Abstract
Background
Children born to women with low thyroid hormone levels have been reported to have decreased cognitive function.
Methods
We conducted a randomized trial in which pregnant women at a gestation of 15 weeks 6 days or less provided blood samples for measurement of thyrotropin and free thyroxine (T4). Women were assigned to a screening group (in which measurements were obtained immediately) or a control group (in which serum was stored and measurements were obtained shortly after delivery). Thyrotropin levels above the 97.5th percentile, free T4 levels below the 2.5th percentile, or both were considered a positive screening result. Women with positive findings in the screening group were prescribed 150 μg of levothyroxine per day. The primary outcome was IQ at 3 years of age in children of women with positive results, as measured by psychologists who were unaware of the group assignments.
Results
Of 21,846 women who provided blood samples (at a median gestational age of 12 weeks 3 days), 390 women in the screening group and 404 in the control group tested positive. The median gestational age at the start of levothyroxine treatment was 13 weeks 3 days; treatment was adjusted as needed to achieve a target thyrotropin level of 0.1 to 1.0 mIU per liter. Among the children of women with positive results, the mean IQ scores were 99.2 and 100.0 in the screening and control groups, respectively (difference, 0.8; 95% confidence interval [CI], −1.1 to 2.6; P = 0.40 by intention-to-treat analysis); the proportions of children with an IQ of less than 85 were 12.1% in the screening group and 14.1% in the control group (difference, 2.1 percentage points; 95% CI, −2.6 to 6.7; P = 0.39). An on-treatment analysis showed similar results.
Conclusions
Antenatal screening (at a median gestational age of 12 weeks 3 days) and maternal treatment for hypothyroidism did not result in improved cognitive function in children at 3 years of age. (Funded by the Wellcome Trust UK and Compagnia di San Paulo, Turin; Current Controlled Trials number, ISRCTN46178175.)
Active secretion of thyroid hormone in the fetus does not start until about 18 to 20 weeks’ gestation.1 Studies in animals suggest that until fetal hormone secretion begins, the fetus is dependent on circulating free thyroxine (T4) in the mother for growth and development, including central nervous system maturation.1 Iodine is essential for free T4 synthesis, and in iodine-deficient populations, an increase in cognitive performance has been observed after iodine supplementation before pregnancy.2-4
High levels of thyrotropin in women during pregnancy have been associated with impaired cognitive development in their offspring. This finding suggests that antenatal screening and treatment of thyroid deficiency may be worth-while.5 We conducted a randomized, controlled trial to assess the effects on cognitive function at 3 years of age in the offspring of women who underwent thyroid screening in early pregnancy and received treatment if they had a high thyrotropin level, a low free T4 level, or both.
Methods
Study Participants
We invited pregnant women to participate at their first antenatal hospital visit. The women were recruited from 10 centers in the United Kingdom and 1 center in Italy. Exclusion criteria were an age of less than 18 years, a gestational age of more than 15 weeks 6 days, twin pregnancies, and known thyroid disease. Approval of the study was obtained from research ethics committees in the United Kingdom and Italy, and all participants provided written informed consent.
Study Procedures
Blood samples were sent to the laboratory at the University Hospital of Wales, Cardiff, or to Ospedale Sant’Anna, Turin, Italy, for measurement of thyrotropin and free T4 levels. On receipt of samples, women were randomly assigned with the use of a computer-generated block design to the screening or control group.
Serum samples from the screening group were immediately assayed for levels of free T4 and thyrotropin (see below). Serum samples from women in the control group, stored at −40°C, were assayed for levels of free T4 and thyrotropin after delivery. Women were classified as positive if the serum thyrotropin concentration was above the 97.5th percentile, the serum free T4 concentration was below the 2.5th percentile, or both. Cutoff values for serum levels of free T4 (the 2.5th percentile) and thyrotropin (the 97.5th percentile) were periodically adjusted according to assay results obtained during the study.
