Abstract
Purpose
Transcatheter mitral valve replacement would represent a major advance in heart valve therapy. Such a device requires a specialized anchoring and sealing technology. This study was designed to test the feasibility of a novel mitral valve replacement device (the sutureless mitral valve [SMV]) designed to anchor and seal in the mitral position without need for sutures.
Description
The SMV is a self-expanding device consisting of a custom-designed nitinol framework and a pericardial leaflet valve mechanism.
Evaluation
Ten sheep underwent successful surgical SMV device implantation. All animals underwent cardiac catheterization 6 hours postoperatively. Hemodynamic, angiographic, echocardiographic and necroscopic data were recorded. The mean aortic cross-clamp time was 9.5 ± 3.1 minutes. Echocardiography and angiography revealed excellent left ventricular systolic function, no significant perivalvular leak, no mitral valve stenosis, no left ventricular outflow tract obstruction, and no aortic valve insufficiency. Necropsy demonstrated that the SMV devices were anchored securely.
Conclusions
This study demonstrates the feasibility and short-term success of sutureless mitral valve replacement using a novel SMV device.
Heart valve replacement therapy is in the midst of a major paradigm shift [1]. Improvements in imaging, catheter technology, and stent design have made transcatheter replacement of the aortic and pulmonic valves clinical realities [2–4]. A transcatheter approach to mitral valve replacement (MVR) would represent a major advance in the treatment of valvular heart disease as approximately 2.4 million Americans suffer from moderate to severe ischemic mitral regurgitation. Many of these patients would benefit from valve replacement but are deemed too sick or debilitated to tolerate standard open heart surgical procedures.
The complex anatomy of the mitral valve (MV) and the high pressures to which it is exposed prevent the application of the current aortic and pulmonic replacement technologies to the treatment of MV disease. Successful transcatheter MVR requires (1) a specialized atraumatic sutureless anchoring mechanism, (2) a perivalvular sealing strategy, and (3) foldability. In extensive preliminary work, our group has developed a novel anchoring and sealing technology that is foldable and will potentially facilitate transcatheter MVR.
In this study, we describe our initial experience with implantation of this device as a sutureless mitral valve (SMV) in an ovine model of open heart surgical MV replacement.
Technology
Sutureless Mitral Valve Replacement Device
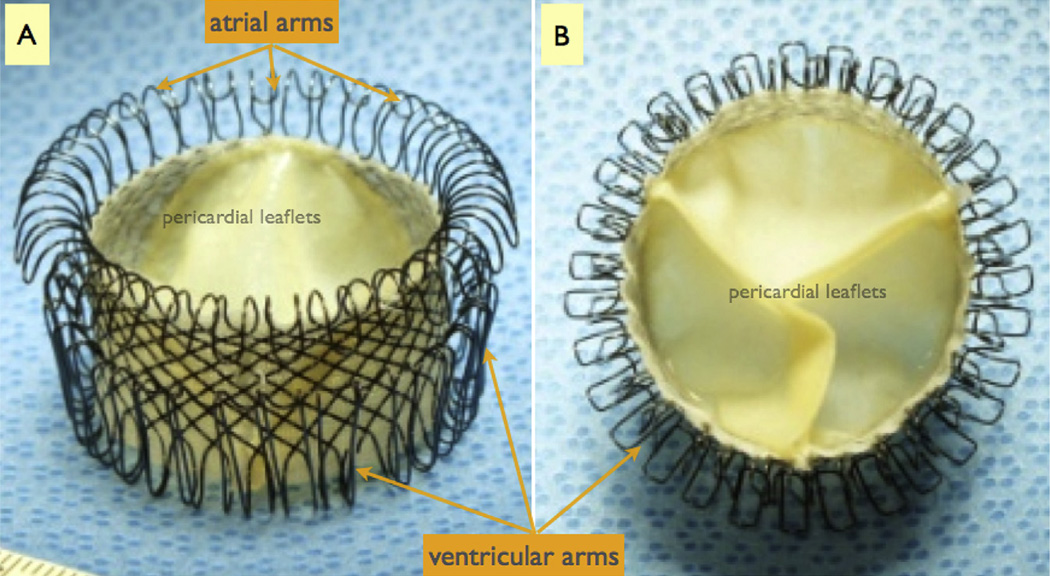
The SMV device is composed of two major components: a supporting frame and a tissue valve mechanism (Fig 1). The frame is constructed from a single nitinol wire (0.012″) woven into a complex three-dimensional shape that provides a combination of anchoring forces, including a radial (expansive) force and a “cinching” or “grasping” force—all while maintaining a hollow lumen for housing the valve mechanism. The radial force is generated by self-expansion of the SMV, which is designed to gently but firmly expand within the mitral annulus. The grasping force is produced in the submitral space by the “ventricular arms,” which are designed to gather the anterior and posterior leaflets of the MV onto the body of the SMV frame. The atrial side of the device is engineered to gently gather the supra-annular tissue centrally. In combination, these forces result in secure anchoring and tight perivalvular seal. The SMV device is shown in Figure 1.
