Abstract
Purpose:
Due to the low rate of screening for prostate cancer in Japan, the incidence rates of cancer are high. We have established a prostate-specific antigen (PSA)-based screening system for prostate cancer in our region. We analyzed recent trends of clinical symptoms and prognosis of prostate cancer patients aged 55 to 69 years old in our institution.
Methods:
Between 2000 and 2007, 162 cases of prostate cancer in patients aged 55 to 69 years old were newly diagnosed. The study population was divided into 119 cases with high PSA without symptoms, 36 cases with urological symptoms, and 7 cases with systemic symptoms. We analyzed the clinical courses of the patients in each group.
Results:
The rate of localized disease was significantly higher in the PSA testing group than in the other groups. The median serum PSA levels were 1,600 ng/mL in the systemic symptom group, 13.3 ng/mL in the urological symptom group, and 7.1 ng/mL in the PSA testing group. The probability of nonrecurrence of the patients in the PSA testing group was significantly higher than in the other groups.
Conclusions:
The rate of prostate cancer patients diagnosed by PSA testing was relatively high in our institution. These patients have better prognosis than those with symptoms.
Keywords: Prostate neoplasms, Clinical symptoms, Prostate-specific antigen screening, Prognosis
INTRODUCTION
Prostate cancer is the most common form of cancer among men in Western countries, and the mortality rate of prostate cancer was high in the 1990s [1]. However, according to the latest cancer registry in the USA, the mortality rate has decreased since 1992, and in 2004 showed a 34% decrease compared with 1990 [2]. Taking into consideration the recent high rate of prostate-specific antigen (PSA) testing in the USA among men aged 50 years or older, the decrease in cancer mortality may be due to the establishment of PSA-based screening systems and subsequent appropriate treatment for screening-detected prostate cancer.
In Japan, the incidence rates of prostate cancer have increased and are estimated to continue to do so in the near future. The number of patients with newly diagnosed prostate cancer will make this the second most common form of cancer among men in Japan following lung cancer [3]. The mortality rate of prostate cancer will also increase in the future, and it has been predicted to reach 2.8 times higher in 2020 than that in 2000 [4]. One of the reasons for these prospects is that the rate of screening for prostate cancer in Japan is still very low compared with the USA and Western Europe. Screening systems for prostate cancer have not been established by the national government and depend on each municipal government in Japan. Moreover, it is difficult to determine the results of cancer screening correctly because the cancer registration systems of the various registries have not been standardized [5]. We have established a PSA-based screening system for prostate cancer by the municipal government in Kanazawa, Japan [6], which has been implemented in the region. To investigate the effectiveness and usefulness of our population-based screening program, the present study was performed to analyze the clinical courses of prostate cancer patients aged 55 to 69 years old in our institution.
MATERIALS AND METHODS
Since 2000, we have performed PSA-based screening for prostate cancer in men aged 55 to 69 years old [6]. Between 2000 and 2007, 423 cases of prostate cancer were newly diagnosed in our institution, and 162 of these cases (38.3%) were patients aged 55 to 69 years old. One hundred of these 162 cases (61.7%) were referred to our institution because of high PSA levels detected on PSA-based screening in the region. Nineteen cases (11.7%) were referred from general practitioners because of high PSA levels without urological symptoms. Thirty-six cases (22.2%) had urological symptoms (including symptoms of benign prostatic hyperplasia), and 7 cases (4.3%) had systemic symptoms at the time of diagnosis. The study population was divided into 119 cases with high PSA without symptoms (PSA testing group), 36 cases with urological symptoms (urological symptom group), and 7 cases with systemic symptoms (systemic symptom group). We have followed-up the patients and analyzed the clinical courses of each group.
Clinical staging was determined in accordance with the unified TNM criteria based on the results of digital rectal examination, transrectal ultrasound, computed tomography, magnetic resonance imaging (MRI), and bone scan [7]. Pathological tumor grading was determined by transrectal biopsy before initiation of any treatment.