Patients in the screening group who had positive results were treated with levothyroxine (recommended starting dose, 150 μg per day). Levels of thyrotropin and free T4 were checked 6 weeks after the start of levothyroxine therapy and at 30 weeks’ gestation, with adjustment of the dose as necessary. The target thyrotropin level was 1.0 mIU per liter. Women in the screening and control groups who had positive test results received standard routine care and were advised to visit their family physician after delivery to determine whether levothyroxine therapy should be continued or initiated, respectively.
In the United Kingdom, levels of serum thyrotropin and free T4 were measured with the use of immunochemiluminescence (ADVIA Centaur, Siemens Healthcare Diagnostics). The 95% range of thyrotropin levels was 0.15 to 3.65 mIU per liter, and the 95% range of free T4 levels was 8.4 to 14.6 pmol per liter (6.6 to 11.4 ng per deciliter). In Turin, levels of serum thyrotropin and free T4 were measured with the use of an immunofluorescence method (AutoDELFIA, PerkinElmer Life and Analytical Sciences). The 95% ranges of thyrotropin and free T4 were 0.11 to 3.50 mIU per liter and 7.15 to 11.34 pg per milliliter, respectively.
The first author vouches for the accuracy and completeness of the reported data and the fidelity of the report to the study protocol, which is available with the full text of this article at NEJM.org.
Outcomes and Assessments
The primary outcome was the IQ, at 3 years of age, of children of the women who tested positive. IQ was assessed with the use of the Wechsler Preschool and Primary Scale of Intelligence, third edition (2003) (Psychological Corporation) by psychologists who visited the children’s home and who were unaware of the group assignments.
Aspects of child behavior that might affect the evaluation of IQ were assessed with the use of the Child Behavior Checklist (CBCL) 2000 (Achenbach System of Empirically Based Assessment, University of Vermont) and the Behavior Rating Inventory of Executive Function, preschool version (Brief-P), 2003 (Psychological Assessment Resources). Information relating to parental education and maternal psychological status (assessed with the use of the Beck Depression Inventory II [Psychological Corporation]) was also collected.
IQ assessments were performed by two psychologists in the United Kingdom and one in Turin. To allow for between-psychologist differences in the assessments, the mean IQ score for the controls was set at 100 for each psychologist. For example, if the mean IQ score in the control group was 105 for a particular psychologist, all IQ scores assessed by that psychologist were reduced by 5 points. An analysis based on z scores (the observed IQ minus the mean, divided by the standard deviation according to psychologist) was also performed.
Statistical Analysis
Baseline characteristics of women with positive findings (a high thyrotropin level, a low free T4 level, or both) in the screening and control groups were compared with the use of t-tests for continuous variables with a Gaussian distribution and the Wilcoxon rank-sum test for those with a non-Gaussian distribution. The chi-square test was used for categorical variables.
The primary analysis was performed according to the intention-to-treat principle. A t-test was used to compare standardized IQ test results of the children between the screening and control groups, and the chi-square test was used to compare the proportion of children with an IQ of less than 85 (the coprimary outcome). To assess a possible trend, we estimated the relative risk of standardized IQ values (and standardized verbal and performance components) of less than 75, 80, 85, 90, and 95 among children in the screening group as compared with those in the control group. A post hoc, on-treatment analysis of IQ scores was also performed, which included the children of women in whom thyrotropin levels decreased by at least 10% and free T4 levels increased by at least 10% from the first blood sample obtained (at approximately 12 weeks’ gestation) to the sample obtained 6 weeks after the initiation of levothyroxine therapy.
The study protocol specified recruitment of 22,000 women with singleton pregnancies, with 440 of the 11,000 women in each study group having positive screening results (a high thyrotropin level, a low free T4 level, or both). Among the women with positive results, 22 of the children in the screening group (5%) were expected to have an IQ of 85 or less, as compared with 66 children in the control group (15%). This expectation was based on the results of a study by Haddow et al.,5 in which women with a high thyrotropin level were three times as likely to have children with an IQ that was less than or equal to 85 as women with normal thyrotropin levels during pregnancy. With an expected 10% loss to follow-up, the power to detect such a difference in child IQ between the screening and control groups is greater than 95% at the 5% significance level (two-sided test).
