Abstract
Conjugated linoleic acid (CLA) supplementation decreases adipose mass and increases bone mass in mice. Recent clinical studies demonstrate a beneficial effect of CLA on reducing weight and adipose mass in humans. This article reviews possible biological mechanisms of action of CLA on bone metabolism, focusing on modulation of nuclear receptor peroxisome proliferator-activated receptor gamma activity to steer mesenchymal stem cell differentiation toward an adipose and away from an osteoblast lineage. Clinical studies of the effects of CLA on bone mass and clinical implications of the effects of CLA on bone health in humans are summarized and discussed.
Keywords: adipose, bone mineral density, conjugated linoleic acid, fatty acids, peroxisome proliferator-activated receptor gamma
Introduction
Osteoporosis is characterized by compromised bone strength, which increases the risk for fracture1 and contributes to a considerable public health burden in the US population.2 The current annual incidence of osteoporotic fracture is estimated at >2 million and the associated direct medical costs at $17 billion per year; these are projected to rise to 3 million fractures costing $25 billion per year in 2025.3
Conjugated linoleic acid (CLA) describes a group of positional and geometric isomers of the 18-carbon polyunsaturated fatty acid (PUFA) octadecadienoic acid, which contains two conjugated double bonds.4 Dietary CLA is found naturally in meat and milk products from cattle, lamb, and goat. It is also available as a dietary supplement purported to cause weight loss. Among 28 different isomers of CLA, the major isomers of CLA found in dietary supplements are an equal ratio of cis-9, trans-11 (c9t11)-CLA (rumenic acid), and t10c12-CLA.5 CLA has anticarcinogenic properties when used in chemically induced models of carcinogenesis in rodents.6,7 Additionally, the t10c12 isomer of CLA reduces adipose mass in mice and humans, while the c9t11 isomer has anti-inflammatory properties.8–12 Recent studies suggest t10c12-CLA induces retention of bone mineral density (BMD) in older mice.13 Further, in growing mice fed a calcium-enriched diet, t10c12-CLA increased ash content as a measure of BMD.14 The aim of this paper is to provide a review of primary literature supporting and opposing the proposal that CLA modulates bone physiology through a mechanism dependent on changes of peroxisome proliferator-activated receptor (PPAR)-γ-mediated induction of adipogenesis of mesenchymal cell differentiation to adipocytes in bone. Additional biological mechanisms and possible clinical implications will be discussed.
Conjugated Linoleic Acid Lowers Adipose Mass
CLA lowers adipose mass in several strains of growing male and female mice. The t10c12-CLA isomer is responsible for the adipose-lowering effects. Demonstrated in differentiated adipocytes, the mechanism of adipose-decreasing activity likely involves inhibition of heparinreleasable lipoprotein lipase, resulting in increased apoptosis of preadipocytes.15–18 The t10c12-CLA isomer appears to inhibit differentiation to mature adipocytes via the downregulation of PPAR-γ.19–21 Addition of a thiazolidinedione, ciglitazone, induced PPAR-γ mRNA and protein expression in a murine preadipocyte cell line, and cotreatment with t10c12-CLA abolished these effects.20 In the same study, mice supplemented with CLA showed decreased gene expression of PPAR-γ in adipose tissue. Physiological concentrations (10–30 μmol/L) of t10c12-CLA decreased, whereas c9t11-CLA increased PPAR-γ expression and downstream target gene expression versus vehicle control in primary cultures of human preadipocytes; however, both isomers antagonized PPAR-γ ligand-dependent activation.21 Inhibition of PPAR-γ expression and target gene expression by t10c12-CLA also occurs in murine and human adipocyte cultures.22
Until recently, the adipose-lowering effect of t10c12-CLA in humans was not demonstrated. Reasons for these negative data include lack of adequately powered studies and short durations of clinical interventions to show measurable changes of adipose mass. By 2002, a few studies of 6–12 months' duration demonstrated a dose-responsive lowering of adipose mass in trials involving men and women.9 A meta-analysis predicted that for each 1 g of CLA consumed, a fat loss of 0.024 kg (0.05 lb) per week could be achieved.12
We recently tested the effect of 6.4 g per day of CLA (of which 3.2 g included the adipose-lowering t10c12-CLA) on alterations in adipose mass in obese women with type 2 diabetes. The study involved 55 postmenopausal women in a crossover double-blinded intervention in which the comparator oil was linoleic-acid-rich safflower oil. Supplementation with CLA lowered body fat over a 16-week period by over 1.5 kg in obese postmenopausal women with type 2 diabetes.23 These data confirmed the earlier observation that t10c12-CLA effectively lowers adipose mass in obese humans.
