Abstract
Background and Aims
Studies of the prognostic value of Ishak fibrosis stage are lacking. We utilized multi-year follow-up of the Hepatitis C Antiviral Long Term Treatment Against Cirrhosis (HALT-C) Trial to determine whether individual Ishak fibrosis stages predicted clinical outcomes in patients with chronic hepatitis C.
Methods
Baseline liver biopsies from 1,050 patients with compensated chronic hepatitis C who had failed combination peginterferon and ribavirin were reviewed by a panel of expert hepatopathologists. Fibrosis was staged with the Ishak scale (ranging from 0=no fibrosis to 6=cirrhosis). Biopsy fragmentation and length as well as number of portal tracts were recorded. We compared rates of pre-specified clinical outcomes of hepatic decompensation and hepatocellular carcinoma across individual Ishak fibrosis stages.
Results
Of 1,050 biopsies 25% were fragmented, 63% >1.5 cm, 69% >10 mm2, and 75% had ≥10 portal tracts. Baseline laboratory markers of liver disease severity were worse and the frequency of esophageal varices higher with increasing Ishak stage (p<.0001). The six-year cumulative incidence of first clinical outcome was 5.6% for stage 2, 16.1% for stage 3, 19.3% for stage 4, 37.8% for stage 5, and 49.3% for stage 6. Among non-fragmented biopsies, the predictive ability of Ishak staging was enhanced; however, no association was observed between Ishak stage and outcomes for fragmented biopsies due to high rates of outcomes for patients with non-cirrhotic stages. Similar results were observed with liver transplantation or liver-related death as the outcome.
Conclusions
Ishak fibrosis stage predicts clinical outcomes, need for liver transplantation, and liver-related death in patients with chronic hepatitis C. Patients with fragmented biopsies with low Ishak stage may be understaged histologically
Keywords: fibrosis, cirrhosis, hepatitis C, liver biopsy, fragmentation
Liver biopsy remains the accepted standard for histologic assessment of liver disease activity and fibrosis, despite such limitations as sampling variability, potential complications of an invasive technique, and subjective scoring. Studies of the natural and treatment-modified histologic progression of liver injury and fibrosis have relied upon the adoption of uniform grading and staging criteria.(1) With the availability of semi-quantitative measurements, investigators have been able to deduce relative rates of histologic progression and to go well beyond the qualitative information available from liver biopsies. Although several histologic scoring systems are used widely in clinical research, prospective studies of the prognostic value of staging of fibrosis— i.e., of the correlation between the fibrosis stage and subsequent outcomes or complications of liver disease—are rare.(2) Among patients with established cirrhosis, clinical complications of chronic liver disease, such as ascites, variceal bleeding, hepatic encephalopathy, coagulopathy, and renal/electrolyte disorders, emerge progressively over time; however, it has not been shown whether the sequential stages of fibrosis (short of cirrhosis) identified by these grading systems predict a higher likelihood of the clinical consequences of chronic liver disease.
Of the scoring systems described in the literature, the most commonly used in the United States are the Knodell histologic activity index (0–4), Batts-Ludwig stage (0–4) and Scheuer (0–4) (3–5) and the METAVIR scheme (0–4) in Europe.(6) The Ishak staging system, which has not enjoyed the general popularity of the others, is a modification of the Knodell system (which lacks a stage 2) and has six stages of fibrosis (0–6).(7) In recent years, the Ishak staging system has become widely used in clinical trials, especially in the United States. Because each Ishak fibrosis stage reflects more scarring than the preceding stage, clinicians and investigators assume that succession from one stage to the next represents progressively more advanced liver disease.
Thus, patients with a higher stage should have an increasing risk of clinical outcomes if each score compared to its lower counterpart represents a significant difference in disease severity. However, the link between fibrosis stages and clinical outcomes has not been validated in a prospective trial.
The HALT-C Trial provided an opportunity to follow prospectively more than 1,000 patients for up to 6 years and to assess the association of Ishak fibrosis stage and clinical outcomes. Moreover, because no difference in clinical outcomes occurred between the maintenance peginterferon-treated and untreated-control arms, the two arms could be combined, doubling the number of subjects for analysis. The demonstration of a stepwise increase in the frequency of clinical outcomes with each increase in histologic fibrosis stage among pre-cirrhotic and cirrhotic patients would help establish the validity of multiple fibrosis staging levels as reflected in the Ishak scoring system.
