Abstract
BACKGROUND
Linezolid has antimycobacterial activity in vitro and is increasingly used for patients with highly drug-resistant tuberculosis.
METHODS
We enrolled 41 patients who had sputum-culture–positive extensively drug-resistant (XDR) tuberculosis and who did not have a response to any available chemotherapeutic option during the previous 6 months. Patients were randomly assigned to linezolid therapy that started immediately or after 2 months, at a dose of 600 mg per day, without a change in their background regimen. The primary end point was the time to sputum-culture conversion on solid medium, with data censored 4 months after study entry. After confirmed sputum-smear conversion or 4 months (whichever came first), patients underwent a second randomization to continued linezolid therapy at a dose of 600 mg per day or 300 mg per day for at least an additional 18 months, with careful toxicity monitoring.
RESULTS
By 4 months, 15 of the 19 patients (79%) in the immediate-start group and 7 of the 20 (35%) in the delayed-start group had culture conversion (P = 0.001). Most patients (34 of 39 [87%]) had a negative sputum culture within 6 months after linezolid had been added to their drug regimen. Of the 38 patients with exposure to linezolid, 31 (82%) had clinically significant adverse events that were possibly or probably related to linezolid, including 3 patients who discontinued therapy. Patients who received 300 mg per day after the second randomization had fewer adverse events than those who continued taking 600 mg per day. Thirteen patients completed therapy and have not had a relapse. Four cases of acquired resistance to linezolid have been observed.
CONCLUSIONS
Linezolid is effective at achieving culture conversion among patients with treatment-refractory XDR pulmonary tuberculosis, but patients must be monitored carefully for adverse events. (Funded by the National Institute of Allergy and Infectious Diseases and the Ministry of Health and Welfare, South Korea; ClinicalTrials.gov number, NCT00727844.)
Linezolid (Zyvox, Pfizer) was approved in 2000 for drug-resistant, gram-positive bacterial infections.1 A member of the oxazolidinone antibiotic class, linezolid inhibits protein synthesis by binding the 23S ribosomal RNA (rRNA) portion of the bacterial 50S ribosomal subunit.2 In adults, linezolid is administered at a dose of 600 mg twice daily, with phase 3 and postmarketing trials showing an acceptable side-effect and adverse-event profile during the FDA-approved 28 days of therapy.3 Data on longer-term use are limited, but serious neuropathies (e.g., peripheral and optic neuropathies), myelosuppression, and hyperlactatemia have been observed4,5 and are considered to be related to the inhibition of mitochondrial protein synthesis.6,7
Linezolid exhibits in vitro bacteriostatic activity against Mycobacterium tuberculosis, including multidrug-resistant (MDR) and extensively drugresistant (XDR) strains, with a minimum inhibitory concentration of less than 1 µg per milliliter.8–11 It has only modest activity in murine models of tuberculosis.12,13 A study of the early and extended bactericidal activity of linezolid showed only minimal early activity (during the initial 2 days) and no late activity (during the subsequent 5 days), with the authors concluding that linezolid had little tissue-sterilizing ability and therefore had a limited role in the treatment of MDR tuberculosis.14 Despite these characteristics, a number of case reports and retrospective studies suggest that linezolid may be effective in treating MDR and XDR tuberculosis.15–21 These studies all have important limitations, including a retrospective design, small numbers, the use of linezolid with multiple other active agents, no controls, and limited follow-up. This apparent discrepancy between preclinical data and clinical observations prompted us to undertake a prospective, randomized trial of linezolid in patients with chronic XDR tuberculosis who did not have a response to all other available chemotherapeutic options.
METHODS
STUDY PATIENTS
From December 2008 through May 2011, we enrolled adults, 20 years of age or older, with chronic XDR pulmonary tuberculosis (positive sputum smear and culture) and with confirmed genotypic or phenotypic resistance to isoniazid, rifampin, kanamycin, ofloxacin, and moxifloxacin or a documented nonresponse to treatment, despite test results showing drug susceptibility. Patients were eligible if they had been treated with an unchanged, failing regimen for 6 months or more before enrollment. Exclusion criteria were previous treatment with linezolid, anticipated surgical treatment, a positive test result for the human immunodeficiency virus (HIV), specific baseline laboratory abnormalities, moderate-to-severe peripheral or optic neuropathy, and need for treatment with contraindicated drugs. Additional details regarding the inclusion and exclusion criteria, regimen changes, dose adjustments, study timeline, and adverse events are provided in the Supplementary Appendix and the study protocol, both of which are available with the full text of this article at NEJM.org.
STUDY DESIGN AND OUTCOME MEASURES
This phase 2a, randomized, two-group study was conducted at the National Masan Hospital in Changwon and the National Medical Center in Seoul, South Korea. Patients were randomly assigned to receive linezolid, at a dose of 600 mg per day, in addition to their existing regimen, either immediately or after a 2-month delay. Permuted-block randomization was performed, with stratification according to status with regard to diabetes mellitus (types 1 and 2 included). A 2-month delay was used to minimize the possibility that study effects other than linezolid could account for observed improvement. All patients continued their existing regimen and were hospitalized from the time of enrollment until sputum-culture conversion. The microbiology staff were unaware of treatment assignments throughout the study.
The primary end point was sputum-culture conversion, with data censored at 4 months. Conversion was defined as negative sputum samples on solid (Löwenstein–Jensen) medium for 3 consecutive weeks; culture on liquid medium was performed with the use of the MB/BacT automated mycobacterial culture system. Patients continued taking linezolid at a dose of 600 mg per day until they had negative sputum smears (Ziehl–Neelsen staining) for 2 consecutive weeks or until they had received 4 months of linezolid treatment, whichever came first. Regimen changes, which were not allowed during the 6 months before enrollment, were allowed after sputum-smear conversion and at least 2 months of treatment with linezolid. After conversion to negative sputum smears (or receipt of 4 months of therapy), patients underwent a second randomization, stratified according to diabetes mellitus status, either to continue receiving linezolid at a dose of 600 mg per day or to receive a lower dose, 300 mg per day, for an additional 18 months or until therapy was stopped owing to side effects or laboratory abnormalities.