Fig 1.
The sutureless mitral valve replacement device consists of a frame constructed from a single nitinol wire that is woven into a complex three-dimensional geometry engineered to provide a housing for the valve mechanism and anchoring forces to ensure secure position within the mitral annulus. (A) The device as seen from the side. (B) The device as seen from the ventricular side. The valve leaflets are constructed from porcine pericardium.
Technique
Sutureless Mitral Valve Implantation Procedure
After approval by the University of Pennsylvania’s Animal Care and Use Committee, 10 adult male sheep were subjected to study. In all animals, a left thoracotomy was performed. Using standard cardiac surgical techniques, cardiopulmonary bypass was instituted, the heart was arrested, and a left atriotomy was created. The SMV was packaged into a custom-made 30F delivery device (Fig 2) and then advanced through a 2-cm left atrial incision into the left ventricle (LV). Then, in a stepwise process, the ventricular end of the SMV was opened within the LV cavity and then slowly withdrawn backward toward the left atrium, seating the ventricular arms so that the anterior and posterior MV leaflets were captured between the body and the ventricular arms of the device. The atrial side of the device was then allowed to blossom on the left atrial side of the annulus. The atrial arms clamped down circumferentially around the MV annulus as they assumed their nominal configuration. Once appropriate positioning was confirmed, the delivery device was removed, the left atrium was closed, and the animals were weaned from cardiopulmonary bypass. Epicardial echocardiography was performed to assess device location and function. Six hours after closure of the chest, animals were brought to the catheterization laboratory for hemodynamic and angiographic assessment.
Fig 2.
The 30F sutureless mitral valve delivery device.
After the cardiac catheterization, all animals were euthanized as per protocol. Necroscopy was performed, including gross inspection of the SMV device in situ and evaluation of the surrounding tissues for evidence of device-related trauma.
Statistical Analysis
Hemodynamic measurements after SMV implantation were summarized using standard descriptive statistics and reported as mean ± standard deviation.
Clinical Experience
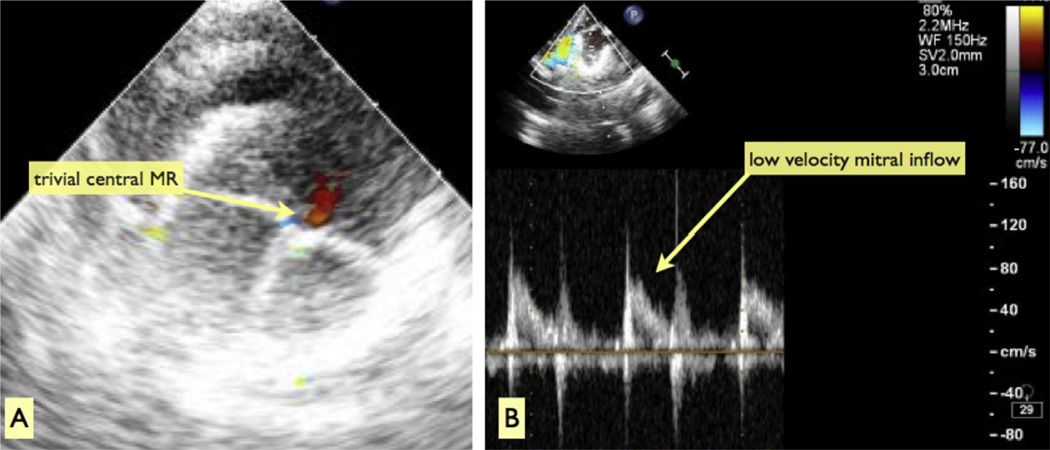
All implant procedures were successful and resulted in secure SMV anchoring in the mitral position. The operative data are summarized in Table 1. The mean cardiopulmonary bypass and aortic cross-clamp times were 63.8 ± 12.1 and 9.5 ± 3.1 minutes, respectively. After SMV implantation, epicardial echocardiography revealed normal LV systolic function, symmetric SMV leaflet excursion, and uniform coaptation with no mitral regurgitation in 7 animals and mild central regurgitation in 3 (Fig 3A). There was no perivalvular leak, no inflow acceleration across the SMV (mean echocardiographic Doppler velocity, 1.3 ± 0.5 m/s; Fig 3B), no left ventricular outflow tract (LVOT) obstruction, and no aortic valve insufficiency.
