Abstract
Purpose:
To evaluate the predictive performance of various parameters derived from volume-adjusted prostate-specific antigen (PSA) values in detecting prostate cancer (PCa) and high-grade (Gleason score≥7) PCa according to treatment with a 5α-reductase inhibitor (5ARI).
Methods:
The results of 3,520 prostate biopsies performed between May 2006 and January 2013 were retrospectively assessed. With adjustment for age, 291 patients who had received 5ARI treatment for more than 6 months were identified and matched 1:3 to patients naïve to 5ARIs, resulting in a total of 873 patients. Peripheral zone (PZ) and transition zone (TZ) volumes were determined by transrectal ultrasonography. Receiver-operating characteristic (ROC) curve analysis was used to compare predictive performances of PSA, PSA density (PSAD; PSA/prostate volume), PZPSAD (PSA/PZ volume), and TZPSAD (PSA/TZ volume) for detecting PCa and high-grade PCa for each group.
Results:
The area under the ROC curve (AUC) was higher for PSAD than for PSA in the 5ARI group (0.751 vs. 0.677) and in the 5ARI-naïve group (0.649 vs. 0.582), respectively (P<0.001). In the 5ARI group, the AUC for PZPSAD was even higher than that for PSAD (0.781 vs. 0.751, P=0.038); in the 5ARI-naïve group, however, PZPSAD failed to achieve significant superiority (0.652 vs. 0.649, P=0.321). All volume-adjusted PSA indexes showed higher predictive accuracies for detecting PCa than did PSA in both groups. For detecting high-grade cancer, PZPSAD also revealed the highest predictive value in the 5ARI group, whereas PSA revealed the highest predictive value in the 5ARI-naïve group.
Conclusions:
The diagnostic performance of PSAD in the detection of PCa is superior to that of PSA. For patients receiving 5ARI for more than 6 months, PZPSAD confers additional benefits for detecting both PCa and high-grade PCa.
Keywords: Density, Prostate cancer, Prostate-specific antigen
INTRODUCTION
Prostate cancer (PCa) is the most common solid organ malignancy and the second most common cause of cancer-related death among men in industrialized nations [1]. Prostate-specific antigen (PSA) is the most widely used serum marker that has revolutionized the early detection and management of PCa [2,3]. However, the relative lack of cancer-specificity, without upper or lower threshold values, is a major drawback that may lead to unnecessary risks and costs [1].
Various attempts have been made to overcome these limitations, namely, the utilization of PSA density (PSAD), percentage of free PSA, PSA velocity, age-specific PSA ranges, complex PSA, and transition zone (TZ) PSAD [4–6]. However, none of these indexes has achieved satisfactory results applicable to everyday clinical practice. The interpretation of PSA is even more complex in patients with benign prostatic hyperplasia who are administered 5α-reductase inhibitors (5ARIs). 5ARIs have been shown to reduce prostate volume (PV) by approximately 20% and to decrease serum PSA levels by about 50% on a 6-month course [7]. It is generally accepted that the sensitivity and the specificity of serum PSA levels can be maintained by doubling the patient’s PSA value to account for the change in PSA [8]. However, this is a rough estimation that does not exactly reflect the biological variability in PSA between individuals.
To address these issues, in the present study, various volume-adjusted PSA parameters were derived and analyzed for predictive performance in the detection of PCa. Furthermore, in an attempt to investigate whether these volume-adjusted indicators may account for the changes in PV, these parameters were analyzed and compared between patients who had been receiving 5ARIs for over 6 months and patients who were naïve to 5ARIs. Our findings indicate that volume-adjusted PSA parameters are more reliable than PSA in discriminating PCa and that such parameters may reflect changes in prostate volumetrics in patients administered 5ARIs.
