Abstract
Purpose:
Prostate-specific antigen (PSA) response rate (>50% PSA decline in pretreatment PSA following chemotherapy) carries a significant survival advantage in castration-resistant prostate cancer (CRPC). We compared PSA response rates in first-, second- and third-line chemotherapy after failure of previous chemotherapy according to chemotherapeutic agents.
Methods:
We retrospectively evaluated the oncological outcomes and PSA response rates of 384 patients with CRPC, who were treated with chemotherapy and had histologically proven adenocarcinoma of the prostate with failure after androgen ablation therapy between 1991 and 2012, at Asan Medical Center.
Results:
In 384 eligible patients, the median age was 67.5 years. The median pretreatment PSA and initial Gleason scores at baseline were 92.4 ng/mL (range, 2.0 to 6,370 ng/mL) and 9 (range, 6 to 10), respectively. The time from first diagnosis of prostate cancer to CRPC was 23 months (range, 1 to 164 months). As first-line chemotherapy, 245 patients (63.8%) received estramustine, 91 (23.7%) received docetaxel, and 39 (10.2%) received mitoxantrone. The PSA response rates were 39.6%, 51.6%, and 46.2%, respectively. Of 169 patients with second-line chemotherapy, estramustine was 15 (8.9%), docetaxel was 84 (49.7%), and mitoxantrone was 52 (30.8%). PSA response rates were 57.1%, 52%, and 28.0%, respectively. Of 81 patients with third-line chemotherapy, estramustine was 18 (22.2%), docetaxel was 16 (19.8%), and mitoxantrone was 28 (34.6%). The PSA response rates were 41.2%, 53.8%, and 11.1%, respectively. Declines in serum PSA levels of at least 50% occurred more frequently after treatment with docetaxel than with other chemo-agents regardless of second-and third-line chemotherapy. Even in third-line chemothrapy, docetaxel maintained the PSA response rate, whereas the PSA response rate of other agents, including mitoxantrone, decreased in patients in whom prior therapy failed.
Conclusions:
Docetacel was the most effective chemotherapeutic agent in second- and third-line trials of chemotherapy in Korean CRPC patients. Although docetaxel is not used as first-line chemotherapy, and new agents are not available for therapy in CRPC patients, we can consider docetaxel a second- or third-line chemotherapy in CRPC.
Keywords: Castration refractory prostate cancer, Chemotherapy, Prostate-specific antigen
INTRODUCTION
Prostate cancer is the most common cancer in men in the United States and the fifth most common cancer in men in Korea [1]. Androgen deprivation therapy (ADT) remains the main treatment for metastatic, hormone-sensitive prostate cancer. Although ADT is effective in lowering PSA in most men, the therapeutic response will eventually wane, and the disease will eventually progress. For many years, development of new therapies and new treatment strategies emerged slowly for prostate cancer in general and for castration-resistant prostate cancer (CRPC) in particular. However, in recent years that has changed with the emergence of various new agents that access several different mechanistic disease pathways. Additional drugs have been approved by the U.S. Food and Drug Administration (FDA) for the treatment of CRPC in the past three years than in the prior three decades. These new treatment options include, among others, a new cytotoxic agent, immunotherapy, and androgen receptor-signaling inhibitors. The newest agents of U.S. FDA approval of were enzalutamide [2], abiraterone [3], and cabazitaxel [4].
Although many new drugs are on the verge of approval, based on the results of recently reported randomized trials, many countries still use docetaxel-based chemotherapy because of socio-economic issues. Docetaxel-based chemotherapy is currently the treatment of choice in patients with CRPC because it prolongs survival rates compared with the previously standard mitoxantrone therapy [5,6]. Several recent studies have demonstrated the feasibility and activity of a single docetaxel rechallenge, thus providing an additional opportunity in clinical practice for treatment of docetaxel-sensitive CRPC patients [7–9]. Currently, in Korea many chemotherapy agents are also administrated to CRPC patients, but only a few reports of clinical outcomes of chemotherapy have been published, and new chemotherapy agents are restricted because of the parameters of the national insurance. Therefore, we undertook this study to investigate the outcomes of chemotherapy in the treatment of CRPC in real life practice in Korean patients. We compared the feasibility and efficacy of chemotherapeutic agents in docetaxel-sensitive CRPC patients in first-, second-, and third-line settings.