Results
Randomization and Baseline Measurements
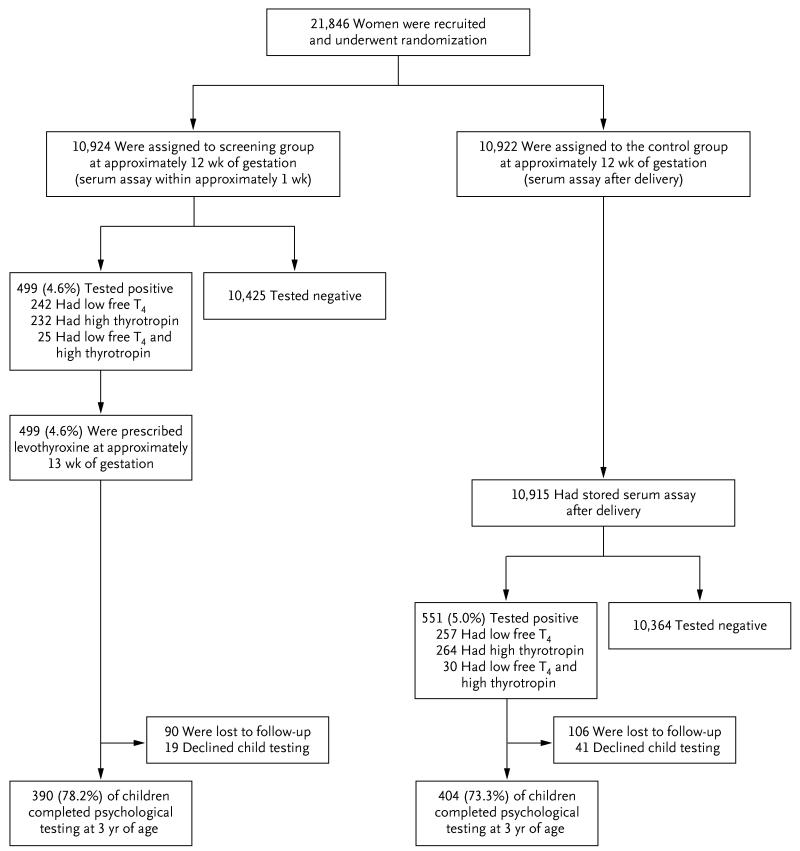
As shown in Figure 1, a total of 21,846 women were recruited and underwent randomization (16,346 women from 10 centers in the United Kingdom and 5500 from 1 center in Italy); 10,924 women were assigned to the screening group and 10,922 were assigned to the control group. The proportions of women classified as having positive screening results were 4.6% in the screening group and 5.0% in the control group. Similar proportions were classified as having positive results on the basis of a high thyrotropin level or a low free T4 level. About 5% of women classified as having positive screening results had both a high thyrotropin level and a low free T4 level. Levothyroxine therapy was started in the screening group at a median gestation of 13 weeks 3 days. Psychological testing was completed in 78.2% of the children of women who tested positive in the screening group and in 73.3% of the children of women who tested positive in the control group.
Figure 1.
Randomization and Follow-up of the Study Participants.
Table 1 shows characteristics of the women with positive test results and their children in the screening (treated) and control groups. The groups were similar with respect to baseline characteristics and socioeconomic characteristics, with the exception that thyrotropin levels were higher in the screening group than in the control group among the women in the United Kingdom.