Table 1.
Procedural Variables
| Variable | Mean ± SD |
|---|---|
| Weight (kg) | 37.9 ± 2.9 |
| Mitral annulus dimensions (mm × mm) | 32 ± 1.8 × 24 ± 1.9 |
| CPB time (min) | 63.8 ± 12.1 |
| Aortic cross-clamp time (min) | 9.5 ± 3.1 |
| MR grade after implantation (0–4 scale) | 0 (n = 7); 1 (n = 3) |
| MV gradient after implantation (m/s) | 1.3 ± 0.5 |
CPB = cardiopulmonary bypass; MR = mitral regurgitation; MV = mitral valve; SD = standard deviation.
Fig 3.
Epicardial echocardiographic assessment after cessation of cardiopulmonary bypass. (A) En face color Doppler interrogation of the sutureless mitral valve device from the left atrium is shown. There is no perivalvular lead and only trivial central mitral regurgitation (MR). (B) Low-velocity flow from the left atrium through the sutureless mitral valve into the left ventricle is shown. There is no stenosis.
Post-SMV implant hemodynamic data, including cardiac output and chamber pressures, were recorded. The mean cardiac output was 3.2 ± 1.7 L/min. There was no significant difference between the mean left atrial and LV end-diastolic pressures (15.4 ± 4.9 versus 15.1 ± 3.6 mm Hg), and there was no LVOT obstruction (LV pressure, 77.1 ± 12.8 mm Hg; ascending aorta pressure, 75.2 ± 14.5 mm Hg).
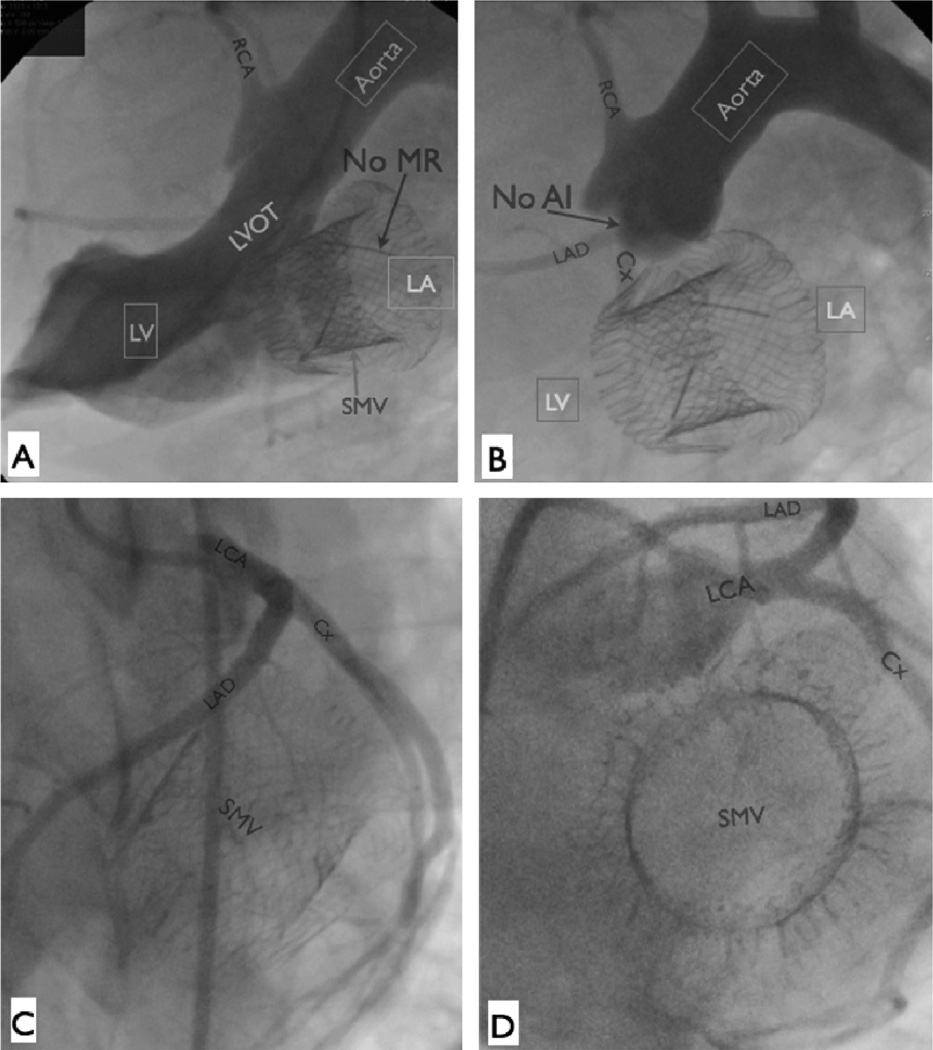
Follow-up angiography revealed stable device position and normal LV function. The absence of LVOT obstruction and aortic insufficiency were again confirmed (Figs 4A, 4B). Selective coronary angiography revealed no evidence of perturbation of the left coronary system (Figs 4C, 4D).