Conjugated Linoleic Acid Alters Bone Mineral Content in Mice
Effects of CLA on bone ash or mineral content in animals vary, depending on the species investigated. Since skeletal bone serves as the major reservoir of minerals, bone ash serves as a valid surrogate measure for bone mass. The CLA-induced increase in bone ash content was observed in chicks24 and mice,25 but not in rats,26–30 and was found variably in pigs.31–33 When data from multiple CLA trials in mice were compiled, Park and Pariza14 suggested an interaction of dietary calcium with the ability of CLA to increase bone mass. These findings are supported by a recent study demonstrating that CLA supplementation increases bone ash, but only in those animals fed a higher level of dietary calcium (1% versus 0.05% or 0.01%).34 Further, the effect was due to the t10c10 rather than the c9t11 isomer.34
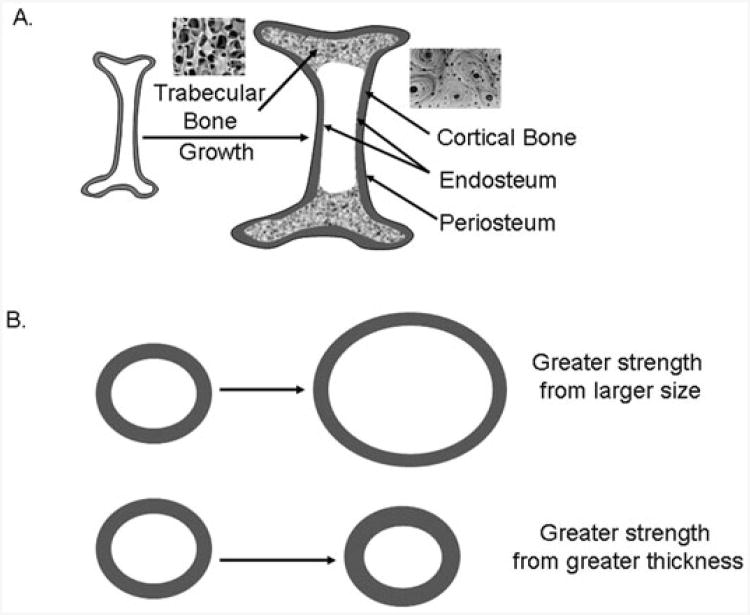
Supplementation of experimental diets with CLA for 14 weeks in young (2-month-old) male mice resulted in increased volumetric bone mineral content, bone area, and BMD of trabecular and cortical bone, despite decreases in body weight gain.35 Notably, bone size increased and was accompanied by greater periosteal perimeter, endocortical perimeter, and cortical thickness. These changes in bone geometry induced by CLA suggest CLA can promote growth of a larger and thicker skeleton that is biomechanically stronger (Figure 1).36 A similar 10-week feeding experiment in middle-aged (12-month-old) female mice showed that CLA increased volumetric BMD of trabecular and cortical bone.13 CLA treatment in both young and middle-aged animals increased cortical thickness and cortical bone area due to increased periosteal bone, but in the middle-aged mice, some bone sites (e.g., femoral neck) showed a decrease in endocortical perimeter, suggesting decreased bone resorption at this bone surface. Exercised middle-aged mice also showed increases in bone mass and size, which was additive in combination with CLA.13
Figure 1. A. Schematic longitudinal section of a long bone showing outer cortical bone and inner trabecular bone tissue.
Cortical bone is denser and is organized in concentric lamellar structures called osteons. Trabecular bone is more porous and is organized as interconnected plates and struts. Representative light microscopy images of human trabecular and cortical bone are shown. Bone growth involves resorption at the endosteal surface and new bone apposition at the periosteal surface, producing greater cortical bone diameters and thickness. B. Schematic cross-section of a long bone. Cortical bone size is an important determinant of bone strength. Bone strength is increased by a greater cortical diameter or greater cortical thickness.
CLA has been shown to affect physiological markers of bone turnover. In middle-aged mice, CLA decreased the proinflammatory cytokines interleukin 6 and tumor necrosis factor-α, decreased the key soluble regulator of osteoclastogenesis, the receptor activator of NF-κβ ligand (RANKL), and decreased the bone resorption biomarker serum tartrate-resistant acid phosphatase 5b.37 CLA supplementation in ovariectomized rats lowers a marker of bone resorption, urinary pyridinium crosslinks.28 Like the isomer specificity of t10c12-CLA to lower adipose, this same isomer may be the bioactive isomer of CLA to alter bone. Supplementation with the t10c12-CLA, rather than the c9t11 isomer, increased whole-body ash content in mice15 and increased osteoblast differentiation in mesenchymal stem cells.38 In summary, animal models provide evidence for a beneficial effect of CLA on bone metabolism in aging.
Potential Mechanisms of Conjugated Linoleic Acid Action on Bone
The most compelling mechanism of action of CLA relates to effects on PPARs, a family of transcription factors within the nuclear hormone receptor superfamily, which also includes estrogen, vitamin D, thyroid, glucocorticoid, and retinoic acid receptors.39 Four isoforms are described, PPAR-α, PPAR-β/δ, PPAR-γ1, and PPAR-γ2. Although many different tissues express PPAR-γ1 at low levels, mature adipocytes, including bone marrow adipocytes, express PPAR-γ2 at high levels. Human primary osteoblasts have been shown to express PPAR-γ and PPAR-β/δ.40 Activity of PPAR as a transcription factor requires partnering as a heterodimer with a retinoid X receptor, with each receptor requiring a ligand for activation and interaction with specific coactivators and corepressors to bind cognate nuclear PPAR response elements.41
PPAR-γ and differentiation of mesenchymal stem cells
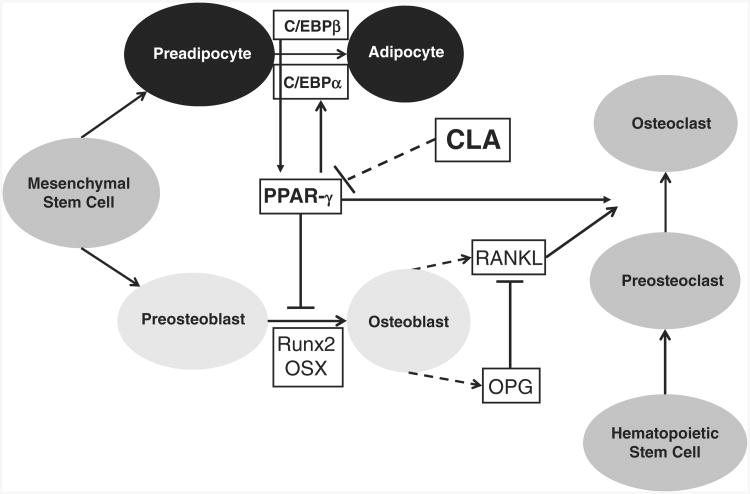
Adipocyte differentiation requires activation of PPAR-γ2. Within bone marrow tissue, differentiation of mesenchymal stem cells is steered toward an adipocyte versus an osteoblast lineage by activation of PPAR-γ2.42,43 Alternatively, osteoblastogenic differentiation requires activation of the transcription factor, core-binding factor A1 (Cbfa1)/runt-related transcription factor (Runx)2.44 Another transcription factor, CCAAT/enhancer-binding protein (C/EBP)β, maintains PPAR-γ expression during adipocytic differentiation, and PPAR-γ upregulates C/EBPα expression, which stimulates adipocyte gene expression.45 PPAR-γ2 downregulates expression and activity of Cbfa1/Runx2,44,46 thereby inhibiting osteoblastogenesis. PPAR-γ2 also suppresses other osteoblast-signaling pathways (Wnt, transforming growth factor-β/bone morphogenetic protein, and insulin growth factor-1) and transcriptional regulators (Osterix,Dlx5).47 A number of transcription factors reciprocally regulate the cell fate of the multipotential bone marrow mesenchymal stem cell. The PPAR-γ1 isoform also promotes lineage commitment of hematopoietic stem cells toward osteoclast development.48 Thus, PPAR-γ upregulation within bone marrow would decrease bone formation, increase bone resorption, and increase marrow adiposity (Figure 2).