If adverse events occurred that were considered to be related to linezolid, a reduction in the dose or a rechallenge at a dose of 300 mg per day was allowed after a limited drug holiday (described in Table 2 in the Supplementary Appendix). Linezolid was administered by means of directly observed therapy during hospitalization. The study staff monitored outpatient adherence by means of videophone or telephone every weekday and also performed pill counts monthly. Patients were treated for at least 18 months after sputum-culture conversion and were followed for an additional 12 months after completing the treatment. Blood was collected for pharmacokinetic analysis22 of linezolid at both doses.
ADVERSE-EVENT MONITORING
Patients underwent baseline and serial safety evaluations (including complete blood counts, blood chemical measurements, and liver-function tests) weekly until 16 weeks, every 2 weeks from 17 through 24 weeks, and then monthly thereafter. A neurologist evaluated all patients at entry with the use of nerve-conduction studies and was available for repeat consultation if any peripheral neuropathy developed. The Subjective Peripheral Neuropathy Screen, a screening tool used in the treatment of HIV infection (Supplementary Material 2 in the Supplementary Appendix),23 and clinical neurologic examinations were performed by the study staff at baseline and monthly thereafter. To monitor patients for linezolid-induced optic neuropathy, the study staff performed testing for visual acuity (using the Han test, which is similar to the Snellen chart), contrast sensitivity, and color vision (using Ishihara plates). Patients with any symptoms or abnormal findings were referred to an ophthalmologist.
STUDY OVERSIGHT
Written informed consent was obtained from all participants. The study was approved by the local institutional review boards and by the U.S. National Institute of Allergy and Infectious Diseases and was conducted in accordance with Good Clinical Practice guidelines. A data and safety monitoring board reviewed adverse events and provided management guidance to investigators. All serious adverse events were reported to the data and safety monitoring board, all institutional review boards, and the U.S. and Korean Food and Drug Administrations, per protocol. The study was monitored by an independent clinical research organization. Pfizer provided linezolid at no cost and reviewed the protocol without comment. The authors are fully responsible for the study design, data collection, analysis, completeness of data reporting, fidelity of this report to the study protocol, and interpretation of the data.
STATISTICAL ANALYSIS
From prior case studies, we estimated a culture-conversion rate of more than 90% with linezolid during the first 4 months of therapy, and on the basis of historical data, we assumed that less than 10% of patients would have spontaneous culture conversion without having received linezolid. Thus, we calculated that a sample of 16 patients per group would provide 92% power, assuming a two-sided type 1 error rate of 0.05 and a 10% discontinuation rate. We planned to recruit 20 patients per group to allow for additional loss to follow-up and death. For the primary analysis of time to culture conversion on solid medium, we used a generalized Wilcoxon test24 and a modified intention-to-treat analysis, which excluded 2 patients who withdrew (owing to baseline neuropathy) before receiving any linezolid. Data from other patients who withdrew were included in the modified intention-to-treat analysis as treatment failures. Ethical concerns regarding the delayed-start group prompted the data and safety monitoring board to request two unplanned interim analyses: one when 16 patients had an end point that could be evaluated and one when 32 had such an end point. For the interim analysis of the primary end point, the Haybittle–Peto rule was used, which specifies a strict significance level of 0.001 as the criterion for stopping the study early because of efficacy and allows for the usual 0.05 level of significance at the final analysis.25 The boundary for early stopping was not met, and follow-up of the 39 patients in the modified intention-to-treat cohort continued until the planned stopping point.
RESULTS
STUDY PATIENTS
A total of 41 patients underwent randomization, with 21 assigned to the immediate-start group and 20 to the delayed-start group (Fig. 1). Owing to preexisting neuropathy, 2 patients in the immediate-start group were withdrawn from the study before receiving any dose of linezolid and were excluded from the modified intention-to-treat analysis. The remaining 39 patients were predominantly men (72%), with a mean age of 41.2 years (range, 20 to 64), and 36% of the patients had diabetes mellitus (Table 1). On the basis of radiologic testing, 77% of the patients were classified as having “far advanced” tuberculosis, which was defined according to the guidelines of the Korea Centers for Disease Control and Prevention26 as the presence of disseminated lesions of slight-to-moderate density exceeding the total volume of one lung, or dense and confluent lesions exceeding one third the volume of one lung, or the presence of cavities greater than 4 cm in diameter. Patients had a median of 5 previous treatment episodes for pulmonary tuberculosis (interquartile range, 3 to 8), and their isolates were resistant to a mean of 11 drugs (range, 6 to 15). The baseline characteristics reported in Table 1 were well balanced between the two groups.