MATERIALS AND METHODS
1. Patients
The results of 3,520 consecutive prostate biopsies performed between May 2006 and January 2013 were retrospectively assessed. The median age of the patients was 67.5 years (range, 31 to 90 years). The inclusion criteria were patients who underwent 12-core to 14-core prostate biopsy. Patients with inadequate transrectal ultrasonography (TRUS) images with an ambiguous boundary between the peripheral zone (PZ) and the TZ, an indefinite history of prior medications, or a pathological diagnosis of prostatic intraepithelial neoplasia or atypical small acinar proliferation were excluded from the study cohort. Patients who had received 5ARIs for more than 6 months were identified and designated as group A. Each group A patient was randomly matched with three patients naïve to 5ARIs with adjustment for age, who were designated as group B. The study was carried out in accordance with the Institutional Review Board practice guidelines.
2. Measurements of PSAD-based parameters
TRUS was used to measure the total PV and the TZ volume (TZV) by use of the formula for a prolate ellipsoid (length× width×height×0.52). The PZ volume (PZV) was measured by subtracting TZV from PV. PSAD, PZPSAD, and TZPSAD were calculated by dividing PSA by PV, PZ, and TZ, respectively.
3. Statistical analysis
Comparisons between groups were performed by using Student t-test. Areas under the ROC curves (AUCs) were used to calculate performances of PSA, PSAD, PZPSAD, and TZPSAD in detecting PCa and high-grade disease, defined as a Gleason score sum ≥7. Pairwise comparisons of ROC curves were used to compare predictive performances between each volume-adjusted PSA parameter. All statistical analyses were performed by using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA) and MedCalc ver. 11.6 (MedCalc Software, Acacialaan, Ostend, Belgium). Statistical significance was set at P<0.05.
RESULTS
1. Demographic data of investigated subjects
Among 1,164 eligible patients, group A consisted of 291 patients (25%), and group B consisted of 873 patients (75%). Overall, PCa was histologically diagnosed in 345 patients (29.6%). The clinical characteristics of the overall cohort are presented in Table 1. Patients in whom PCa was diagnosed were older; had higher PSA, PSAD, PZPSAD, and TZPSAD; and had significantly lower PV, PZV, and TZV than did patients with benign pathology. The clinical characteristics of the patients according to group stratification are shown in Table 2. PCa was histologically confirmed in 65 group A patients (22.3%) and in 280 group B patients (32.1%).
Table 1.
Clinical characteristics of the overall cohort
| Parameter | Total | Group A | Group B | P-value |
|---|---|---|---|---|
| Patients | 1,164 | 291 (25) | 873 (75) | |
| Age (yr) | 67.5 (31–90) | 67.3 (31–90) | 67.8 (39–90) | 0.474 |
| PSA (ng/mL) | 6.5 (2.6–19.8) | 6.4 (2.6–19.4) | 6.6 (2.7–19.8) | 0.707 |
| PSAD | 0.15 (0.02–0.75) | 0.13 (0.02–0.72) | 0.15 (0.02–0.75) | 0.001 |
| PV (mL) | 43.8 (8.1–263.9) | 51.1 (8.1–188.7) | 40.1 (12.5–263.9) | <0.001 |
| PZV (mL) | 18.9 (1.4–93.4) | 20.6 (3.4–93.4) | 18.1 (1.4–79.1) | 0.001 |
| TZV (mL) | 23.4 (4.2–184.8) | 26.6 (4.7–118.3) | 21.3 (4.2–184.8) | <0.001 |
| PZPSAD | 0.36 (0.04–2.81) | 0.39 (0.04–1.54) | 0.35 (0.04–2.81) | 0.024 |
| TZPSAD | 0.26 (0.03–2.32) | 0.23 (0.03–1.46) | 0.28 (0.03–2.32) | 0.001 |
| DRE (+) | 209 (17.9) | 45 (15.5) | 164 (18.8) | 0.072 |
| TRUS (+) | 141 (12.1) | 36 (12.4) | 105 (12.1) | 0.456 |
Values are presented as number (%) or median (range).
PSA, prostate-specific antigen; PSAD, PSA density; PV, prostate volume; PZV, peripheral zone volume; TZV, transition zone volume; PZPSAD, peripheral zone PSAD; TZPSAD, transition zone PSAD; DRE, digital rectal examination; TRUS, transrectal ultrasonography.
Table 2.