Figure 2. Possible differentiation of mesenchymal stem cells toward an adipocytic or osteoblastic lineage.
Activation of transcription factor PPAR-γ and C/EBP promotes an adipocyte cell fate, whereas activation of transcription factor Cbfa1/Runx2 steers lineage allocation toward an osteoblast fate. PPAR-γ stimulates C/EBPα, and C/EBPβ induces PPAR-γ. PPAR-γ also inhibits Run×2 and promotes osteoclast formation from hematopoietic stem cells. Osteoblast-derived RANKL induces osteoclastogenesis, and OPG inhibits RANKL.
Abbreviations: PPAR-γ, peroxisome proliferator-activated receptor gamma; C/EBP, CCAAT/enhancer-binding proteins; Cbfa1, core-binding factor A1; Runx2, runt-related transcription factor 2; RANKL, receptor activator of nuclear transcription factor κβ ligand; OPG, osteoprotegerin.
Whereas homozygous PPAR-γ deletion is lethal early in gestation (day 10–10.5), PPAR-γ haploinsufficient mice demonstrate a phenotype of high bone mass, increased osteoblastogenesis from bone marrow progenitors, and low bone marrow adiposity.49 The inverse relationship of increased adiposity and diminished bone mass that commonly occurs in older-age individuals may be mediated by PPAR-γ activation steering lineage allocation of mesenchymal bone marrow stem cells toward adipogenesis and away from osteoblastogenesis.50 The PPAR-γ agonist medication class, thiazolidinediones (TZDs), such as rosiglitazone and pioglitazone, increase tissue sensitivity to the action of insulin and are commonly used pharmacologic agents in the treatment of type 2 diabetes mellitus. Rosiglitazone treatment of a mesenchymal stem cell line transfected with PPAR-γ inhibited osteoblastogenesis and promoted adipogenesis.51 In mice, rosiglitazone52 and darglitazone53 (at 20- and 100-fold increased potency relative to rosiglitazone and pioglitazone) decreased bone mass and bone formation rate and increased bone marrow fat mass. TZDs have been recently been associated with BMD loss in prospective cohort54 and clinical trials.55,56 Especially worrisome are recent clinical reports that TZD use is associated with fragility fractures of upper and lower extremities in observational studies,57,58 clinical trials,59,60 and a meta-analysis.61
Naturally occurring PPAR-γ ligands include arachidonic acid derivatives, various prostaglandins, and long-chain PUFAs. Some specific examples of highaffinity naturally occurring ligands for PPAR-γ include 15-deoxy-Δ12,14-prostaglandin J2,62 9(2)-HODE,63 15-hydroxy-eicosatetraenoic acid (15-HETE),64 and 8-S-hydroxyeicosatetraenoic acid (8S-HETE). Several PUFAs and eicosanoids are promiscuous ligands that interact with two or more PPARs. Leukotriene B4 activates all PPARs, especially PPAR-α.65 Both c9t11-CLA and t10c12-CLA isomers are potent ligands and activators of PPAR-α,66 while both are modest activators of PPAR-γ.67 Most PUFAs are moderately potent activators of PPAR-γ.
All three PPAR isoforms have been detected in cultures of human osteoclasts.68 Specific agonists of PPAR isoforms as well as nonspecific PPAR activators (bezafibrate and fenofibrate) inhibited osteoclastogenesis. However, there were different effects of isoform-specific agonists on osteoclast function. PPAR-γ agonist (ciglitazone) inhibited, PPAR-β/δ agonist (L165041) stimulated, and PPAR-α agonist (GW9578) had a neutral effect on bone resorption.68 PPAR-β/δ and PPAR-γ but not PPAR-α have been identified in human primary osteoblasts.40
Indirect effects of PPAR-γ on bone via adipokines
Of interest to bone physiology, activation of PPAR-γ by TZD may indirectly influence bone metabolism via adipokine secretion. A cross-sectional population study reported an inverse relationship between BMD and concentration of serum adiponectin, an adipocyte-derived hormone.69 In contrast, adiponectin dose-dependently promoted proliferation and differentiation in cultured osteoblasts.70 Serum concentrations of leptin, another adipocyte-derived hormone, reflect the mass of adipose in mice and in humans.71 In leptin-deficient ob/ob mice and leptin-resistant db/db mice, bone mass of vertebral trabecular sites is higher while bone mass at femoral cortical and trabecular sites is lower than in nonmutated littermates fed similar diets.72 Leptin inhibits bone formation via a hypothalamic pathway. Intracerebroventricular infusion of leptin in hypoleptinemic and wild-type mice activates hypothalamic sympathetic nerve fibers innervating osteoblasts via the B2-adrenergic receptor (ADRB2).72 ADRB2 signaling in osteoblasts decreases bone formation and increases bone resorption, with the latter occurring via upregulation of RANKL.73 In a parabiosis experiment, leptin administered via intracerebroventricular infusion did not cross into systemic circulation and decreased bone mass only in the leptintreated animals.74 The ADBR2-null mouse demonstrated increased trabecular bone mass at the vertebrae and distal femur and increased cortical bone at the mid femur.75 CLA treatment decreased serum leptin concentration in mice37 and lowered serum and white adipose tissue leptin concentration in rats.76 Leptin can directly affect bone metabolism by steering differentiation of a bone marrow stromal cell line toward an osteoblast pathway without altering expression of the osteoblastogenic transcription factor Cbfa-1/Runx2 or the adipogenic transcription factor PPAR-γ.77 Finally, bone marrow adipocytes produce adiponectin and leptin under the control of PPAR-γ78 and osteoblasts express receptor for adiponectin79 and leptin,73 suggesting that bone remodeling may also be modulated locally.
