Abstract
Salen Mn complexes, including EUK-134, EUK-189 and a newer cyclized analog EUK-207, are synthetic SOD/catalase mimetics that have beneficial effects in many models of oxidative stress. As oxidative stress is implicated in some forms of delayed radiation injury, we are investigating whether these compounds can mitigate injury to normal tissues caused by ionizing radiation. This review describes some of this research, focusing on several tissues of therapeutic interest, namely kidney, lung, skin, and oral mucosa. These studies have demonstrated suppression of delayed radiation injury in animals treated with EUK-189 and/or EUK-207. While an antioxidant mechanism of action is postulated, it is likely that the mechanisms of radiation mitigation by these compounds in vivo are complex and may differ in the various target tissues. Indicators of oxidative stress are increased in lung and skin radiation injury models, and suppressed by salen Mn complexes. The role of oxidative stress in the renal injury model is unclear, though EUK-207 does mitigate. In certain experimental models, salen Mn complexes have shown “mito-protective” properties, that is, attenuating mitochondrial injury. Consistent with this, EUK-134 suppresses effects of ionizing radiation on mitochondrial function in rat astrocyte cultures. In summary, salen Mn complexes could be useful to mitigate delayed radiation injury to normal tissues following radiation therapy, accidental exposure, or radiological terrorism. Optimization of their mode of delivery and other key pharmaceutical properties, and increasing understanding of their mechanism(s) of action as radiation mitigators, are key issues for future study.
Keywords: salen manganese complex, radiation injury, radiation mitigation, catalytic antioxidant
II. Rationale for testing Salen Mn complexes as radiation mitigators
Radiation therapy, besides destroying the tumor cells that are its intended target, can also cause delayed injuries, some very serious or even lethal, to normal tissues. There is a need for therapeutic agents to prevent such normal tissue injury. Preferably, these agents would be administered well after irradiation, so as not to risk interfering with the anti-tumor effects of the radiation therapy [1]. While the mechanisms of delayed radiation injury are not well understood, evidence suggests that oxidative stress is involved [2–4]. Therefore, we and others have studied synthetic agents that neutralize the reactive oxygen species (ROS) superoxide and hydrogen peroxide as potential therapeutic agents to mitigate normal tissue injury resulting from ionizing radiation. This review focuses primarily on radiation mitigation studies employing one class of synthetic ROS scavenger, a class of synthetic metal-containing compounds known as salen Mn complexes [5].
It has long been known that exposure of biological materials to ionizing radiation produces a burst of reactive oxygen species (ROS), including superoxide, hydrogen peroxide, hydroxyl radical, and singlet oxygen. These highly reactive species oxidize cellular macromolecules, such as DNA, producing lethal damage [6]. However, what has been less appreciated until recently is the potential role of chronically generated ROS in delayed radiation-induced damage [2–4]. Such injury, including fibrosis, necrosis, atrophy, and vascular damage, occurs months to years after exposure to radiation. Clinically relevant target tissues for radiation injury include the skin, kidney, lung, oral mucosa and brain. There is evidence for cumulative ROS and reactive nitrogen species (RNS)-mediated damage, for example lipid peroxidation and protein tyrosine nitration, in such target tissues following irradiation. There is also evidence for proinflammatory processes, namely acute activation of stress-sensitive signaling transcriptional events and cytokine production [4, 7]. Indeed, infiltrating inflammatory cells would serve as one likely source of damaging ROS. Another potential source of ROS is the mitochondria, especially when dysfunctional. Evidence indicates not only that radiation leads to mitochondrial injury, but also that delivery of antioxidant enzymes to the mitochondria through gene therapy techniques can protect against delayed radiation damage in vivo [2], and a mitochondrially targeted antioxidant reduces radiation injury in cell culture [8]. Therefore, both proinflammatory processes and mitochondrial dysfunction are implicated in delayed radiation injury. These findings suggest that agents that interrupt these damaging subcellular processes might have considerable therapeutic benefit against the damaging effects of radiation exposure.
Such considerations have led to our investigation of salen Mn complexes as potential mitigators of delayed radiation injury. As discussed further below, prior data showed that treatment with these compounds suppressed proinflammatory processes, inhibited mitochondrial damage, and reduced oxidative changes in various experimental models not involving radiation injury.
Currently the only FDA-approved drug for preventing radiation injury is amifostine (Ethylol®), which is usually administered intravenously or subcutaneously and, according to current clinical guidelines, prior to radiation exposure [9]. It acts as a free radical scavenger, but may have additional mechanisms of action such as modulation of antioxidant enzymes [9, 10]. No agent has yet been FDA-approved to prevent radiation injury when administered after radiation, a property referred to as “radiation mitigation”. In the radiation countermeasures area, mitigators refer to therapies begun after irradiation but prior to clinical evidence of injury. This is to distinguish them from protectors, which are given prior to irradiation, and from treatment agents, which are used after there is clinical evidence of injury [11]. Salen Mn complexes, while not yet approved for human use, are interesting candidates for development with potential advantages over amifostine. One major advantage is their ability, as summarized in this review, to mitigate delayed radiation injury when given days to weeks after irradiation.
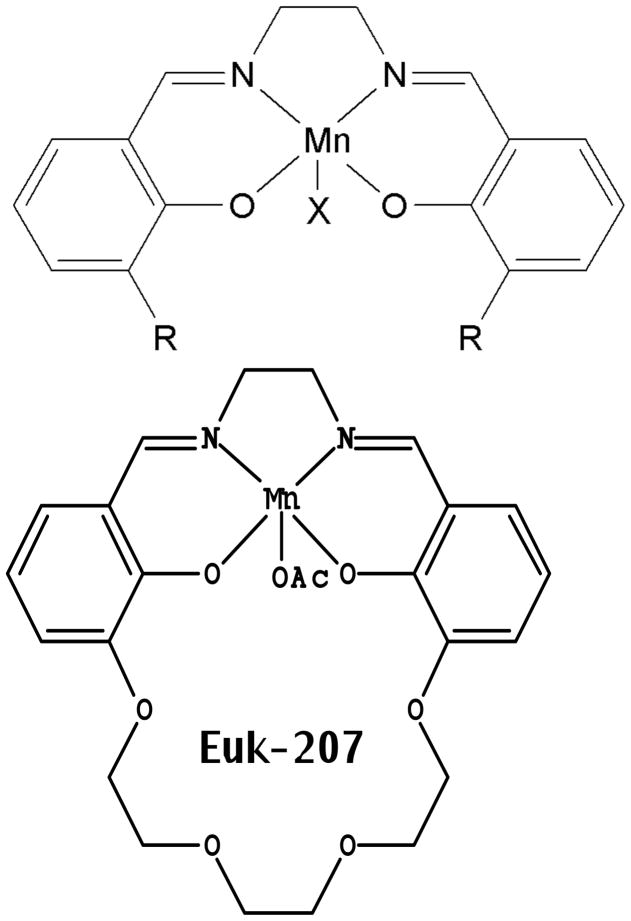
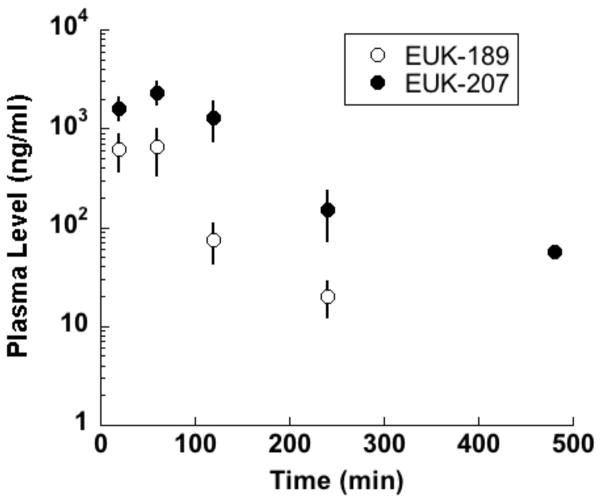
Salen Mn complexes are a class of synthetic low molecular weight agents that mimic the antioxidant enzymes superoxide dismutase (SOD) and catalase, scavenging superoxide and hydrogen peroxide, respectively [5]. Data, including structure-activity relationship findings in vitro and in vivo, indicate that the hydrogen peroxide scavenging property, namely catalase activity, is a more important parameter than SOD activity in determining certain cytoprotective effects of salen Mn complexes, though other factors such as pharmacokinetics and cytotoxicity are also important [5]. In addition, it’s been shown that salen Mn complexes can scavenge reactive nitrogen species (RNS) through mechanisms analogous to their catalase activity [12], a property of potential relevance to their ability to attenuate protein nitration in oxidative injury models [13, 14]. Overall, this combination of properties (low molecular weight, catalytic scavenging mechanism, and activity against multiple damaging species) provides advantages for salen Mn complexes over other antioxidants, such as noncatalytic ROS scavengers or proteinacious antioxidant enzymes [15, 16]. Prototype salen Mn complexes EUK-8 and the improved catalase mimetics EUK-134 and EUK-189 (Fig 1), are effective in a wide range of models for diseases involving oxidative stress [6, 17]. In many of these models, salen Mn complexes were not only functionally protective, but also suppressed biochemical indicators of oxidative stress, such as oxidative modifications of protein, lipids and nucleic acids [13, 18, 19]. EUK-207 (Fig 1) is a “second generation” cyclized salen Mn complex that has catalytic properties equivalent to EUK-134 and EUK-189, but was designed for greater stability [17, 18, 20]. Consistent with this, we have found that EUK-207 attains much higher plasma levels than EUK-189 (e.g., when given subcutaneously to rats (Fig 2)). Both EUK-189 and EUK-207 are highly effective at improving a behavioral indicator of cognitive function and suppressing markers for brain oxidative stress (e.g. nucleic acid oxidation) in a mouse model for age-associated mild cognitive impairment [18, 21]. While structure-activity relationship data and other relevant properties have been published for these compounds, Table 1 is included to facilitate their comparison. The Table summarizes key properties of EUK-189 and EUK-207, as well as Mn porphyrin compounds that are discussed in section IV.