METHODS
The HALT-C Trial study design and main results have been described in detail.(8, 9) Briefly, 1,050 patients with compensated liver disease related to hepatitis C-related (that is Child- Turcotte-Pugh [CTP] score of 5 or 6), who had not had a sustained virological response to an adequate course of peginterferon and ribavirin were randomized to receive maintenance therapy with peginterferon alfa-2a in a dose of 90 µ g per week or no treatment for 3 and a half years. Written informed consent was obtained from each patient, and the protocol had a priori approval by the institutional review committee of each participating center. All patients had local histological interpretation of protocol biopsies by individual study pathologists followed by central reading (see below). Fibrosis was staged according to the Ishak fibrosis scale of 0 to 6.(1, 7, 10) In this system, a score of 2 is defined as fibrous expansion of most portal areas, with or without short fibrous septa; 3 fibrous expansion of most portal areas with occasional portal-to-portal bridging; 4 fibrous expansion of most portal areas with marked bridging (both portal-to-portal and portal-to-central); 5 incomplete cirrhosis characterized by marked bridging and occasional nodules; and 6 probable or definite cirrhosis. To enter the trial, a study participant had to have had a current or a past liver biopsy interpreted by the local clinical-site study pathologist as demonstrating an Ishak fibrosis score of 3 or greater. A subsequent central reassessment of the biopsy through a consensus evaluation at a multiheaded microscope by the pathology reading group (composed of pathologists from the individual centers) could result in a change of the stage. By the conclusion of the central reading process, 79 baseline biopsies were scored as stage 2; 53 of these biopsies had been scored as 3 or greater when interpreted at the local site, and 26 were from patients whose current biopsies were staged locally as 2 but who had had an earlier biopsy scored as stage 3 or greater. The central, not the local, reading has been used to establish the fibrosis stage for all HALT-C Trial manuscripts, including this one. According to the HALT-C Trial protocol, the biopsy required for study entry and stratification into the bridging fibrosis group versus the cirrhosis group could have been performed as long as 12 months prior to the baseline clinical visit and as long as 2 years prior to randomization for a few patients who had virological relapse after a full course of therapy.(8)
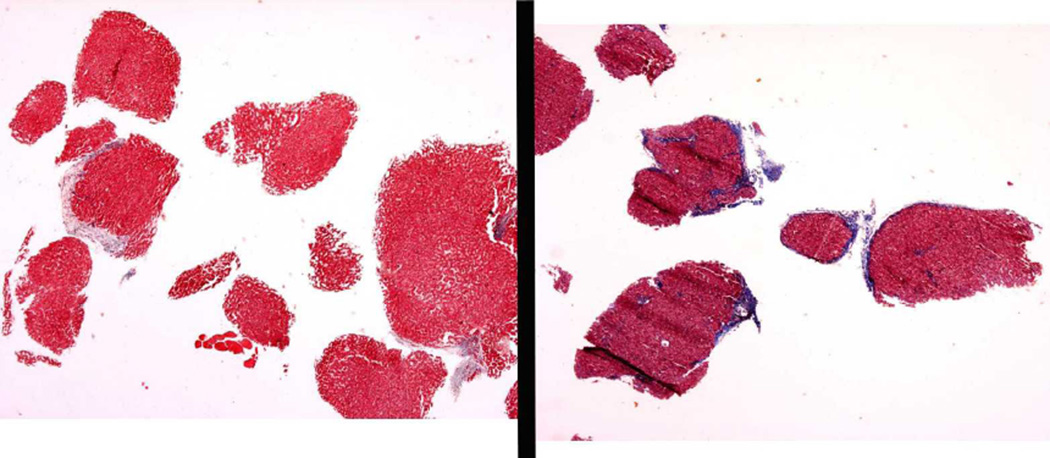
Due to the difficulty in interpreting the severity of fibrosis correctly from an inadequate biopsy, we also examined characteristics of biopsy quality. Biopsy length was determined at the time of central reading. For two ancillary studies, total biopsy area was measured on 1,004 baseline biopsies and number of portal tracts recorded on 1,002 biopsies. Biopsy fragmentation was determined by a single study pathologist, who judged as fragmented multiple small pieces of tissue with rounded contours, usually not more than 2 mm in size. When two biopsy passes had been made with one fragmented and the other not fragmented (of reasonable size), the biopsy was categorized as non-fragmented. Examples of fragmented biopsies are shown in Figure 1.
Figure 1.
Examples of fragmented biopsies. Specimen on left, which only has fibrosis focally along the edge of one fragment, was interpreted as Ishak stage 3. Specimen on right has fibrosis enveloping several fragments and was interpreted as Ishak stage 6.
Following the conclusion of the randomized phase, HALT-C participants continued scheduled semi-annual visits that included ascertainment of study outcomes. For this analysis, the first liver-related clinical outcome within 6 years of randomization was used as the primary endpoint. About 80% if the original cohort who had not had a primary outcome continued to be seen through 6 years. Because treatment had no impact on clinical outcomes,(9) we grouped treated and untreated-control subjects together. The predefined primary clinical outcomes included an increase in CTP score to at least 7 on two successive study visits at least 3 months apart, ascites, encephalopathy, bleeding esophageal or gastric varices, hepatocellular carcinoma, or death. For these analyses, however, we excluded deaths that were considered to be unrelated to liver disease. Secondary outcomes included liver transplantation and liver-related death.
Statistical methods
We compared rates of predefined liver-related clinical outcomes across Ishak fibrosis stages. Cumulative incidence over time of outcomes were calculated with Kaplan-Meier survival estimates and comparison of outcomes by fibrosis stage with the Cox proportional hazards model. Results are reported as hazard ratios (HR) and 95% confidence intervals. Stage 2 and 3 cases were not compared by Cox analysis because of the small number of events for patients with stage 2 and because the model assumption of a constant HR across time could not be met.
Analysis of variance, logistic regression, and the chi-square test for trend were used to assess the relationship between biopsy characteristics, demographic and laboratory variables. SAS version 9.1 was used for all analyses (SAS Institute, Cary, NC), and a p value of < 0.05 was considered to be statistically significant. No adjustments were made for multiple comparisons.
RESULTS
A total of 1,050 patients were eligible to be followed for clinical outcomes. The mean (± standard deviation) time from biopsy to randomization was 269 ± 177 days, uniform across all Ishak fibrosis stages, except for Ishak stage 4, which was longer at 303 ± 175 days (Table 1). Similar proportions of patients were randomized to treatment across Ishak scores (p=0.55). Several characteristics of the biopsies were examined in addition to Ishak stage. Sixty-three percent of biopsies were at least 1.5 cm long, 69% had an area of at least 10 mm2, and 75% contained at least 10 portal triads; none of these biopsy characteristics varied substantially across Ishak score (test for trend, p >0.10). Among the biopsy characteristics, fragmentation was associated most strongly with Ishak stage (p=0.0002), occurring more commonly among patients with cirrhosis.
Table 1.