Table 1. Characteristics of Women with Positive Screening Results and Their Children Who Completed Psychological Testing.✲.
| Characteristic | Screening Group (N = 390) |
Control Group (N = 404) |
|---|---|---|
| Gestational age at screening (weeks, days) | ||
| Median | 12, 3 | 12, 3 |
| Interquartile range | 11, 6–13, 6 | 11, 6–13, 5 |
| Thyrotropin (mIU/liter)† | ||
| United Kingdom‡ | ||
| Median | 3.8 | 3.2 |
| Interquartile range | 1.5–4.7 | 1.2–4.2 |
| Turin | ||
| Median | 3.1 | 2.4 |
| Interquartile range | 1.3–4.0 | 1.3–3.9 |
| Free T4† | ||
| United Kingdom (pmol/liter) | ||
| Median | 11.1 | 11.2 |
| Interquartile range | 10.5–13.3 | 10.5–13.2 |
| Turin (pg/ml) | ||
| Median | 7.4 | 7.4 |
| Interquartile range | 7.1–8.6 | 7.2–8.3 |
| Maternal smokers (%) | 17 | 14 |
| Maternal weight (kg) | ||
| Median | 68 | 67 |
| Interquartile range | 59–78 | 59–80 |
| Age when mother left full-time education (%)¶ | ||
| ≤16 yr | 34 | 33 |
| 17–18yr | 27 | 26 |
| ≥19 yr | 39 | 41 |
| Age when father left full-time education (%)¶ | ||
| ≤16 yr | 51 | 42 |
| 17–18yr | 18 | 23 |
| ≥19 yr | 31 | 35 |
| Maternal age at delivery (yr) | 30±5.4 | 31±5.3 |
| Paternal age at delivery (yr)¶ | 32±5.9 | 33±6.3 |
| Male children (%) | 55 | 51 |
| Age at psychological testing (yr) | ||
| Median | 3.2 | 3.2 |
| Interquartile range | 3.2–3.3 | 3.2–3.3 |
Plus–minus values are means ±SD. In the screening group, the women were assigned to treatment with levothyroxine. The conversion factor for free thyroxine (T4) is 1.287.
Values for thyrotropin and free T4 levels are provided separately for the United Kingdom and Turin because different assays were used (ADVIA Centaur in the United Kingdom and AutoDELFIA in Turin).
The difference between the screening and control groups was significant (P=0.009); there were no other significant differences.
Data shown are for the United Kingdom only (parents in Turin, Italy, were not asked about educational status or age at delivery).
There were no significant differences between the screening and control groups with respect to gestational age at delivery (median, 40.1 and 40.2 weeks, respectively; P = 0.10), rates of preterm birth (<37 weeks’ gestation, 5.6% and 7.9%; P = 0.20), and birth weight (mean, 3.5 kg and 3.3 kg; P = 0.15). Table 1 in the Supplementary Appendix (available at NEJM.org) shows the numbers of women with positive screening results (and the numbers enrolled) according to center.
cognitive function
Table 2 shows the outcome based on an intention-to-treat analysis. The mean standardized IQ in children at 3 years of age in the control group was 100.0 (by definition), and in the screening group it was 99.2 (P = 0.40). The proportions of children with an IQ of less than 85 were 12.1% in the screening group and 14.1% in the control group (P = 0.39). An analysis adjusting for initial thyrotropin measurements (log-transformed) did not show a significant association between thyrotropin and IQ and yielded results that were not materially different. Table 2 in the Supplementary Appendix shows the observed, nonstandardized IQ scores according to randomization group and psychologist.
Table 2. Standardized Full-Scale Child IQ and Scores on the Child Behavior Checklist (CBCL) and the Behavior Rating Inventory of Executive Function, Preschool Version (Brief-P), According to Study Group.✲.
| Test | Screening Group (N = 390) |
Control Group (N = 404) |
Difference (95% CI) (Control Group–Screening Group)† |
P Value |
|---|---|---|---|---|
| IQ | ||||
| Mean | 99.2±13.3 | 100.0±13.3 | 0.8 (−1.1 to 2.6) | 0.40 |
| <85 (% of children) | 12.1 | 14.1 | 2.1 (−2.6 to 6.7) | 0.39 |
| CBCL T score‡ | ||||
| Mean | 44.4±12.4 | 45.1±13.6 | 0.7 (−1.2 to 2.5) | 0.49 |
| Brief-P T score§ | ||||
| Median | 40 | 40 | 0 | 0.59 |
| Interquartile range | 47–55 | 47–55 |
Plus–minus values are means ±SD. The full-scale child IQ test was standardized so that for each psychologist, the mean score among the children in the control group whom they tested was 100. In the screening group, the women were assigned to treatment with levothyroxine.