Fig 1. Structures of salen Mn complexes: EUK-8, EUK-134, EUK-189 and EUK-207.
SOD and catalase activities, cytoprotective, and other properties of EUK-207 [17, 20] and the non-cyclized salen Mn complexes [5] have been described. The structures of the non-cyclized salen Mn complexes are: EUK-8, R=H, X=Cl; EUK-134, R = methoxy, X = acetoxy; EUK-189, R = ethoxy; X = acetoxy; EUK-207: OAc = acetoxy
Fig 2. Plasma levels of EUK-207 and EUK-189 following sc injection in rats.
Compounds (50 mg/kg and 62.5 mg/kg, respectively) were given as a single sc injection to WAG/RijCmcr rats and blood samples collected by orbital bleeding, collected into heparinized tubes. Plasma was extracted and quantitated for salen Mn complexes using LC-MS/MS as described previously [70]. Mean ± SD of n = 4 and 5, respectively. EUK-189 was undetectable at the last time point.
Table 1. Summary of properties of selected synthetic SOD/catalase mimetics.
Relevant properties, discussed further at various places in the text, are summarized here. EUK-207 and EUK-189 are salen Mn complexes (Fig 1) and EUK-423, EUK-418, and EUK-451 are orally available Mn porphyrin complexes, with structures as reported by Rosenthal et al. [70]. Superoxide dismutase (SOD), catalase (CAT) and peroxidase (PX) activities were assayed as described [70]. SOD activity is represented as an IC50 (μM), so lower values represent higher SOD activity. CAT and PX are expressed as reaction rates (μM/minute). LogP values were determined by octanol-water partitioning, as described [70]. Stability to synthetic gastric solution (SGF), determined as described [70], shows the percent of compound remaining after 90 min at 37°C. Stability to EDTA, determined as described [17], shows the percent of compound remaining after incubation at ambient temperature in a 114-fold molar excess of EDTA for 3.2 or 70 hr, as indicated. All data are reprinted from the cited articles. Error bars represent SD for triplicate determinations. nd indicates not done
| Compound | EUK-189 | EUK-207 | EUK-451 | EUK-418 | EUK-423 |
|---|---|---|---|---|---|
| SOD | 0.37 | 0.48 | 3.36 | 1.73 | 6.36 |
| CAT | 3.15±0.01 | 3.21±0.04 | 0.64±0.25 | 0.47±0.10 | 0.45±0.01 |
| PX | 3.61±0.05 | 4.43±0.01 | 0.59±0.03 | 0.63±0.08 | 0.61±0.05 |
| logP | −0.90±0.01 | −1.41±0.15 | −0.66±0.01 | 0.55±0.01 | 0.91±0.01 |
| SGF | 14 | 72 | 100 | 92 | 91 |
| EDTA (3.2h) | 95.8 | 99.2 | nd | nd | nd |
| EDTA (70h) | 38.1 | 82.7 | nd | nd | nd |
Endogenous antioxidant defense enzymes [6] include three types of superoxide dismutases (SOD). The cytosolic and extracellular forms of superoxide dismutase (sod1 and sod3, respectively) are Cu and Zn containing enzymes. The mitochondrial form (sod2, or MnSOD) instead has Mn in its active site. Mitochondria-targeted expression of either sod2 or sod1 protects against radiation injury in a mouse esophagitis model [2, 22] and mice deficient in sod2 (sod2−/+ mice) are more vulnerable to radiation lung injury [23]. A mutation leading to increased production of mitochondria-derived ROS has recently been shown to make mammalian cells (hamster fibroblasts) substantially more sensitive to ionizing radiation, and it’s been hypothesized that radiation-induced disruptions in mitochondrial oxygen metabolism contributes to radiation injury [24]. Such findings suggest that a synthetic antioxidant reaching the mitochondria should have therapeutic benefits in radiation injury. Although salen Mn complexes were not designed specifically to target the mitochondria, several studies have shown that the compounds are, indeed, “mito-protective”. They prolong survival up to 3-fold, protect mitochondrial enzymes, and prevent oxidative pathologies in sod2−/− mice, an in vivo model for severe mitochondrial oxidative stress [25, 26]. EUK-189 and EUK-207 were much more effective than other agents tested in sod2−/− mice, namely, the Mn porphyrin MnTBAP, the reported SOD mimetic M40403, and the mitochondrial metabolites, with reported mitoprotective properties, alpha lipoic acid and L-acetyl-carnitine [17]. Since salen Mn complexes, unlike targeted agents such as MitoQ [27] and others [8] are not designed to exclusively reach the mitochondria, they might have broader therapeutic potential because, presumably, they can also address non-mitochondrial injury mechanisms, such as proinflammatory processes.
Irradiation of late-responding tissues leads to acute activation of stress-responsive transcriptional events, leading to production of inflammatory cytokines mediating an aberrant chronic inflammatory cascade that ultimately leads to fibrosis or necrosis [4, 7] Production of such mediators is controlled by “stress-induced” transcriptional factors including those, e.g. NF-kB and AP-1, which are believed to be activated by increased ROS. Such events are reminiscent of proinflammatory processes that mediate tissue injury in many other pathological conditions, such as infection, ischemia/excitotoxicity, and environmental stress. Salen Mn complexes are markedly protective against such forms of tissue injury in vivo, while concomitantly suppressing activation of “stress-induced” transcription factors in target tissues [13, 28, 29]. For example, EUK-134 inhibited the excitotoxic activation of AP-1 and NF-κB secondary to kainic acid-induced seizures, while preventing hippocampal neuronal death [13]. EUK-189 inhibited AP-1 activation in aged rats subjected to mild heat-stress, while concomitantly preventing severe liver injury that occurs in aged, but not young, rats in this experimental system [30]. Salen Mn complex treatment increased the mean survival time of allogeneic C57Bl/6 skin grafted into BALB/c mice, reduced pro-inflammatory type 1 alloresponse (IFN-γ) while promoting anti inflammatory type 2 alloimmunity (IL-4 and IL-5) [31]. Tissue transglutamininase 2 is activated in a pro-oxidative environment, inducing degradation of PPAR-γ, NF-κB activation and inflammation [32] and treatment with EUK-134 restored PPAR-γ, levels [33]. Therefore, the suppressive effects of salen Mn complexes against proinflammatory, injury-associated transcriptional processes provided another rationale, in addition to their “mito-protective” properties, for investigating their benefits in radiation injury models.
Taken together, such findings led to the hypothesis that salen Mn complexes would mitigate radiation injury to normal tissues. Proposed mechanisms might involve preventing mitochondrial injury, suppressing “proinflammatory” processes, inhibiting other forms of oxidative injury to cellular constituents or most likely, complex combinations thereof.