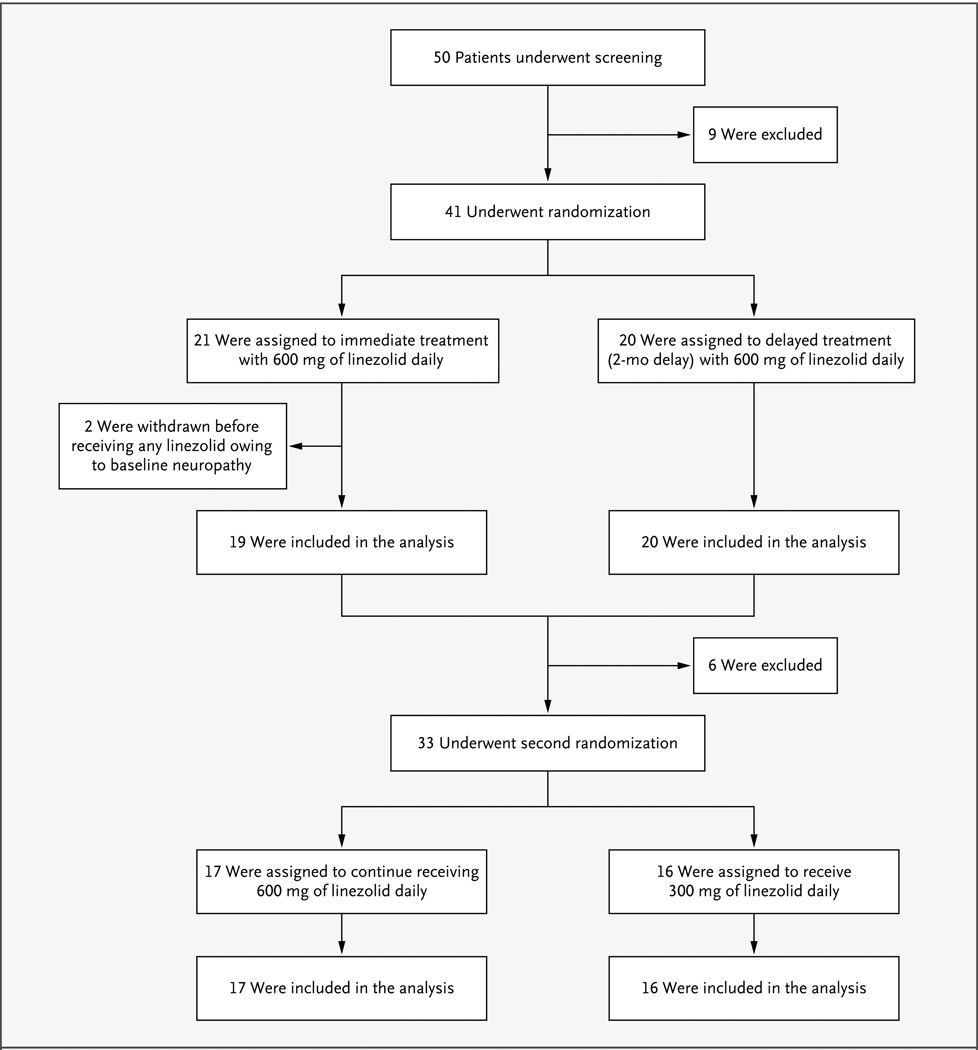
Figure 1. Enrollment, Randomization, and Follow-Up of the Study Patients.
Between December 2008 and May 2011, a total of 50 patients were screened for eligibility and 41 underwent randomization. Two patients were subsequently withdrawn owing to preexisting peripheral neuropathy that was discovered during the baseline examination; the remaining 39 patients were included in the modified intention-to-treat analysis. Two other patients who withdrew before culture conversion were considered to have treatment failure: 1 patient, who had an adverse event requiring a drug holiday, was withdrawn 79 days after starting treatment with linezolid because the drug holiday exceeded the protocol-specified window (28 days before sputum-culture conversion and 42 days after sputum-culture conversion); the other patient was withdrawn 32 days after study entry because of a diagnosis of advanced colon cancer (this patient was in the delayed-start group and had not received any linezolid). Thirty-three patients underwent the second randomization; 17 patients were randomly assigned to continue receiving linezolid at a dose of 600 mg per day, and 16 to receive the reduced dose of 300 mg per day. The 6 patients who did not undergo the second randomization included 4 who had dose reductions due to adverse events before culture conversion and the 2 withdrawn patients mentioned above who were included in the modified intention-to-treat analysis as having treatment failure.
Table 1.
Baseline Characteristics of the Study Participants According to Treatment Group.*
| Characteristic | Immediate-Start Group (N = 19) |
Delayed-Start Group (N = 20) |
Total (N = 39) |
|---|---|---|---|
| Age — yr | |||
| Mean | 42.1 | 40.4 | 41.2 |
| Range | 20–64 | 23–63 | 20–64 |
| Male sex — no. (%) | 12 (63) | 16 (80) | 28 (72) |
| Body-mass index† | |||
| Mean | 19.6 | 20.5 | 20.0 |
| Range | 14.9–25.7 | 14.4–28.1 | 14.4–28.1 |
| Diabetes mellitus — no. (%) | 7 (37) | 7 (35) | 14 (36) |
| BCG vaccination scar — no. (%) | 14 (74) | 17 (85) | 31 (79) |
| Radiographic findings — no. (%) | |||
| Far advanced tuberculosis‡ | 15 (79) | 15 (75) | 30 (77) |
| Cavitary tuberculosis | 9 (47) | 8 (40) | 17 (44) |
| Bilateral lesions | 18 (95) | 20 (100) | 38 (97) |
| No. of previous treatment episodes for tuberculosis | |||
| Median | 5.0 | 5.0 | 5.0 |
| Interquartile range | 3.0–8.5 | 4.0–7.0 | 3.0–7.3 |
| No. of resistant drugs§ | |||
| Mean | 11.6 | 10.4 | 11.0 |
| Range | 8–15 | 6–14 | 6–15 |
There were no significant between-group differences. BCG denotes bacille Calmette–Guérin.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
Far advanced tuberculosis was defined according to the guidelines of the Korea Centers for Disease Control and Prevention26 as the presence of disseminated lesions of slight-to-moderate density exceeding the total volume of one lung, or dense and confluent lesions exceeding one third the volume of one lung, or the presence of cavities greater than 4 cm in diameter.