The mean follow-up time was 37.1 months. Biochemical recurrence was defined as two consecutive increases in serum PSA levels. Freedom from biochemical and/or clinical recurrence was calculated from the date of pathological diagnosis. Kaplan-Meier analysis was used for estimation of recurrence and cause-specific survival, and the significance of differences between each group was determined using the log-rank test. Other statistical assessments were performed using the χ2 and Fisher exact tests, and P<0.05 was considered statistically significant.
RESULTS
Age, clinical stage, Gleason score of prostate biopsy specimens, and serum PSA levels at diagnosis of the patients are presented in Table 1. The patients recruited into this study ranged in age from 55 to 69 years, and there were no significant differences in age between the three groups. All of the patients in the systemic symptom group had distant and/or lymph node metastases. The clinical stages of the patients in the urological symptom group and in the PSA testing group were T1c and T2 for 24 and 103 patients, T3 for 3 and 6, T4 for 3 and 3, and N1 and/or M1 for 6 and 7, respectively. The rate of localized disease was significantly higher in the PSA testing group than in the other groups. The median Gleason scores of biopsy specimens were as follows: systemic symptom group, 9; urological symptom group, 6.5; and PSA testing group, 6. The Gleason score of biopsy specimens in the PSA testing group was significantly lower than in the other groups. In terms of serum PSA level at diagnosis, as we set the PSA cutoff to 2.0 ng/mL in our screening [6], there were 15 patients with serum PSA levels under 4.0 ng/mL. The median serum PSA levels were 1,600 ng/mL in the systemic symptom group, 13.3 in the urological group, and 7.1 in the PSA testing group. The serum PSA levels at diagnosis in the PSA testing group were significantly lower than those in the other groups.
Table 1.
Characteristics of the groups of patients divided by clinical symptoms
| Variable | Systemic symptom | Urological symptom | PSA testing |
|---|---|---|---|
| No of patients | 7 | 36 | 119 |
| Median age (yr) | 66 | 65.5 | 65 |
| Clinical stage | |||
| T1c, T2 | 0 | 24 | 103 |
| T3 | 0 | 3 | 6 |
| T4 | 0 | 3 | 3 |
| N1 and/or M1 | 7 | 6 | 7 |
| Gleason score | |||
| ≤6 | 0 | 18 | 70 |
| 7 | 2 | 9 | 34 |
| 8–10 | 5 | 9 | 15 |
| Serum PSA (ng/mL) | |||
| ≤4 | 0 | 1 | 15 |
| 4.1–10 | 0 | 13 | 66 |
| 10.1–20 | 0 | 7 | 15 |
| ≥20.1 | 7 | 15 | 23 |
PSA, prostate-specific antigen.
Table 2 shows the variety of treatment methods used. All of the patients in the systemic symptom group were treated with primary androgen deprivation therapy. In contrast, 24 of 36 patients (66.7%) in the urological symptom group and 93 of 119 (78.2%) in the PSA testing group were treated with surgery and radiation.
Table 2.
Primary treatments for each group
| Treatment | Systemic symptom | Urological symptom | PSA testing |
|---|---|---|---|
| Radical prostatectomy | 0 | 15 | 48 |
| High dose brachyterapy | 0 | 8 | 37 |
| Permanent implant brachyterapy | 0 | 0 | 6 |
| External beam radiation | 0 | 1 | 1 |
| Primary androgen deprivation | 7 | 12 | 21 |
| Watchful waiting | 0 | 0 | 5 |
| HIFU | 0 | 0 | 1 |
PSA, prostate-specific antigen; HIFU, high-intensity focused ultrasound.
Serum PSA levels could be obtained for all patients at a mean postdiagnosis follow-up of 37.1 months. Five patients (3.1%) died of prostate cancer, and clinical and/or biochemical recurrence occurred in 19 patients (11.7%). Cancer-specific survival rates for each group are shown in Fig. 1. The probabilities of survival at 5 years were 98.2% and 96.3% for the PSA testing and urological symptom groups, respectively. In contrast, the corresponding percentage for the patients in the systemic symptom group was 40.0%; this difference was statistically significant.