III. Mitigation of radiation injury by salen Mn complexes
Cell culture systems
Mitochondrial dysfunction in astrocytes as a model for central nervous system (CNS) radiation injury
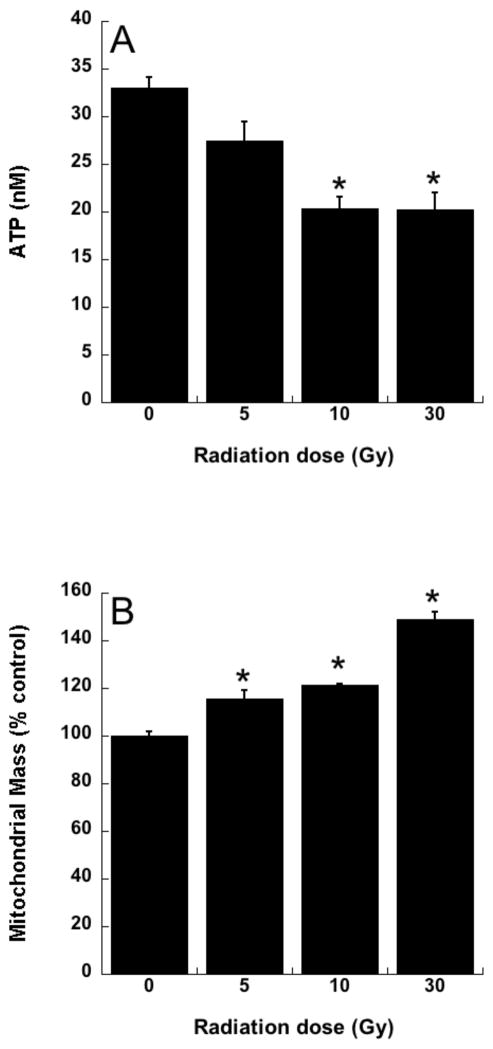
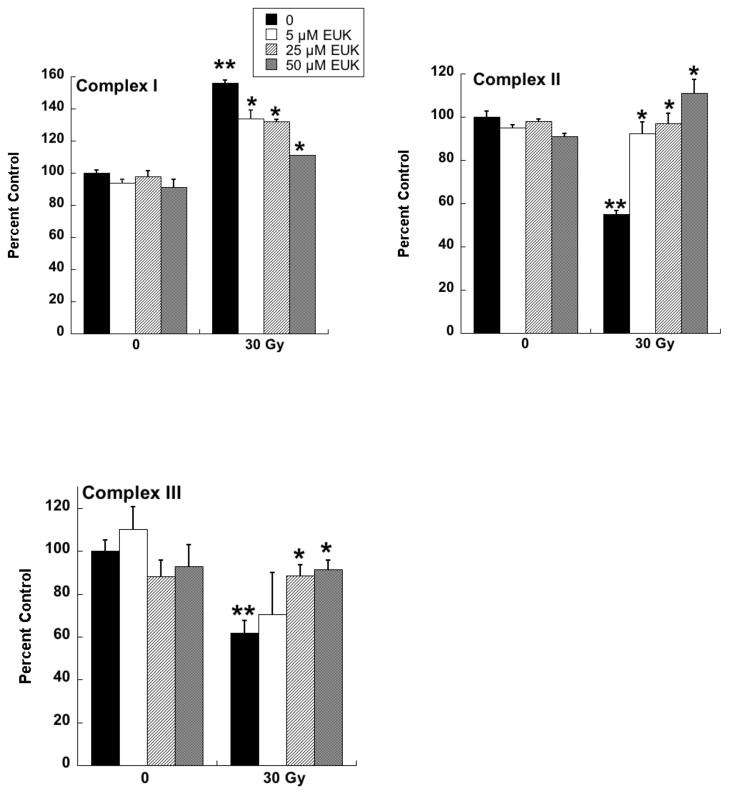
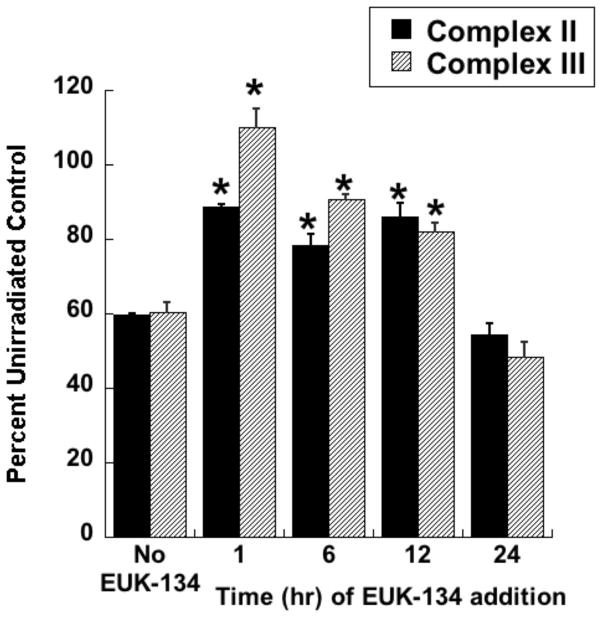
Radiotherapy continues to be a primary treatment for malignant brain tumors as well as other neoplasms located in or near the CNS. Consequently, radiation-induced injury to normal CNS tissue is of significant concern and is the major dose-limiting factor in the treatment of gliomas and other head and neck tumors. The CNS damage resulting from irradiation is most often characterized by vascular abnormalities, demyelination and, ultimately, necrosis, which can be expressed years after radiotherapy [34]. Although this form of radiation-induced normal tissue injury has been well described in terms of histological and functional criteria [35, 36], its pathogenesis remains poorly understood. Delayed cell death and tissue injury can result from a number of reactive processes, but accumulating data suggest that an initiating source of these delayed effects can be mitochondrial dysfunction. Mitochondrial damage has been implicated in a number of neurological conditions, namely trauma, ischemia, excitotoxic insults and neurodegeneration, and CNS lesions following radiation share some similarity to these other forms of injury [37]. The ultimate consequences of mitochondrial injury include insufficient ATP production and increased ROS generation. With its high energy demands and rate of oxidative metabolism, the CNS is particularly susceptible to these impairments. Rat astrocyte cultures were used to investigate the role of mitochondrial dysfunction in CNS radiation injury. Astrocytes are the most prevalent phenotype in the CNS, outnumbering neurons by approximately 9:1. They have numerous functions in the normal CNS [38] including a critical role in the protection of endothelial cells, oligodendrocytes and neurons from oxidative or excitotoxic injury [39–41]. Given their key functions, it would be expected that the response of astrocytes to radiation would have a major impact on the radiation response of the CNS as a whole. The astrocytes, which are normally relatively non-proliferative in vivo, were tested for radiation injury as confluent cultures, thus eliminating the complicating variables of cell cycle delay and redistribution. These cells, irradiated at 5, 10, or 30 Gy, showed no significant loss of viability, consistent with their non-proliferative state (data not shown). However, the astrocyte cultures developed mitochondrial abnormalities, particularly at the higher radiation doses. These abnormalities included decreased ATP levels and increased mitochondrial mass per cell (Fig 3), both known consequences of mitochondrial impairment. The irradiated astrocytes also developed changes in respiratory chain activities, namely decreases in complex II and III and, atypical of other forms of mitochondrial toxicity, an increase in complex I (Fig 4). Generally, these changes began to appear 1 day post-irradiation, persisting for at least 7 days (data not shown). When given to the cells immediately after 30 Gy irradiation, the salen Mn complex EUK-134 attenuated, in a dose-dependent manner, radiation-induced effects on respiratory complex activities (Fig 4). This “mito-protection” was significant even when EUK-134 (50 μM) was added to cultures up to 12 hr after irradiation (30 Gy) (Fig 5). These data provide direct evidence for radiation-induced nonlethal mitochondrial abnormalities in astrocytes, potentially sufficient to cause sustained ROS production and other injurious effects. Furthermore, these data support the use of “mito-protective” salen Mn complexes to mitigate such injury. Whether these findings extend to key target cells for delayed radiation injury in other tissues remains to be investigated. However, Vorotnikova et al. [42] showed that several compounds with SOD and catalase activities, including the salen Mn complexes EUK-189 and EUK-207, inhibited radiation-induced apoptosis in bovine adrenal capillary endothelial cell cultures. While the mechanism of such anti-apoptosis is not well understood, previous findings have shown that staurosporine induced apoptosis, also inhibited by salen Mn complexes, is mediated by oxidative stress [43]. More recently, EUK-207 was found to prevent several radiation-induced injuries in human microvascular endothelial cell cultures [44]. These findings may be relevant to the potentially broad applicability of these agents as radiation mitigators, since the vascular endothelial cell is regarded as being an important target for radiation injury in several normal tissues [45].
Fig 3. Radiation-induced mitochondrial abnormalities in rat astrocytes.
Rat astrocytes were cultured and exposed to ionizing radiation as described previously [101] A. ATP levels, assayed 4 days after irradiation using the luciferin/luciferase assay [102]. B. Mitochondrial mass, assayed 4 days after irradiation, using MitoTracker Green fluorescence measured with flow cytometry, as described [103]. Data are means ± SEM for n = 3; * indicates significantly different from unirradiated group (p<0.05).
Fig 4. Dose-dependent mitigation of respiratory chain effects in irradiated astrocytes by EUK-134.
Rat astrocytes were cultured and exposed to ionizing radiation as described for Fig. 3. EUK-134 was added immediately after irradiation, where indicated. On day 4 after irradiation, cells were harvested and respiratory chain complex activities measured as previously described for Complex I [104], Complex II [105], and Complex III [106]. Y axis indicates percent of the activity of the unirradiated control cells. Data are means ± SEM for n=3. ** indicates significantly different from unirradiated (no EUK-134); * indicates significantly different from 30 Gy (no EUK-134) (p<0.05).
Fig 5. Time course of EUK-134 mitigation of mitochondrial effects in irradiated astrocytes.