MATERIALS AND METHODS
1. Patients
We retrospectively evaluated the oncological outcomes and PSA response rates in 412 patients with CRPC, who were treated with chemotherapy between 1991 and 2012, at Asan Medical Center. Patient records were retrospectively reviewed to determine base-line characteristics and clinical efficacy. The sample comprised 384 eligible patients with histologically proven adenocarcinoma of the prostate and documented disease progression (documented locoregional or distant metastases and/or PSA increase) after failure of androgen ablation therapy or second hormonal treatment. Progressive disease was defined by an increase in PSA levels as determined by 2 consecutive measurements at least 2 weeks apart, an increase in the size of a measurable lesion by computed tomography or any newly developed bony metastasis with hot uptake by bone scan. To investigate differences in prior chemotherapy, patients were classified into three groups: the first-line group consisted of patients who had undergone no prior chemotherapy; the second-line group had undergone first-line chemotherapy; and the third-line group had undergone previous second-line chemotherapy. The first-line therapy for each CRPC patient in this study was determined at the physicians’ discretion, based on cancer-related symptoms, rising PSA, extent of metastasis and performance status. The second-and third-line therapy was determined based on treatment response to and progression on prior chemotherapy treatment as well as drug toxicity. Patients treated with androgen-deprivation therapy during the interval from prior chemotherapy to next chemotherapy were included and patients treated with new agents in clinical trials were excluded from this study.
2. PSA response and overall survival
The criteria used for determining responses were based on the guidelines of the PSA working group [10]. A PSA decline of 50%, confirmed by a second evaluation at least three weeks later, was considered a PSA response with no evidence of disease progression in imaging in the available patients and progression was defined as the increase in PSA. Baseline PSA was defined as the PSA value obtained within a two-week period prior to starting the chemotherapy in the study. Because the chemotherapy agents mainly used in this study were estramustine-based chemotherapy, docetaxel and mitoxantrone, our analysis focused on these agents. The group of estramustine-based chemotherapy (estramustine only, estramustine+etoposide, estramustine+hormone therapy, etc.) was considered the estramustine group. However, estramustine combined with docetaxel group was considered the docetaxel group in this study. Overall survival was defined as the time between the administration of the first chemotherapy and death.
3. Statistical analysis
The major statistical endpoints of this study consisted of PSA response and overall survival. We also investigated differences between these endpoints in first, second- and third-line groups. Overall survival curves were produced using the Kaplan-Meier method. Differences between the first-, second- and third-line groups were compared using the log-rank test. The T test (or Mann-Whitney test fornon-parametric variables) was used for continuous variables. The chi-square (or Fisher exact test for nonparametric variables) was used for categorical variables. P-values <0.05 (2-sided) were considered statistically significant, and confidence intervals were set at the 95% level. All analyses were performed using the IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA).
RESULTS
1. Patient characteristics
A total of 412 patients were enrolled between October 1991 and January 2012. Twenty-eight patients did not meet the study`s criteria: 16 had missing documentation of PSA levels; 7 had inadequate baseline laboratory studies; and 5 were eliminated for miscellaneous reasons. The baseline characteristics of the 384 patients are listed in Table 1. Of the 384 study patients, the median age was 68.0 years (range, 44 to 87 years). The median pretreatment PSA and initial Gleason score at baseline in the overall population were 96 ng/mL (range, 2 to 6,370 ng/mL) and 9 (range, 6 to 10), respectively. The time from first diagnosis of prostate cancer to CRPC was 22 months (range, 0 to 165 months). In terms of metastases, 76 patients (25.0%) had visceral metastasis and most other patients had bone or lymph node metastasis. The comparison analysis among baseline tumor characteristics according to each chemotherapy-line showed no significant difference.