Patient and biopsy characteristics according to Ishak fibrosis stage; and laboratory information at enrollment and presence of esophageal varices according to Ishak fibrosis stage and fragmentation.*
| Ishak Stage | |||||||
|---|---|---|---|---|---|---|---|
| All | 2 | 3 | 4 | 5 | 6 | ||
| Number of randomized patients |
1,050 | 79 | 355 | 188 | 231 | 197 | p-value for trend |
| Mean days from baseline biopsy to randomization |
269 (177) |
277 (192) |
273 (187) |
303 (175) |
245 (161) |
253 (166) |
0.05 |
| Randomized to treatment |
49.2% | 51.9% | 48.7% | 50.5% | 51.1% | 45.7% | 0.55 |
| Biopsy characteristics | |||||||
| Length ≥1.5 cm | 62.9% | 60.8% | 67.6% | 59.6% | 62.8% | 58.4% | 0.11 |
| Area ≥10 (n=1004) | 69.0% | 70.5% | 70.5% | 66.1% | 74.8% | 61.5% | 0.23 |
| Portal triads ≥10 (n=1,002) |
74.7% | 65.3% | 71.5% | 84.2% | 76.8% | 72.6% | 0.21 |
| Fragmented (%) | 23.5% | 8.9% | 22.5% | 17.6% | 29.9% | 29.4% | 0.0002 |
| Laboratory Information |
|||||||
| Days from biopsy to laboratory testing |
94 (137) |
108 (131) |
90 (126) |
113 (153) |
78 (139) |
95 (140) |
0.47 |
| All biopsies | |||||||
| Platelet count (103/mm3) |
165 (66) |
221 (59) |
187 (62) |
169 (59) |
143 (60) |
125 (51) |
<0.0001 |
| AST/ALT ratio | 0.88 (0.29) |
0.78 (0.26) |
0.84 (0.28) |
0.87 (0.28) |
0.90 (0.29) |
0.98 (0.32) |
<0.0001 |
| Total Bilirubin (mg/dl) |
0.79 (0.40) |
0.68 (0.29) |
0.71 (0.35) |
0.78 (0.37) |
0.85 (0.41) |
0.92 (0.49) |
<0.0001 |
| Albumin (g/dl) | 3.87 (0.39) |
4.02 (0.31) |
3.94 (0.35) |
3.93 (0.35) |
3.81 (0.40) |
3.69 (0.46) |
<0.0001 |
| INR | 1.04 (0.11) |
0.99 (0.08) |
1.02 (0.12) |
1.02 (0.10) |
1.07 (0.10) |
1.08 (0.11) |
<0.0001 |
| Esophageal varices (%) (n=1016) |
25.7 | 10.5 | 13.5 | 23.1 | 34.5 | 45.3 | <0.0001 |
| Non-fragmented Biopsies | |||||||
| Platelet count** (103/mm3) |
171 (67) |
225 (59) |
196 (60) |
172 (59) |
148 (64) |
121 (49) |
<0.0001 |
| AST/ALT ratio** | 0.87 (0.30) |
0.78 (0.26) |
0.82 (0.27) |
0.86 (0.29) |
0.88 (0.29) |
1.02 (0.34) |
<0.0001 |
| Total Bilirubin (mg/dl)** |
0.77 (0.38) |
0.67 (0.29) |
0.68 (0.34) |
0.77 (0.36) |
0.82 (0.36) |
0.94 (0.46) |
<0.0001 |
| Albumin (g/dl) | 3.89 (0.38) |
4.03 (0.31) |
3.96 (0.32) |
3.94 (0.33) |
3.84 (0.38) |
3.68 (0.47) |
<0.0001 |
| INR | 1.03 (0.11) |
0.99 (0.08) |
1.01 (0.11) |
1.02 (0.10) |
1.07 (0.10) |
1.09 (0.11) |
<0.0001 |
| Esophageal varices (%) |
24.0 | 11.4 | 10.3 | 22.5 | 34.2 | 47.1 | <0.0001 |
| Fragmented Biopsies | |||||||
| Platelet count (103/mm3) |
144 (57) |
176 (47) |
157 (61) |
155 (61) |
131 (50) |
133 (54) |
0.0006 |
| AST/ALT ratio | 0.90 (0.28) |
0.72 (0.27) |
0.88 (0.30) |
0.88 (0.25) |
0.95 (0.29) |
0.91 (0.24) |
0.10 |
| Total Bilirubin (mg/dl) |
0.85 (0.46) |
0.72 (0.27) |
0.80 (0.38) |
0.81 (0.41) |
0.93 (0.50) |
0.85 (0.56) |
0.21 |
| Albumin (g/dl) | 3.82 (0.43) |
4.01 (0.35) |
3.89 (0.42) |
3.87 (0.40) |
3.76 (0.45) |
3.74 (0.44) |
0.007 |
| INR | 1.06 (0.12) |
1.00 (0.08) |
1.05 (0.15) |
1.05 (0.10) |
1.08 (0.10) |
1.07 (0.11) |
0.08 |
| Esophageal varices (%) |
31.1 | 0.0 | 24.4 | 27.3 | 35.3 | 41.1 | 0.009 |
Mean (SD) or %
Mean ± SD time from biopsy to enrollment laboratory testing was 94 ± 137 days and did not differ by Ishak fibrosis stage (test for trend, p-value =0.47) (Table 1). Laboratory markers that reflect severity of liver disease (lower platelet count and albumin; higher AST/ALT ratio, bilirubin, and INR ) and the frequency of esophageal varices were increased proportionately to increased Ishak stage (p<0.0001 for all these variables). These trends were examined further according to whether or not the biopsy was fragmented. Patients with fragmented biopsies had lower platelet counts than those with non-fragmented biopsies (p<0.0001). In addition, the decline in platelet count with increasing Ishak stage was steeper among patients with non-fragmented biopsies than among patients with fragmented biopsies (test for interaction, p<0.0001). AST/ALT ratio (p=0.06), total bilirubin (p=0.02), and INR (p=0.001) were higher, and albumin (p=0.17) was slightly lower for the fragmented than non-fragmented biopsies, but the interaction with Ishak score was not statistically significant for AST/ALT ratio (p=0.09), bilirubin (p=0.05), and albumin (p=0.32), yet was for INR (p=0.01). The prevalence of esophageal varices was higher for patients with fragmented (31.1%) than non-fragmented biopsies (24.0%) (p=0.001). As was the case for platelets and INR, the prevalence of varices was higher in patients with fragmented than with non-fragmented biopsies across Ishak stages, especially for Ishak stages 2 and 3 (test for interaction p-value = 0.02).