For percentages of children with an IQ below 85, the absolute (percentage-point) differences are shown.
For the CBCL, a T score above the 98th percentile is indicative of a clinically significant problem.
For the Brief-P, a T score above the 65th percentile is indicative of a clinically proven significant problem.
We also performed an analysis based on z scores (the observed IQ score minus the mean, divided by the standard deviation according to psychologist) and found no significant difference between groups with respect to the mean IQ z score or the proportion of children with an IQ z score of less than −1 (Table 3 in the Supplementary Appendix). There were likewise no significant between-group differences in the results of other psychological assessments (CBCL and Brief-P scores) (Table 2). One woman in each of the screening and control groups was classified as having mild depression according to the Beck Depression Inventory II and was referred to her community health nurse; the children of these women were included in all analyses.
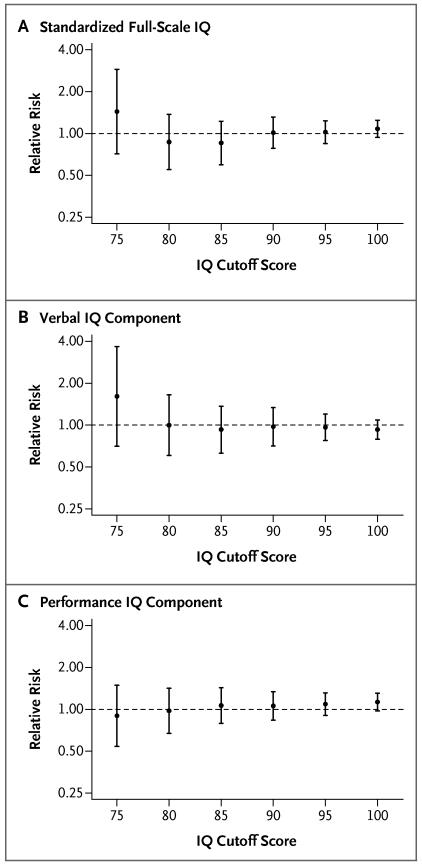
Figure 2 shows the relative risk of an IQ below various specified scores (75, 80, 85, 90, 95, and 100), for the verbal and performance components and for the full-scale IQ, in the screening group as compared with the control group. The relative risks were not significant for any of the specified cutoff scores, and there was no significant trend according to cutoff score (for details, see Table 4 in the Supplementary Appendix).
Figure 2. Relative Risk of an IQ Score below Specified Cutoff Scores in the Screening Group as Compared with the Control Group, According to the Intention-to-Treat Analysis.
I bars indicate 95% confidence intervals.
Among women who received levothyroxine, the thyrotropin level decreased, on average, by a factor of about 10 from baseline to 20 weeks’ gestation, and the free T4 level increased by about 35% (for changes in thyrotropin and free T4 levels over time, see Fig. 1, and Table 5 in the Supplementary Appendix).