Table 1.
Baseline characteristics of the patients
| Characteristic | Total patient | Nondocetaxel era (<2004 yr) | Docetaxel era (≥2004 yr) |
|---|---|---|---|
| No. | 384 | 94 | 290 |
| Age (yr) | 68 (44–87) | 69 (44–87) | 67.5 (45–87) |
| PSA at initial diagnosis (ng/mL) | 96 (2–6,370) | 89 (4–4,830) | 94.6 (2.0–6,370.0) |
| PSA at CRPC (ng/mL) | 28.2 (0.2–3707.0) | 41.4 (0.6–3707.0) | 24.2 (0.2–2510.0) |
| Baseline Hb (mg/dL) | 11.6 (7.1–15.8) | 11.3 (7.1–15.2) | 11.9 (7.4–15.8) |
| Baseline ALP (IU/L) | 124 (41–7,604) | 167.5 (54.0–7,604.0) | 106 (41–1,160) |
| Gleason score | |||
| ≤6 | 10 (3) | 6 (6) | 4 (1.4) |
| 7 | 58 (15) | 14 (15) | 44 (15) |
| ≥8 | 316 (82) | 74 (79) | 242 (83) |
| Disease extension at baseline | |||
| Bone metastases | 308 (85.4) | 77 (82.1) | 251 (86.7) |
| Visceral metastases | 76 (25.0) | 26 (29.3) | 70 (24.3) |
| ECOG | 1 (0–3) | 1 (0–3) | 1 (0–3) |
| Significant pain | 161 (42) | 22 (23) | 139 (48) |
| Period from diagnosis to chemotherapy | 22 (0–165) | 17 (0–103) | 26 (0–165) |
Values are presented as median (range) or number (%).
PSA, prostate-specific antigen; CRPC, castration-resistant prostate cancer; Hb, hemoglobin; ALP, alkaline phosphatase; ECOG, Eastern Cooperative Oncology Group.
The number of eligible patients was divided into two groups, based on the year 2004 (i.e., before and after the docetaxel era). The patients’ characteristics after 2004, in the docetaxel era, were similar to those before the docetaxel era, except for baseline alkaline phosphatase levels, significant pain, and the time from initial diagnosis to first-line chemotherapy (Table 1). Although initial PSA levels of the patients treated before the doxetaxel era tended to be high compared with those of patients treated after the docetaxel era, there was no significant difference. At initial chemotherapy, of the 384 patients, 245 (63.8%) received estramustine, 91 (23.7%) received docetaxel, and 39 (10.2%) received mitoxantrone. Of the 169 patients with second chemotherapy, 88 (42%) received docetaxel, 51 (25%) received mitoxantrone, and 21 (10%) received estramustine. Of 84 patients with third-line chemotherapy, 28 (34.6%) received mitoxantrone, 18 (22.2%) received estramutine, and 16 (19.8%) received docetaxel (Table 2).
Table 2.
The distributions of chemotherapy agents in all patients
| Variable | First-line therapy | Second-line therapy | Third-line therapy |
|---|---|---|---|
| Estramustine | 245 (63.8) | 15 (8.9) | 18 (22.2) |
| Docetaxel | 91 (23.7) | 84 (49.7) | 16 (19.8) |
| Mitoxantrone | 39 (10.2) | 52 (30.8) | 28 (34.6) |
| Vincristine + cyclophosphamide | 5 (1.3) | 13 (7.7) | 12 (14.3) |
| Etc. | 4 (1.0) | 5 (2.9) | 10 (11.9) |
| Total | 384 (100) | 169 (100) | 84 (100) |
Values are presented as number (%).
