Abstract
Purpose:
The National Institutes of Health classification of prostatitis reported the proportion of chronic bacterial prostatitis, especially category II, at 3% to 10%. Because of the polymerase chain reaction (PCR) diagnosis technique, chronic prostatitis syndrome (CPS) with a known bacterial origin has increased recently. In this study, we evaluated the proportion of chronic bacterial prostatitis in a general hospital and a primary care clinic (PCC) in addition to the distribution of the microorganism in chronic bacterial prostatitis in Korea.
Methods:
Two hundred and ninety-three patients were enrolled in this study. One hundred and five patients in the general hospital and 188 patients in the PCC were enrolled in the study. Using a questionnaire, all patients were checked for symptoms of urinalysis, expressed prostate secretion (EPS), EPS or V3 culture and PCR of EPS or VB3 for Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, Mycoplasma genetalia, and Trichomatis vaginalis.
Results:
In routine EPS or VB3 culture, 12 of 105 patients (11.4%) in the general hospital showed positive culture, but 77 of 188 patients (40.9%) in the PCC showed a positive culture. Escherichia coli, Streptococcus faecalis, Staphylococcus epidermidis, Staphylococcus hemolyticus, Staphylococcus aureus, and Pseudomonas were isolated in routine culture. In the PCR diagnosis, 37 of 105 patients (35.2%) in the general hospital were PCR positive, and 65 of 188 patients (34.5%) in the PCC were PCR positive. In the general hospital, C. trachomatis was the most common (49%), followed by U. urealyticum (24%), M. genetalia (16%), M. hominis (10%), and T. vaginalis (2%). In the PCC, U. urealyticum was the most common (45%), followed by C. trachomatis (34%), M. hominis (13%), M. genetalia (7%) and T. vaginalis (1%). The proportions of chronic bacterial prostatitis were 46.6% (49/105) and 67.5% (127/188) in the general hospital and PCC, respectively.
Conclusions:
The total portion of chronic bacterial prostatitis was 59.3% (174/293). Culture-positive patients in the PCC were significantly higher than in the general hospital, but the number of PCR positive patients in the PCC was the same as in the general hospital.
Keywords: Chronic bacterial prostatitis, Etiology, Diagnosis
INTRODUCTION
Prostatitis is a disease diagnosed by clinical symptoms and signs evident in the microscopy of expressed prostatic secretion (EPS), and in the culture of EPS and segmented samples, according to Meares and Stamey [1,2]. The generally accepted classification of prostatitis syndrome differentiates the following: 1) acute bacterial prostatitis; 2) chronic bacterial prostatitis; 3) chronic pelvic pain syndrome (CPPS); inflammatory CPPS, white blood cells (WBCs) in semen (EPS)/voided bladder urine (VB3), noninflammatory CPPS, no WBCs in semen (EPSs)/VB3; 4) asymptomatic inflammatory prostatitis.
Symptoms of prostatitis are vague and involve pain in the pelvic region, urethral symptoms, voiding dysfunction, sexual disorder, and possibly considerable psychosocial distress [3]. In addition to these clinical problems, the pathogenesis and etiology of prostatitis are only partially understood and remain controversial. Nickel et al. [4] reported that “prostatitis has a prevalence of 6% to 8%, is responsible for 25% of all male genitourinary clinic visits, and is the most common urological disorder in men aged under 50”. In a cross-sectional Finnish study, Mehik et al. [5] reported a 14% of lifetime prevalence in which 27% of Finnish men reported symptoms at least once per year, and 16% complained of persistent prostatitis symptoms.
The disease entities in primary care clinics (PCCs) and tertiary centers are different. The causative pathogens and pathogen distributions might differ even in one disease entity. Furthermore, disease entities differ between country and region. This difference is important because in epidemics, the treatment of causative organisms differs accordingly.
The aim of this study was to investigate the etiology and the proportion of chronic bacterial prostatitis in a general hospital and a PCC in addition to the distribution of microorganisms in chronic bacterial prostatitis in Korea.