Fig 4.
Angiographic assessment of the sutureless mitral valve (SMV) device, 6 hours postoperatively. The device is securely anchored. There is no mitral regurgitation (MR; A), no left ventricular outflow tract (LVOT) obstruction (A), and no aortic valve insufficiency (AI; B). (C, D) Selective left coronary angiography after sutureless mitral valve device implantation. The left coronary system is undisturbed by the sutureless mitral valve device. (Cx = circumflex artery; LA = left atrium; LAD = left anterior descending artery; LCA = left coronary artery; LV = left ventricle; RCA = right coronary artery.)
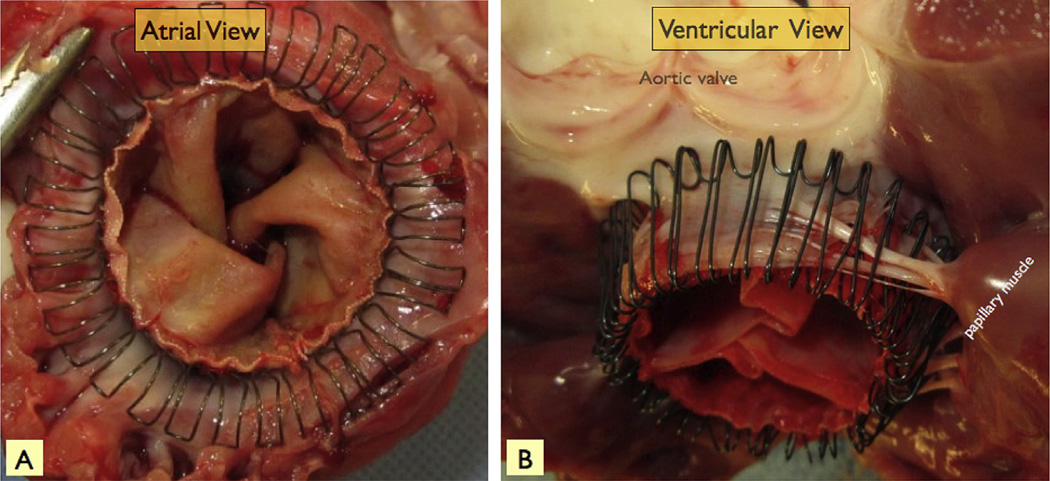
After euthanasia, necropsy demonstrated that the SMV devices were anchored securely, with the ventricular arms of the device enmeshed with the submitral chordae and MV leaflets as intended in all animals (Fig 5). There was a complete circumferential seal around the SMV and, on removal of the SMV from the mitral annulus, no evidence for significant device-related trauma.
Fig 5.
Postmortem pictures of the sutureless mitral valve device from the left atrial (A) and left ventricular (B) perspectives. The atrial arms gather the supraannular tissue centrally, and the ventricular arms entrap the mitral leaflets between the arms and the body of the sutureless mitral valve. The sutureless mitral valve ventricular arms also enmesh with the mitral chordae.
Comment
In this report, we describe our initial preclinical experience with sutureless MVR using a custom-made device designed to achieve stable, atraumatic anchoring and perivalvular seal. The flexibility of the nitinol wire-weave stent body design allows the device to gently conform to the complex MV geometry, creating a perivalvular seal without impinging on the LVOT. We have found that the optimal anchoring, seal, and avoidance of LVOT impingement occurs when the device is sized (length and diameter) to remain within and conform snugly to the conically shaped region defined by the mitral annulus and leaflets (Figs 4, 5).