2. Serum PSA response
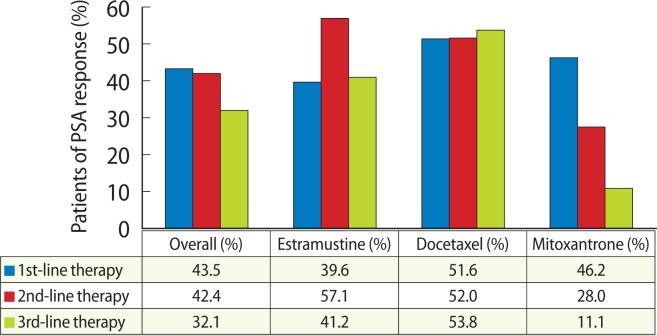
Overall PSA responses (>50% PSA decline in pretreatment PSA following chemotherapy) were as follows: 43.5% at first-line chemotherapy, 42.4% at second-line chemotherapy, and 32.1% at third-line chemotherapy (Fig. 1). Declines in serum PSA levels of at least 50% occurred more frequently after treatment with docetaxel and estramustine than after treatment with mitoxantrone but this was not significant (P =0.615) at first line chemotherapy. However, at second- and third-line chemotherapy, PSA responses were significantly more frequently demonstrated in the docetaxel and estramustine groups compared with the mitoxantrone group (second, P= 0.017; third, P =0.010). When PSA responses were analyzed based on the year 2004, there was no statistically significant difference (first, 47.9% vs. 42.1%, P =0.193; second, 26.9% vs. 45.3%, P=0.062; third, 33.3% vs. 31.9%, P=0.631).
Fig. 1.
Prostate-specific antigen (PSA) responses (>50% PSA decline in pretreatment PSA following chemotherapy) according to chemotherapy agents at first-, second- and third-line chemotherapy.
3. Survival
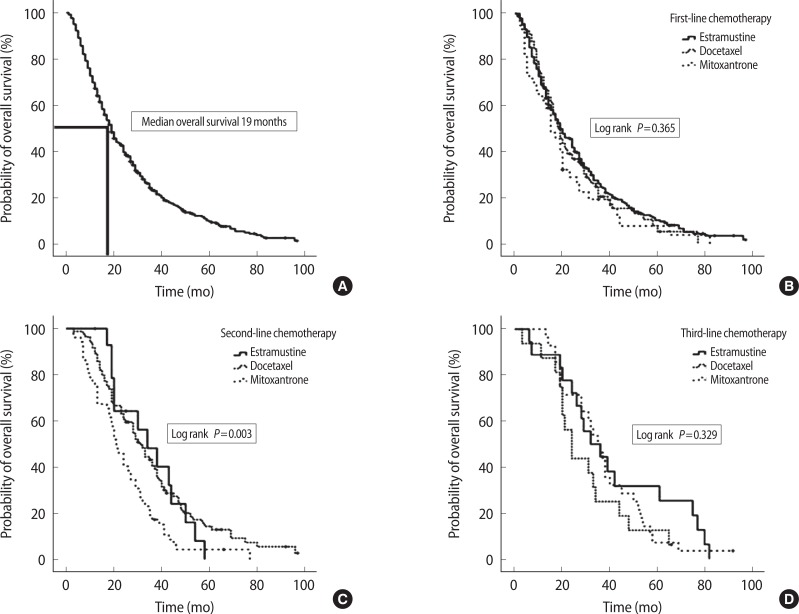
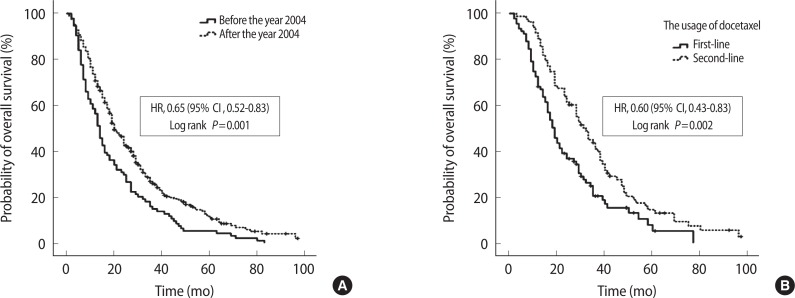
Median overall survival was 19.0 months (range, 1 to 99 months). The overall survival curve is presented in Fig. 2A. According to first-line and third-line chemotherapy agents, there were no differences in median survival (P=0.365 and P=0.329). However, according to second-line chemotherapy agents, the docetaxel and estramustine group had longer median survival than the mitoxantrone group did (median value, 30 months vs. 21 months vs. 19 months, P=0.003) (Fig. 2B–D). When overall survivor rates were analyzed based on the year 2004, the patients after docetaxel era (after 2004) showed significantly longer overall survival (median value, 14 months vs. 20 months; P=0.001; hazard ratio [HR], 0.65; 95% confidence interval [CI], 0.52 to 0.83) (Fig. 3A). The median overall survival was 31.5 months in patients treated with docetaxel chemotherapy in second-line chemotherapy and 19.5 months in patients treated with docetaxel chemotherapy in first-line chemotherapy (P=0.002) (Fig. 3B). The corresponding hazard ratio for death was 0.60 (95% CI, 0.43 to 0.83).
Fig. 2.