Clinical Studies of Conjugated Linoleic Acid on Bone
A search of medical literature was undertaken using PubMed with the following search details: “conjugated”[All Fields] AND (“linoleic acid”[MeSH Terms] OR (“linoleic”[All Fields] AND “acid”[All Fields]) OR “linoleic acid”[All Fields]) AND (“bone and bones” [MeSH Terms] OR (“bone”[All Fields] AND “bones”[All Fields]) OR “bone and bones”[All Fields] OR “bone”[All Fields]). Of the 52 papers identified, only the in vivo clinical studies in human subjects were included in this review. There were four studies with bone turnover biomarkers and five studies with dual X-ray absorptiometry (DXA) data. The latter group is summarized in Table 1.
Table 1.
Clinical data on the effects of conjugated linoleic acids (CLA) on bone.
| Study | Study population | CLA formulation | Study type/duration | Outcome |
|---|---|---|---|---|
| Brownbill et al. (2005)88 | 136 postmenopausal women; 68.6 years; BMI 26.0 | Dietary CLA by 3-day food diary | Cross-sectional | Dietary CLA correlated with hip BMD |
| Gaullier et al. (2004)89 and (2005)90 | 31 men, 149 women; 44.5–48.0 years; BMI 27.7–28.3 (extension study, 134 subjects) | CLA-FFA: 4.5 g of 80% CLA (3.46 g CLA, 39% c9t11, 41% t10c12); CLA-TG: 4.5 g of 76% CLA (3.4 g CLA, 38% c9, t11, 38% t10, c12); Placebo: 4.5 g olive oil | Placebo-controlled randomized trial, 12 months with 12-month open-label extension | Total body bone mineral mass decreased in the CLA-FFA group at first year but returned to baseline at second year |
| Gaullier (2007)91 | 21 men, 84 women; mean age 45.8–48.7 years; BMI 32–35 | CLA: 4.5 g of 80% CLA (3.4 g CLA, 37.5% c9t11, 38% t10c12); Placebo: 4.5 g olive oil | Placebo-controlled randomized trial, 6 months | Total body bone mineral content did not change between or within groups |
| Racine (2010)92 | 31 boys, 22 girls; ages 6–10 years; BMI >85th percentile | Clarinol™ 3.0 g (80% CLA, 50% c9t11, 50% t10c12); Placebo: sunflower oil | Placebo-controlled randomized trial, 7 months | Total body bone mineral content accrual was decreased in CLA group |
Abbreviations: CLA-FFA, CLA-free fatty acid; CLA-TG, CLA triacylglycerol; c9t11, cis-9, trans-11 CLA; t10c12, t10c12 CLA.
In an 8-week intervention study in 60 men, supplementation with CLA versus placebo did not affect bone formation biomarkers (serum osteocalcin and bonespecific alkaline phosphatase), bone resorption biomarkers (serum C-telopeptide, urinary N-telopeptide, urinary pyridinoline, and urinary deoxypuridinoline), serum calcium concentrations, or urinary calcium concentrations.80 Likewise, other short-term studies involving 1–6 months of strength training plus CLA versus placebo did not reveal changes in bone turnover markers.81–83
It is well established that decreasing body weight is associated with declining BMD in clinical trials of weight loss84,85 and in prospective epidemiological studies.86,87 Although CLA reduces body weight by decreasing fat mass, bone mass is not compromised, and there is some evidence to support a beneficial effect of CLA on bone. Brownbill et al.88 assessed the relationship between dietary CLA consumption (c9t11-CLA) and BMD in a cross-sectional study of 136 subjects. Dietary CLA assessed using 3-day diet records was positively associated with BMD at the Ward's triangle component of the proximal hip. BMD at the distal forearm was greater in subjects with a higher CLA intake. This is the only clinical study in postmenopausal women (mean age, 68.6 years; mean BMI, 26) using site-specific bone densitometry. The largest and longest human intervention study of CLA with skeletal outcomes data is reported by Gaullier et al.,89 who randomized 180 men and women to CLA-free fatty acid (CLA-FFA), CLA-triacylglycerol (CLA-TG, 3.4 g of equal parts c9t11 and t10c12), or placebo. The CLA groups exhibited a 6.9–8.7% decrease in body fat mass versus placebo. During a follow-up in a 1-year open-label extension study in which all subjects took CLA-TG supplements, decrements in body fat mass previously observed in the CLA groups were maintained, and the placebo group (switching to CLA) experienced a comparable decrease in body fat mass.90 The CLA-TG group and the placebo group showed no changes in bone mineral mass (BMM) by body composition DXA at 0, 1, and 2 years. The CLA-FFA group showed a small 1.4% decrease in BMM from baseline to year 1, which returned to baseline by the second year. In a subsequent clinical trial to determine which body compartments accounted for loss in body fat, CLA supplementation (3.4 g of active isomer) led to a decrease in fat mass, mainly in the leg; however, total body bone mineral content did not change.91 Assessment of specific skeletal sites was not performed. Recently, CLA supplementation (3 g/day of 80% t10c12-CLA and c9t11-CLA in equal proportion) versus placebo in overweight and obese 6- to 10-year-old children attenuated increases in BMI and body fat but decreased accrual of total body bone mineral content (CLA + 0.05 ± 0.03 kg versus placebo 0.07 ± 0.03 kg, P = 0.04).92 Notably, this study did not assess bone size, which was increased in a preclinical study13 Further study using 3D assessment of bone geometry would be needed to clarify this potential safety issue in the growing human skeleton exposed to CLA supplementation. Since these interventional studies were primarily designed to evaluate the effect of CLA on weight loss, the effect on changes in bone mass are inconclusive. Of note, in these clinical trials, assessment of bone mass is limited to measures of total body bone mass using body composition DXA.