Clinical characteristics of group A (patients on 5ARI) and group B (patients naïve to 5ARI)
| Parameter | Group A (n=291) | Group B (n=873) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Cancer | Benign | P-value | Cancer | Benign | P-value | |
| Patients | 65 (22.3) | 226 (77.7) | 280 (32.1) | 593 (68.5) | ||
| Low grade | 30 (46.2) | 135 (48.2) | ||||
| High grade | 35 (53.8) | 145 (51.8) | ||||
| Age (yr) | 69.3 (51–81) | 66.2 (31–90) | 0.002 | 68.5 (39–87) | 65.1 (39–90) | <0.001 |
| Low grade | 69.0 (51–81) | 0.186 | 67.9 (47–80) | 0.009 | ||
| High grade | 70.2 (56–81) | 0.029 | 69.8 (39–87) | <0.001 | ||
| PSA (ng/mL) | 6.9 (3.3–18.5) | 6.3 (2.6–19.4) | 0.001 | 7.0 (3.1–19.5) | 6.3 (2.7–19.8) | 0.001 |
| Low grade | 6.8 (3.3–18.5) | 0.376 | 6.1 (3.1–19.1) | 0.498 | ||
| High grade | 8.1 (4.1–17.8) | 0.071 | 8.1 (3.3–19.5) | <0.001 | ||
| PSAD | 0.17 (0.04–0.61) | 0.12 (0.02–0.72) | <0.001 | 0.18 (0.04–0.75) | 0.14 (0.02–0.72) | <0.001 |
| Low grade | 0.19 (0.06–0.53) | 0.002 | 0.17 (0.04–0.71) | 0.052 | ||
| High grade | 0.17 (0.04–0.61) | <0.001 | 0.22 (0.05–0.75) | <0.001 | ||
| PV (mL) | 40.1 (8.1–112.7) | 53.9 (17.1–188.7) | 0.004 | 36.5 (12.5–146.1) | 44.4 (13.6–263.9) | <0.001 |
| Low grade | 40.4 (15.3–112.7) | 0.008 | 36.9 (12.5–86.1) | <0.001 | ||
| High grade | 39.4 (8.1–97.1) | 0.002 | 36.2 (12.7–146.1) | 0.002 | ||
| PZV (mL) | 15.6 (3.4–33.9) | 21.2 (6.1–93.4) | <0.001 | 16.4 (1.4–44.1) | 19.1 (3.6–79.1) | <0.001 |
| Low grade | 16.2 (8.9–30.7) | 0.035 | 16.2 (1.4–44.1) | <0.001 | ||
| High grade | 15.3 (3.4–33.9) | <0.001 | 16.7 (5.9–43.1) | <0.001 | ||
| TZV (mL) | 22.7 (4.7–87.7) | 30.3 (4.8–118.3) | 0.042 | 20.1 (4.9–103.0) | 24.6 (4.2–184.8) | 0.001 |
| Low grade | 21.9 (6.4–87.7) | 0.011 | 20.7 (6.7–56.1) | 0.001 | ||
| High grade | 23.1 (4.7–63.1) | 0.002 | 19.7 (4.9–103.0) | 0.021 | ||
| PZPSAD | 0.49 (0.1–1.54) | 0.31 (0.04–1.42) | 0.001 | 0.43 (0.11–2.81) | 0.32 (0.04–1.95) | <0.001 |
| Low grade | 0.54 (0.17–1.12) | 0.001 | 0.38 (0.11–2.81) | 0.004 | ||
| High grade | 0.42 (0.12–1.54) | <0.001 | 0.51 (0.14–1.88) | <0.001 | ||
| TZPSAD | 0.30 (0.07–1.03) | 0.22 (0.03–1.46) | 0.001 | 0.37 (0.06–1.46) | 0.25 (0.03–2.32) | <0.001 |
| Low grade | 0.33 (0.09–1.03) | 0.011 | 0.32 (0.06–1.19) | 0.025 | ||
| High grade | 0.29 (0.07–1.02) | 0.010 | 0.42 (0.07–1.46) | <0.001 | ||
| DRE (+) | 20 (30.8) | 25 (11.1) | <0.001 | 93 (33.2) | 71 (11.9) | <0.001 |
| Low grade | 9 (45.0) | 39 (41.9) | ||||
| High grade | 11 (55.0) | 54 (58.1) | ||||
| TRUS (+) | 22 (33.8) | 14 (6.2) | <0.001 | 73 (26.1) | 32 (5.4) | <0.001 |
| Low grade | 13 (59.1) | 32 (43.8) | ||||
| High grade | 9 (40.9) | 41 (56.2) | ||||
Values are presented as number (%) or median (range).