Kaplan-Meier estimates of overall survival among men with androgen-independent prostate cancer treated with chemotherapy agents. (A) Median overall survival was 19.0 months in total eligible patients. (B–D) According to first and third-line chemotherapy agents, there were no differences in median survival by the log rank test (P=0.365 and P=0.329). However, with second-line chemotherapy agents, docetaxel and estramustine group were longer in median survival than mitoxantrone group (P=0.003). HR, hazard ratio; CI, confidence interval.
Fig. 3.
Kaplan-Meier estimates of overall survival according to docetaxel era (A) and the usage of docetaxel as first-line or second-line chemotherapy (B). (A) The patients after docetaxel era (after 2004) showed significantly longer overall survival (P=0.001; hazard ratio [HR], 0.65; 95% confidence interval [CI], 0.52 to 0.83). (B) The overall survival was longer in patients treated with docetaxel chemotherapy as second-line chemotherapy than in the patients treated with docetaxel chemotherapy as first-line chemotherapy. (P=0.002; HR, 0.60; 95% CI, 0.43 to 0.83).
DISCUSSION
In the last three decades, various kinds of chemotherapies for CRPC have been tried in order to improve understanding of the pathogenesis of CRPC. To assess the treatment outcomes of new agents in CRPC, the Response Evaluation Criteria in Solid Tumors (RECIST) group recommended both tumor shrinkage (objective response) and disease progression as useful endpoints in clinical trials [11]. A key question considered by the RECIST Working Group in developing RECIST 1.1 was whether it was appropriate to move from an anatomic unidimensional assessment of tumor burden to either a volumetric anatomical assessment or a functional assessment with positron emission tomography or magnetic resonance imaging. However, 80% to 90% of CRPC patients do not have bidimensionally measurable disease. The Prostate Cancer Working Group 2 recommended that using the same methods used at enrolment, investigators should measure early-response outcomes by the changes in the individual disease manifestations present initially for both cytotoxic and noncytotoxic drugs [12]. In this study, to evaluate therapeutic outcomes, PSA response rates (>50% PSA decline in pretreatment PSA following chemotherapy) were used. Because many clinicians were involved in this study, a long-period retrospective study was designed, and various chemotherapy agents were used, PSA response rates as markers of chemotherapy response could be applied to all cases objectively.