The SMV device does not rely solely on radial force for anchoring strength. Anchoring is facilitated by a combination of gentle radial expansion and grasping arms that emanate from the ventricular aspect of the stent. These arms have been designed to insinuate themselves around the leaflets and chordae when the device is exposed to systolic LV pressures. This design serves to harness the LV pressure to help seat and maintain the valve in the correct position (Figs 4, 5). As the LV exerts pressure on the valve mechanism, the arms are pushed up behind the anterior and posterior leaflets. This mechanism allows the leaflets to be gently trapped between the stent body and the arms. In the region of the commissures where leaflet tissue can be sparse, especially in sheep, the arms tend to grasp chordae up near the annulus. These forces combine to form a remarkably strong, yet atraumatic seal.
Compared with traditional prosthetic valve devices, the SMV device allows for a more rapid replacement of the MV; however, the implications of this work are more significant than just expediting surgical valve replacement. The design as presented can be folded easily and reproducibly to allow delivery through a 30F applicator device. Additionally, as yet unreported, improvements in the design facilitate even more compact folding. With the appropriately designed delivery system, such devices may ultimately be used to replace the MV by means of an off-pump, minimally invasive thoracotomy procedure or even a percutaneous, transvenous, transatrial septal antegrade approach. This is the frontier of MV therapy, and our experience with the SMV prototype adds to the growing literature supporting the feasibility of catheter-based MVR [5–10].
This was a proof-of-principle study designed to test the feasibility of sutureless MVR using a novel self-expanding device engineered to anchor and seal within the MV annulus in an atraumatic fashion. This was an acute study; therefore, we cannot comment on the durability and functionality of the SMV device in the longer term. Long-term studies are currently under way.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute of the National Institutes of Health, Bethesda, MD (HL63954, HL73021, and HL108330), and the Gorman Cardiovascular Research Group Discretionary Fund. R. Gorman and J. Gorman were supported by individual Established Investigator Awards from the American Heart Association, Dallas, TX.
Footnotes
Disclosures and Freedom of Investigation
All authors had full control of the study design, methods used, outcome parameters, analysis of the data, and production of the written report. The valves tested in the study were designed and developed at the University of Pennsylvania by Drs R. Gorman, J. Gorman, and Gillespie.
Disclaimer
The Society of Thoracic Surgeons, the Southern Thoracic Surgical Association, and The Annals of Thoracic Surgery neither endorse nor discourage use of the new technology described in this article.
References
- 1.Stuge O, Liddicoat J. Emerging opportunities for cardiac surgeons within structural heart disease. J Thorac Cardiovasc Surg. 2006;132:1258–1261. doi: 10.1016/j.jtcvs.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 3.Boudjemline Y, Bonhoeffer P. Steps toward percutaneous aortic valve replacement. Circulation. 2002;105:775–778. doi: 10.1161/hc0602.103361. [DOI] [PubMed] [Google Scholar]
- 4.Cribier A, Eltchaninoff H, Tron C, et al. Early experience with percutaneous transcatheter implantation of heart valve prosthesis for the treatment of end-stage inoperable patients with calcific aortic stenosis. J Am Coll Cardiol. 2004;43:698–703. doi: 10.1016/j.jacc.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 5.Ma L, Tozzi P, Huber CH, Taub S, Gerelle G, von Segesser LK. Double-crowned valved stents for off-pump mitral valve replacement. Eur J Cardiothorac Surg. 2005;28:194–199. doi: 10.1016/j.ejcts.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 6.Banai S, Jolicoeur EM, Schwartz M, et al. Tiara: a novel catheter-based mitral valve bioprosthesis: initial experiments and short-term pre-clinical results. J Am Coll Cardiol. 2012;60:1430–1431. doi: 10.1016/j.jacc.2012.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudjemline Y, Agnoletti G, Bonnet D, et al. Steps toward the percutaneous replacement of atrioventricular valves an experimental study. J Am Coll Cardiol. 2005;46:360–365. doi: 10.1016/j.jacc.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 8.Lutter G, Quaden R, Osaki S, et al. Off-pump transapical mitral valve replacement. Eur J Cardiothorac Surg. 2009;36:124–128. doi: 10.1016/j.ejcts.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Lutter G, Quaden R, Iino K, et al. Mitral valved stent implantation. Eur J Cardiothorac Surg. 2010;38:350–355. doi: 10.1016/j.ejcts.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Iino K, Boldt J, Lozonschi L, et al. Off-pump transapical mitral valve replacement: evaluation after one month. Eur J Cardiothorac Surg. 2012;41:512–517. doi: 10.1093/ejcts/ezr106. [DOI] [PubMed] [Google Scholar]