Experiment was conducted as described for Fig 4, except that EUK-134 (50 uM) was added at the indicated time, in hours, after irradiation. * indicates significantly different from 30 Gy (no EUK-134).
Animal Models
Pulmonary radiation injury
The lung is one of the most susceptible organs to potentially debilitating radiation injury. The functional effects of pulmonary radiation injury are normally separated into two phases. Radiation pneumonitis generally occurs within 2 to 4 months and fibrosis tends to develop 4 to 6 months after irradiation [46]. Acute pneumonitis is treated with steroids, but can be life-threatening even with treatment. Lung fibrosis is permanent and progressive and can ultimately lead to respiratory distress, pulmonary hypertension, right heart failure and even death. Though the mechanisms are not well understood, extensive data suggest that pneumonitis and fibrosis may result from a cycle of chronic inflammation and oxidative damage initiated by radiation exposure to the lung [4, 7, 47, 48].
In Sprague-Dawley rats subjected to partial lung irradiation, EUK-189 given by a single subcutaneous (sc) injection suppressed micronucleus formation, an indicator of DNA damage in lung fibroblasts, measured 18 hr later [49]. This suppression of micronuclei counts did not persist without subsequent EUK-189 injections, but it was observed even if the EUK-189 injection was delayed until two weeks after irradiation. These findings were consistent with micronucleus formation resulting from an ongoing cycle of injury and repair, consistent with (as discussed above) a continuous “cascade” of ROS formation after irradiation. This theory also implies that sustained treatment with an ROS scavenger such as a salen Mn complex, would be required in order to have a significant impact on lung injury. Thus, despite an intriguing report that a single injection of EUK-189 enhanced hematopoietic survival in lethally irradiated mice [50], the single injections of EUK-189, regardless of timing, did not have a substantial effect on the clinical outcome of radiation lung injury in the rats [49]. Similarly, in a mouse CNS radiation injury model, a single injection of EUK-134 failed to show radioprotective efficacy in preventing loss of proliferating neuronal precursors [51]. Consequently, most subsequent studies with salen Mn complexes have increased drug exposure through longer periods of therapy, either by daily sc injection or by continuous sc infusion utilizing Alzet osmotic pumps.
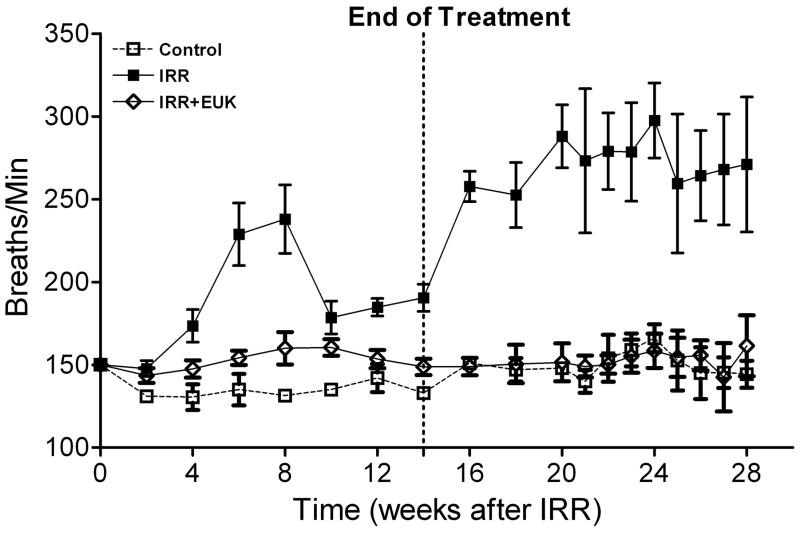
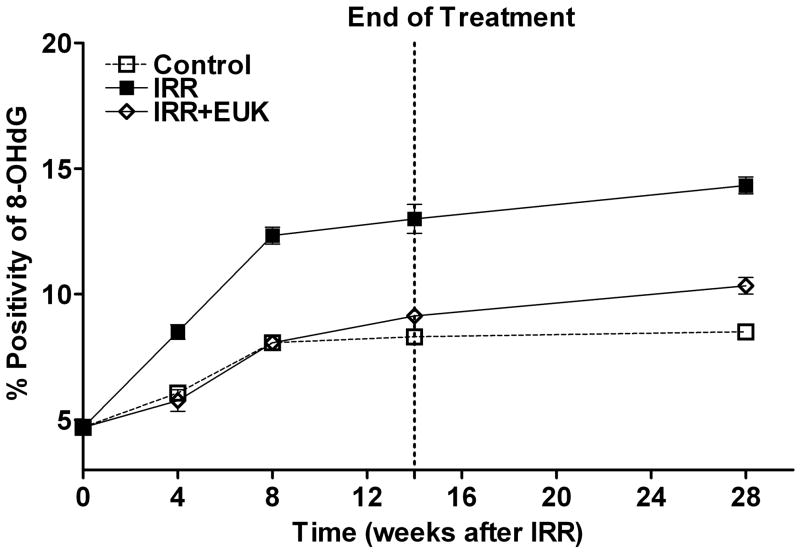
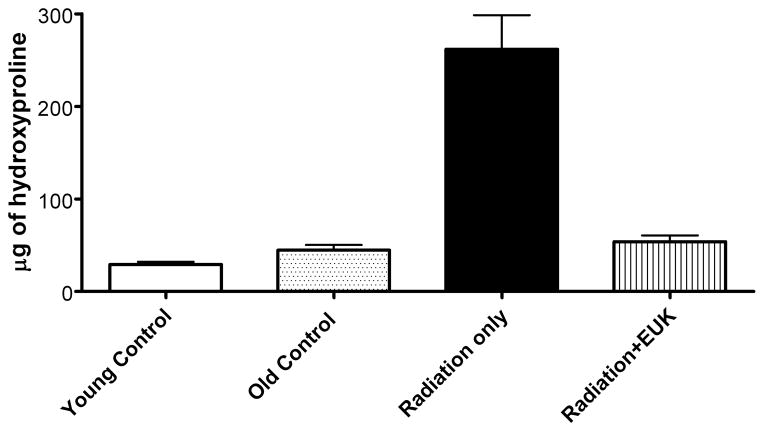
One such study [52], also in Sprague-Dawley rats, utilized the newer generation compound EUK-207, given by continuous sc infusion. Rats received 10 Gy thoracic irradiation and were monitored for indicators of lung injury for 28 weeks, with animals sacrificed for tissue collection at various time points (0, 4, 8 and 28 weeks). They received EUK-207 (~8 mg/kg-day continuous sc infusion) for 14 wks, beginning 1 hr after irradiation. As shown in Fig 6, compared to the unirradiated controls, irradiated rats showed an increase in breathing rate, essentially consisting of two rises, and persisting throughout the analysis period. EUK-207-treated irradiated rats, in contrast, showed normalized breathing rates. It was of interest that the normalization of breathing rate persisted until at least 28 wks, even though drug treatment stopped at 14 wks. A well-established biochemical indicator of DNA damage due to oxidative stress, the oxidized nucleic acid 8-OHdG, was significantly higher in lungs from irradiated, versus unirradiated, rats at all time points (Fig 7). EUK-207 treated irradiated rats showed substantially lower 8-OHdG levels, relative to irradiated control rats, indicating suppression of DNA damage due to oxidative injury. It was also noted that even unirradiated controls showed a slight increase in 8-OHdG over time, perhaps due to some age-related changes. As an indicator of lung fibrosis, collagen levels were monitored in lung tissue using Masson Trichrome stain imaging (not shown) and hydroxyproline content (Fig 8). As the figure shows, lungs from untreated irradiated rats had greatly increased hydroxyproline levels at 28 wks, and the levels were significantly reduced by EUK-207 treatment. Similar results were obtained with Masson Trichrome staining [52]. Interestingly, and of possible relevance to an anti-fibrotic effect, the lungs from EUK-207 treated irradiated rats also had significantly lower TGF-beta levels, comparable to those of unirradiated rats, at 28 wks [52]. The ED-1 stain for activated macrophages was also significantly decreased by treatment of EUK-207 compared to the elevated level of ED-1 stain in irradiated rats only. The effects of drug treatment on other cytokines (IL-1 alpha and beta, IL-6, and TNF-alpha) were less marked, though there were some decreases [52]. Along with EUK-207, Mahmood and colleagues also tested the effects of a diet of genistein, a soy isoflavone with antioxidant and anti-inflammatory properties, and combined genistein with EUK-207 in a third treatment group. Although there were slight differences among them, all treatment groups showed similar effects on lung injury and oxidative stress parameters [52]. Thus, two chemically quite different agents, hypothesized to have similar mechanisms of action, showed significant mitigating effects against radiation injury to the lung.
Fig. 6. Breathing rates in rat lung injury model.