Many contemporary studies have used PSA as a marker of response, even though there is no consensus about the magnitude and duration of decline in PSA levels. Although PSA is used as a rapid screening tool to test new agents for activity, there is conflicting evidence about the role of PSA as a response marker. However, it has been reproducibly shown that >50% PSA decline in pretreatment PSA following therapy had a significant advantage in survival [13]. An improved PSA response was also associated with prolonged survival in the TAX 327 study, with a median survival of 33 months when the PSA was normalized ( <4 ng/mL) versus 15.8 months for an abnormal PSA. This study also showed that a PSA response was not a surrogate marker for survival. Even though the same PSA response rate was found in both docetaxel arms (45%), improved survival only occurred with a regime of docetaxel three times a week. According to the most recent evaluation of the TAX 327 and SWOG 99-16 studies, a PSA decrease of >30% is associated with a significant survival benefit [14,15]. In this study, regardless of first-line chemotherapy agents, the overall survival rate significantly increased according to the PSA response rate (not shown).
The emergence of docetaxel as an effective therapy, and the development of a new generation of agents for patients with CRPC have altered the treatment paradigm for this patient population. The Korean FDA also approved docetaxel for the treatment of CRPC in 2005. Therefore, docetaxel is actively being administrated to many patients throughout Korea. Joung et al. [16] reported the first results for the efficacy of docetaxel in Korean patients. They included patients with progressive disease despite prior chemotherapy; i.e., mitoxantrone-resistant and estramustine resistant cases. That study compared the efficacy of docetaxel chemotherapy in Korean patients with hormone refractory prostate cancer between first- and second-line docetaxel. PSA response was more common in the first-line group, but this was not statistically different. However, the first-line group showed a longer time to PSA progression (4 months vs. 2 months, P=0.015) and survival (17 months vs. 10 months, P =0.037) than the second-line group did. At our institution, we recently reported the efficacy and safety of docetaxel plus prednisolone chemotherapy for metastatic hormone-refractory prostate adenocarcinoma [17]. In that study, a PSA response was seen in 51% of 63 evaluable patients at 12 weeks, maximal PSA decline ≥50% in 59% of 70 evaluable patients. Tumor response was evaluated in 13 patients, 4 patients achieved partial response, and 5 patients had stable disease with a response rate of 31%. Median overall survival was 22.8 months (95% CI, 16.6 to 29.1).
It is well known that chemotherapy with docetaxel is currently the standard first-line cytotoxic treatment in CRPC. The clinical efficacy of docetaxel-based chemotherapy administered three times a week in patients with CRPC has been demonstrated in two randomized phase III trials (TAX-327 study; SWOG99-16 study). The results showed a median survival benefit of 2 to 3 months compared with mitoxantrone and prednisone [5,6,18,19]. Recently, some studies reported that docetaxel rechallenge showed preserved antitumor activity and tumor response in first-line chemotherapy. Docetaxel has been suggested as an indicator for activity of docetaxel rechallenges [20–22]. Heck et al. [22] reported that in first-line docetaxel chemotherapy, 36 patients (82%) achieved a reduction in PSA level of ≥50%. In docetaxel chemotherapy rechallenge, 10 patients (28%) responded with a reduction of ≥50% for the second time. The median (95% CI) PSA-progression free survival was 5.9 months (95% CI, 3.5 to 6.8), and the median overall survival was 21.8 months (95% CI, 19.9 to 23.7) at docetaxel rechallenge. A few studies have examined the outcomes of docetaxel chemotherapy in Asian countries, especially as a second-line treatment [23,24]. In our study, the PSA response rate ( >50%) of docetaxel chemotherapy as a second-line treatment was 45%. The first study of a docetaxel rechallenge by Eymard et al. [7] reported a similar PSA response rate (48%) with manageable toxicity. In our study, we did not show the toxicity of docetaxel in second-line chemotherapy, but several studies of second-line or rechallenge docetaxel chemotherapy reported that toxicities were tolerable. Notably, Di Lorenz et al. [9] reported that the side effects were moderate, and the main hematological grade 3 to 4 toxicities were neutropenia in 24.5%, thrombocytopenia in 11.1%, and anemia in 6.7% of patients. The main nonhematological grades 3 to 4 toxicities were nausea/vomiting and hypertension in 6.6% and 6.6% of patients, respectively. The authors concluded that docetaxel rechallenge preserved anti-tumor activity and was well tolerated in a selected population of pretreated patients with CRPC.