5ARI, 5α-reductase inhibitor; PSA, prostate-specific antigen; PSAD, PSA density; PV, prostate volume; PZV, peripheral zone volume; TZV, transition zone volume; PZPSAD, peripheral zone PSAD; TZPSAD, transition zone PSAD; DRE, digital rectal examination; TRUS, transrectal ultrasonography.
2. Logistic regression analysis on predictive values by use of volume-adjusted PSA parameters
Univariate logistic analyses were performed for volume-adjusted PSA parameters (Table 3). All volume-adjusted PSA parameters were significant predictors for the detection of PCa in both cohorts. Multivariate regression analysis revealed that PSA (odds ratio [OR], 0.959; P=0.021) and PSAD (OR, 84.81; P=0.033) were significant independent predictors for detecting PCa for the overall population. In group A, PZPSAD (OR, 43.18; P =0.017) remained the only independent predictor, whereas in group B, PSA (OR, 0.957; P =0.038) was an independent parameter (Table 4).
Table 3.
Univariate logistic regression analyses for volume-adjusted PSA parameters of each group
| Parameter | Group A | Group B | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| Age | 1.07 | 1.03–1.11 | <0.001 | 1.06 | 1.03–1.08 | <0.001 |
| PSA | 1.03 | 1.01–1.04 | 0.011 | 1.01 | 1.00–1.02 | 0.006 |
| PSAD | 5.81 | 2.13–15.78 | 0.001 | 2.57 | 1.63–4.04 | <0.001 |
| PZPSAD | 2.43 | 1.47–3.99 | <0.001 | 1.51 | 1.23–1.83 | <0.001 |
| TZPSAD | 2.11 | 1.33–3.34 | 0.001 | 1.45 | 1.19–1.78 | <0.001 |
PSA, prostate-specific antigen; CI, confidence interval; PSAD, PSA density; PZPSAD, peripheral zone PSAD; TZPSAD, transition zone PSAD.
Table 4.
Volume-adjusted PSA parameters which showed significant predictive values in multivariate logistic regression analyses of each group
| Parameter | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Overall population | |||
| PSA | 0.959 | 0.927–0.993 | 0.021 |
| PSAD | 84.81 | 1.421–5054.7 | 0.033 |
| Group A | |||
| PZPSAD | 43.18 | 1.784–1045.1 | 0.017 |
| Group B | |||
| PSA | 0.957 | 0.917–0.998 | 0.038 |
PSA, prostate-specific antigen; CI, confidence interval; PSAD, PSA density; PZPSAD, peripheral zone PSAD.
3. Analysis by ROC curves
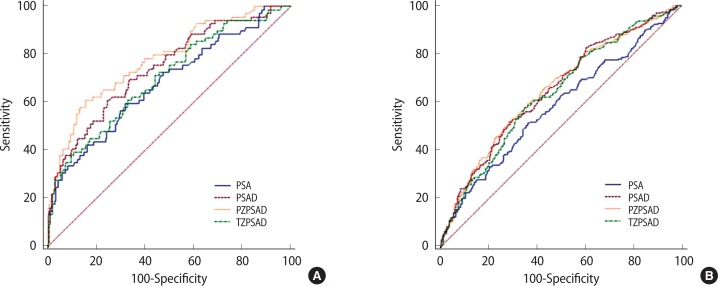
ROC analyses of volume-adjusted PSA parameters in the detection of PCa are shown in Table 5 and Fig. 1. The ROC curves of group A showed that PZPSAD had the highest accuracy for discriminating PCa, followed by PSAD, TZPSAD, and PSA. PSAD and PZPSAD revealed significantly higher AUCs than that of PSA, whereas the superiority of PZPSAD compared with PSAD was statistically significant (P=0.039). The sensitivities of the two highest predictors, i.e., PSAD and PZPSAD, at a set specificity of 40%, were 84% and 88%, respectively. In group B, PSAD and PZPSAD showed significantly higher AUCs than did PSA (P<0.001); however, the AUC of PZPSAD failed to significantly surpass that of PSAD (P=0.321). TZPSAD showed no better accuracy than PSA. The sensitivities of the two highest predictors, i.e., PSAD and PZPSAD, at a set specificity of 40%, were 81% and 79%, respectively.