Breathing rate (mean breaths per minute) is given as a function of time after irradiation (10 Gy) for rats receiving no radiation (Control), radiation only (IRR) or radiation plus EUK-207 (IRR + EUK). Additional experimental details are described in the text and in the original study [52]. Drug treatments started immediately after irradiation and finished at 14 weeks. Each point represents the mean (±SEM) for all rats available for analysis at the different times. Statistical analysis (Tukey’s method, p<0.05) showed that the breathing rate in IRR + EUK rats did not differ from that of controls and that both differed from IRR.
Fig 7. Nucleic acid oxidation (8OHdG levels) in rat lung injury model.
Experimental details of the lung injury model, drug treatment, and statistical analysis are as described for Fig 6. Lung tissue was extracted and analyzed by immunohistochemistry for 8-OHdG as described [52]. The assays were performed at 0, 4, 8, 14 and 28 weeks following irradiation. Percent positivity is the ratio of positive pixels/total number of positive and negative pixels in the tissue section (air spaces excluded). Each bar (A) or each point (B) represents the mean (±SEM). The 8OHdG levels in the EUK+IRR treated group was significantly lower than in IRR, and did not differ from that of the Control group, at all time points following irradiation (4 - 28 wks).
Fig 8. Collagen synthesis (hydroxyproline content) in rat lung injury model.
Experimental details of the lung injury model and drug treatment are as described for Fig 6. Hydroxyproline content (μg of hydroxyproline/100 mg of wet lung tissue) was determined as described [52] in lung tissue collected at 28 weeks following irradiation. Each bar represents the mean (±SEM). Data from unirradiated controls were obtained from lungs from rats with ages corresponding to the time of radiation (age 7–8 wks), “young” and 28 wks later, “old”. The hydroxyproline content of the “Radiation only” group was significantly higher than that of the other groups, which did not differ from one another.
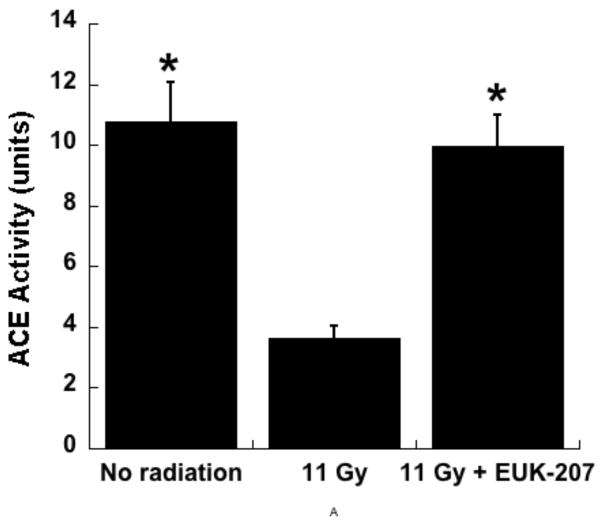
In another type of rat model for radiation pneumonitis, WAG/RijCmcr rats were exposed to 11Gy total body irradiation (TBI) followed by syngeneic bone marrow transplant (BMT). This protocol avoids death caused by hematopoietic injury. Under such conditions, the animals develop pneumonitis by 6 weeks post-irradiation and those surviving the lung injury go on to develop radiation-induced renal injury, as discussed further in the following section. When this strain of rats is exposed to whole thoracic radiation, the resulting pulmonary injury is indicated by increased breathing rate, right ventricular hypertrophy, increased pulmonary vascular resistance and other vascular abnormalities [53, 54]. This radiation-induced pneumonitis is mitigated by the angiotensin converting enzyme (ACE) inhibitor captopril [55]. Using the TBI/BMT protocol, a combination therapy of a suboptimal dose of captopril (100 mg/m2/day) and EUK-207 (1.8 mg/m2/day), starting 7–10 days post irradiation and continuing for 8 weeks, showed promise in mitigating radiation-induced pneumonitis [56]. The radiation- induced increase in right ventricular hypertrophy and pulmonary vascular resistance were decreased by EUK-207 and captopril, either alone or in combination [57]. In addition, while isolated perfused lungs from irradiated rats exhibit lower ACE activity than sham (unirradiated) controls, this radiation-induced drop in ACE activity was prevented by EUK-207 (Fig 9). As shown previously using whole thoracic irradiation protocols, ACE activity measured in perfused lungs is lower after irradiation, suggesting a decrease in the number of endothelial cells or an endothelial cell dysfunction [53, 58]. The finding that EUK-207 therapy prevents the loss of ACE activity is consistent with a preservation of functional vasculature in the irradiated rats, perhaps via prevention of endothelial cell loss. Consistent with this hypothesis, Ghosh et al. [53] demonstrated, using imaging techniques, substantial loss of vessel density in irradiated lungs. Other options such as a direct induction of ACE expression by EUK-207 are, in addition, possible.
Fig 9. EUK-207 mitigates radiation induced loss of angiotensin converting enzyme (ACE) activity in the rat lung.
Experiment in WAGRijCmcr rats subjected to TBI (11 Gy) followed by BMT was conducted as described in the text and ACE activity was measured in isolated perfused lungs 6 wks after irradiation as described. Total lung ACE activity is represented as the mean surface area product [ml/min] based on a spectrophotometrically monitored substrate cleavage [53]. The data are means ± SEM, for n= 9 (No radiation), 7 (11 Gy), and 8 (11 Gy+EUK-207). * indicates significant difference from the 11 Gy group (one way ANOVA followed by Bonferroni’s t test).
Similar to the findings in rats, EUK-207 has mitigating effects in mouse models for radiation lung injury. Williams et al [59] have developed mouse models in which C57BL/6J or CH3/HeJ mice receive 5Gy to total body followed by 10Gy to lung only. This method causes lethal lung injury while minimizing death due to hematopoietic injury. The response of these two strains to radiation is being studied by monitoring indicators of pulmonary injury and plasma cytokine levels for up to 15 months. When administered by daily sc injection using an acute (days 1 to 7 post-irradiation) or chronic (from wk 8 to 26) administration, EUK-207 (30 mg/kg-day) improved survival in both strains of mouse, especially when co-administered with simvastatin. Survival was associated with a decrease in inflammatory infiltration and down-regulation of the proinflammatory cytokines IL-1-beta and MCP-1 [59].
Renal radiation injury
Months to several years after undergoing bone marrow transplant requiring TBI, some patients develop renal failure and there is considerable evidence that irradiation plays a causal role [60]. As discussed above, a TBI/BMT protocol induces radiation nephropathy in WAG/RijCmcr rats that is delayed in onset by weeks to months. Loss of renal function in this model is indicated by the primary clinical endpoints including azotemia (measured as increased Blood Urea Nitrogen, BUN), proteinuria (and the urine protein to creatinine ratio, UP/UC), and hypertension. Moulder and colleagues have reported that captopril and other modulators of the renin-angiotensin system mitigate renal injury [61, 62], while a variety of other agents, including several antioxidants [63] are ineffective. Despite the lack of effect of other antioxidant approaches, including a genistein diet [63], several experiments were conducted to evaluate salen Mn complexes as potential mitigators in the rat renal injury model [64].
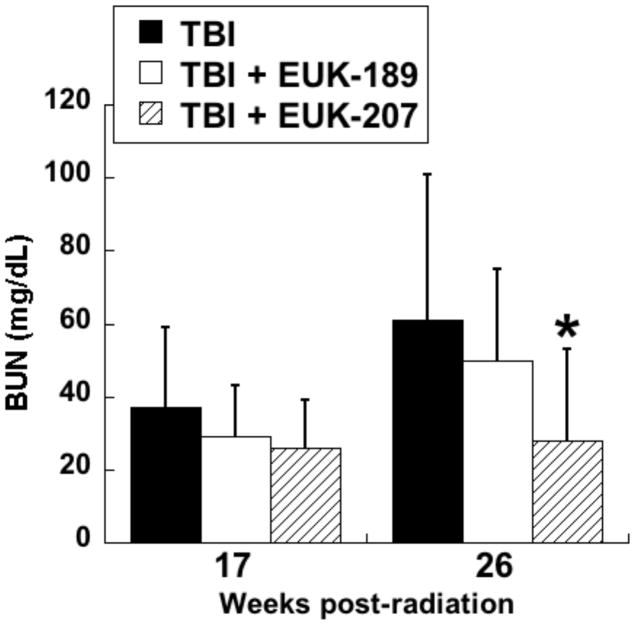
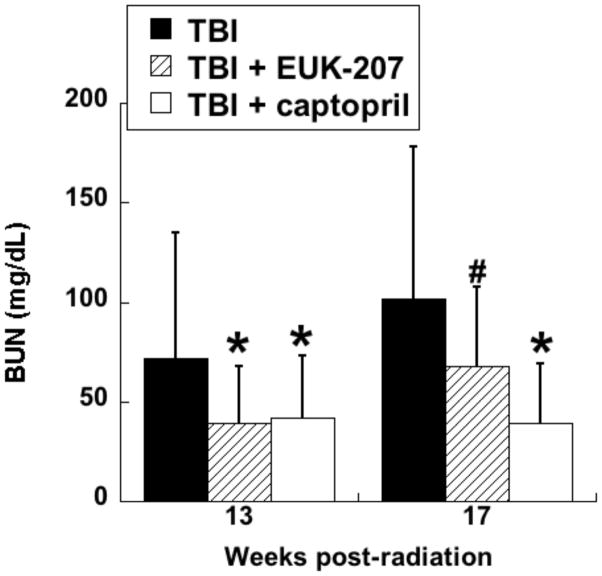
In the first series of experiments, the compounds were administered by continuous sc infusion; therapy was for 12 wks, beginning 3 wks after irradiation, with Alzet osmotic pumps delivering a constant volume, resulting in a dose of 8 to 10 mg/kg-day. The first experiment tested only EUK-189, and it was found to have no significant mitigating effect, consistent with what was seen with other antioxidant approaches in the radiation nephropathy model [63]. A second experiment compared EUK-189 and EUK-207. As shown in Fig 10, EUK-207 mitigated renal injury caused by 9 Gy TBI, while, again, EUK-189 had no significant effect. It is interesting to note that this mitigation was observed 26 wk after irradiation, 11 wks after drug treatment had ended. In a third experiment, employing 10 Gy TBI to accelerate renal injury, EUK-207 was compared to captopril, the latter given orally in the drinking water (12 to 15 mg/kg-day), in the same schedule as that used for EUK-207. As shown in Fig 11, mitigation by EUK-207 was comparable to that of captopril when measured at 13 wks, though with a lesser degree of effectiveness at 17 wks, 2 wks after termination of drug therapy.