Drug-susceptibility testing for 15 drugs was performed: isoniazid, para-aminosalicylic acid, streptomycin, ethambutol, rifampin, protionamide, cycloserine, kanamycin, amikacin, ofloxacin, levofloxacin, pyrazinamide, rifabutin, moxifloxacin, and capreomycin.
PRIMARY OUTCOME
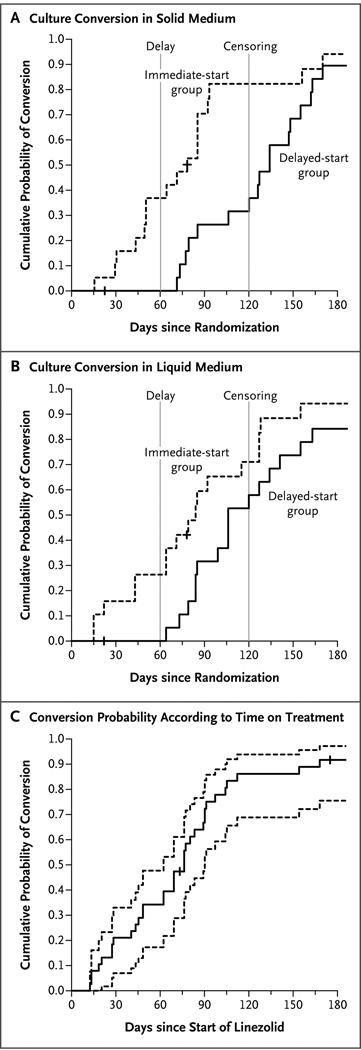
The primary outcome was the time to sputum-culture conversion, with data censored at 4 months. By 4 months, 15 of the 19 patients (79%) in the immediate-start group and 7 of the 20 (35%) in the delayed-start group had conversion to negative sputum cultures on solid medium (P = 0.001) (Fig. 2A). Although culture conversion on solid medium is historically the standard used to gauge the effectiveness of a treatment, we also monitored culture results on liquid medium. With data censored at 4 months, 12 of the 19 patients (63%) in the immediate-start group and 11 of the 20 (55%) in the delayed-start group had culture conversion on liquid medium (P = 0.07) (Fig. 2B). Liquid culture medium is thought to have higher sensitivity and more reproducible results than solid-culture medium.27 In our study, however, the culture results on liquid medium had only borderline significance at the per-protocol censored time point (4 months), owing to the higher-than-expected rate of conversion to negative cultures on liquid medium in the delayed-start group.
Figure 2. Kaplan–Meier Curves for Culture Conversion According to Time since Randomization.
Panel A shows the results for solid culture medium, and Panel B the results for liquid culture medium. In both panels, the dashed vertical lines indicate the start of treatment (at 2 months) in the delayed-treatment group and the time of data censoring (at 4 months). Panel C shows the time to culture conversion on solid medium (solid line) along with the 95% confidence interval (dashed lines) for the 38 participants who received linezolid, according to the duration of linezolid therapy. Tick marks indicate the censored observations at the time of the last follow-up visit with culture results.
Combining the two groups, we observed that 34 of the 38 patients who received linezolid (89%) had culture conversion on solid medium by 6 months (Fig. 2C), at a median of 75 days after the start of treatment with linezolid. One patient withdrew before receiving the study drug, owing to a diagnosis of metastatic colon cancer; data from this person were included in the modified intention-to-treat analysis as a treatment failure. As of May 1, 2012, of the 38 patients who received linezolid, 17 were still receiving the treatment per protocol, and 13 had completed treatment, including 6 with no relapse during the treatment period, 4 with no relapse at the 6-month follow-up, and 3 with no relapse at the 12-month follow-up (end of study). Eight patients withdrew early: 4 patients owing to treatment failure, 1 for personal reasons, and 3 owing to adverse events (Fig. 4 in the Supplementary Appendix).
SAFETY
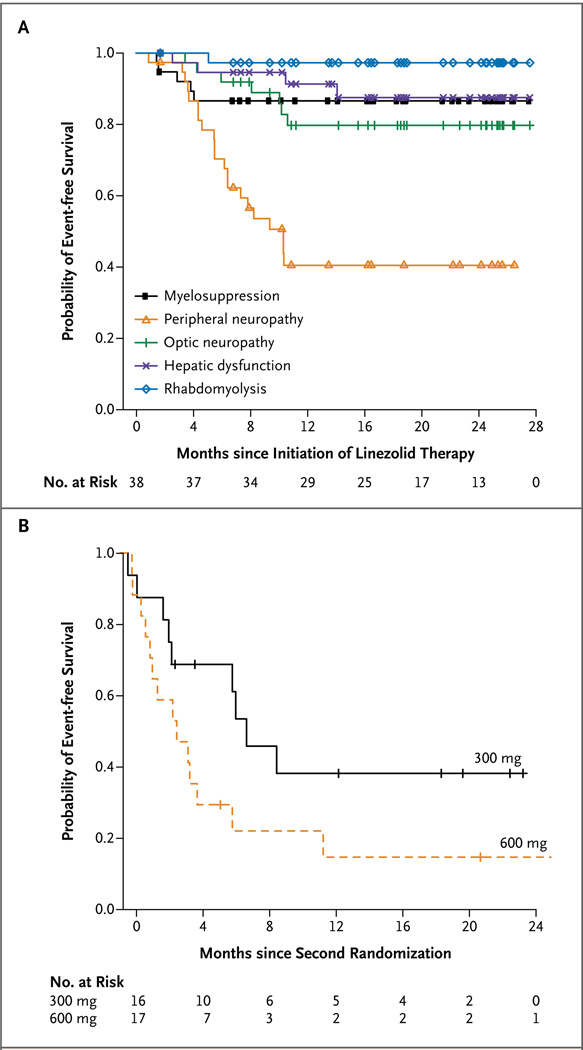
Of the 38 patients who received linezolid, 33 (87%) had clinically significant adverse events; 31 patients (82%) had events that were possibly or probably related to linezolid (Table 2 in the Supplementary Appendix). Most adverse events resolved relatively quickly, and only 3 patients permanently discontinued linezolid owing to drug toxicity (2 patients because of optic neuropathy, and 1 because of anemia). We observed seven episodes of myelosuppression, including anemia and neutropenia, which occurred primarily within the first 5 months (Fig. 3A, and Table 3 in the Supplementary Appendix). In addition, we observed 7 cases of optic neuropathy, 21 cases of peripheral neuropathy, and 1 case of rhabdomyolysis,28 with these events occurring during the first year of treatment.