Prognostic significance of Ishak fibrosis score on primary outcomes
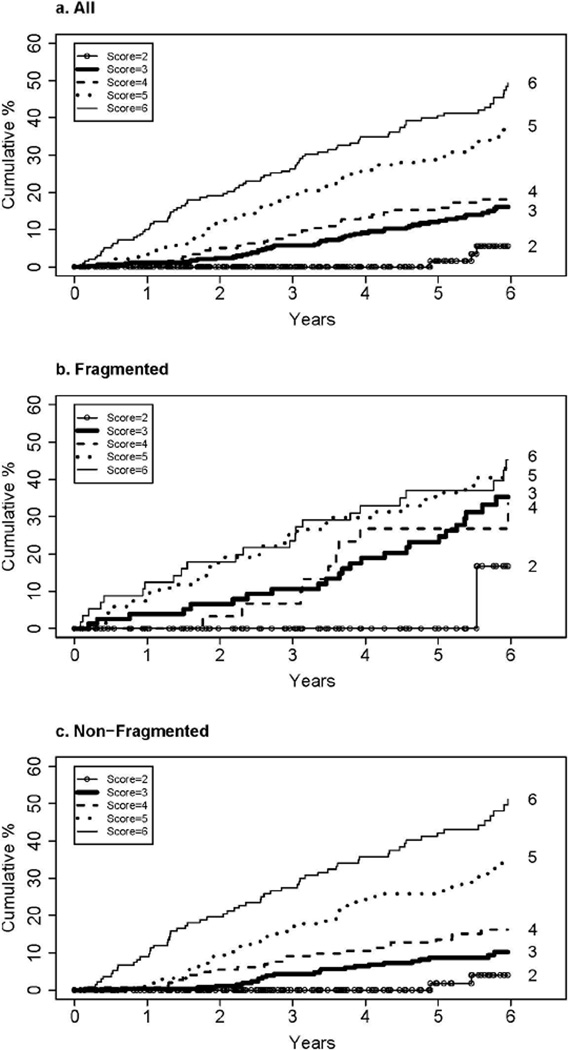
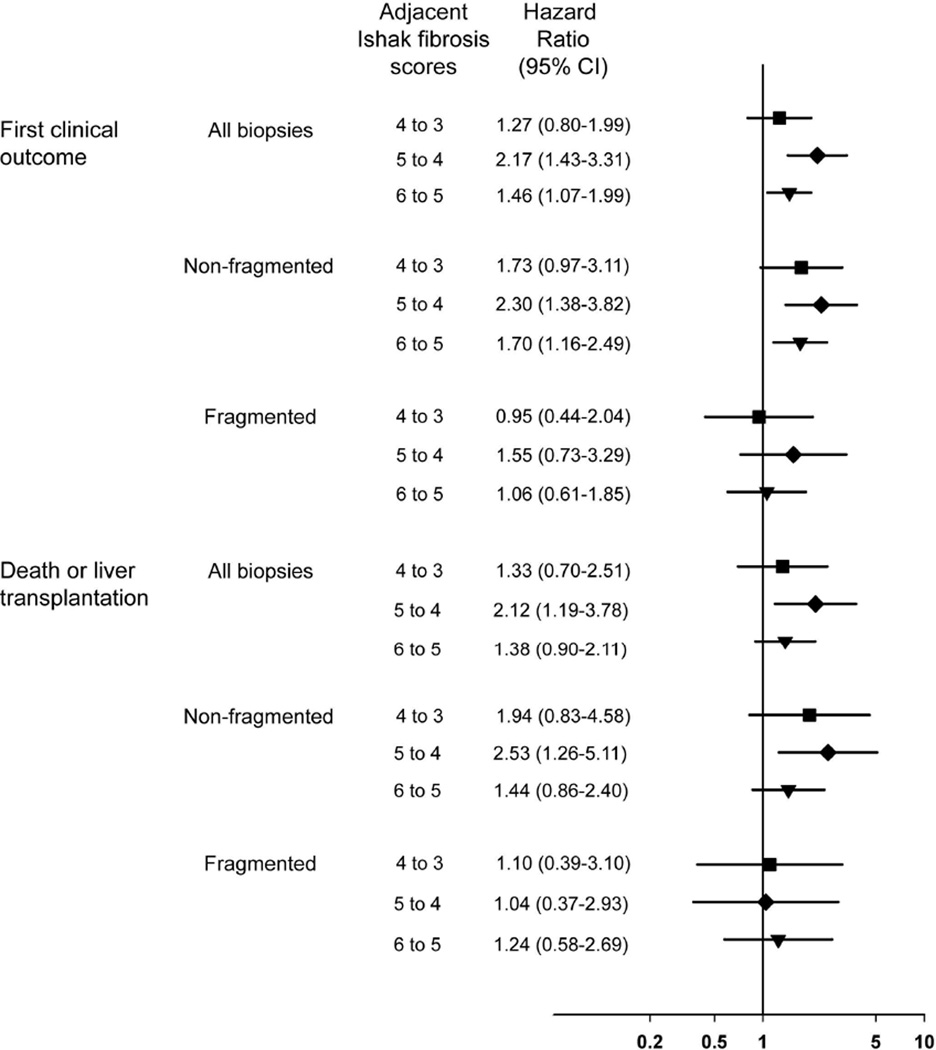
The cumulative 6-year incidence of a first clinical outcome was 27.0% for all patients and ranged from 5.6% for Ishak stage 2 to 49.3% for Ishak stage 6 (Table 2 and Figure 2a). For patients with an Ishak stage of 6, clinical events began to occur shortly after randomization and rose by about 8% per year (Figure 2a). A progressive lag in events was observed for the patients with lower stage disease, most pronounced for patients with an Ishak fibrosis stage of 2, none of whom had an event until nearly 5 years after randomization (and only three after that). When the rate of clinical outcomes was compared for each Ishak stage with the rate for the next lower stage, the outcome rate was statistically significantly higher for Ishak 6 compared with Ishak 5, for Ishak 5 compared with Ishak 4, but not for Ishak 4 compared with Ishak 3 (top of Figure 3). Treatment assignment had no effect on the results.
Table 2.