MATERIALS AND METHODS
1. Patients
From November 2006 to August 2007, 105 patients in a general hospital and 188 patients in a PCC, 293 patients were prospectively enrolled for evaluation. The inclusion criteria were as follows: 1) the disease duration was least 3 months; 2) antibiotic treatment for the same prostatitis symptoms within the last 3 months; 3) a bacterial count of 103 CFU/mL or more (only if gram-positive cocci are found in EPS; a bacterial count of 104 CFU/mL or more is required), and 10 or more WBC per high-power field in EPS or VB3; 4) 10 times as many bacteria in EPS and urine bladder samples collected immediately after prostate massage than in samples of first voided urine or midstream urine. Nonbacterial prostatitis patients were excluded during the evaluation protocol. The purpose and method were explained to the patients before enrollment. All subjects that decided to participate in this study provided written informed consent.
2. Methods
The following data were obtained for each patient: clinical history, symptom questionnaire, clinical status of digital rectal examination, urinalysis, urethral swap specimens, EPS, EPS or VB3 culture according to the four-glass localization technique, and PCR of EPS or VB3.
The Korean version of National Institutes of Health Chronic Prostatitis Symptom Index (NIH-CPSI) questionnaire was used for subjective assessment of the patients’ symptoms.
Urethral swab samples were analyzed for the microbiological evaluation of Chlamydia trachomatis, Ureaplasma urealyticum, Mycoplasma hominis, Mycoplasma genitalia, and Trichomonas vaginalis. We used 1 mL specimens to determine the number of WBCs and gram-positive and gram-negative organisms. EPS and urine samples were examined for the presence of C. trachomatis, U. urealyticum, M. hominis, M. genitalia, and T. vaginalis immediately after prostatic massage.
We used complementary approaches to the polymerase chain reaction (PCR) technique. First, we developed specific PCR assays for each pathogen previously implicated, and then validated these assays for prostate tissue specimens. These specific PCR probes were directed at C. trachomatis, U. urealyticum, M. hominis, M. genitalia, and T. vaginalis probes. Second, we used broad-spectrum PCR assays to identify bacterial DNA sequences. Primer/probes were directed at two targets: common tetracycline resistant genes and bacterial ribosomal encoding genes. Tetracycline resistant genes are common in urogenital bacteria. Bacterial ribosomal encoding genes are distinct from mammalian ribosomal genes, so they are easily distinguished from human DNA. The 475 base pair products were then cloned in an attenuated strain of E. coli K12 and sequenced. Homology searches were performed to compare the identified sequences with the available existing data.
For the PCR, the inclusion criteria for chronic prostatitis caused by Chlamydia trachomatis was the presence of C. trachomatis in EPS or VB3, absence of C. trachomatis in urethral swabs and the absence of other possible pathogens of chronic prostatitis in EPS or VB3. The inclusion criteria for chronic prostatitis caused by Ureaplasma urealyticum and Mycoplasma hominis were the presence of U. urealyticum, M. hominis in EPS or VB3, absence of U. urealyticum or M. hominis in urethral swabs and the absence of other possible pathogens of chronic prostatitis in EPS or VB3. The inclusion criteria for M. genitalia and T. vaginalis were the same as those of described above. The criteria for noninflammatory chronic prostatitis and CPPS were the presence of clinical symptoms of prostatitis and the absence of white cells in EPS or VB3.
3. Statistical analysis
The Fisher exact chi-square test was used to assess statistical significance; P <0.05 was considered significant. Two-sided tests of significance were performed for all analyses. Statistical analyses were performed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA).
RESULTS
1. Patient demographics
The mean ages of the patients were 45.4±12.7 and 43.8±11.3 in the PCC and general hospital, respectively (P=0.592) The mean treatment period for chronic bacterial prostatitis was 13.4±5.2 weeks 11.8±4.4 weeks in the PCC and general hospital, respectively (P =0.274). For the responses to the NIHCPSI questionnaire, the mean pain scores were 9.6±5.2 and 9.4±5.0. in the PCC and in general hospital, respectively (P= 0.574). The mean voiding symptom scores were 4.7±3.1 and 4.6±3.0 in PCC and the general hospital, respectively (P=0.291). The mean scores for quality of life were 7.2±2.5 and 7.2±3.1 in the PCC and general hospital, respectively (P=0.591) The total NIH-CPSI scores in the PCC and the tertiary general hospital were 21.0±5.5 and 21.5±7.4, respectively (P =0.557) (Table 1).