Fig 10. Mitigation of radiation renal injury by EUK-207 but not EUK-189.
WAG/RijCmcr rats were subjected to TBI (9 Gy) followed by BMT and renal injury monitored as described in the text. Salen Mn complexes were administered by continuous sc infusion for 12 wks, beginning 3 wk after irradiation, as described in the text. The infusion was based on constant volume delivery, resulting in a dose of 8 to 10 mg/kg-day. The data are mean BUN for n=6 and error bars indicate 95% confidence intervals. * is significantly different from TBI only (p<0.01, Mann-Whitney). Normal range of BUN in these rats is 18 to 22 mg/dL.
Fig 11. Mitigation of radiation renal injury by EUK-207 and captopril.
Experiment was conducted as described for Fig 10, except that the TBI dose was 10 Gy. EUK-207 was given as described for Fig 10. Captopril (12 - 15 mg/kg-day) was administered in the drinking water over the same treatment period. The data are mean BUN for n=6 to 7 and error bars indicate 95% confidence intervals. *, # denotes difference from TBI only (p<0.01, #p<=.07, Mann-Whitney). Normal range of BUN in these rats is 18 to 22 mg/dL.
As discussed above, captopril and other agents acting on the renin-angiotensin system are regarded as the “gold standards” for mitigating radiation-induced renal injury, whereas a variety of other antioxidant agents have shown no benefit. Thus, the activity of EUK-207 in the kidney is of particular interest, as is the lack of effect of EUK-189. This finding differs from those in a mouse age-associated cognitive impairment model, in which EUK-189 and EUK-207 showed comparable efficacy, including dose-dependency [21]. Perhaps in the renal model the greater plasma half-life of EUK-207, versus that of EUK-189 (Fig. 2), is important for mitigating renal injury, supporting a vascular site of action for the compounds in the radiation nephropathy model. It was noted during the sc infusion studies that the dose of EUK-207 employed in the pumps caused a skin reaction in a subset of irradiated rats, in some cases requiring pump removal. Lower doses (about 2 mg/kg-day) were found to be ineffective in mitigating radiation nephropathy. This is contrast to the lung and skin models in the same strain of rats, where this lower dose range of EUK-207 did cause substantial mitigation of radiation injury.
To test an alternative method of delivery to the infusion pumps, an experiment was conducted using sc daily injections of EUK-207, beginning 48 hr after irradiation and continuing for 10 wks. As shown in Table 2, EUK-207 at 30 mg/kg per day showed significant mitigating activity, whereas lower doses did not. The effective dose of EUK-207, although well tolerated in other rodent models, also caused localized skin reaction hindering its repeated injection at the same site. Apparently, the compromised skin in TBI-treated WAG/RijCmcr rats is vulnerable to this reaction, the mechanism of which is not yet understood. In another study similar to that described in Table 2, EUK-207 was combined with captopril at sub-optimal doses (10 mg/kg-day sc and 150 mg/l in drinking water, respectively). The combination showed no more activity than the captopril alone, suggesting a lack of additivity or synergy between the two treatments. Such data suggest that the lower dose of EUK-207 given by daily injection, 10 mg/kg-day, is ineffective. Nonetheless, the higher dose (30 mg/kg-day sc injected or 8–10 mg/kg-day by continuous sc infusion) does have substantial mitigating activity in the renal injury model.
Table 2. Mitigation of radiation renal injury with EUK-207 (sc daily).
Renal injury was induced in WAG/Rij rats using TBI/BMT as described in the text. The radiation dose was 10 Gy. Rats treated with EUK-207, at the indicated daily doses, received sc injections beginning 48 hr after irradiation and continuing for 10 wks. Proteinurea (UP/UC) was measured at 9 and 17 weeks. BUN was measured at 9, 17, 21 weeks and at sacrifice. Blood pressure (BP) was measured at 17 weeks. The median survival and range are shown in the first data column. The data for other parameters are means (with 95% confidence levels); n = 9–10. Results of statistical testing (Mann-Whitney, corrected for multiple comparisions):
| Group | Median survival (days)* | BUN at 17 wks (±95% CI) | UP/UC at 9 wks (±95% CI) | BP at 17 wks (±95% CI) |
|---|---|---|---|---|
| TBI alone | 106 (95–120)a | 140 (120–163)a | 17 (13–23)a | 152 (132–171)a |
| 30 mg/kg/day | 140 (136–152)a,b | 59 (43–81)a,b | 0.8 (0.6–1.1)b | 139 (131–147)a |
| 10 mg/kg/day | 113 (109–128)a | 124 (96–156)a | 19 (10–38)a | 148 (137–159)a |
| 3.3 mg/kg/day | 115 (93–127)a | 124 (82–186)a | 25 (22–30)a | 154 (136–172)a |
| 1.1 mg/kg/day | 96 (75–101)a | 191 (118–309)a | 24 (21–28)a | 160 (150–170)a |
| 0.33 mg/kg/day | 99 (79–112)a | 152 (112–205)a | 25 (20–32)a | 161 (152–171)a |
p<0.05 vs age-matched normal;
p<0.05 vs TBI alone; all other comparisons p>0.10
time to BUN=120 (with range)
However, efficacy of salen Mn complexes would not necessarily have been predicted because the mechanism of renal injury in this model, particularly the involvement of oxidative stress, is in question. A study examining several indicators of oxidative injury, including 8-OHdG and protein carbonyl, failed to find evidence for increased oxidative stress in irradiated, versus unirradiated rats in this TBI/BMT model [65]. As the authors concluded, “if chronic oxidative stress is part of the pathogenesis of radiation nephropathy, it does not leave widespread or easily detectable evidence behind”. Results in the radiation nephropathy model contrast with those in the radiation-induced pulmonary injury model [52], where there was a clear increase in 8OHdG levels in lungs from irradiated rats, and a substantial decrease with EUK-207 treatment. In addition, as noted above, EUK-207 and genistein had comparable effects in the lung injury model [52], whereas genistein and several other antioxidants were ineffective in the renal model [63]. Such observations, while not excluding its involvement, bring into question the importance of oxidative stress, and its mitigation, in the renal injury model. The mitigating effects of EUK-207 might involve another mechanism altogether but, in any event, EUK-207 was not as effective as captopril in this model. Further supporting its use as a mitigator of radiation renal injury, captopril is an FDA-approved drug that has been used widely as an antihypertensive, and its safety has been established in patients undergoing TBI in preparation for bone marrow transplantation [66]. While EUK-207 may also be of therapeutic use to mitigate renal radiation injury, its use as an adjunct or alternative therapy to captopril is not, to date, well-supported by the rodent model data.