Cumulative 6-year incidence (and hazard ratios for fragmentation) of first clinical outcome by baseline Ishak fibrosis stage according to biopsy characteristic and treatment assignment.
| Ishak Fibrosis Stage | ||||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | ||
| Cumulative Incidence of 1st outcome (N with outcomes) | p-value for trend |
|||||
| All biopsies | 5.6% (3) | 16.1% (47) | 19.3% (31) | 37.8% (74) | 49.3% (83) | <0.0001 |
| Fragmentation | ||||||
| Non-fragmented (NF) |
4.0% | 10.2% | 16.2% | 35.6% | 51.1% | <0.0001 |
| Fragmented (F) | 16.7% | 35.3% | 33.5% | 43.0% | 45.2% | 0.05 |
| F vs NF: HR* (95% CI) & p- value |
-- |
3.9 (2.2–6.8) <0.0001 |
2.1 (0.95–4.5) 0.07 |
1.4 (0.9–2.2) 0.18 |
0.9 (0.5–1.4) 0.52 |
-- |
| Time randomized from biopsy |
Cumulative Incidence of 1st outcome | |||||
| < 200 days | 7.2% | 22.2% | 13.5% | 41.4% | 49.9% | <0.0001 |
| ≥200 days | 4.2% | 9.9% | 24.1% | 33.4% | 48.4% | <0.0001 |
| Randomized | ||||||
| Treatment | 3.1% | 19.2% | 17.8% | 32.8% | 50.2% | <0.0001 |
| Control | 8.2% | 13.0% | 20.5% | 43.1% | 48.6% | <0.0001 |
| Biopsy Length | ||||||
| ≥1.5 cm | 3.5% | 13.7% | 15.4% | 27.5% | 50.9% | <0.0001 |
| <1.5 cm | 8.6% | 21.3% | 24.5% | 53.5% | 47.0% | <0.0001 |
| Biopsy Area | ||||||
| Area ≥10 mm2 | 3.1% | 15.8% | 18.5% | 31.1% | 49.1% | <0.0001 |
| Area < 10 mm2 | 11.9% | 15.4% | 22.1% | 43.6% | 48.8% | <0.0001 |
| Portal triads | ||||||
| ≥10 | 2.9% | 15.3% | 18.8% | 34.1% | 47.0% | <0.0001 |
| <10 | 11.1% | 17.3% | 23.8% | 48.8% | 59.6% | <0.0001 |
Hazard ratio. Ishak 2 not compared because of too few outcomes.
Figure 2.
Cumulative, 6-year incidence of first clinical liver disease outcome according to baseline Ishak fibrosis stage. a) all (non-fragmented and fragmented) biopsies, b) non-fragmented biopsies and c) fragmented biopsies.
Figure 3.
Hazard rate ratios and 95% confidence intervals for clinical outcomes over 6 years for Ishak fibrosis stage relative to the next lower stage.
Effect of fragmentation on first primary outcome
The same analysis was performed according to whether or not the biopsy was fragmented. The cumulative, 6-year incidence of clinical liver-disease outcomes according to baseline Ishak fibrosis stage and fragmentation is shown in Figure 2b (non-fragmented) and 2c (fragmented). Overall, patients with fragmented biopsies were more likely to have clinical outcomes than patients with non-fragmented biopsies (HR = 1.9; 95% CI = 1.4–2.4); however, this observed difference in clinical outcomes was confined to patients with Ishak fibrosis stages 3 and 4. Thus, at Ishak stages less than 5, fragmentation was associated with increased rates of outcomes, a finding not seen in patients with cirrhosis, stages 5 and 6 (test for interaction, p<0.0001). In the comparison of rates of outcomes between one Ishak stage and the next lower stage, the hazard ratios were statistically significant for patients with non-fragmented biopsies (Figure 3) (test for trend p<0.0001). Specifically, when we compared the rate of clinical outcomes for each Ishak fibrosis stage with the rate for the next lower stage, we found that the outcome hazard ratio for each comparison was higher for patients with non-fragmented biopsies than with fragmented biopsies (Figure 3). If the biopsy was fragmented, only a borderline association of Ishak stage with outcomes was observed (test for trend, p=0.05). This association was a result of a modest prognostic value (HR = 1.6; 95% CI= 1.004–2.4, p=0.05) for the presence of cirrhosis (Ishak stage 5 or 6) relative to the presence of less severe fibrosis (Ishak stage 4 or less)
Other markers of biopsy quality
Other markers of biopsy quality did not have an appreciable influence on the results. Thus, in general, patients with longer biopsies, larger biopsy areas, or more numerous portal tracts did not have different rates of outcomes than patients with lower quality biopsies (Table 2). The only exception was biopsy length among patients with an Ishak fibrosis stage 5; in this single subset, patients with biopsy length of <1.5 cm had cumulative outcomes of 53.5% versus a cumulative incidence of 28.5% among patients with longer biopsies (p=0.0003). Unlike fragmentation, these measures of biopsy quality did not interact significantly with Ishak stage in relation to outcomes.