Table 1.
Patient demographics
| Variable | Primary care clinic | General hospital | P-value |
|---|---|---|---|
| Age (yr) | 45.4±12.7 | 43.8±11.3 | 0.592 |
| Treatment period (wk) | 13.4±5.2 | 11.8±4.4 | 0.274 |
| NIH-CPSI questionnaire | |||
| Pain score | 9.6±5.2 | 9.4±5.0 | 0.574 |
| Voiding score | 4.7±3.1 | 4.6±3.0 | 0.291 |
| QoL score | 7.2±2.5 | 7.2±3.1 | 0.591 |
| Total score | 21.0±5.5 | 21.5±7.4 | 0.557 |
Values are presented as mean±standard deviation.
NIH-CPSI, National Institutes of Health Chronic Prostatitis Symptom Index; QoL, quality of life.
2. Microbiological analysis with EPS culture analysis
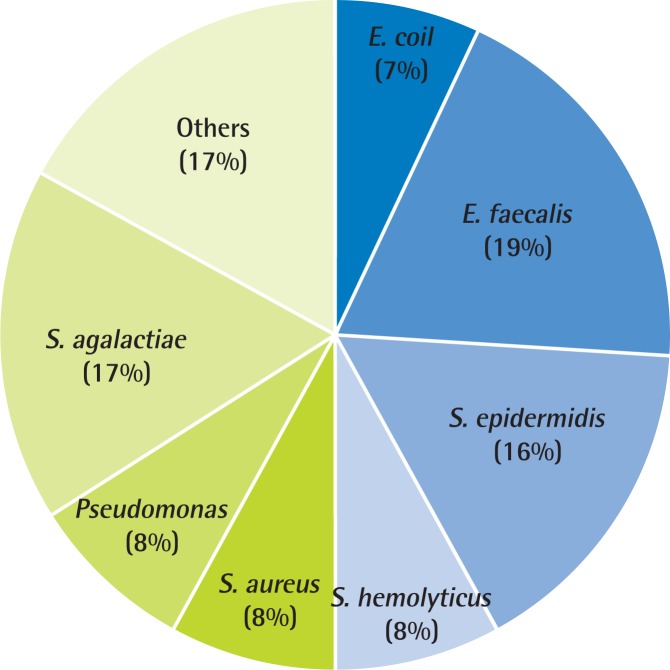
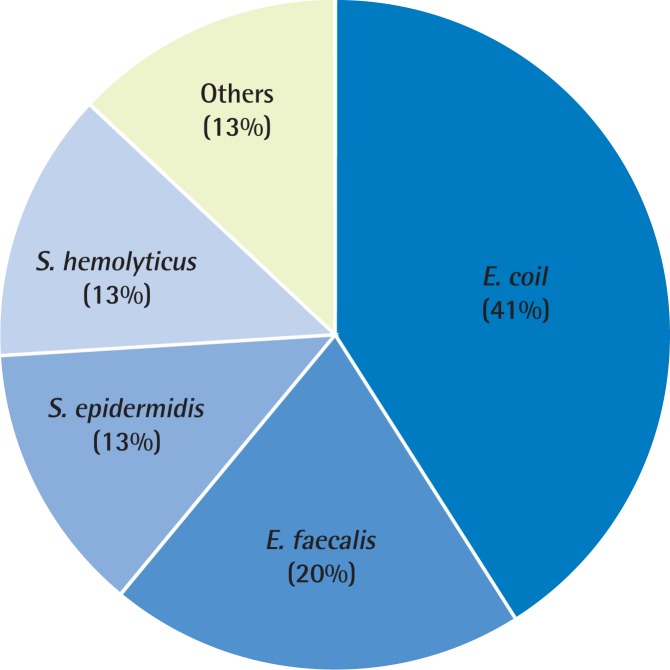
In routine EPS or VB3 culture, 12 of 105 patients (11.4%) in the general hospital were culture positive. However, 77 of 188 patients (40.9%) in the PCC were culture positive (Table 2). The microorganisms obtained from culture were E. coli, S. faecalis, S. epidermidis, S. hemolyticus, S. aureus, and Pseudomonas species. The most common pathogen in the PCC was E. faecalis (19%), followed by Streptococcus agalactiae (17%), S. epidermidis (16%), and S. hemolyticus (8%), S. aureus (8%), Pseudomonas (8%) (Fig. 1). The analysis of the data from the general hospital found that the most common pathogen was E. coli (41%), followed by E. faecalis (20%), S. epidermidis (13%), and S. hemolyticus (13%) (Fig. 2).