Figure 3. Probability of Event-free Survival over Time.
The Kaplan–Meier curves in Panel A show the time to the onset of clinically significant adverse events that resulted in a drug holiday or dose adjustment during the study. Symbols indicate data-censoring points for patients remaining in the study (see Table 3 in the Supplementary Appendix for detailed risk estimates corrected for person-years of exposure). The curves in Panel B show the time to the first adverse event in patients after the second randomization to either a continuation of the 600-mg daily dose or a reduced dose of 300 mg per day. Tick marks indicate data-censoring points for individual patients who continued to receive the study drug.
Of the 38 patients who received linezolid, 33 (87%) underwent the scheduled second randomization (1 patient had an adverse event requiring withdrawal from the study and 4 had an adverse event requiring dose reduction before undergoing this randomization) (Fig. 1). In the second randomization, 17 patients were assigned to continue receiving the 600-mg daily dose, and 16 to receive 300 mg per day. Of the 17 patients who continued taking 600 mg per day, 15 (88%) had an adverse event related to the study drug, with 11 subsequently taking the reduced dose of 300 mg per day; of the 16 patients who received 300 mg per day, 11 (69%) had an adverse event related to the study drug. A Cox proportional-hazards analysis showed that, after the second randomization, the group receiving the 600-mg dose was 2.7 times (95% confidence interval, 1.1 to 6.5) as likely to have an adverse event as the group receiving the 300-mg dose (P = 0.03) (Fig. 3B, and Fig. 3 in the Supplementary Appendix).
DRUG RESISTANCE
Of the four patients who did not have a response to treatment, three (two in the 300-mg group, and one in the 600-mg group) did not have confirmed culture conversion; the sputum smears and cultures for these three patients improved initially, but they did not have a consistent negative-culture status and were classified as having treatment failure rather than treatment relapse. The fourth patient, who was in the 600-mg group, had a treatment relapse (cultures became negative but turned positive again after 1 year of treatment).
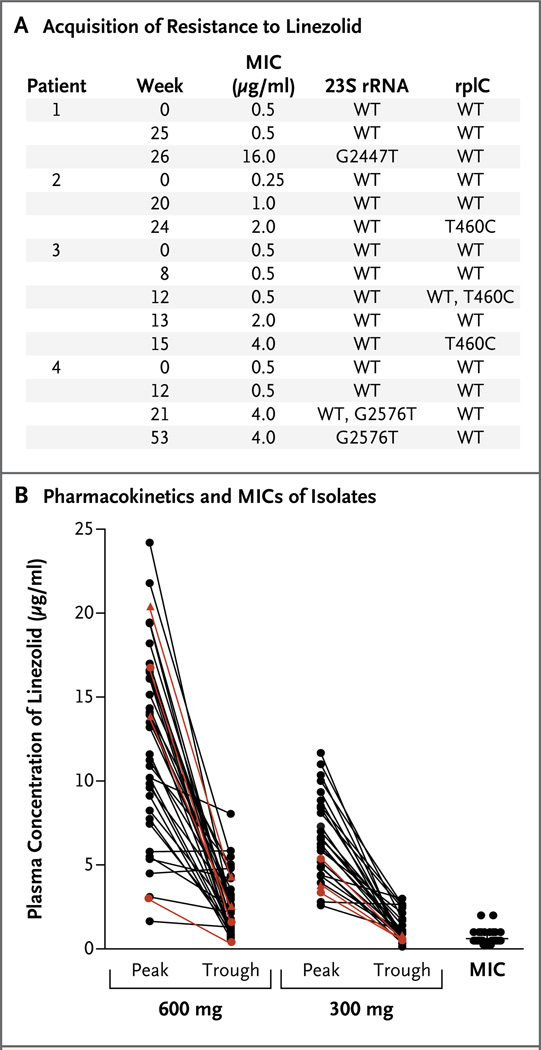
The minimum inhibitory concentration of the corresponding isolates for these four patients increased by a factor of 8 to a factor of 32, as compared with baseline (Fig. 4A). DNA sequencing of these isolates revealed mutations, either in 23S rRNA or in the ribosomal protein L3, in all four patients (Fig. 4A). The observed mutations have previously been reported in association with linezolid resistance from M. smegmatis29 and M. tuberculosis.30,31
Figure 4. Resistance to and Pharmacokinetics of Linezolid.
Acquired resistance to linezolid was observed in the isolates from four patients; mutations in the linezolid-binding site of the 23S ribosomal RNA (rRNA) developed in two patients, and mutations in the mycobacterial ribosomal protein L3 gene (rplC) in the other two (Panel A). Numbers for 23S rRNA indicate the location of mutations according to Escherichia coli numbering. The pharmacokinetics of exposure to linezolid were measured for all patients while they were receiving 600 mg per day and for all patients who subsequently received 300 mg per day (Panel B). Plasma concentrations for the four patients in whom resistant organisms developed are shown in red, and the dose the patient was taking at the time of resistance detection is indicated by triangles (600 mg) or circles (300 mg). The minimum inhibitory concentration (MIC) for linezolid in the initial isolates from the patients and the mean MIC (horizontal line) are shown at the right.