Oral mucosa
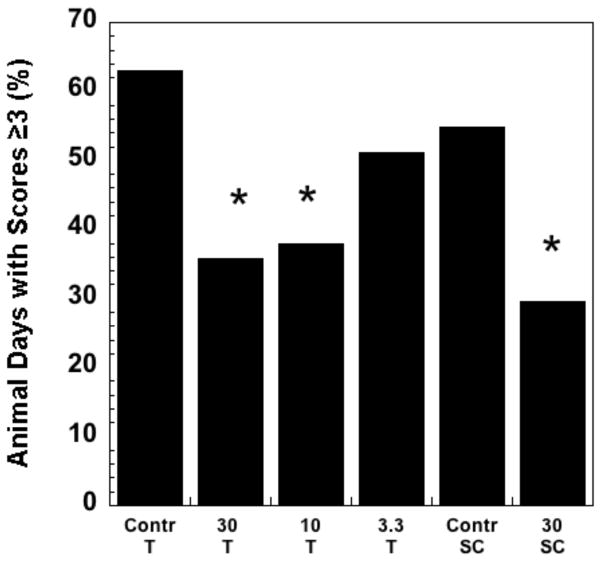
Oral mucositis is a dose-limiting toxicity in patients receiving chemotherapy or localized radiation treatment for head and neck cancers [67, 68]. ROS have been an interventional target for the prevention and treatment of mucositis, with N-acetylcysteine as one agent under study. The ability of EUK-189 to mitigate radiation-induced mucositis was tested in a hamster model, performed as described previously [69]. EUK-189, as compared to EUK-207, is particularly attractive as a topically administered agent because of its greater lipophilicity (Table 1) as well as its shorter plasma half-life (Fig. 2). The latter property should help to minimize any unwanted systemic effects in an agent intended to act locally. Briefly, the cheek pouches of anesthetized hamsters were everted to expose the oral mucosa and the surface was irradiated with a single exposure of 35 Gy. This resulted in severe to moderate oral mucositis by 15 days. EUK-189 was administered by two methods in this study. One was a daily sc injection (30 mg/kg), given immediately after irradiation and each day thereafter, through day 14 (with day 0 being the day of irradiation). A second method utilized topical administration. EUK-189 solutions in water, at three different concentrations (3.3, 10 and 30 mg/ml) were applied four times on each day (from day 0 til 14) in the following manner: one observed application, where 0.2 ml EUK-189 solution was applied directly to the mucosa of an anesthetized hamster; three blind applications, where 0.3 ml of EUK-189 solution was delivered into the cheek pouch of the conscious animal. Vehicle (water) was administered to control groups in an analogous manner. Exposed buccal pouches were photographed on alternate days, beginning at day 6 and photographs scored blindly for mucositis severity, as described [69]. Using this standard 6-point severity score, a score of greater than 3 in this animal model corresponds to National Cancer Institute or WHO clinical scores of ≥3 [69], indicating a clinically significant ulcerative mucositis. Consequently, the data are presented as the prevalance of a mucositis score of ≥3 [69]. As shown in Fig 12, both methods of EUK-189 administration resulted in a significant decrease in mucositis severity. With the topical administration protocol, the two higher doses of EUK-189 were effective. This protocol was included because it mimics the way the drug might be given clinically, with a lozenge or mouth rinse the likely formulations for patients. The sc administration was included as a “positive control”, since this route of EUK-189 delivery has shown efficacy in other pharmacological models. Though some of the “topical” EUK-189 rinse was probably also swallowed by the animals, it is not likely that this added to its effect, as EUK-189 is not orally bioavailable [70].
Fig 12. Improvement of radiation-induced ulcerative mucositis in the hamster by EUK-189.
The hamster mucositis model and scoring system are described in the text and in a previous study [69]. EUK-189 dosing was topical (T) or subcutaneous (SC), using protocols described in the text. The figure shows the percent animal days with a mucositis score ≥ 3. Group sizes were n = 8. * indicates statistically different from corresponding vehicle control group (chi square). The T and SC control groups were not significantly different from one another.
It is interesting to note that the prolonged, 15 day, administration of EUK-189 was effective using both dosing protocols. In contrast, efficacy in attenuating oral mucositis by the SOD mimetic M40403, a Mn macrocyclic complex reported to lack catalase activity [71], was dose-schedule dependent in this model [69]. While M40403, given by ip injections (30 mg/kg) twice per day, from day -1 to day 3, mitigated oral mucositis, the mitigation effect was absent when the compound was given from day -1 to day 15. Also, administration of M40403 beginning on day 0 was more effective than beginning before irradiation (day -1), with a day 0 through 3 dosing having the greatest effect. One can speculate on a number of possible explanations for the apparent differences in schedule dependence between these two compounds. One hypothesis is that excess hydrogen peroxide could prolong mucositis by impairing healing, similar to the complex role that this ROS is known to play in skin wound healing [72]. Possibly, extended dosing with the SOD mimetic M40403 resulted in later increases in hydrogen peroxide, whereas EUK-189 scavenges both superoxide and hydrogen peroxide. Nonetheless, both mucositis studies do support the potential value of both classes of ROS scavenging Mn complexes in mitigating radiation-induced oral mucositis. Given the commonality in underlying pathogenesis [69], it is also likely that both compounds would also be efficacious in attenuating chemotherapy-induced mucositis.
Cutaneous radiation injury
Radiation dermatitis is a consequence of radiation therapy, for example in breast and lung cancer patients, and cutaneous radiation exposure can impair wound healing. The cutaneous injury after radiation exposure involves a combination of inflammatory processes and alteration of cellular proliferation involving release of pro-inflammatory cytokines, growth factors, and adhesion molecules [73]. Furthermore, fibrinogenesis and angiogenesis are directly inhibited by ionizing radiation. Previous studies indicate that ionizing radiation causes fibroblasts to show reduced proliferation, atypical migration, and abnormalities in collagen synthesis [74, 75]. Several studies reported changes in the extracellular matrix composition after radiation injury affecting the healing time of wounded skin [76, 77]. Moreover, a growing body of evidence supports a causative role of oxidative stress in the development of cutaneous radiation syndrome and delayed healing response following radiation exposure [3, 7]. Such data imply that salen Mn complexes could have beneficial effects in models for cutaneous radiation injury. Thus, EUK-207 was tested in a rat model for combined radiation dermatitis and skin wound healing [78–80]. In this model, WAG/RijCmcr rats were irradiated dorso-ventral with a 15kVp soft x-ray beam that has a steep dose gradient, a protocol enabling delivery of high doses (10 to 40 Gy, defined at the upper dermis) to the skin without significant exposure to internal organs. Rats received full thickness wounds by punch biopsy within one hour of irradiation. Dermatitis was scored weekly using a semi-quantitative scoring system for skin injury [81] and wound closure was monitored by image analysis of traced wound areas. Irradiated rats developed, in a radiation dose-dependent manner, cutaneous radiation reactions ranging in severity from transient erythema (onset 24 hours, duration 1–2 days) to large deep scabs and non-healing ulcers (onset day 21, duration up to 90 days), plus alopecia (onset day 20, duration up to 90 days) and skin fibrosis (onset day 30, duration entire period of study). There was markedly impaired wound healing, also dependent on radiation dose. The peak of acute skin reactions was ~ 30 days. As reported [78–80]. a number of other assessments demonstrated radiation-induced changes in extracellular matrix deposition, blood vessel proliferation, gene expression changes and oxidative stress indicators in the skin.
EUK-207 was evaluated in this cutaneous combined injury model, using the 30 Gy radiation dose that, without drug treatment, induced severe cutaneous injury and wounds that failed to heal over the 90 day observation period. EUK-207 (~1.8 mg/kg-day) or vehicle (water) were given by sc Alzet infusion pumps beginning 48 hr after irradiation and continuing for up to 90 days. As reported [78, 80], there was a dramatic mitigation effect of EUK-207 on radiation injury, including improvement of wound healing. Within one month, EUK-207 treated rats had reduced moist desquamation, reduced tissue inflammation, and increased wound contraction. Unlike the vehicle treated groups, those treated with EUK-207 showed only slight tissue fibrosis at 90 days post-irradiation, and interestingly, there was even growth of new hair within the center of the radiation field. Also unlike the vehicle treated animals, those in the EUK-207 treatment groups had wounds that were fully healed.
While, based on its other in vivo effects, the mitigation of radiation dermatitis by EUK-207 was not unexpected, the beneficial effect of an ROS scavenger on wound healing in this combined-injury scenario would not necessarily have been predicted. ROS, particularly hydrogen peroxide, have been reported to play key signaling roles to promote wound healing, and localized transfection of catalase delays healing in rodents [72, 82]. Yet, in other experiments, excess hydrogen peroxide impaired healing [72] and transfection with sod2 improved wound healing [83] as did chronic administration of a mitochondrially targeted antioxidant to aged mice [84]. Thus, the role of ROS, particularly hydrogen peroxide, and of redox regulation in cutaneous wound healing is highly complex. Despite this, our study and others support the notion that selected ROS-scavenging agents such as EUK-207, given under the right circumstances, can mitigate radiation-induced skin injury, including facilitating wound healing.