Table 2.
Comparison of culture-positive patients in EPS and PCR culture in primary care clinic and general hospital
| Primary care clinic | General hospital | P-value | |
|---|---|---|---|
| EPS/VB3 culture (+) | 40.9 | 11.4 | 0.038 |
| PCR (+) | 34.5 | 35.2 | 0.275 |
EPS, expressed prostate secretion; PCR, polymerase chain reaction.
Fig. 1.
Evidence of chronic bacterial prostatitis in primary care clinic (n=77). The microbial pathogen distributions in a primary care clinic in Korea. The most common pathogen in primary care clinic was Enterococcus faecalis (19%), followed by Streptococcus agalactiae (17%), Staphylococcus epidermidis (16%), Staphylococcus hemolyticus (8%), Staphylococcus aureus (8%), and Pseudomonas (8%). E. coli, Escherichia coli.
Fig. 2.
Evidence of chronic bacterial prostatitis in general hospital (n=12). The microbial pathogen distributions in a general hospital in Korea. The most common pathogen was Escherichia coli (41%), followed by Enterococcus faecalis (20%), Stwaphylococcus epidermidis (13%), and Staphylococcus hemolyticus (13%).
3. Microbiological analysis with PCR analysis
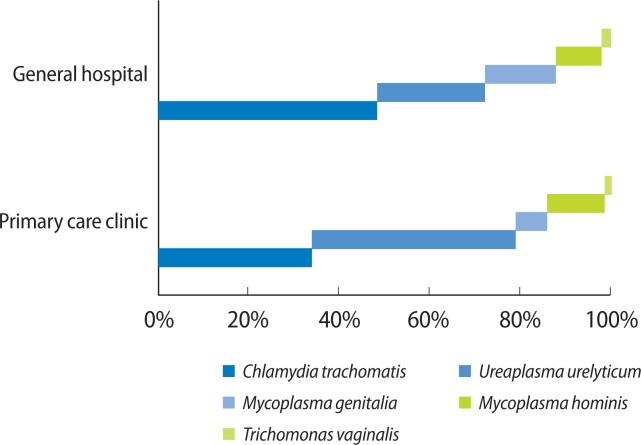
The PCR analysis showed that 37 of 105 patients (35.2%) in the general hospital were PCR positive, and 65 of 188 patients (34.5%) in the PCC were PCR positive (Table 2). In the general hospital, C. trachomatis was the most common pathogen (49%), followed by U. urealyticum (24%), M. genitalia (16%), M. hominis (10%), and T. vaginalis (2%) (Fig. 2). However, in the PCC, U. urealyticum is the most common pathogen (45%), followed by C. trachomatis (34%), M. hominis (13%), M. genitalia (7%), and T. vaginalis (1%) (Fig. 3).
Fig. 3.
Comparison of polymerase chain reaction (PCR) analyses of chronic bacterial prostatitis in primary care clinic (n=65) and general hospital (n=37). Comparison of the PCR analyses of chronic prostatitis in a general hospital and primary care clinic. The pathogenic distribution was different. The most common pathogen in the general hospital was C. trachomatis. In the primary care clinic, it was U. urealyticum.
The proportion of chronic bacterial prostatitis in the general hospital was 46.6% (49/105), but in the PCC, it was 67.5% (127/188). The proportion of culture-positive chronic bacterial prostatitis patients in the entire population sample was 59.3%.