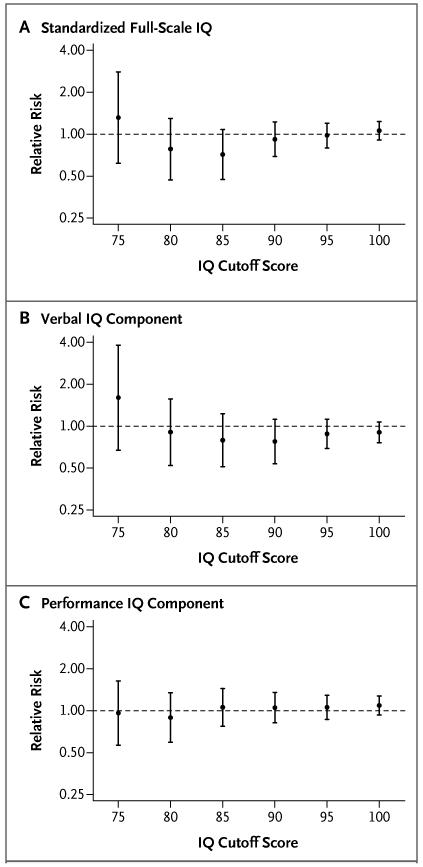
A total of 79.0% of women in the screening group were found to have complied with treatment. Figure 3 shows the results of the on-treatment analysis. As in the intention-to-treat analysis, the relative risks of an IQ below each cutoff score were not significant (for details, see Table 6 in the Supplementary Appendix).
Figure 3. Relative Risk of an IQ Score below Specified Cutoff Scores in the Screening Group as Compared with the Control Group, According to the On-Treatment Analysis.
A total of 308 women (79%) were found to have complied with treatment (i.e., they had a decrease of at least 10% in the thyrotropin level and an increase of at least 10% in the free thyroxine level). I bars indicate 95% confidence intervals.
The average dose of levothyroxine at the start of treatment was 147 μg (most women received 150 μg), and 85% percent of women continued to receive their starting dose after their checkup 6 weeks after screening. In 10% of women, the dose was lowered, in most cases to 125 μg, because of a very low thyrotropin level, a high free T4 level, or minor side effects (palpitations); in 5% of women, the dose was increased, in most cases to 175 μg, because the target thyrotropin level was not reached.
Post hoc analyses were performed in the following subgroups: women with positive screening results according to thyrotropin level only, women with positive screening results according to free T4 level only, women with positive screening results according to both thyrotropin and free T4 levels, women who began to receive treatment before 14 weeks’ gestation, women who began to receive treatment at 14 weeks’ gestation or later, women in whom the target thyrotropin level was achieved 6 weeks after screening, and women in whom the target thyrotropin level was achieved at 30 weeks’ gestation. There were no significant differences in IQ scores between the screening and control groups in these subgroups, and no significant interactions were found (Table 7 in the Supplementary Appendix).
Discussion
We found no significant difference in IQ scores between 3-year-old children born to women who were randomly assigned to the screening group at about 12 weeks’ gestation and treated for reduced thyroid function before 20 weeks’ gestation (median, 13 weeks 3 days) and children born to women with reduced thyroid function who were randomly assigned to the control group. There were also no significant between-group differences in analyses limited to the women who were compliant with treatment.
Although our trial showed no benefit, prior observational studies have shown associations between low maternal thyroid hormone levels in pregnancy and impaired cognitive development in children. A study in 1971 described impaired intellectual development in children born to women with non–iodine-deficient hypothyroidism during pregnancy.6 A subsequent study showed an IQ level of less than 85 in 19% of the children of women with a high thyrotropin level, as compared with 5% of the children of euthyroid women in the control group (P = 0.005).5 Decreased neurologic development at 2 years of age in children born to women with subclinical hypothyroidism during pregnancy as compared with women who were euthyroid during pregnancy was later reported in a Dutch study7 and in a study from China.8
These observational studies may have been subject to confounding. In our trial, randomization effectively avoided this problem. Indeed, baseline characteristics were similar in the screening and control groups, with one exception: the median thyrotropin level in women recruited in the United Kingdom was slightly higher in the screening group than in the control group (3.8 mIU vs. 3.2 mIU per liter). This difference may have arisen from the periodic adjustments to the 97.5th percentile value used to define a positive result (adjustments that were made to obtain an overall 2.5% positive rate for thyrotropin as defined by the 97.5th percentile). This difference is unlikely to have biased our results, given that it was small and that an analysis adjusted for thyrotropin levels yielded similar results.
