(9536):687–93. doi: 10.1016/S0140-6736(06)69253-2. [DOI] [PubMed] [Google Scholar]
- 3.Elkayam U, Akhter MW, Singh H, et al. Pregnancy-associated cardiomyopathy: clinical characteristics and a comparison between early and late presentation. Circulation. 2005;111(16):2050–5. doi: 10.1161/01.CIR.0000162478.36652.7E. [DOI] [PubMed] [Google Scholar]
- 4.Mielniczuk LM, Williams K, Davis DR, et al. Frequency of peripartum cardiomyopathy. Am J Cardiol. 2006;97(12):1765–8. doi: 10.1016/j.amjcard.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 5.Ritchie C. Clinical contributions to the pathology, diagnosis and treatment of certain chronic diseases of the heart. Edinburg Med Surg J. 1849;12:333. [PMC free article] [PubMed] [Google Scholar]
- 6.Meadows WR. Post-partum heart disease. Am J Cardiol. 1960;6:788. doi: 10.1016/0002-9149(60)90229-0. [DOI] [PubMed] [Google Scholar]
- 7.Demakis JG, Rahimtoola SH. Peripartum cardiomyopathy. Circulation. 1971;44(5):964–8. doi: 10.1161/01.cir.44.5.964. [DOI] [PubMed] [Google Scholar]
- 8.Melvin KR, Richardson PJ, Olsen EG, Daly K, Jackson G. Peripartum cardiomyopathy due to myocarditis. N Engl J Med. 1982;307(12):731–4. doi: 10.1056/NEJM198209163071207. [DOI] [PubMed] [Google Scholar]
- 9.Midei MG, DeMent SH, Feldman AM, Hutchins GM, Baughman KL. Peripartum myocarditis and cardiomyopathy. Circulation. 1990;81(3):922–8. doi: 10.1161/01.cir.81.3.922. [DOI] [PubMed] [Google Scholar]
- 10.Rizeq MN, Rickenbacher PR, Fowler MB, Billingham ME. Incidence of myocarditis in peripartum cardiomyopathy. Am J Cardiol. 1994;74(5):474–7. doi: 10.1016/0002-9149(94)90906-7. [DOI] [PubMed] [Google Scholar]
- 11.Sanderson JE, Olsen EG, Gatei D. Peripartum heart disease: an endomyocardial biopsy study. Br Heart J. 1986;56(3):285–91. doi: 10.1136/hrt.56.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sliwa K, Forster O, Libhaber E, et al. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J. 2006;27(4):441–6. doi: 10.1093/eurheartj/ehi481. [DOI] [PubMed] [Google Scholar]
- 13.Sliwa K, Skudicky D, Bergemann A, Candy G, Puren A, Sareli P. Peripartum cardiomyopathy: analysis of clinical outcome, left ventricular function, plasma levels of cytokines and Fas/APO-1. J Am Coll Cardiol. 2000;35(3):701–5. doi: 10.1016/s0735-1097(99)00624-5. [DOI] [PubMed] [Google Scholar]
- 14.Bultmann BD, Klingel K, Nabauer M, Wallwiener D, Kandolf R. High prevalence of viral genomes and inflammation in peripartum cardiomyopathy. Am J Obstet Gynecol. 2005;193(2):363–5. doi: 10.1016/j.ajog.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 15.Lamparter S, Pankuweit S, Maisch B. Clinical and immunologic characteristics in peripartum cardiomyopathy. Int J Cardiol. 2007;118(1):14–20. doi: 10.1016/j.ijcard.2006.04.090. [DOI] [PubMed] [Google Scholar]
- 16.Demakis JG, Rahimtoola SH, Sutton GC, et al. Natural course of peripartum cardiomyopathy. Circulation. 1971;44(6):1053–61. doi: 10.1161/01.cir.44.6.1053. [DOI] [PubMed] [Google Scholar]
- 17.Brar SS, Khan SS, Sandhu GK, et al. Incidence, mortality, and racial differences in peripartum cardiomyopathy. Am J Cardiol. 2007;100(2):302–4. doi: 10.1016/j.amjcard.2007.02.092. [DOI] [PubMed] [Google Scholar]
- 18.Chee KH, Azman W. Prevalence and outcome of peripartum cardiomyopathy in Malaysia. Int J Clin Pract. 2007 doi: 10.1111/j.1742-1241.2007.01449.x. [DOI] [PubMed] [Google Scholar]
- 19.Fett JD, Christie LG, Carraway RD, Murphy JG. Five-year prospective study of the incidence and prognosis of peripartum cardiomyopathy at a single institution. Mayo Clin Proc. 2005;80(12):1602–6. doi: 10.4065/80.12.1602. [DOI] [PubMed] [Google Scholar]
- 20.Isezuo SA, Abubakar SA. Epidemiologic profile of peripartum cardiomyopathy in a tertiary care hospital. Ethn Dis. 2007;17(2):228–33. [PubMed] [Google Scholar]
- 21.Desai D, Moodley J, Naidoo D. Peripartum cardiomyopathy: experiences at King Edward VIII Hospital, Durban, South Africa and a review of the literature. Trop Doct. 1995;25(3):118–23. doi: 10.1177/004947559502500310. [DOI] [PubMed] [Google Scholar]
- 22.Ford RF, Barton JR, O’Brien JM, Hollingsworth PW. Demographics, management, and outcome of peripartum cardiomyopathy in a community hospital. Am J Obstet Gynecol. 2000;182(5):1036–8. doi: 10.1067/mob.2000.105402. [DOI] [PubMed] [Google Scholar]
- 23.Hilfiker-Kleiner D, Sliwa K, Drexler H. Peripartum cardiomyopathy: recent insights in its pathophysiology. Trends in cardiovascular medicine. 2008;18(5):173–9. doi: 10.1016/j.tcm.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Yancy CW. Does race matter in heart failure? Am Heart J. 2003;146(2):203–6. doi: 10.1016/S0002-8703(03)00241-2. [DOI] [PubMed] [Google Scholar]