In summary, CLA maintains an overall neutral effect on bone mass despite inducing loss of adiposity and body weight. Total body BMM rather than BMD was reported, and site-specific measurements of BMD such as lumbar spine, proximal hip, and distal forearm have not been reported, which limits interpretation of the clinical effects of CLA on bone outcomes. Menopausal status of women has not reported in previous studies but could contribute to confounding issues with interpreting the data from previous studies. It could be presumed that many subjects in previous studies were premenopausal, since the mean age was 44 to 48 years.
Conclusion
Preclinical data support a positive effect of CLA on bone health, whereas clinical data in human subjects have yet to show convincing evidence of a benefit. If CLA-induced decrement in adipose mass and weight is taken into account, a decrease in BMD might be expected; in contrast, maintenance of BMD with CLA supplementation has been observed.89,90,91 Future clinical trials in specific populations, e.g., in older women to prevent postmenopausal bone loss, are necessary to elucidate the proper role of CLA supplementation to enhance skeletal health.
Acknowledgments
The authors thank Dr Carolyn Gunther for her helpful feedback regarding the content of this manuscript.
Funding. This work was partially funded by grants 1R21AT003520 (to M.A. Belury).
Footnotes
Declaration of interest. The authors have no relevant interests to declare.
Contributor Information
Steven W Ing, Division of Endocrinology, Diabetes, & Metabolism, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, Ohio, USA.
Martha A Belury, Department of Human Nutrition, The Ohio State University, Columbus, Ohio, USA.
References
- 1.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795. [Google Scholar]
- 2.National Osteoporosis Foundation. America's Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation. Washington, DC: National Osteoporosis Foundation; 2002. [Google Scholar]
- 3.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–475. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 4.Pariza MW, Park Y, Cook ME. The biologically active isomers of conjugated linoleic acid. Prog Lipid Res. 2001;40:283–298. doi: 10.1016/s0163-7827(01)00008-x. [DOI] [PubMed] [Google Scholar]
- 5.Pariza MW. Perspective on the safety and effectiveness of conjugated linoleic acid. Am J Clin Nutr. 2004;79(Suppl):S1132–S1136. doi: 10.1093/ajcn/79.6.1132S. [DOI] [PubMed] [Google Scholar]
- 6.Pariza MW, Ashoor SH, Chu FS, Lund DB. Effects of temperature and time on mutagen formation in pan-fried hamburger. Cancer Lett. 1979;7:63–69. doi: 10.1016/s0304-3835(79)80097-x. [DOI] [PubMed] [Google Scholar]
- 7.Pariza MW, Hargraves WA. A beef-derived mutagenesis modulator inhibits initiation of mouse epidermal tumors by 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1985;6:591–593. doi: 10.1093/carcin/6.4.591. [DOI] [PubMed] [Google Scholar]
- 8.Bassaganya-Riera J, Hontecillas R, Beitz DC. Colonic anti-inflammatory mechanisms of conjugated linoleic acid. Clin Nutr. 2002;21:451–459. doi: 10.1054/clnu.2002.0594. [DOI] [PubMed] [Google Scholar]
- 9.Belury MA. Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action. Annu Rev Nutr. 2002;22:505–531. doi: 10.1146/annurev.nutr.22.021302.121842. [DOI] [PubMed] [Google Scholar]
- 10.Evans M, Brown J, McIntosh M. Isomer-specific effects of conjugated linoleic acid (CLA) on adiposity and lipid metabolism. J Nutr Biochem. 2002;13:508. doi: 10.1016/s0955-2863(02)00211-5. [DOI] [PubMed] [Google Scholar]
- 11.Whigham LD, Higbee A, Bjorling DE, Park Y, Pariza MW, Cook ME. Decreased antigen-induced eicosanoid release in conjugated linoleic acid-fed guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1104–R1112. doi: 10.1152/ajpregu.00075.2001. [DOI] [PubMed] [Google Scholar]
- 12.Whigham LD, Watras AC, Schoeller DA. Efficacy of conjugated linoleic acid for reducing fat mass: a meta-analysis in humans. Am J Clin Nutr. 2007;85:1203–1211. doi: 10.1093/ajcn/85.5.1203. [DOI] [PubMed] [Google Scholar]
- 13.Banu J, Bhattacharya A, Rahman M, Fernandes G. Beneficial effects of conjugated linoleic acid and exercise on bone of middle-aged female mice. J Bone Miner Metab. 2008;26:436–445. doi: 10.1007/s00774-008-0863-3. [DOI] [PubMed] [Google Scholar]
- 14.Park Y, Pariza MW. Cosupplementation of dietary calcium and conjugated linoleic acid (CLA) improves bone mass in mice. J Food Sci. 2008;73:C556–C560. doi: 10.1111/j.1750-3841.2008.00861.x. [DOI] [PubMed] [Google Scholar]
- 15.Park Y, Storkson JM, Albright KJ, Liu W, Pariza MW. Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 1999;34:235–241. doi: 10.1007/s11745-999-0358-8. [DOI] [PubMed] [Google Scholar]
- 16.Brodie AE, Manning VA, Ferguson KR, Jewell DE, Hu CY. Conjugated linoleic acid inhibits differentiation of pre- and post-confluent 3T3-L1 preadipocytes but inhibits cell proliferation only in preconfluent cells. J Nutr. 1999;129:602–606. doi: 10.1093/jn/129.3.602. [DOI] [PubMed] [Google Scholar]
- 17.Satory DL, Smith SB. Conjugated linoleic acid inhibits proliferation but stimulates lipid filling of murine 3T3-L1 preadipocytes. J Nutr. 1999;129:92–97. doi: 10.1093/jn/129.1.92. [DOI] [PubMed] [Google Scholar]