Table 5.
Receiver operating characteristic curve analyses of PSA, PSAD, PZPSAD, and TZPSAD in detecting prostate cancer according to each group
| Parameter | AUC | 95% CI |
|---|---|---|
| Group A | ||
| PZPSAD | 0.781 | 0.712–0.839 |
| PSAD | 0.751 | 0.679–0.811 |
| TZPSAD | 0.717 | 0.645–0.782 |
| PSA | 0.677 | 0.603–0.745 |
| Group B | ||
| PZPSAD | 0.652 | 0.614–0.689 |
| PSAD | 0.649 | 0.611–0.686 |
| TZPSAD | 0.637 | 0.598–0.674 |
| PSA | 0.582 | 0.543–0.621 |
The volume-adjusted PSA parameters are listed the order of their predictive performance. Group A: PZPSAD vs. PSAD, P=0.038; PSAD vs. TZPSAD, P<0.001; TZPSAD vs. PSA, P=0.554. Group B: PZPSAD vs. PSAD, P=0.321; PSAD vs. TZPSAD, P=0.058; TZPSAD vs. PSA, P=0.756.
PSA, prostate-specific antigen; PSAD, PSA density; PZPSAD, peripheral zone PSAD; TZPSAD, transition zone PSAD; AUC, area under the curve; CI, confidence interval.
Fig. 1.
Receiver operating characteristic curves comparing the performances of PSA, PSAD, PZPSAD, and TZPSAD in the detection of prostate cancer in group A (A) and group B (B). The receiver-operating characteristic area under the curve and comparisons of each parameter are shown in Table 5. PSA, prostate-specific antigen; PSAD, PSA density; PZPSAD, peripheral zone PSAD; TZPSAD, transition zone PSAD.
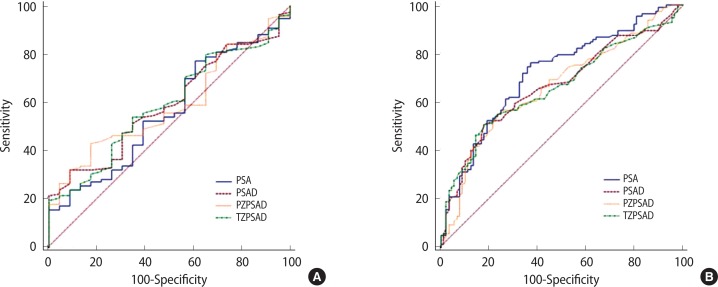
ROC analyses of volume-adjusted PSA parameters in detecting high-grade PCa are shown in Table 6 and Fig. 2. PZPSAD revealed the highest AUC in group A but did not meet statistical significance compared with PSAD, which revealed the second highest AUC. A notable finding was that PSA was significantly inferior to all volume-adjusted parameters for detecting PCa. The sensitivities of the two highest predictors, i.e., PSAD and PZPSAD, at a set specificity of 40%, were 85% and 87%, respectively. In group B, PSA showed the highest AUC for discriminating high-grade disease. The sensitivity of PSA at 40% specificity was revealed to be 76%.
Table 6.