DISCUSSION
In the United States, prostatitis is diagnosed in 2,000,000 visits annually, including 8% of all visits to urologists and 1% of all visits to primary care physicians [6]. Men with prostatitis symptoms appear to be at increased risk for persistent symptoms and recurrent episodes. Many studies have evaluated the risk factors for recurrent prostatitis. Lifestyle, diet, smoking, gastrointestinal disease, anorectal disease, and even coitus seem to affect the prevalence of the disease [7–9]. However, we think that the risk factors for recurrent prostatitis are still controversial. In addition, over time, prostatitis-like symptoms result in a substantial number of physician visits. Nickel et al. [10] reported that 60% of patients with prostatitis-like symptoms sought medical help. Moreover, patients with a previous diagnosis of prostatitis had a substantially higher cumulative probability of subsequent episodes [9,11].
The treatment of the disease begins with its diagnosis. If the physician diagnoses the disease accurately, then the treatment can be applied immediately. However, in the chronic prostatitis disease entity, the treatment might not be applicable. Because of diverse pathogens and pathogenesis [12,13], recurrence is more common so the treatment period should be longer than in other diseases. Furthermore, in Korea, data on nation-wide prostatitis pathogens has not been available to physicians. The variability of pathogens in regional primary-tertiary hospitals also remains unknown. Hence, the results of this study provide an important foundation for further research, particularly comparisons between PCCs and tertiary general hospitals.
In our study, the results of the EPS culture showed significant discrepancies between the PCC and the general hospital. The EPS culture-positive rate for the general hospital was only about 11%. However, in the PCC it about 40%. The PCC culture-positive rate for EPS was higher than in other reports. de la Rosette et al. [14] reported an EPS culture-positive rate of 10.4%, and Krieger et al. [15] reported an EPS culture-positive rate of 7%. Because most microorganisms in the PCC were gram-positive, the potential reasons for the high culture-positive rate in the PCC could be skin contamination and inadequate sample acquisition. Furthermore, bias in the selection of the patients might account for the difference between these two medical institutions. To understand this result, we should understand the Korean healthcare system.
In Korea, the governmental referral system divides the medical institution into primary medical offices, secondary hospitals, and tertiary general hospitals. In this referral system, it is recommended that patients visit their primary care physician first, but there is no restriction. Because the PCC is easy to access, patients can visit freely whenever they have symptoms. If symptoms persist after treatment in the PCC, then patients seek treatment in specialized hospital clinics, followed by a tertiary general hospital [16]. Because the patients in the present sample first received treatment at the PCC, the culture-positive rate for the general hospital is lower than the PCC rate. However, according to the PCR analysis results, the number of patients showing positive PCR results almost equaled those in the PCC and the general hospital. Therefore, the results indicated that the chronic prostatitis pathogens in the PCC and the general hospital share a similar origin.
The extraordinary finding in our study was the discrepancy in pathogens between the PCC and the general hospital. Un-like the results of studies in western countries [15,17–19], PCR showed that the most common pathogen in the PCC was U. urealyticum. It accounted for 45% of all chronic bacterial prostatitis pathogens in the PCC. However, the results of the PCR showed that the most common pathogen in the general hospital was C. trachomatis, which accounted for 49% of all bacterial prostatitis pathogens. Because of the difference in the prevalence of pathogens, the treatment should differ accordingly. Although worldwide treatment guidelines are important [20,21], the disease entity might be different in each continent and each country. Therefore, the treatments should differ. The results of our Korean study showed that the most common chronic bacterial prostatitis pathogen in the PCC is Chlamydia, which and can be managed with azithromycin, doxycycline, or levofloxacin. In the general hospital, the most common pathogen in chronic bacterial prostatitis is Urea-plasma, which also can be treated. The role of U. urealyticum in nonbacterial prostatitis is uncertain [22]. These microorganisms are part of normal male urethral flora [23]. Therefore, the quantification of U. urealyticum is obligatory, thus allowing comparison of the numbers of these microorganisms in EPS. Furthermore, in chronic bacterial prostatitis patients with clinical symptoms, U. urealyticum should be treated with doxycycline or tetracycline. Furthermore, because doxycycline is not a primary treatment of choice for chronic bacterial prostatitis, the physician should be aware of the patient’s symptoms and treatment history.