Two large-scale randomized phase III trials recently tested the efficacy of new drugs as a second-line treatment after docetaxel failure: cabazitaxel, a new taxane that led to better survival than mitoxantrone [4]; and Abiraterone, a new CYP17 inhibitor that was superior to placebo in terms of survival [3]. In 2010, phase III data in the TROPIC trial of cabazitaxel-based chemotherapy, 755 patients were randomized to receive either cabazitaxel 25 mg/m2 every three weeks plus prednisone or mitoxantrone 12 mg/m2 every three weeks plus prednisone. Eighteen patients in each arm received up to 10 cycles of treatment. OS (the primary end-point) was significantly longer in the cabazitaxel arm (15.1 months) than in the control arm (12.7 months) (HR, 0.70; 95% CI, 0.59 to 0.83). Subgroup analysis showed a benefit even in heavily pretreated patients. Interestingly, in our study, the median overall survival was longer in patients treated with docetaxel chemotherapy as second-line chemotherapy than in the patients treated with docetaxel chemotherapy as first-line chemotherapy. However, the comparison analysis between baseline patient characteristics showed that patients with docetaxel chemotherapy as second-line chemotherapy had good prognosis factors, such as higher levels of hemoglobin and lower Gleason scores at initial diagnosis (not shown). We were impressed that docetaxel chemotherapy as second-line chemotherapy was more effective than the use of docetaxel administered to the chemo-naïve patients. We will further analyze this result in a future study with a larger population sample.
One limitation of the present study is its retrospective design of PSA response criteria. A second limitation is that the first-line therapy for CRPC patients was determined at a physician’s discretion. Heterogeneous patients that did not satisfy the inclusion criteria of the new CPRC definition (castrate serum levels of testosterone [testosterone <50 ng/dL], antiandrogen withdrawal for at least four weeks for flutamide and for at least six weeks for bicalutamide) were included in this study because these patients were treated before the concept of CRPC was established. Nevertheless, the merits of the present study are that it analyzes a large cohort Koreans. The relatively few existing studies used small cohorts to examine the outcomes of chemotherapy in Asian countries.
In conclusion, doxetacel showed superior PSA response rates compared to other agents as second-line chemotherapy in Korean CRPC patients. If docetaxel is not used as first-line chemotherapy and new agents is not available for therapy in CRPC patients, docetaxel could be considered a second-line chemotherapy in CRPC. Nevertheless, these retrospective data need confirmation in future prospective studies.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Kim SJ, Kim SI. Incidence, epidemiology and patterns of progression of prostate cancer. J Korean Med Assoc. 2010;53:92–7. [Google Scholar]
- 2.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 3.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 5.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 6.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 7.Eymard JC, Oudard S, Gravis G, Ferrero JM, Theodore C, Joly F, et al. Docetaxel reintroduction in patients with metastatic castration-resistant docetaxel-sensitive prostate cancer: a retrospective multicentre study. BJU Int. 2010;106:974–8. doi: 10.1111/j.1464-410X.2010.09296.x. [DOI] [PubMed] [Google Scholar]
- 8.Loriot Y, Massard C, Gross-Goupil M, Di Palma M, Escudier B, Bossi A, et al. The interval from the last cycle of docetaxel-based chemotherapy to progression is associated with the efficacy of subsequent docetaxel in patients with prostate cancer. Eur J Cancer. 2010;46:1770–2. doi: 10.1016/j.ejca.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Di Lorenzo G, Buonerba C, Faiella A, Rescigno P, Rizzo M, Autorino R, et al. Phase II study of docetaxel re-treatment in docetaxel-pretreated castration-resistant prostate cancer. BJU Int. 2011;107:234–9. doi: 10.1111/j.1464-410X.2010.09498.x. [DOI] [PubMed] [Google Scholar]