PHARMACOKINETICS
The maximal and minimal plasma concentrations that we observed for linezolid (Fig. 4B) are generally in agreement with published pharmacokinetic data in patients with other infectious diseases.22,32,33 Considering that the plasma protein binding of linezolid is approximately 30%, plasma levels of free linezolid were above the measured minimum inhibitory concentration for each isolate during the entire dosing interval in almost all patients taking 600 mg per day. Among those taking 300 mg per day, the trough level was lower than the mean minimum inhibitory concentration in nine patients, including the two in whom linezolid resistance developed during treatment with that dose. The two doses provided proportional exposures, with a mean (±SD) area under the curve of 180.4±89 µg per milliliter per hour for the 600-mg dose and 91.1±43 µg per milliliter per hour for the 300-mg dose. Using Cox regression, we found no association between the time to culture conversion (measured from the date of the start of treatment with linezolid) and either the peak level (P = 0.93) or the trough level (P = 0.92), measured after at least 2 weeks of linezolid treatment.
DISCUSSION
In this analysis involving 39 patients with XDR pulmonary tuberculosis who had not had a response to any standard treatment regimen for 6 months or more, we found that the immediate addition of linezolid at a dose of 600 mg per day to the ongoing background treatment regimen had a significant beneficial effect on the time to sputum-culture conversion on solid medium, as compared with the delayed addition of linezolid at the same dose. During the first 6 months of treatment, 34 of the 39 patients (87%) had confirmed culture conversion, at a median of 76 days.
In studies conducted immediately after the discovery of streptomycin, which is also a protein-synthesis inhibitor, the rate of culture conversion at 3 months was only 19%,34 whereas in the present study, 60% of patients who received linezolid had negative sputum cultures at 3 months. Monotherapy with isoniazid was also studied throughout the 1950s, and in those studies, less than 32% of patients had sputum-culture conversion during the first 3 months of treatment.35 First-line quadruple drug therapy (isoniazid, rifampin, pyrazinamide, and ethambutol) has been associated with a mean time to culture conversion on solid medium of 30 to 40 days.36,37 In patients with MDR tuberculosis who were treated with second-line agents, the conversion rate was substantially lower, and the time to conversion was longer. For example, in a recent study that used a five-drug regimen (kanamycin, ofloxacin, ethionamide, pyrazinamide, and cycloserine), less than 10% of patients had a negative culture after 2 months.38 In larger studies involving patients with MDR tuberculosis, the estimated median time to culture conversion was 63 days.39 Linezolid alone would therefore appear to be similar to the five-drug chemotherapy regimen currently used as second-line treatment, and the incorporation of linezolid into second-line regimens may substantially improve culture-conversion rates.
A major concern in undertaking this trial was the emergence of acquired resistance to linezolid because we were adding a single active drug to a failing regimen. In early studies of streptomycin as monotherapy, 35 of 41 patients (85%) had resistant organisms at a mean of 53 days after the initiation of therapy.34 Likewise, by 3 months, resistant bacilli developed in 83% of the patients who received isoniazid monotherapy.35 In our study, 4 of the 38 patients who received linezolid for 6 months or more (11%) had apparent acquired resistance. This low frequency of acquisition of resistance may be related to the low rate of observed mutation to linezolid resistance in vitro(estimated at 1×10−9).30 The dose of 600 mg per day also maintained linezolid levels above the published mutant-prevention concentration of 0.6 µg per milliliter40 and may have played a role in reducing the incidence of acquired resistance. The relatively small number of clinical isolates with reported linezolid resistance is consistent with this observation.41
The significant beneficial effect of linezolid was tempered, as expected, by the high rates of drug-related adverse events, although almost all events resolved quickly with a reduction in the dose or the temporary cessation of linezolid, and only three patients discontinued the study owing to adverse events. As we also expected, adverse events were significantly reduced in patients who subsequently received the reduced dose of 300 mg per day. The pharmacokinetic profile of the 300-mg dose, as compared with the minimum inhibitory concentration of the isolates, showed that this dose was sufficient to maintain serum levels above the minimum inhibitory concentration in most patients, although it is worrisome that three of the patients in whom resistance developed had relatively low exposures while receiving the 300-mg dose (Fig. 4B). Whether the lower dose, which is associated with fewer adverse events, has sufficient potency will need to be further evaluated, along with the possible role of therapeutic drug monitoring. Linezolid shows good pulmonary penetration42,43 and has been shown to have favorable distribution in infected soft tissue.44,45 These pharmacokinetic properties may also play a role in maintaining linezolid concentrations that are high enough to prevent the emergence of resistant organisms.
This study is limited by the small number of patients evaluated, particularly the small number of patients who did not have a response to treatment or in whom resistance developed. Although the small numbers of treatment failure and cases of acquired resistance are encouraging, they also preclude more in-depth analyses of the associated risk factors. Balancing the long-term risk– benefit ratio of linezolid requires identifying a dose with sufficient potency but less toxicity.
Supplementary Material
Acknowledgments
Supported by the Intramural Research Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by the Ministry of Health and Welfare, South Korea.
We thank all the study participants; Ji-Im Lee, M.S., Yumi Park, Ph.D., Sung-Hwa Seo, M.S., Jeong-Ae Park, M.S., Youngran Kim, R.N., Hyemi Park, B.S., and Nayoung Jang, B.S., of the International Tuberculosis Research Center for technical and other assistance during the study; Steven M. Holland, M.D., and Ying Cai, M.S., of the National Institute of Allergy and Infectious Diseases for encouragement and valuable suggestions regarding study design and data management and analysis, respectively; and Soohee Hwang, M.D., Hyungseok Kang, M.D., Doh Hyoung Kim, M.D., Jin Hong Min, M.D., Doosoo Jeon, M.D., and Myung-Hee Lee, R.N., of the National Masan Hospital for assistance in patient care and sample collection.