The PCR technique greatly affects the pathogenic diagnosis of bacterial prostatitis. Many patients have numerous previous courses of antibiotics, which could interfere with the cultivation of microorganisms. Many organisms seldom grow in most refined culture conditions. Although a higher proportion of cultivable microorganisms are known in humans, this source still contains a significant number of uncharacterized species that grow poorly on conventional media [24,25]. In spite of these shortcomings, the PCR technique does not need micro-organisms to survive in laboratory conditions. Therefore, by using this technique, we can identify the causative organisms directly and rapidly.
The limitation of this study is the relatively small population sample. First, for epidemic evaluation, the enrollment of a greater number of patients is needed for analysis. The second limitation is the bias in the selection of tertiary hospital patients. Because the patient population in general hospitals is clearly different from that in PCCs, the clinical impact of this study is limited. In spite of these shortcomings, this report compares the recent status of chronic bacterial prostatitis in PCCs and general hospitals. The results, especially the pathogen results, are unique. Compared with the results of studies in other countries, our results for chronic bacterial prostatitis are specific to conditions in Korean PCCs and general hospitals.
In conclusion, the proportion of patients that were culture-positive for chronic bacterial prostatitis was significantly higher in the PCC than in the general hospital. However, the results of the PCR were the same for both the PCC and the general hospital. The proportion of patients in the entire population that were culture-positive for chronic bacterial prostatitis patients was 59.3%.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Schneede P, Hofstetter AG, Naber KG, Vahlensieck W, Ludwig M, Bach D, et al. European Association of Urology guidelines on urinary and male genital tract infections. Urologe A. 2003;42:104–12. doi: 10.1007/s00120-002-0262-7. [DOI] [PubMed] [Google Scholar]
- 2.Meares EM, Stamey TA. Bacteriologic localization patterns in bacterial prostatitis and urethritis. Invest Urol. 1968;5:492–518. [PubMed] [Google Scholar]
- 3.Egan KJ, Krieger JN. Psychological problems in chronic prostatitis patients with pain. Clin J Pain. 1994;10:218–26. doi: 10.1097/00002508-199409000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Nickel JC, Teichman JM, Gregoire M, Clark J, Downey J. Prevalence, diagnosis, characterization, and treatment of prostatitis, interstitial cystitis, and epididymitis in outpatient urological practice: the Canadian PIE Study. Urology. 2005;66:935–40. doi: 10.1016/j.urology.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 5.Mehik A, Hellstrom P, Lukkarinen O, Sarpola A, Jarvelin M. Epidemiology of prostatitis in Finnish men: a population-based cross-sectional study. BJU Int. 2000;86:443–8. doi: 10.1046/j.1464-410x.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 6.Collins MM, Stafford RS, O’Leary MP, Barry MJ. How common is prostatitis? A national survey of physician visits. J Urol. 1998;159:1224–8. [PubMed] [Google Scholar]
- 7.Bartoletti R, Cai T, Mondaini N, Dinelli N, Pinzi N, Pavone C, et al. Prevalence, incidence estimation, risk factors and characterization of chronic prostatitis/chronic pelvic pain syndrome in urological hospital outpatients in Italy: results of a multicenter case-control observational study. J Urol. 2007;178:2411–5. doi: 10.1016/j.juro.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 8.Alexander RB, Trissel D. Chronic prostatitis: results of an Internet survey. Urology. 1996;48:568–74. doi: 10.1016/s0090-4295(96)00234-8. [DOI] [PubMed] [Google Scholar]