- 18.Evans M, Geigerman C, Cook J, Curtis L, Kuebler B, McIntosh M. Conjugated linoleic acid suppresses triglyceride accumulation and induces apoptosis in 3T3-L1 preadipocytes. Lipids. 2000;35:899–910. doi: 10.1007/s11745-000-0599-6. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi Y, Kushiro M, Shinohara K, Ide T. Dietary conjugated linoleic acid reduces body fat mass and affects gene expression of proteins regulating energy metabolism in mice. Comp Biochem Physiol B Biochem Mol Biol. 2002;133:395–404. doi: 10.1016/s1096-4959(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 20.Kang K, Liu W, Albright KJ, Park Y, Pariza MW. trans-10,cis-12 CLA inhibits differentiation of 3T3-L1 adipocytes and decreases PPAR gamma expression. Biochem Biophys Res Commun. 2003;303:795–799. doi: 10.1016/s0006-291x(03)00413-3. [DOI] [PubMed] [Google Scholar]
- 21.Brown JM, Boysen MS, Jensen SS, et al. Isomer-specific regulation of metabolism and PPARgamma signaling by CLA in human preadipocytes. J Lipid Res. 2003;44:1287–1300. doi: 10.1194/jlr.M300001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Granlund L, Juvet LK, Pedersen JI, Nebb HI. Trans10, cis12-conjugated linoleic acid prevents triacylglycerol accumulation in adipocytes by acting as a PPARgamma modulator. J Lipid Res. 2003;44:1441–1452. doi: 10.1194/jlr.M300120-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Norris LE, Collene AL, Asp ML, et al. Comparison of dietary conjugated linoleic acid with safflower oil on body composition in obese postmenopausal women with type 2 diabetes mellitus. Am J Clin Nutr. 2009;90:468–476. doi: 10.3945/ajcn.2008.27371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook ME, Jerome DL, Pariza M. Broilers fed conjugated linoleic acid had enhanced bone ash. Poult Sci. 1997;76(Suppl 1):S41. [Google Scholar]
- 25.Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW. Effect of conjugated linoleic acid on body composition in mice. Lipids. 1997;32:853–858. [Google Scholar]
- 26.Li Y, Seifert MF, Ney DM, et al. Dietary conjugated linoleic acids alter serum IGF-I and IGF binding protein concentrations and reduce bone formation in rats fed (n-6) or (n-3) fatty acids. J Bone Miner Res. 1999;14:1153–1162. doi: 10.1359/jbmr.1999.14.7.1153. [DOI] [PubMed] [Google Scholar]
- 27.Kelly O, Cusack S, Jewell C, Cashman KD. The effect of poly-unsaturated fatty acids, including conjugated linoleic acid, on calcium absorption and bone metabolism and composition in young growing rats. Br J Nutr. 2003;90:743–750. doi: 10.1079/bjn2003951. [DOI] [PubMed] [Google Scholar]
- 28.Kelly O, Cashman KD. The effect of conjugated linoleic acid on calcium absorption and bone metabolism and composition in adult ovariectomised rats. Prostaglandins Leukot Essent Fatty Acids. 2004;71:295–301. doi: 10.1016/j.plefa.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Weiler H, Austin S, Fitzpatrick-Wong S, et al. Conjugated linoleic acid reduces parathyroid hormone in health and in polycystic kidney disease in rats. Am J Clin Nutr. 2004;79(Suppl):S1186–S1189. doi: 10.1093/ajcn/79.6.1186S. [DOI] [PubMed] [Google Scholar]
- 30.Burr LL, Taylor CG, Weiler HA. Dietary conjugated linoleic acid does not adversely affect bone mass in obese fa/fa or lean Zucker rats. Exp Biol Med (Maywood) 2006;231:1602–1609. doi: 10.1177/153537020623101004. [DOI] [PubMed] [Google Scholar]
- 31.Thiel-Cooper RL, Parrish FC, Jr, Sparks JC, Wiegand BR, Ewan RC. Conjugated linoleic acid changes swine performance and carcass composition. J Anim Sci. 2001;79:1821–1828. doi: 10.2527/2001.7971821x. [DOI] [PubMed] [Google Scholar]
- 32.Demaree SR, Gilbert CD, Mersmann HJ, Smith SB. Conjugated linoleic acid differentially modifies fatty acid composition in subcellular fractions of muscle and adipose tissue but not adiposity of postweaning pigs. J Nutr. 2002;132:3272–3279. doi: 10.1093/jn/132.11.3272. [DOI] [PubMed] [Google Scholar]
- 33.Ostrowska E, Suster D, Muralitharan M, et al. Conjugated linoleic acid decreases fat accretion in pigs: evaluation by dual-energy X-ray absorptiometry. Br J Nutr. 2003;89:219–229. doi: 10.1079/BJN2002765. [DOI] [PubMed] [Google Scholar]
- 34.Park Y, Terk M. Interaction between dietary conjugated linoleic acid and calcium supplementation affecting bone and fat mass. J Bone Miner Metab. 2010 doi: 10.1007/s00774-010-0212-1. Available at http://www.springerlink.com/content/f7g52685u439x8rn/. [Epub ahead of print 2010 Aug 10] [DOI] [PubMed]
- 35.Banu J, Bhattacharya A, Rahman M, O'Shea M, Fernandes G. Effects of conjugated linoleic acid and exercise on bone mass in young male Balb/C mice. Lipids Health Dis. 2006;5:7. doi: 10.1186/1476-511X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouxsein ML, Karasik D. Bone geometry and skeletal fragility. Curr Osteoporos Rep. 2006;4:49–56. doi: 10.1007/s11914-006-0002-9. [DOI] [PubMed] [Google Scholar]