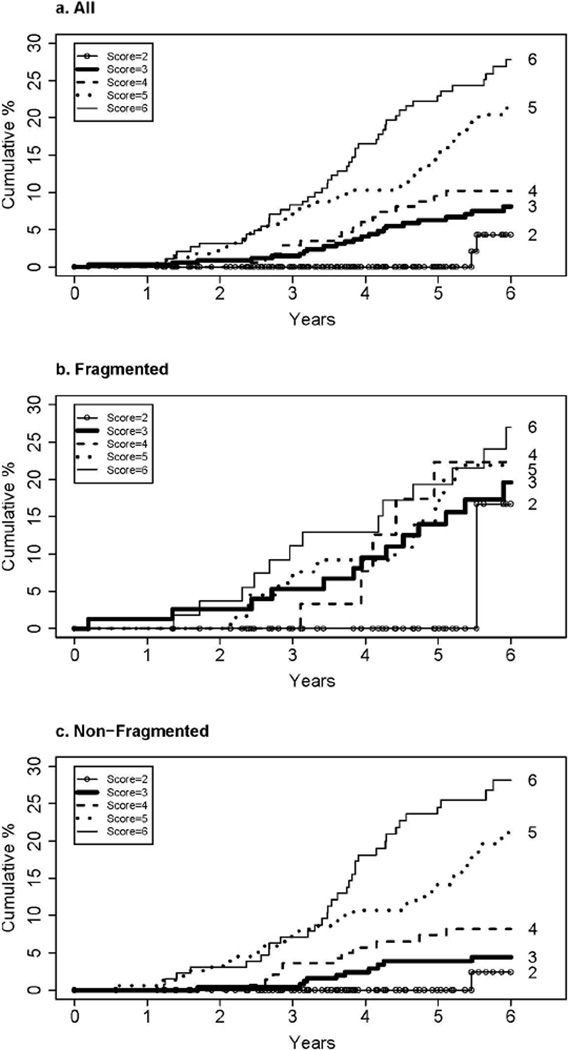
Prognostic significance of Ishak fibrosis stage for liver transplantation or liver-related death
Nearly half of all first clinical events (48.3%) were increases in CTP score to 7 or more, primarily achieved through deterioration in laboratory components of the score (albumin, bilirubin and prothrombin time/INR). To assess the impact of Ishak fibrosis stage on secondary clinical outcomes of liver transplantation and liver-related deaths, the same analyses were performed as described above for primary clinical outcomes. Sixty-eight patients underwent liver transplantation, and another 58 patients died from liver disease without liver transplantation (half from end-stage liver disease and half from hepatocellular carcinoma). The cumulative 6- year incidence of these outcomes was 14.3%. As was the case for primary clinical outcomes, there was a strong association between successive Ishak fibrosis stages and liver-related death and liver transplantation (Figure 4a and Table 3). In individual comparisons between fibrosis stages, there was a statistically significant difference in secondary clinical outcomes between Ishak 4 (marked bridging but no nodules) and 5 (bridging with occasional nodules), but not for 3 (occasional bridging) versus 4 or 5 versus 6 (cirrhosis) (Figure 3, lower half). As with the primary outcomes, there was an effect of fragmentation on the association between Ishak stage and outcome (Figure 4b and 4c) (test for interaction, p=0.0005). This effect was most striking with Ishak stage 3; for this subgroup, if the biopsy was fragmented, the frequency of liver-related death or liver transplantation was nearly as high as that for patients with stage 6.
Figure 4.
Cumulative incidence of transplantation or liver related death according to baseline Ishak fibrosis stage. a) all (non-fragmented and fragmented) biopsies, b) non-fragmented
Table 3.
Cumulative 6-year incidence and hazard ratios of liver transplantation or liver-related death according to Ishak fibrosis stage and biopsy fragmentation
| Ishak Stage | ||||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | ||
| Cumulative Incidence of transplant or death (N with outcomes) | p-value for trend |
|||||
| All biopsies | 4.3% (2) | 8.1 % (23) | 10.2 % (16) | 21.3 % (40) | 27.8% (45) | <0.0001 |
| Non- fragmented (NF) |
2.4% | 4.4% | 8.2% | 21.1% | 28.1% | <0.0001 |
| Fragmented (F) |
16.7% | 20.0% | 22.3% | 21.9% | 27.0% | 0.31 |
| F vs NF: HR* (95% CI) & p- value |
-- | 4.4 (1.9–10.1) 0.0004 |
2.4 (0.8–7.0) 0.10 |
1.1 (0.6–2.1) 0.83 |
0.9 (0.5–1.7) 0.79 |
-- |
Hazard ratio. Ishak 2 not compared because too few outcomes.
Complexities of the HALT-C Trial design that could have affected the results
Two design issues of the HALT-C Trial that could have affected the results were examined: 1) the difference in time between the initial biopsy and the beginning of observation for clinical outcomes and 2) randomization assignment to treatment or control. From the date of the initial baseline biopsy, the time when observation for clinical outcomes began varied widely, ranging from 10 to 918 days. Because only patients who did not have an outcome between the baseline biopsy and randomization could be followed for clinical outcomes, we could not determine directly whether the variable delay between biopsy and randomization could have affected the results, and patients with cirrhosis did have a trend to shorter biopsy-to-randomization interval (test for trend, p=0.05, Table 1). When we compared outcomes for patients whose randomization date was either > or ≤200 days (close to the median of 198 days), the trend to more frequent outcomes with increasing Ishak stage was not affected by the time to randomization (Table 2). Overall, when we controlled for Ishak stage, patients with longer times between the baseline biopsy and randomization had a statistically insignificant lower rate of outcomes (HR 0.79, 95% CI 0.61–1.03, p=0.08) than patients with shorter biopsy-to-randomization times. This observation was most apparent for patients with Ishak 3 stage (Table 2); however, in light of the fact that the rate of clinical outcomes within a year of randomization for Ishak stage 3 was only 1.7%, the delay prior to randomization was unlikely to have substantially affected the results. Most important, no marked evidence was apparent for an interaction of time to randomization with Ishak stage on outcomes (p=0.08). Similarly, because randomization was based on fibrosis stratum (precirrhotic fibrosis group versus cirrhosis group), no statistically significant difference in Ishak stages was observed between treated and untreated patients (Table 1, p=0.55), and treatment had no effect on outcomes (p=0.75). Thus treatment did not appear to influence the relationship between Ishak fibrosis stage and outcomes (test for interaction, p=0.53).
DISCUSSION
In designing this study, we pursued two principal questions: 1) do the individual Ishak fibrosis stages have prognostic value, and 2) does consideration of the presence of liver-biopsy tissue fragmentation enhance the staging of bridging fibrosis? Our analysis indicates that the answer to both questions is yes.
Numerical scoring systems for evaluating liver biopsies came into widespread use in clinical trials in the 1990s as new treatments for hepatitis B and C were developed. The four systems used most often are the Knodell score,(3) which has only three stages of fibrosis (portal, bridging, and cirrhosis), the Batts-Ludwig and Scheuer(4, 5) with four stages (portal, periportal, septal with nodularity and cirrhosis) and METAVIR,(6) with four stages (portal, few septa, numerous septa, and cirrhosis). The Ishak system,(7) with six stages of fibrosis, has been used less often, but, because of its finer distinctions of fibrosis and architectural remodeling, we chose the it as the primary system for the HALT-C Trial. Therefore, the current analysis addressed the issue of whether, in fact, the Ishak fibrosis staging system provided clinically useful prognostic information.