Fig. 1.
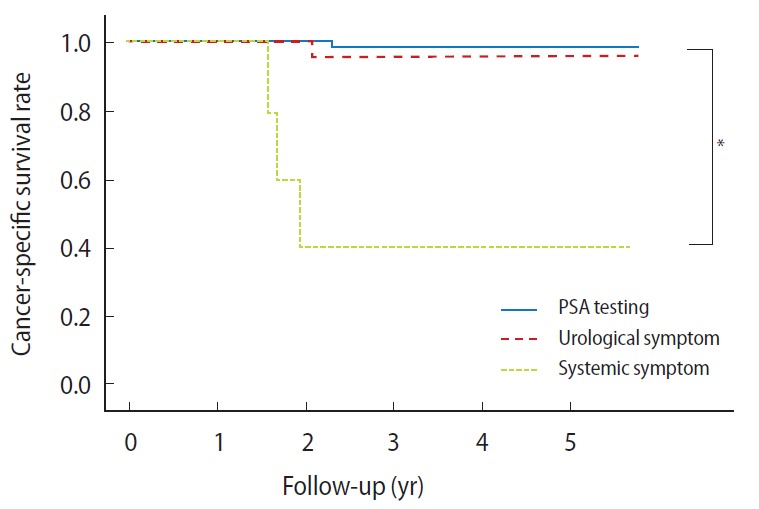
Kaplan-Meier plots of cancer-specific survival rates of prostate cancer patients in prostate-specific antigen (PSA) testing, urological symptom, and systemic symptom groups. *P<0.01.
Recurrence-free survival rates for each group are shown in Fig. 2. The probabilities of nonrecurrence at 5 years were 91.6%, 70.8%, and 47.6% in PSA testing, urological symptom, and systemic symptom groups, respectively. The probability of non-recurrence in the PSA testing group was significantly higher than those in the other groups.
Fig. 2.
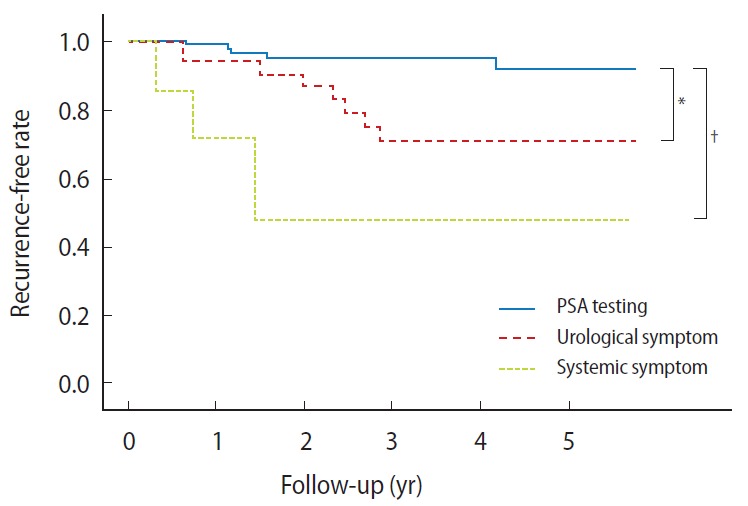
Kaplan-Meier plots of recurrence-free rates of prostate cancer patients in prostate-specific antigen (PSA) testing, urological symptom, and systemic symptom groups. *P<0.05, †P<0.01.
DISCUSSION
The effectiveness of PSA-based mass screening for improving the prognosis of prostate cancer patients remains controversial [8,9]. However, some recent studies indicated good prognosis of prostate cancer patients in countries and regions with established mass screening systems [10,11]. The results of the Tyrol screening project indicated a 70% decrease in metastatic prostate cancer in the area following the introduction of free PSA testing, and the exposure rate was high at 86.6% [10].