- 37.Rahman MM, Bhattacharya A, Banu J, Fernandes G. Conjugated linoleic acid protects against age-associated bone loss in C57BL/6 female mice. J Nutr Biochem. 2007;18:467–474. doi: 10.1016/j.jnutbio.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 38.Platt ID, El-Sohemy A. Regulation of osteoblast and adipocyte differentiation from human mesenchymal stem cells by conjugated linoleic acid. J Nutr Biochem. 2009;20:956–964. doi: 10.1016/j.jnutbio.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 40.Maurin AC, Chavassieux PM, Meunier PJ. Expression of PPAR-gamma and beta/delta in human primary osteoblastic cells: influence of polyunsaturated fatty acids. Calcif Tissue Int. 2005;76:385–392. doi: 10.1007/s00223-004-0108-y. [DOI] [PubMed] [Google Scholar]
- 41.Greene ME, Pitts J, McCarville MA, et al. PPARgamma: observations in the hematopoietic system. Prostaglandins Other Lipid Mediat. 2000;62:45–73. doi: 10.1016/s0090-6980(00)00075-7. [DOI] [PubMed] [Google Scholar]
- 42.Rosen ED, Sarraf P, Troy AE, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 43.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 44.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 45.Rosen ED, Hsu CH, Wang X, et al. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeon MJ, Kim JA, Kwon SH, et al. Activation of peroxisome proliferator-activated receptor-gamma inhibits the Runx2-mediated transcription of osteocalcin in osteoblasts. J Biol Chem. 2003;278:23270–23277. doi: 10.1074/jbc.M211610200. [DOI] [PubMed] [Google Scholar]
- 47.Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J Cell Biochem. 2009;106:232–246. doi: 10.1002/jcb.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 49.Akune T, Ohba S, Kamekura S, et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell. 2004;3:379–389. doi: 10.1111/j.1474-9728.2004.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lecka-Czernik B, Gubrij I, Moerman EJ, et al. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- 52.Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145:401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li M, Pan LC, Simmons HA, et al. Surface-specific effects of a PPARgamma agonist, darglitazone, on bone in mice. Bone. 2006;39:796–806. doi: 10.1016/j.bone.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz AV, Sellmeyer DE, Vittinghoff E, et al. Thiazolidinedi-one use and bone loss in older diabetic adults. J Clin Endo-crinol Metab. 2006;91:3349–3354. doi: 10.1210/jc.2005-2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grey A, Bolland M, Gamble G, et al. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone decreases bone formation and bone mineral density in healthy postmenopausal women: a randomized, controlled trial. J Clin Endocrinol Metab. 2007;92:1305–1310. doi: 10.1210/jc.2006-2646. [DOI] [PubMed] [Google Scholar]
- 56.Glintborg D, Andersen M, Hagen C, Heickendorff L, Hermann AP. Association of pioglitazone treatment with decreased bone mineral density in obese premenopausal patients with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2008;93:1696–1701. doi: 10.1210/jc.2007-2249. [DOI] [PubMed] [Google Scholar]
- 57.Meier C, Kraenzlin ME, Bodmer M, Jick SS, Jick H, Meier CR. Use of thiazolidinediones and fracture risk. Arch Intern Med. 2008;168:820–825. doi: 10.1001/archinte.168.8.820. [DOI] [PubMed] [Google Scholar]
- 58.Dormuth CR, Carney G, Carleton B, Bassett K, Wright JM. Thiazolidinediones and fractures in men and women. Arch Intern Med. 2009;169:1395–1402. doi: 10.1001/archinternmed.2009.214. [DOI] [PubMed] [Google Scholar]
- 59.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 60.Kahn SE, Zinman B, Lachin JM, et al. Rosiglitazone-associated fractures in type 2 diabetes: an Analysis from A Diabetes Outcome Progression Trial (ADOPT) Diabetes Care. 2008;31:845–851. doi: 10.2337/dc07-2270. [DOI] [PubMed] [Google Scholar]
- 61.Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009;180:32–39. doi: 10.1503/cmaj.080486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- 63.Knouff C, Auwerx J. Peroxisome proliferator-activated receptor-gamma calls for activation in moderation: lessons from genetics and pharmacology. Endocr Rev. 2004;25:899–918. doi: 10.1210/er.2003-0036. [DOI] [PubMed] [Google Scholar]
- 64.Bendixen AC, Shevde NK, Dienger KM, Willson TM, Funk CD, Pike JW. IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor gamma 1. Proc Natl Acad Sci U S A. 2001;98:2443–2448. doi: 10.1073/pnas.041493198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giaginis C, Tsantili-Kakoulidou A, Theocharis S. Peroxisome proliferator-activated receptors (PPARs) in the control of bone metabolism. Fundam Clin Pharmacol. 2007;21:231–244. doi: 10.1111/j.1472-8206.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 66.Moya-Camarena SY, Vanden Heuvel JP, Blanchard SG, Leesnitzer LA, Belury MA. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J Lipid Res. 1999;40:1426–1433. [PubMed] [Google Scholar]
- 67.Houseknecht KL, Vanden Heuvel JP, Moya-Camarena SY, et al. Dietary conjugated linoleic acid normalizes impaired glucose tolerance in the Zucker diabetic fatty fa/fa rat. Biochem Biophys Res Commun. 1998;244:678–682. doi: 10.1006/bbrc.1998.8303. [DOI] [PubMed] [Google Scholar]