More than 100 papers have been published on the natural history of and prognostic factors in patients with cirrhosis resulting from hepatitis C and other liver diseases.(11) Only a few studies have incorporated numerical scoring systems to assess fibrosis progression in repeated liver biopsies to estimate rates of fibrosis progression to cirrhosis in hepatitis C.(11–17) To our knowledge, however, in only one study have investigators examined the utility of a contemporary fibrosis scoring system (the Ishak system) along with other clinical and laboratory factors in predicting clinical outcomes in patients with chronic hepatitis C.(18) In that single-region study, 131 clinically compensated patients with advanced fibrosis, defined as Ishak stages 4 (marked bridging) to 6 (cirrhosis), were followed for a median of 51 months, during which 25% died or underwent liver transplantation. The authors did not find an association of Ishak stage with prognosis, but the sample was much smaller than that of the HALT-C Trial (for example, only 26 patients had Ishak fibrosis stage 4), and deaths were not restricted to those related to liver disease. In contrast, in the present study of 1,050 patients who were followed clinically for up to 6 years, we found statistically significant differences between successive Ishak stages 4 through 6 for the likelihood of developing clinical outcomes. Overall, when all study patients were assessed, we observed no significant difference in clinical outcomes between patients with stages 3 (occasional bridging) and 4 (marked bridging); however, in patients with baseline biopsies interpreted as stage 3, those with fragmented biopsies had both indicators of poorer liver function and portal hypertension and much higher rates of outcomes than patients with non-fragmented stage 3 biopsies. A reasonable inference is that many of the fragmented stage 3 biopsies were understaged and actually cirrhotic. Excluding fragmented biopsies from the analysis, we found that the difference in outcomes between stages 3 and 4 was much closer to achieving statistical significance (Figure 3). These findings reflect the difficulties for pathologists in interpretation of biopsies that are not intact cores. The pathologist cannot assume that fragmentation equals cirrhosis; rather, he or she evaluates the amount of collagen present as well as other subtle features of cirrhotic remodeling. In very small and fragmented biopsies, there may be very little of either.
A confident histologic diagnosis of cirrhosis requires remodeled vascular architecture as assessed by the observation of nodules of hepatocytes surrounded by fibrous tissue.(19) In the absence of this direct criterion, fragmentation of a biopsy specimen obtained by suction biopsy technique has been recognized to provide strong suggestive evidence that the patient may have underlying cirrhosis;(20) therefore, especially in this setting, correlation with clinical and laboratory findings can help establish a clinical-pathologic diagnosis of advanced liver disease with probable cirrhosis.(19, 21, 22) To prevent bias in clinical trials, however, histologic reviews must be blinded to clinical information and laboratory findings and different biopsy techniques from various centers cannot be avoided. Accordingly, even though a fragmented biopsy is suspicious for cirrhosis, unless the specimen contains at least some fragments surrounded by a thin rim of fibrous tissue, the patient’s fibrosis stage will be underscored. The fact that specimen fragmentation introduces a systematic source of error in liver biopsy interpretation emphasizes the need for use of standardized biopsy techniques in clinical trials to avoid this problem. For studies of fibrosis, liver biopsies obtained with cutting biopsy needles have been shown to be superior to those obtained with suction needles and are more likely to yield the correct diagnosis of cirrhosis.(23–25)
The current findings have an important implication for the design of clinical trials of new forms of therapy for hepatic fibrosis and remodeling. The six-stage Ishak system distinguishes between early or incomplete cirrhosis and established or advanced cirrhosis, but the other histologic scoring systems do not. Our results show that the difference between early or incomplete (stage 5) cirrhosis and established (stage 6) cirrhosis is clinically significant; therefore, in trials of antifibrotic therapy, the Ishak system can be considered superior to other systems that do not include this distinction.
The implications of our findings extend to clinical practice. When reporting the results of liver biopsies obtained from patients with chronic hepatitis C, many pathologists provide histologic stage on a limited scale of only 0 to 4; biopsies consistent with cirrhosis, whether early or advanced, are categorized as being stage 4 in the scoring systems in most common use. In clinical practice, whether fibrosis is staged on a limited 4-point or more finely discriminative 6- point scale, a more meaningful and precise pathology report should include an accompanying narrative that states whether the cirrhosis is early or incomplete or established, which are more precise distinctions that carry prognostic implications for the patient. In such clinical settings, the narrative is superior to and conveys more meaningful information than a simple numerical stage.
Although our data allow us to conclude that, in the absence of tissue fragmentation, Ishak fibrosis stages of 3, 4, 5 and 6 represent clinically progressive liver disease, a limitation of this study is the small number of patients with Ishak stages of 2 or less. In the HALT-C Trial, only patients with advanced hepatic fibrosis were enrolled in order to include a population in which clinical outcomes could be anticipated during the period of monitoring; the rate of clinical progression in patients with milder degrees of fibrosis would have been too low for this assessment. Therefore, studies of serial biopsies over time in patients with mild fibrosis will be necessary to determine if prognostic information can be derived from early as well as from late Ishak fibrosis stages. Nevertheless, in the HALT-C trial, patients with Ishak fibrosis stage 2 had no clinical outcomes until nearly 5 years after randomization (Figure 2) and closer to 6 years after the baseline biopsy.
Thus in patients with advanced hepatic fibrosis, the Ishak fibrosis stage provides prognostically meaningful distinctions between and among stages in technically adequate, non-fragmented biopsy specimens. Attempts to optimize specimen quality and to avoid tissue fragmentation will improve the accuracy and value of fibrosis scoring. Alternatively, if fragmentation has occurred and is accompanied by pertinent clinical and laboratory findings, then there is a high likelihood of cirrhosis (stage 6). Such patients need more accurate assessments of disease severity and appropriate follow-up surveillance for development of hepatic decompensation and hepatocellular carcinoma.