There may be more specific cognitive impairments associated with maternal hypothyroidism that we did not assess. A low free T4 level (without an increased level of thyrotropin) has been associated with psychomotor deficit in infancy9 and at the age of 2 to 3 years,10 as well as with delays in the development of expressive language.11 Other studies have shown a decrement of orientation12 (the ability to attend to visual and auditory stimuli and overall alertness), vision abnormalities,13 and behavioral changes14 in children born to women with hypothyroidism in pregnancy. The neurodevelopmental effects of maternal hypothyroidism may be specific to certain neural systems. For example, defects in memory, visual attention, and processing have been described in the children of women with untreated low free T4 levels during pregnancy.15 In our study, the Brief-P subscore for memory did not differ significantly between the children in the screening group and those in the control group. Detailed assessment of visuospatial development was not performed.
A possible explanation for the negative results of our study is that screening was performed and levothyroxine therapy initiated too late in gestation to have a major influence on brain development (up to 20 weeks’ gestation; median, 13 weeks 3 days). The timing of screening in our study was chosen because it is at this time that routine antenatal care in hospitals is initiated in the majority of women. However, in post hoc analyses, we likewise did not find a benefit of screening and treatment in the subgroup of women who started treatment earlier, although it should be acknowledged that our trial was not powered for this assessment. It is also possible that IQ assessment at 3 years of age is insensitive to the effects of maternal levothyroxine treatment; in the study by Haddow et al.,5 which showed an association between hypothyroidism in women and a lower IQ in their children, psychological testing was performed at 7 years. Additional randomized trials are needed to determine whether earlier antenatal screening or later childhood cognitive assessment could show a benefit of thyroxine replacement in pregnancy. An ongoing randomized trial is assessing the effect of screening and treatment on IQ at 5 years of age in the children of women with elevated thyrotropin or reduced free T4 levels in blood samples provided between 8 and 20 weeks’ gestation.16
A limitation of our study is that about 24% of the women were lost to follow-up. Most of these women could not be contacted, with similar proportions in the screening and control groups, but 19 women from the screening group and 41 from the control group declined to have their child assessed. A sensitivity analysis indicated that the higher rate of declined assessment in the control group than in the screening group is extremely unlikely to explain our results. Twelve percent of the children of women in the screening group had an IQ of less than 85. If this percentage was the same among the children of women in the screening group who declined testing, at least 44% of the children of women in the control group who declined testing would have to have had an IQ of less than 85 for the difference between the screening and control groups to be significant.
In our study, most of the women with positive screening results had a positive result based on just one of the two thyroid tests performed (free T4 or thyrotropin); only 5% of women had both a high thyrotropin level and a low free T4 level. Post hoc analyses revealed no significant differences between the screening and control groups in any of these subgroups, including the subgroup with both elevated thyrotropin and reduced free T4 levels; however, conclusions regarding these findings are limited by the small numbers of women and the post hoc nature of the analyses.
Current guidelines do not recommend routine antenatal screening for hypothyroidism in pregnancy.17 Our study provides support for these guidelines, since we found no benefit of routine screening for maternal hypothyroidism at about 12 to 13 weeks’ gestation in the prevention of impaired childhood cognitive function.
Supplementary Material
Includes supplementary tables 1-7 and supplementary figure 1.
Acknowledgments
We thank Lisa Bass, Sarah Lee, Catherine Angel, and Lyn Taylor for technical assistance; all the obstetricians for their help; and the mothers and children who participated in this study.