Footnotes
No potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Leach KL, Brickner SJ, Noe MC, Miller PF. Linezolid, the first oxazolidinone antibacterial agent. Ann N Y Acad Sci. 2011;1222:49–54. doi: 10.1111/j.1749-6632.2011.05962.x. [DOI] [PubMed] [Google Scholar]
- 2.Ippolito JA, Kanyo ZF, Wang D, et al. Crystal structure of the oxazolidinone antibiotic linezolid bound to the 50S ribosomal subunit. J Med Chem. 2008;51:3353–3356. doi: 10.1021/jm800379d. [DOI] [PubMed] [Google Scholar]
- 3.Vinh DC, Rubinstein E. Linezolid: a review of safety and tolerability. J Infect. 2009;59(Suppl 1):S59–S74. doi: 10.1016/S0163-4453(09)60009-8. [DOI] [PubMed] [Google Scholar]
- 4.Di Paolo A, Malacarne P, Guidotti E, Danesi R, Del Tacca M. Pharmacological issues of linezolid: an updated critical review. Clin Pharmacokinet. 2010;49:439–447. doi: 10.2165/11319960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Lee E, Burger S, Shah J, et al. Linezolid-associated toxic optic neuropathy: a report of 2 cases. Clin Infect Dis. 2003;37:1389–1391. doi: 10.1086/379012. [DOI] [PubMed] [Google Scholar]
- 6.Beekmann SE, Gilbert DN, Polgreen PM. Toxicity of extended courses of line-zolid: results of an Infectious Diseases Society of America Emerging Infections Network survey. Diagn Microbiol Infect Dis. 2008;62:407–410. doi: 10.1016/j.diagmicrobio.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Nagiec EE, Wu L, Swaney SM, et al. Oxazolidinones inhibit cellular proliferation via inhibition of mitochondrial protein synthesis. Antimicrob Agents Chemother. 2005;49:3896–3902. doi: 10.1128/AAC.49.9.3896-3902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashtekar DR, Costa-Periera R, Shrinivasan T, Iyyer R, Vishvanathan N, Rittel W. Oxazolidinones, a new class of synthetic antituberculosis agent: in vitro and in vivo activities of DuP-721 against Mycobacterium tuberculosis. Diagn Microbiol Infect Dis. 1991;14:465–471. doi: 10.1016/0732-8893(91)90002-w. [DOI] [PubMed] [Google Scholar]
- 9.Barbachyn MR, Hutchinson DK, Brickner SJ, et al. Identification of a novel oxazolidinone (U-100480) with potent antimycobacterial activity. J Med Chem. 1996;39:680–685. doi: 10.1021/jm950956y. [DOI] [PubMed] [Google Scholar]
- 10.Tato M, de la Pedrosa EG, Cantóon R, et al. In vitro activity of linezolid against Mycobacterium tuberculosis complex, including multidrug-resistant Mycobacterium bovis isolates. Int J Antimicrob Agents. 2006;28:75–78. doi: 10.1016/j.ijantimicag.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Zurenko GE, Yagi BH, Schaadt RD, et al. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother. 1996;40:839–845. doi: 10.1128/aac.40.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cynamon MH, Klemens SP, Sharpe CA, Chase S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1999;43:1189–1191. doi: 10.1128/aac.43.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams KN, Stover CK, Zhu T, et al. Promising antituberculosis activity of the oxazolidinone PNU-100480 relative to that of linezolid in a murine model. Antimicrob Agents Chemother. 2009;53:1314–1319. doi: 10.1128/AAC.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietze R, Hadad DJ, McGee B, et al. Early and extended early bactericidal activity of linezolid in pulmonary tuberculosis. Am J Respir Crit Care Med. 2008;178:1180–1185. doi: 10.1164/rccm.200806-892OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anger HA, Dworkin F, Sharma S, Munsiff SS, Nilsen DM, Ahuja SD. Linezolid use for treatment of multidrug-resistant and extensively drug-resistant tuberculosis, New York City, 2000–06. J Antimicrob Chemother. 2010;65:775–783. doi: 10.1093/jac/dkq017. [DOI] [PubMed] [Google Scholar]
- 16.Condos R, Hadgiangelis N, Leibert E, Jacquette G, Harkin T, Rom WN. Case series report of a linezolid-containing regimen for extensively drug-resistant tuberculosis. Chest. 2008;134:187–192. doi: 10.1378/chest.07-1988. [DOI] [PubMed] [Google Scholar]
- 17.Fortún J, Martín-Dávila P, Navas E, et al. Linezolid for the treatment of multi-drug-resistant tuberculosis. J Antimicrob Chemother. 2005;56:180–185. doi: 10.1093/jac/dki148. [DOI] [PubMed] [Google Scholar]
- 18.Park IN, Hong SB, Oh YM, et al. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multi-drug-resistant tuberculosis. J Antimicrob Chemother. 2006;58:701–704. doi: 10.1093/jac/dkl298. [DOI] [PubMed] [Google Scholar]
- 19.Schecter GF, Scott C, True L, Raftery A, Flood J, Mase S. Linezolid in the treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2010;50:49–55. doi: 10.1086/648675. [DOI] [PubMed] [Google Scholar]
- 20.Singla R, Caminero JA, Jaiswal A, et al. Linezolid, an effective, safe and cheap drug in MDR-TB treatment failure patients in India. Eur Respir J. 2012;39:956–962. doi: 10.1183/09031936.00076811. [DOI] [PubMed] [Google Scholar]
- 21.von der Lippe B, Sandven P, Brubakk O. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB) — a report of ten cases. J Infect. 2006;52:92–96. doi: 10.1016/j.jinf.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Stalker DJ, Jungbluth GL. Clinical pharmacokinetics of linezolid, a novel oxazolidinone antibacterial. Clin Pharmacokinet. 2003;42:1129–1140. doi: 10.2165/00003088-200342130-00004. [DOI] [PubMed] [Google Scholar]