In Japan, as screening systems for prostate cancer have not been established by the national government and the awareness of the importance of PSA testing is very low among healthy people, the exposure rate is thought to be about 10% among men aged 50 years or older. Although screening for prostate cancer, including PSA testing, has been performed by the municipal governments and in “human dry-dock” programs in general hospitals since the 1990s, the rates of screening for candidates are different between municipal governments and are especially very low in metropolitan areas. Recently, we established a PSA-based screening system for prostate cancer, which has been implemented in Kanazawa, Japan [6]. The rate of screening is about 20% for men aged 55 to 69 years old in the city. Moreover, as general practitioners are aware of the importance of PSA testing, it is implemented in their male patients without urological symptoms. Therefore, the rate of PSA testing in our region has increased above the average for Japan. In this study, we showed that 119 of 162 patients (73.5%) aged 55 to 69 years old in our institution were identified by PSA testing. Although we cannot evaluate whether the rate is high because there have been no recent analyses of chief complaints in middle-aged patients diagnosed with prostate cancer, the rate of patients diagnosed by PSA testing may increase with the widespread adoption of PSA-based mass screening.
We showed that the rate of patients with local disease in the PSA testing group was higher than those in the symptomatic groups. Moreover, the patients in the PSA testing group had a better prognosis. As mentioned in some recent reports [10,11], the mortality rate of prostate cancer may also decrease in the regions of Japan with established mass screening programs.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Ohno Y, Nakamura T, Murata K. Prediction of the future incidence of cancer in Japan. In: Oshima A, Kuroishi T, Tajima K, editors. White paper on cancer and statistics-incidence/mortality/prognosis-2004 (in Japanese) Tokyo: Shinohara-Shuppan; 2004. pp. 201–17. [Google Scholar]
- 4.Kuroishi T, Hirose K, Tominaga Y. Prediction of the future mortality of cancer in Japan. In: Oshima A, Kuroishi T, Tajima K, editors. White paper on cancer and statistics-incidence/mortality/prognosis-2004 (in Japanese) Tokyo: Shinohara-Shuppan; 2004. pp. 219–34. [Google Scholar]
- 5.Sobue T. Current activities and future directions of the cancer registration system in Japan. Int J Clin Oncol. 2008;13:97–101. doi: 10.1007/s10147-008-0761-7. [DOI] [PubMed] [Google Scholar]
- 6.Kobori Y, Kitagawa Y, Mizokami A, Komatsu K, Namiki M. Free-to-total prostate-specific antigen (PSA) ratio contributes to an increased rate of prostate cancer detection in a Japanese population screened using a PSA level of 2.1–10.0 ng/ml as a criterion. Int J Clin Oncol. 2008;13:229–32. doi: 10.1007/s10147-007-0742-2. [DOI] [PubMed] [Google Scholar]
- 7.International Union Against Cancer . Urologic tumors: prostate. In: Sobin LH, Wittekind CH, editors. TNM classification of malignant tumours. 5th ed. New York: John Wiley & Sons; 1997. pp. 170–3. [Google Scholar]
- 8.Andriole GL, Crawford ED, Grubb RL, 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 10.Bartsch G, Horninger W, Klocker H, Pelzer A, Bektic J, Oberaigner W, et al. Tyrol Prostate Cancer Demonstration Project: early detection, treatment, outcome, incidence and mortality. BJU Int. 2008;101:809–16. doi: 10.1111/j.1464-410X.2008.07502.x. [DOI] [PubMed] [Google Scholar]
- 11.Bouchardy C, Fioretta G, Rapiti E, Verkooijen HM, Rapin CH, Schmidlin F, et al. Recent trends in prostate cancer mortality show a continuous decrease in several countries. Int J Cancer. 2008;123:421–9. doi: 10.1002/ijc.23520. [DOI] [PubMed] [Google Scholar]