Supported by the Wellcome Trust and Compagnia di San Paulo, Turin.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.de Escobar GM, Obregón MJ, del Rey FE. Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract Res Clin Endocrinol Metab. 2004;18:225–48. doi: 10.1016/j.beem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Pharoah PO, Buttfield IH, Hetzel BS. Neurological damage to the fetus resulting from severe iodine deficiency during pregnancy. Lancet. 1971;1:308–10. doi: 10.1016/s0140-6736(71)91040-3. [DOI] [PubMed] [Google Scholar]
- 3.Velasco I, Carreira M, Santiago P, et al. Effect of iodine prophylaxis during pregnancy on neurocognitive development of children during the first two years of life. J Clin Endocrinol Metab. 2009;94:3234–41. doi: 10.1210/jc.2008-2652. [DOI] [PubMed] [Google Scholar]
- 4.Berbel P, Mestre JL, Santamaría A, et al. Delayed neurobehavioral development in children born to pregnant women with mild hypothyroxinemia during the first month of gestation: the importance of early iodine supplementation. Thyroid. 2009;19:511–9. doi: 10.1089/thy.2008.0341. [DOI] [PubMed] [Google Scholar]
- 5.Haddow JE, Palomaki GE, Allan WC, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 6.Man EB, Jones WS, Holden RH, Mellits ED. Thyroid function in human pregnancy: retardation of progeny aged 7 years; relationships to maternal age and maternal thyroid function. Am J Obstet Gynecol. 1971;111:905–16. [PubMed] [Google Scholar]
- 7.Smit BJ, Kok JH, Vulsma T, Briët JM, Boer K, Wiersinga WM. Neurologic development of the newborn and young child in relation to maternal thyroid function. Acta Paediatr. 2000;89:291–5. [PubMed] [Google Scholar]
- 8.Li Y, Shan Z, Teng W, et al. Abnormalities of maternal thyroid function during pregnancy affect neuropsychological development of their children at 25-30 months. Clin Endocrinol. 2010;72:825–9. doi: 10.1111/j.1365-2265.2009.03743.x. [DOI] [PubMed] [Google Scholar]
- 9.Pop VJ, Kuijpens JL, van Baar AL, et al. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol. 1999;50:149–55. doi: 10.1046/j.1365-2265.1999.00639.x. [DOI] [PubMed] [Google Scholar]
- 10.Pop VJ, Brouwers EP, Vader HL, et al. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol. 2003;59:282–8. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 11.Henrichs J, Bongers-Schokking JJ, Schenk JJ, et al. Maternal thyroid function during early pregnancy and cognitive functioning in early childhood: the Generation R study. J Clin Endocrinol Metab. 2010;95:4227–34. doi: 10.1210/jc.2010-0415. [DOI] [PubMed] [Google Scholar]
- 12.Kooistra L, Crawford S, van Baar AL, Brouwers EP, Pop VJ. Neonatal effects of maternal hypothyroxinemia during early pregnancy. Pediatrics. 2006;117:161–7. doi: 10.1542/peds.2005-0227. [DOI] [PubMed] [Google Scholar]
- 13.Mirabella G, Westall CA, Asztalos E, Perlman K, Koren G, Rovet J. Development of contrast sensitivity in infants with prenatal and neonatal thyroid hormone insufficiencies. Pediatr Res. 2005;57:902–7. doi: 10.1203/01.PDR.0000157681.38042.B4. [DOI] [PubMed] [Google Scholar]
- 14.Ghassabian A, Bongers-Schokking JJ, Henrichs J, et al. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the Generation R study. Pediatr Res. 2011;69:454–9. doi: 10.1203/PDR.0b013e3182125b0c. [DOI] [PubMed] [Google Scholar]
- 15.Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: clinical observations and experimental findings. J Neuroendocrinol. 2004;16:809–18. doi: 10.1111/j.1365-2826.2004.01243.x. [DOI] [PubMed] [Google Scholar]
- 16.Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Thyroid therapy for mild thyroid deficiency in pregnancy (TSH) National Library of Medicine; Bethesda, MD: http://www.clinicaltrials.gov/ct/show/NCT00388297. [Google Scholar]
- 17.Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thryoid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid. 2011;21:1081–1125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Includes supplementary tables 1-7 and supplementary figure 1.