- 9.Roberts RO, Lieber MM, Rhodes T, Girman CJ, Bostwick DG, Jacobsen SJ. Prevalence of a physician-assigned diagnosis of prostatitis: the Olmsted County Study of Urinary Symptoms and Health Status Among Men. Urology. 1998;51:578–84. doi: 10.1016/s0090-4295(98)00034-x. [DOI] [PubMed] [Google Scholar]
- 10.Nickel JC, Downey J, Hunter D, Clark J. Prevalence of prostatitis-like symptoms in a population based study using the National Institutes of Health chronic prostatitis symptom index. J Urol. 2001;165:842–5. [PubMed] [Google Scholar]
- 11.Turner JA, Ciol MA, Von Korff M, Berger R. Prognosis of patients with new prostatitis/pelvic pain syndrome episodes. J Urol. 2004;172:538–41. doi: 10.1097/01.ju.0000132797.63480.44. [DOI] [PubMed] [Google Scholar]
- 12.Skerk V, Krhen I, Schonwald S, Cajic V, Markovinovic L, Roglic S, et al. The role of unusual pathogens in prostatitis syndrome. Int J Antimicrob Agents. 2004;24(Suppl 1):S53–6. doi: 10.1016/j.ijantimicag.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Weidner W, Ludwig M. Common organisms in urogenital infections with special impact on prostatitis. Eur Urol Suppl. 2003;2:15–8. [Google Scholar]
- 14.de la Rosette JJ, Hubregtse MR, Meuleman EJ, Stolk-Engelaar MV, Debruyne FM. Diagnosis and treatment of 409 patients with prostatitis syndromes. Urology. 1993;41:301–7. doi: 10.1016/0090-4295(93)90584-w. [DOI] [PubMed] [Google Scholar]
- 15.Krieger JN, Ross SO, Riley DE. Chronic prostatitis: epidemiology and role of infection. Urology. 2002;60(6 Suppl):8–12. doi: 10.1016/s0090-4295(02)02294-x. [DOI] [PubMed] [Google Scholar]
- 16.Choi KS, Cho WH, Lee S, Lee H, Kim C. The relationships among quality, value, satisfaction and behavioral intention in health care provider choice: a South Korean study. J Bus Res. 2004;57:913–21. [Google Scholar]
- 17.Weidner W, Schiefer HG, Krauss H, Jantos C, Friedrich HJ, Altmannsberger M. Chronic prostatitis: a thorough search for etiologically involved microorganisms in 1,461 patients. Infection. 1991;19(Suppl 3):S119–25. doi: 10.1007/BF01643680. [DOI] [PubMed] [Google Scholar]
- 18.Brunner H, Weidner W, Schiefer HG. Studies on the role of Ureaplasma urealyticum and Mycoplasma hominis in prostatitis. J Infect Dis. 1983;147:807–13. doi: 10.1093/infdis/147.5.807. [DOI] [PubMed] [Google Scholar]
- 19.Schneider H, Ludwig M, Hossain HM, Diemer T, Weidner W. The 2001 Giessen Cohort Study on patients with prostatitis syndrome: an evaluation of inflammatory status and search for microorganisms 10 years after a first analysis. Andrologia. 2003;35:258–62. [PubMed] [Google Scholar]
- 20.Bjerklund Johansen TE, Gruneberg RN, Guibert J, Hofstetter A, Lobel B, Naber KG, et al. The role of antibiotics in the treatment of chronic prostatitis: a consensus statement. Eur Urol. 1998;34:457–66. doi: 10.1159/000019784. [DOI] [PubMed] [Google Scholar]
- 21.Grabe M, Bishop M, Bjerklund-Johansen TE, Botto H, Cek M, Lobel B, et al. Guidelines on urological infections. Arnhem: European Association of Urology. 2010:110. [Google Scholar]
- 22.Weidner W, Schiefer HG. Urethro-adnexitis des mannes und sexuell übertragbare erreger: ein erfahrungsbericht der giessener arbeitsgruppe. Der Urologe Ausgabe A. 1988;27:123–31. [PubMed] [Google Scholar]
- 23.Brunner H, Weidner W, Schiefer HG. Quantitative studies on the role of Ureaplasma urealyticum in non-gonococcal urethritis and chronic prostatitis. Yale J Biol Med. 1983;56:545–50. [PMC free article] [PubMed] [Google Scholar]
- 24.Krieger JN, Riley DE, Roberts MC, Berger RE. Prokaryotic DNA sequences in patients with chronic idiopathic prostatitis. J Clin Microbiol. 1996;34:3120–8. doi: 10.1128/jcm.34.12.3120-3128.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dojka MA, Hugenholtz P, Haack SK, Pace NR. Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl Environ Microbiol. 1998;64:3869–77. doi: 10.1128/aem.64.10.3869-3877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]