- 68.Chan BY, Gartland A, Wilson PJ, et al. PPAR agonists modulate human osteoclast formation and activity in vitro. Bone. 2007;40:149–159. doi: 10.1016/j.bone.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 69.Richards JB, Valdes AM, Burling K, Perks UC, Spector TD. Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab. 2007;92:1517–1523. doi: 10.1210/jc.2006-2097. [DOI] [PubMed] [Google Scholar]
- 70.Luo XH, Guo LJ, Yuan LQ, et al. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res. 2005;309:99–109. doi: 10.1016/j.yexcr.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 71.Shimizu H, Shimomura Y, Hayashi R, et al. Serum leptin concentration is associated with total body fat mass, but not abdominal fat distribution. Int J Obes Relat Metab Disord. 1997;21:536–541. doi: 10.1038/sj.ijo.0800437. [DOI] [PubMed] [Google Scholar]
- 72.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 73.Elefteriou F, Ahn JD, Takeda S, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 74.Takeda S, Karsenty G. Molecular bases of the sympathetic regulation of bone mass. Bone. 2008;42:837–840. doi: 10.1016/j.bone.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 75.Bonnet N, Pierroz DD, Ferrari SL. Adrenergic control of bone remodeling and its implications for the treatment of osteoporosis. J Musculoskelet Neuronal Interact. 2008;8:94–104. [PubMed] [Google Scholar]
- 76.Yamasaki M, Mansho K, Ogino Y, Kasai M, Tachibana H, Yamada K. Acute reduction of serum leptin level by dietary conjugated linoleic acid in Sprague-Dawley rats. J Nutr Biochem. 2000;11:467–471. doi: 10.1007/978-94-017-0728-2_81. [DOI] [PubMed] [Google Scholar]
- 77.Thomas T, Gori F, Khosla S, Jensen MD, Burguera B, Riggs BL. Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology. 1999;140:1630–1638. doi: 10.1210/endo.140.4.6637. [DOI] [PubMed] [Google Scholar]
- 78.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 79.Shinoda Y, Yamaguchi M, Ogata N, et al. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem. 2006;99:196–208. doi: 10.1002/jcb.20890. [DOI] [PubMed] [Google Scholar]
- 80.Doyle L, Jewell C, Mullen A, Nugent AP, Roche HM, Cashman KD. Effect of dietary supplementation with conjugated linoleic acid on markers of calcium and bone metabolism in healthy adult men. Eur J Clin Nutr. 2005;59:432–440. doi: 10.1038/sj.ejcn.1602093. [DOI] [PubMed] [Google Scholar]
- 81.Kreider RB, Ferreira MP, Greenwood M, Wilson M, Almada AL. Effects of conjugated linoleic acid supplementation during resistance training on body composition, bone density, strength, and selected hematological markers. J Strength Cond Res. 2002;16:325–334. [PubMed] [Google Scholar]
- 82.Tarnopolsky M, Zimmer A, Paikin J, et al. Creatine monohydrate and conjugated linoleic acid improve strength and body composition following resistance exercise in older adults. PLoS ONE. 2007;2:e991. doi: 10.1371/journal.pone.0000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cornish SM, Candow DG, Jantz NT, et al. Conjugated linoleic acid combined with creatine monohydrate and whey protein supplementation during strength training. Int J Sport Nutr Exerc Metab. 2009;19:79–96. doi: 10.1123/ijsnem.19.1.79. [DOI] [PubMed] [Google Scholar]
- 84.Avenell A, Richmond PR, Lean ME, Reid DM. Bone loss associated with a high fibre weight reduction diet in postmenopausal women. Eur J Clin Nutr. 1994;48:561–566. [PubMed] [Google Scholar]
- 85.Ricci TA, Chowdhury HA, Heymsfield SB, Stahl T, Pierson RN, Jr, Shapses SA. Calcium supplementation suppresses bone turnover during weight reduction in postmenopausal women. J Bone Miner Res. 1998;13:1045–1050. doi: 10.1359/jbmr.1998.13.6.1045. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen TV, Sambrook PN, Eisman JA. Bone loss, physical activity, and weight change in elderly women: the Dubbo Osteoporosis Epidemiology Study. J Bone Miner Res. 1998;13:1458–1467. doi: 10.1359/jbmr.1998.13.9.1458. [DOI] [PubMed] [Google Scholar]
- 87.Hannan MT, Felson DT, Dawson-Hughes B, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–720. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 88.Brownbill RA, Petrosian M, Ilich JZ. Association between dietary conjugated linoleic acid and bone mineral density in postmenopausal women. J Am Coll Nutr. 2005;24:177–181. doi: 10.1080/07315724.2005.10719463. [DOI] [PubMed] [Google Scholar]
- 89.Gaullier JM, Halse J, Hoye K, et al. Conjugated linoleic acid supplementation for 1 y reduces body fat mass in healthy overweight humans. Am J Clin Nutr. 2004;79:1118–1125. doi: 10.1093/ajcn/79.6.1118. [DOI] [PubMed] [Google Scholar]
- 90.Gaullier JM, Halse J, Hoye K, et al. Supplementation with conjugated linoleic acid for 24 months is well tolerated by and reduces body fat mass in healthy, overweight humans. J Nutr. 2005;135:778–784. doi: 10.1093/jn/135.4.778. [DOI] [PubMed] [Google Scholar]
- 91.Gaullier JM, Halse J, Hoivik HO, et al. Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. Br J Nutr. 2007;97:550–560. doi: 10.1017/S0007114507381324. [DOI] [PubMed] [Google Scholar]
- 92.Racine NM, Watras AC, Carrel AL, et al. Effect of conjugated linoleic acid on body fat accretion in overweight or obese children. Am J Clin Nutr. 2010;91:1157–1164. doi: 10.3945/ajcn.2009.28404. [DOI] [PMC free article] [PubMed] [Google Scholar]