- 23.McArthur JH. The reliability and validity of the subjective peripheral neuropathy screen. J Assoc Nurses AIDS Care. 1998;9:84–94. doi: 10.1016/S1055-3290(98)80048-4. [DOI] [PubMed] [Google Scholar]
- 24.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc Ser A. 1972;135:185–207. [Google Scholar]
- 25.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44:793–797. doi: 10.1259/0007-1285-44-526-793. [DOI] [PubMed] [Google Scholar]
- 26.Guidelines for tuberculosis control, 2010. Seoul, South Korea: Korea Centers for Disease Control and Prevention; 2010. (In Korean.) [Google Scholar]
- 27.Davies GR. Early clinical development of anti-tuberculosis drugs: science, statistics and sterilizing activity. Tuberculosis (Edinb) 2010;90:171–176. doi: 10.1016/j.tube.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Carroll MW, Choi H, Min S, et al. Rhabdomyolysis in a patient treated with linezolid for extensively drug-resistant (XDR) tuberculosis. Clin Infect Dis. 2012;54:1624–1627. doi: 10.1093/cid/cis293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sander P, Belova L, Kidan YG, Pfister P, Mankin AS, Böttger EC. Ribosomal and non-ribosomal resistance to oxazolidinones: species-specific idiosyncrasy of ribosomal alterations. Mol Microbiol. 2002;46:1295–1304. doi: 10.1046/j.1365-2958.2002.03242.x. [DOI] [PubMed] [Google Scholar]
- 30.Hillemann D, Rusch-Gerdes S, Richter E. In vitro-selected linezolid-resistant Mycobacterium tuberculosis mutants. Antimicrob Agents Chemother. 2008;52:800–801. doi: 10.1128/AAC.01189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beckert P, Hillemann D, Kohl TA, et al. rplC T460C identified as a dominant mutation in linezolid-resistant Mycobacterium tuberculosis strains. Antimicrob Agents Chemother. 2012;56:2743–2745. doi: 10.1128/AAC.06227-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh WJ, Kwon OJ, Gwak H, et al. Daily 300 mg dose of linezolid for the treatment of intractable multidrug-resistant and extensively drug-resistant tuberculosis. J Antimicrob Chemother. 2009;64:388–391. doi: 10.1093/jac/dkp171. [DOI] [PubMed] [Google Scholar]
- 33.McGee B, Dietze R, Hadad DJ, et al. Population pharmacokinetics of linezolid in adults with pulmonary tuberculosis. Antimicrob Agents Chemother. 2009;53:3981–3984. doi: 10.1128/AAC.01378-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Streptomycin treatment of pulmonary tuberculosis. BMJ. 1948;2:769–782. [PMC free article] [PubMed] [Google Scholar]
- 35.Comparative trial of isoniazid alone in low and high dosage and isoniazid plus pas in the treatment of acute pulmonary tuberculosis in East Africans. Tubercle. 1960;41:83–102. [Google Scholar]
- 36.Rustomjee R, Lienhardt C, Kanyok T, et al. A Phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int J Tuberc Lung Dis. 2008;12:128–138. [PubMed] [Google Scholar]
- 37.Dorman SE, Johnson JL, Goldberg S, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. Am J Respir Crit Care Med. 2009;180:273–280. doi: 10.1164/rccm.200901-0078OC. [DOI] [PubMed] [Google Scholar]
- 38.Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360:2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- 39.Yew WW, Chan CK, Leung CC, et al. Comparative roles of levofloxacin and ofloxacin in the treatment of multidrugn-resistant tuberculosis: preliminary results of a retrospective study from Hong Kong. Chest. 2003;124:1476–1481. doi: 10.1378/chest.124.4.1476. [DOI] [PubMed] [Google Scholar]
- 40.Rodríguez JC, Cebrián L, López M, Ruiz M, Jimínez I, Royo G. Mutant prevention concentration: comparison of fluoroquinolones and linezolid with Mycobacterium tuberculosis. J Antimicrob Chemother. 2004;53:441–444. doi: 10.1093/jac/dkh119. [DOI] [PubMed] [Google Scholar]
- 41.Richter E, Rüsch-Gerdes S, Hillemann D. First linezolid-resistant clinical isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2007;51:1534–1236. doi: 10.1128/AAC.01113-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boselli E, Breilh D, Rimmelé T, et al. Pharmacokinetics and intrapulmonary concentrations of linezolid administered to critically ill patients with ventilator-associated pneumonia. Crit Care Med. 2005;33:1529–1533. doi: 10.1097/01.ccm.0000168206.59873.80. [DOI] [PubMed] [Google Scholar]
- 43.Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. Intrapulmonary pharmacokinetics of linezolid. Antimicrob Agents Chemother. 2002;46:1475–1480. doi: 10.1128/AAC.46.5.1475-1480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saralaya D, Peckham DG, Hulme B, et al. Serum and sputum concentrations following the oral administration of linezolid in adult patients with cystic fibrosis. J Antimicrob Chemother. 2004;53:325–328. doi: 10.1093/jac/dkh072. [DOI] [PubMed] [Google Scholar]
- 45.Traunmüller F, Schintler MV, Spendel S, et al. Linezolid concentrations in infected soft tissue and bone following repetitive doses in diabetic patients with bacterial foot infections. Int J Antimicrob Agents. 2010;36:84–86. doi: 10.1016/j.ijantimicag.2010.03.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.