Receiver operating characteristic curve analyses of PSA, PSAD, PZPSAD, and TZPSAD in detecting high grade cancer according to each group
| Parameter | AUC | 95% CI |
|---|---|---|
| Group A | ||
| PZPSAD | 0.625 | 0.487–0.731 |
| PSAD | 0.614 | 0.476–0.742 |
| TZPSAD | 0.601 | 0.462–0.731 |
| PSA | 0.562 | 0.425–0.695 |
| Group B | ||
| PSA | 0.715 | 0.647–0.776 |
| PSAD | 0.667 | 0.597–0.731 |
| PZPSAD | 0.662 | 0.593–0.727 |
| TZPSAD | 0.661 | 0.591–0.726 |
The volume-adjusted PSA parameters are listed the order of their predictive performance. Group A: PZPSAD vs. PSAD, P=0.716; PSAD vs. TZPSAD, P=0.581; TZPSAD vs. PSA, P=0.038. Group B: PSA vs. PSAD, P=0.041; PSAD vs. PZPSAD, P=0.803; PZPSAD vs. TZPSAD, P=0.956.
PSA, prostate-specific antigen; PSAD, PSA density; PZPSAD, peripheral zone PSAD; TZPSAD, transition zone PSAD; AUC, area under the curve; CI, confidence interval.
Fig. 2.
Receiver operating characteristic curves comparing the performances of PSA, PSAD, PZPSAD, and TZPSAD in the detection of high-grade cancer in group A (A) and group B (B). The receiver-operating characteristic area under the curve and comparisons of each parameter are shown in Table 6. PSA, prostate-specific antigen; PSAD, PSA density; PZPSAD, peripheral zone PSAD; TZPSAD, transition zone PSAD.
DISCUSSION
It is generally accepted that PSA provides the highest diagnostic performance for PCa and that its application to clinical practice has revolutionized the management of this disease [2]. However, the major drawback is its lack of cancer specificity and the lack of an upper or lower threshold value [9,10]. False elevations in noncancerous conditions not only result in unnecessary biopsies that lead to potential complications, but often mask aggressive forms of cancer that may lead to substantial harm [1]. This ongoing clinical challenge has aroused scientific challenges to evaluate novel biomarkers sensitive to PCa, namely, genetic-based, serologic, and urinary markers [11]. However, to date, none of these biomarkers has clearly outweighed diagnostic benefits against drawbacks. The present study did not seek to settle these concerns, but to raise the possibility that controlling for confounding conditions that can affect PSA values, such as benign prostatic enlargement, may confer additional diagnostic value. Indeed, developing a strategy of utilizing clinical parameters that are routinely evaluated, i.e., serum PSA and PV measurement by TRUS, could be of benefit in terms of cost, time, and treatment decision making.
The present study utilized volume-adjusted PSA-based parameters in an attempt to enhance the predictive performance of PSA. Kalish et al. [12] first introduced the concept of utilizing the volume-adjusted PSA-based parameter TZPSAD to show its superiority in discriminating PCa compared with PSAD. Validation studies have been performed by Kang et al. [13] showing that TZPSAD may be more effective in patients with intermediate PSA levels. Furthermore, Djavan et al. [14] reported that TZPSAD was more useful in patients with prostates larger than 30 g. However, Tanaka et al. [15], along with several other studies, showed that TZPSAD has an AUC similar to that of PSAD and disproved the usefulness of TZPSAD.
Alongside these investigations, our study is the first of its kind to utilize PZPSAD in addition to PSAD and TZPSAD as a potential predictive indicator for PCa and to compare these indexes between groups stratified according to 5ARI administration. We demonstrated that TZPSAD has a similar predictive value to PSAD in patients naïve to 5ARI and that its value is significantly below the level of PSAD in patients receiving 5ARIs. We further demonstrated that PZPSAD, among all volume-adjusted PSA parameters, had the significantly highest predictive value for detecting PCa in the 5ARI administration group. PZPSAD was also shown to be the most useful in the 5ARI-naïve group; however, it did not reach significance. In line with previous studies, PSA showed the lowest AUC compared with volume-adjusted PSA parameters.
The effectiveness of PSA and PSAD in detecting high-grade PCa (herein defined as a Gleason score sum ≥7) has been controversial. Elliot et al. [16] reported a trend toward improved performance of PSA for both Gleason ≥7 and Gleason ≥8 diseases. Our study is consistent with previous results showing that PSA demonstrates the highest AUC for predicting high-grade PCa in patients naïve to hormonal manipulation [17]. However, our study demonstrated the novel finding that in patients administered 5ARIs, the AUC for PSA falls significantly below that for all other volume-adjusted PSA parameters.