ACKNOWLEDGMENTS
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Researchand Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Adrian M. Di Bisceglie, MD, Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR- 01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, Cara C. Gooch
University of Colorado School of Medicine, Denver, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01) Gregory T. Everson, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR- 00827) Timothy R. Morgan, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) William M. Lee, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Karen L. Lindsay, MD, MMM, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Anna S. Lok, MD, Robert J. Fontana, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Mitchell L. Shiffman, MD, Richard K. Sterling, MD, Melissa J. Contos, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: T. Jake Liang, MD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Linda Massey, Margaret C. Bell, MS, MPH
Armed Forces Institute of Pathology, Washington, DC: Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia- Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
The authors appreciate the assistance of Neal Freedman, PhD of National Cancer Institute and Hae-Young Kim, MS, DrPH of New England Research Institutes for assistance in creating manuscript figures.
Abbreviations
- HALT-C
Hepatitis C Antiviral Long Term Treatment Against Cirrhosis
- HR
hazard ratio
Footnotes
Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows: J.C. Hoefs is on the speaker’s bureau; and H.L. Bonkovsky receives research support. Authors with no financial relationships related to this project are: J.E. Everhart, E.C. Wright, Z.D. Goodman, J.L. Dienstag, D.E. Kleiner, M.G. Ghany, A.S. Mills, S.R. Nash, S. Govindarajan, T.E. Rogers, J.K. Greenson, E.M. Brunt, C. Morishima, and H.J. Litman.
References
- 1.Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology. 2000 Jan;31(1):241–246. doi: 10.1002/hep.510310136. [DOI] [PubMed] [Google Scholar]
- 2.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009 Mar;49(3):1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 3.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981 Sep;1(5):431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 4.Batts KP. Acute and chronic hepatic allograft rejection: pathology and classification. Liver Transpl Surg. 1999 Jul;5(4) Suppl 1:S21–S29. doi: 10.1053/JTLS005s00021. [DOI] [PubMed] [Google Scholar]
- 5.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991 Nov;13(3):372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 6.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996 Aug;24(2):289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 7.Ishak K, Baptista A, Bianchi L, Callea F, De GJ, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995 Jun;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 8.Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, et al. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004 Oct;25(5) doi: 10.1016/j.cct.2004.08.003. 472- [DOI] [PubMed] [Google Scholar]
- 9.Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, et al. Prolonged therapy of advanced chronic hepatitis C with low-dose peginterferon. N Engl J Med. 2008 Oct;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol. 2007 Oct;47(4):598–607. doi: 10.1016/j.jhep.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 11.D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006 Jan;44(1):217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Lagging LM, Westin J, Svensson E, Aires N, Dhillon AP, Lindh M, et al. Progression of fibrosis in untreated patients with hepatitis C virus infection. Liver. 2002 Apr;22(2):136–144. doi: 10.1034/j.1600-0676.2002.01623.x. [DOI] [PubMed] [Google Scholar]
- 13.Zarski JP, Mc HJ, Bronowicki JP, Sturm N, Garcia-Kennedy R, Hodaj E, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol. 2003 Mar;38(3):307–314. doi: 10.1016/s0168-8278(02)00387-2. [DOI] [PubMed] [Google Scholar]
- 14.Ghany MG, Kleiner DE, Alter H, Doo E, Khokar F, Promrat K, et al. Progression of 4 fibrosis in chronic hepatitis C. Gastroenterology. 2003 Jan;124(1):97–104. doi: 10.1053/gast.2003.50018. [DOI] [PubMed] [Google Scholar]
- 15.Ryder SD, Irving WL, Jones DA, Neal KR, Underwood JC. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004 Mar;53(3):451–455. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson LE, Torbenson M, Astemborski J, Faruki H, Spoler C, Rai R, et al. Progression of liver fibrosis among injection drug users with chronic hepatitis C. Hepatology. 2006 Apr;43(4):788–795. doi: 10.1002/hep.21091. [DOI] [PubMed] [Google Scholar]
- 17.Levine RA, Sanderson SO, Ploutz-Snyder R, Murray F, Kay E, Hegarty J, et al. Assessment of fibrosis progression in untreated irish women with chronic hepatitis Ccontracted from immunoglobulin anti-D. Clin Gastroenterol Hepatol. 2006 Oct;4(10):1271–1277. doi: 10.1016/j.cgh.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Lawson A, Hagan S, Rye K, Taguri N, Ratib S, Zaitoun AM, et al. The natural history of hepatitis C with severe hepatic fibrosis. J Hepatol. 2007 Jul;47(1):37–45. doi: 10.1016/j.jhep.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Scheuer PJ, Lefkowitch JH. Cirrhosis. Liver biopsy Interpretation. 7th ed. Philadelphia: Elsevier Saunders; 2006. pp. 171–190. [Google Scholar]
- 20.Malik AH, Kumar KS, Malet PF, Jain R, Prasad P, Ostapowicz G. Correlation of percutaneous liver biopsy fragmentation with the degree of fibrosis. Aliment Pharmacol Ther. 2004 Mar 1;19(5):545–549. doi: 10.1111/j.1365-2036.2004.01882.x. [DOI] [PubMed] [Google Scholar]
- 21.Desmet VJ, Roskams T. Reversal of cirrhosis: evidence-based medicine? Gastroenterology. 2003 Aug;125(2):629–630. doi: 10.1016/s0016-5085(03)00973-9. [DOI] [PubMed] [Google Scholar]
- 22.Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J Hepatol. 2004 May;40(5):860–867. doi: 10.1016/j.jhep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Colombo M, Del NE, de FR, De FC, Festorazzi S, Ronchi G, et al. Ultrasound-assisted percutaneous liver biopsy: superiority of the Tru-Cut over the Menghini needle for diagnosis of cirrhosis. Gastroenterology. 1988 Aug;95(2):487–489. doi: 10.1016/0016-5085(88)90509-4. [DOI] [PubMed] [Google Scholar]
- 24.Sherman KE, Goodman ZD, Sullivan ST, Faris-Young S. Liver biopsy in cirrhotic patients. Am J Gastroenterol. 2007 Apr;102(4):789–793. doi: 10.1111/j.1572-0241.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- 25.De HA, Loredo ML, Martinez-Rios MA, Gil MR, Kuri J, Cardenas M. Transjugular liver biopsy in 52 patients with an automated Trucut-type needle. Dig Dis Sci. 1999 Jan;44(1):177–180. doi: 10.1023/a:1026678806368. [DOI] [PubMed] [Google Scholar]