- 10.Scher HI, Eisenberger M, D’Amico AV, Halabi S, Small EJ, Morris M, et al. Eligibility and outcomes reporting guidelines for clinical trials for patients in the state of a rising prostate-specific antigen: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 2004;22:537–56. doi: 10.1200/JCO.2004.07.099. [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 12.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DC, Dunn RL, Strawderman MS, Pienta KJ. Change in serum prostate-specific antigen as a marker of response to cytotoxic therapy for hormone-refractory prostate cancer. J Clin Oncol. 1998;16:1835–43. doi: 10.1200/JCO.1998.16.5.1835. [DOI] [PubMed] [Google Scholar]
- 14.Petrylak DP, Ankerst DP, Jiang CS, Tangen CM, Hussain MH, Lara PN, Jr, et al. Evaluation of prostate-specific antigen declines for surrogacy in patients treated on SWOG 99-16. J Natl Cancer Inst. 2006;98:516–21. doi: 10.1093/jnci/djj129. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong AJ, Garrett-Mayer E, de Wit R, Tannock I, Eisenberger M. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res. 2010;16:203–11. doi: 10.1158/1078-0432.CCR-09-2514. [DOI] [PubMed] [Google Scholar]
- 16.Joung JY, Jeong IG, Han KS, Kim TS, Yang SO, Seo HK, et al. Docetaxel chemotherapy of Korean patients with hormone-refractory prostate cancer:comparative analysis between 1st-line and 2nd-line docetaxel. Yonsei Med J. 2008;49:775–82. doi: 10.3349/ymj.2008.49.5.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JL, Kim JE, Ahn JH, Lee DH, Lee J, Kim CS, et al. Efficacy and safety of docetaxel plus prednisolone chemotherapy for metastatic hormone-refractory prostate adenocarcinoma: single institutional study in Korea. Cancer Res Treat. 2010;42:12–7. doi: 10.4143/crt.2010.42.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berthold DR, Pond GR, Roessner M, de Wit R, Eisenberger M, Tannock AI, et al. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin Cancer Res. 2008;14:2763–7. doi: 10.1158/1078-0432.CCR-07-0944. [DOI] [PubMed] [Google Scholar]
- 19.Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–5. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 20.Mountzios I, Bournakis E, Efstathiou E, Varkaris A, Wen S, Chrisofos M, et al. Intermittent docetaxel chemotherapy in patients with castrate-resistant prostate cancer. Urology. 2011;77:682–7. doi: 10.1016/j.urology.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Caffo O, Pappagallo G, Brugnara S, Caldara A, di Pasquale MC, Ferro A, et al. Multiple rechallenges for castration-resistant prostate cancer patients responding to first-line docetaxel: assessment of clinical outcomes and predictive factors. Urology. 2012;79:644–9. doi: 10.1016/j.urology.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 22.Heck MM, Thalgott M, Retz M, Wolf P, Maurer T, Nawroth R, et al. Rational indication for docetaxel rechallenge in metastatic castration-resistant prostate cancer. BJU Int. 2012;110(11 Pt B):E635–40. doi: 10.1111/j.1464-410X.2012.11364.x. [DOI] [PubMed] [Google Scholar]
- 23.Miyoshi Y, Uemura H, Nakamura M, Hasumi H, Sugiura S, Makiyama K, et al. Treatment of androgen-independent, hormone-refractory prostate cancer with docetaxel in Japanese patients. Int J Clin Oncol. 2005;10:182–6. doi: 10.1007/s10147-005-0490-0. [DOI] [PubMed] [Google Scholar]
- 24.Akaza H, Moore MA, Chang SJ, Cheng C, Choi HY, Esuvaranathan K, et al. The 5th Conference on Asian Trends in Prostate Cancer Hormone Therapy. Asian Pac J Cancer Prev. 2007;8:3–12. [PubMed] [Google Scholar]