A logical question that could be raised by our findings is, “Why would PZPSAD be a better indicator for PCa in patients administered 5ARIs?” A possible answer is that patients receiving 5ARIs would have larger PV and higher PSA levels owing to the relative enlargement of TZV. To the best of our knowledge, no study to date has investigated changes in differential prostate zonal volumes in patients administered 5ARIs. However, it can be presumed that 5ARI has a relatively greater effect on the reduction of TZV and a modest effect on PZV, because TZ accounts for a greater proportion of the total PV in benign prostatic enlargement [18]. This volume reduction effect may have suppressed PSA owing to benign prostatic hyperplasia and thus led to a greater separation in PZPSAD values compared with values in those who actually harbored PCa. Indeed, PCa detected by a conventional PZ biopsy scheme as used in the present study would mostly reflect PZ cancers rather than TZ cancers. To clearly define the underlying mechanisms of these observations, investigations on relative zonal volume reductions according to administration of 5ARI should be undertaken.
The present study had several limitations that should be mentioned. First, the study was retrospective in nature. To confirm our findings, prospective and randomized studies with larger populations will be needed. Second, we could not exactly determine whether a patient harbors PCa because a prostate biopsy may miss 20% of PCa considered to be clinically significant and, conversely, may detect insignificant cancers [19,20]. Third, volume-adjusted PSA measurements necessitate PV determinations that are not always available in clinical practice. Last, our study cohort was not based on a general population as a whole but on a database of patients obtained from a single, tertiary institution. Therefore, our results may include a selection bias in which the results may not be applicable to the whole population.
In conclusion, volume-adjusted PSA parameters could be more useful than PSA in detecting PCa. For patients receiving 5ARI for more than 6 months, PZPSAD conferred the highest diagnostic performance in predicting PCa and high-grade disease. In patients naïve to 5ARIs, PSA remained superior to PSAD or PZPSAD in predicting high-grade disease but showed the lowest value for discriminating PCa.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.O’Rourke ME. The prostate-specific antigen screening conundrum: examining the evidence. Semin Oncol Nurs. 2011;27:251–9. doi: 10.1016/j.soncn.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Schroder FH. Landmarks in prostate cancer screening. BJU Int. 2012;110(Suppl 1):3–7. doi: 10.1111/j.1464-410X.2012.011428.x. [DOI] [PubMed] [Google Scholar]
- 3.Byun HK, Sung YH, Kim W, Jung JH, Song JM, Chung HC. Relationships between prostate-specific antigen, prostate volume, and components of metabolic syndrome in healthy Korean men. Korean J Urol. 2012;53:774–8. doi: 10.4111/kju.2012.53.11.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aganovic D, Prcic A, Kulovac B, Hadziosmanovic O. Influence of the prostate volume, prostate specific antigen density and number of biopsy samples on prostate cancer detection. Med Arh. 2012;66:41–4. doi: 10.5455/medarh.2012.66.41-44. [DOI] [PubMed] [Google Scholar]
- 5.Qi TY, Chen YQ, Jiang J, Zhu YK, Yao XH, Wang XJ. Utility of the transition zone index for identification of prostate cancer in Chinese men with intermediate PSA levels. Int Urol Nephrol. 2012;44:807–15. doi: 10.1007/s11255-011-0119-3. [DOI] [PubMed] [Google Scholar]
- 6.Segawa N, Gohji K, Iwamoto Y, Ueda H, Katsuoka Y. Prostate cancer detection by prostate-specific antigen-related parameters. Hinyokika Kiyo. 2003;49:405–10. [PubMed] [Google Scholar]
- 7.Choi YH, Cho SY, Cho IR. The different reduction rate of prostate-specific antigen in dutasteride and finasteride. Korean J Urol. 2010;51:704–8. doi: 10.4111/kju.2010.51.10.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oderda M, Zitella A, Richiardi L, Tizzani A, Gontero P. Effect of finasteride on the sensitivity of PSA to detect prostate cancer in rebiopsy series. Arch Ital Urol Androl. 2010;82:135–8. [PubMed] [Google Scholar]
- 9.Basch E, Oliver TK, Vickers A, Thompson I, Kantoff P, Parnes H, et al. Screening for prostate cancer with prostate-specific antigen testing: American Society of Clinical Oncology Provisional Clinical Opinion. J Clin Oncol. 2012;30:3020–5. doi: 10.1200/JCO.2012.43.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avery KN, Metcalfe C, Vedhara K, Lane JA, Davis M, Neal DE, et al. Predictors of attendance for prostate-specific antigen screening tests and prostate biopsy. Eur Urol. 2012;62:649–55. doi: 10.1016/j.eururo.2011.12.059. [DOI] [PubMed] [Google Scholar]
- 11.Gamagedara S, Kaczmarek AT, Jiang Y, Cheng X, Rupasinghe M, Ma Y. Validation study of urinary metabolites as potential biomarkers for prostate cancer detection. Bioanalysis. 2012;4:1175–83. doi: 10.4155/bio.12.92. [DOI] [PubMed] [Google Scholar]
- 12.Kalish J, Cooner WH, Graham SD., Jr Serum PSA adjusted for volume of transition zone (PSAT) is more accurate than PSA adjusted for total gland volume (PSAD) in detecting adenocarcinoma of the prostate. Urology. 1994;43:601–6. doi: 10.1016/0090-4295(94)90170-8. [DOI] [PubMed] [Google Scholar]
- 13.Kang SH, Bae JH, Park HS, Yoon DK, Moon DG, Kim JJ, et al. Prostate-specific antigen adjusted for the transition zone volume as a second screening test: a prospective study of 248 cases. Int J Urol. 2006;13:910–4. doi: 10.1111/j.1442-2042.2006.01439.x. [DOI] [PubMed] [Google Scholar]
- 14.Djavan B, Zlotta AR, Byttebier G, Shariat S, Omar M, Schulman CC, et al. Prostate specific antigen density of the transition zone for early detection of prostate cancer. J Urol. 1998;160:411–8. [PubMed] [Google Scholar]
- 15.Tanaka N, Fujimoto K, Chihara Y, Torimoto M, Hirao Y, Konishi N, et al. Prostatic volume and volume-adjusted prostate-specific antigen as predictive parameters for prostate cancer patients with intermediate PSA levels. Prostate Cancer Prostatic Dis. 2007;10:274–8. doi: 10.1038/sj.pcan.4500957. [DOI] [PubMed] [Google Scholar]
- 16.Elliott CS, Shinghal R, Presti JC., Jr The performance of prostate specific antigen, prostate specific antigen density and transition zone density in the era of extended biopsy schemes. J Urol. 2008;179:1756–61. doi: 10.1016/j.juro.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 17.Baade PD, Youlden DR, Cramb SM, Dunn J, Gardiner RA. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int. 2013;1:47–58. doi: 10.12954/PI.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohigashi T, Kanao K, Kikuchi E, Nakagawa K, Nakashima J, Marumo K, et al. Prostate specific antigen adjusted for transition zone epithelial volume: the powerful predictor for the detection of prostate cancer on repeat biopsy. J Urol. 2005;173:1541–5. doi: 10.1097/01.ju.0000154636.24375.76. [DOI] [PubMed] [Google Scholar]
- 19.Thompson IM, Pauler Ankerst D, Chi C, Goodman PJ, Tangen CM, Lippman SM, et al. Prediction of prostate cancer for patients receiving finasteride: results from the Prostate Cancer Prevention Trial. J Clin Oncol. 2007;25:3076–81. doi: 10.1200/JCO.2006.07.6836. [DOI] [PubMed] [Google Scholar]
- 20.Jeong IG, Lim JH, Hwang SS, Kim SC, You D, Hong JH, et al. Nomogram using transrectal ultrasound-derived information predicting the detection of high grade prostate cancer on initial biopsy. Prostate Int. 2013;1:69–75. doi: 10.12954/PI.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]