IV. Future directions and challenges
Drug delivery methods
As discussed in this review, salen Mn complexes, especially EUK-207, mitigate radiation injury to a number of normal tissues. One important issue to be addressed in their further development as mitigating agents is the method of drug delivery. Evidence indicates that systemic use for a number of days, or even weeks, may be needed for optimal effectiveness. With the chronic, continuous nature of the oxidative stress that is implicated in delayed radiation injury [7], this is not at all surprising. It is promising, however, that mitigating effects of EUK-207 in pulmonary and renal models persist weeks after drug therapy has ended. Currently, daily injection and continuous sc infusion are the most effective means of delivery for salen Mn complexes. Such methods of delivery, even portable infusion pumps such as those delivering insulin, are tolerated by patients for certain chronic diseases, but are not the most desirable. Oral administration, instead, would be very convenient for some of these indications. Salen Mn complexes, even EUK-207 which has high stability to gastric solutions (Table 1), are not orally available in rodents [70]. We [70, 85] and others [86] have described low molecular weight uncharged Mn porphyrins which have ROS scavenging properties and also can be orally delivered to rodents. The series that we have studied, in particular compounds known as EUK-418, EUK-423 and EUK-451, are, like EUK-189 and EUK-207, anti-apoptotic in a PC12 cell culture system [70] and mitigate apoptosis of capillary endothelial cell cultures induced by ionizing radiation [42]. While such studies suggest that these “EUK-400 series” compounds may be promising for mitigation of radiation injury in vivo they are, as a class, more cytotoxic than EUK-189 and EUK-207. Their efficacy in both cell culture systems was limited by their toxicity, with EUK-451 being the most effective Mn porphyrin and the least toxic. But the salen Mn complexes EUK-189 and EUK-207 were even less toxic, displaying no cytotoxicity at any of the doses tested. Of course, an increased toxicity would be tolerable if the compound were substantially more potent, but these Mn porphyrins were only slightly more (about 3-fold) potent than salen Mn complexes in the cellular models [42, 70]. In a preliminary in vivo study, EUK-451, administered to WAG/RijCmcr rats via drinking water and, as a positive control, by sc osmotic infusion pumps, did not mitigate radiation renal injury (Fish, Moulder et al., unpublished data). However, studies to optimize dosing and pharmacokinetics remain to be conducted. In addition, the renal injury model, in which, as discussed above, very few agents show any mitigating activity and the role of oxidative stress is questionable, is not necessarily the best choice for testing an unknown agent hypothesized to act as an ROS scavenger. An orally available Mn porphyrin developed by another group [87] does show neuroprotective efficacy in a rodent Parkinson’s disease model [86] suggesting that the approach of investigating low molecular weight Mn porphyrins still has the potential to lead to interesting orally bioavailable therapeutic agents. For further discussion of Mn porphyrins, other chapters in this volume review the use of this class of Mn-ligand complex to mitigate radiation injury, including compounds that, like salen Mn complexes, are given by injection or infusion. For example, a catalytic Mn porphyrin ROS scavenger known as AEOL 10150 mitigates lung injury caused by thoracic irradiation in the rat when given by continuous sc infusion [88, 89].
For some normal tissue radiation mitigation indications, a topical means of delivering salen Mn complexes, if effective, would be therapeutically practical. Some data with these compounds are promising in this regard. In the hamster mucositis model, a topical dosing regimen, consisting of direct application of EUK-189 to the cheek pouch mucosa, showed efficacy equivalent to that of sc injection. In patients, a lozenge or mouth rinse could be useful to mitigate oral mucositis. In the cutaneous injury studies described here, only systemic EUK-207 was tested. However, EUK-189 and EUK-207 can be formulated into a topical preparation that reduces ear inflammation in the mouse (Doctrow et al., unpublished data). Furthermore, topical application of an EUK-134 preparation to the skin of human volunteers was shown to prevent skin lipid peroxidation caused by UVA exposure [90]. Such data support the future testing of topical preparations of salen Mn complexes to mitigate cutaneous radiation injury. Inhaled methods of EUK-207 delivery are also under investigation, especially for pulmonary radiation injury (Williams, Finkelstein, Doctrow and colleagues).
Mechanism of action: role of ROS scavenging in mitigation
As discussed throughout this review, the mechanisms of action of salen Mn complexes, as with any other mitigator of delayed radiation injury, are likely to be complex. Increased understanding of these mechanisms could help in future development of analogs, as well as improved drug delivery and dosing protocols, optimized for each specific radiation injury indication. Data in astrocyte cultures support the hypothesis that the compounds’ “mito-protective” properties are relevant to delayed radiation injury, though this should be further studied in other cell types as well as in vivo. Findings in certain models support the hypothesis that salen Mn complexes are acting as ROS scavengers in vivo. For example, in one rat lung injury model [52], EUK-207 treatment mitigated signs of pneumonitis and fibrosis, while concomitantly decreasing lung levels of oxidized nucleic acid. Similar mitigation of oxidative-stress associated markers was reported in the cutaneous injury model [78, 80]. While such associations do not prove causality they are promising indicators that the ROS scavenging properties of these agents measured in vitro extend to an ability to mitigate oxidative stress in vivo. In the renal injury model, however, the role of oxidative stress is in question [63, 65]; and EUK-207 mitigated renal injury, while numerous antioxidant agents, including EUK-189, did not. Therefore, for the renal injury model, other mechanisms, including some hypothetical modulation of the renin-angiotensin system, should be addressed in future research. More generally, however, it seems likely that combined therapies using two or more agents with distinct mechanisms of action are likely to be more effective than one agent alone for a complex indication such as delayed radiation injury [59].
The pro-oxidative effects of redox cycling agents, including ROS scavenging compounds, can potentially cause toxicities or confound understanding of the mechanism of action in biological systems [6]. Any ability of an ROS scavenger to also generate ROS might be regarded as a “pro-oxidative” activity. As discussed elsewhere in this review, the ability of a “pure” SOD mimetic, or the enzyme SOD, to generate hydrogen peroxide can potentially cause damaging effects. In an intestinal cell culture model, EUK-8 had a protective effect against injury caused by acidosis, whereas bovine CuZnSOD alone exacerbated injury, an effect that was reversed by the addition of bovine catalase [91]. In general, while pro-oxidative effects of salen Mn complexes have not been ruled out in all biological contexts, the toxicity of some analogs in cell culture is not associated with their inherent catalytic properties but, instead, with other parameters such as instability and release of toxic metabolites of the ligand [5]. In a wide range of in vivo models, as reviewed earlier, salen Mn complexes decrease, rather than increase, biochemical indicators of oxidative stress such as oxidized nucleic acids and protein tyrosine nitration. While they suppress signals of ROS “probes” in many instances, under some conditions salen Mn complexes cause increased fluorescence of the commonly used “ROS-indicator” dye dichlorofluoroscein (DCF) [51, 92]. This does not appear to be an ROS-generating effect. Instead, this effect is likely due to the peroxidase activity of salen Mn complexes, that is, their ability to catalyze oxidation of substrates in the presence of hydrogen peroxide. Peroxidase enzymes are among the arsenal of endogenous antioxidant defenses [6] and peroxidase activity is an inherent component of the hydrogen peroxide scavenging mechanisms of salen Mn complexes [5], Mn porphryins [93], and mammalian catalase enzymes [94]. Though it can be oxidized by other ROS, DCF is considered to be an intracellular “ROS indicator” primarily because it is an oxidizable substrate whose fluorescence is increased by endogenous cellular peroxidases acting in the presence of hydrogen peroxide [6]. In cultured rat neural progenitor cells, Limoli et al [51] found that EUK-189 caused an increased DCF fluorescence in either irradiated or unirradiated cells. This experiment was repeated with two other salen Mn complexes having comparable SOD activities; EUK-172, an analog with a high catalase/peroxidase activity, stimulated DCF fluorescence, while EUK-163, with no significant catalase/peroxidase activity, did not. If generation of hydrogen peroxide had been the reason for increased DCF fluorescence, EUK-163, with SOD but no catalase activity, would have been expected to cause higher, not lower, fluorescence than EUK-172. This observation supported the hypothesis that salen Mn complexes can act as functional peroxidases inside cells acting on oxidizable substrates such as DCF. Essentially, the compounds are metabolizing, rather than generating, hydrogen peroxide in the process and, therefore, are not behaving as “pro-oxidative” agents. Such observations also illustrate the need to understand the potential artifacts associated with any fluorescent probe when using it as an indicator substance.
Reaching sites of action: lipophilicity, stability and other properties
The cellular or subcellular target for a given form of radiation injury should have considerable influence on the properties required of a mitigator. As discussed throughout this review, potential cellular and subcellular sites of action include the vascular endothelial cell and the mitochondria, respectively. An extravascular site of action, if needed for a given indication, would require the agent to transit the vascular wall and permeate tissues. Efforts to improve biological efficacy of salen Mn complexes have included increasing lipophilicity which should enhance delivery into cells and across vascular barriers. As a class, salen Mn complexes are more hydrophilic than lipophilic, though a modest increase in lipophilicity has been found to increase their effectiveness in certain experimental models. For example, EUK-189, while having the same ROS scavenging activities as EUK-134, is more lipophilic, due to its having ethyoxy rather than methoxy side chains [5]. This increased lipophilicity is hypothesized to explain why EUK-189 is more potent than EUK-134 at preventing neuronal apoptosis [43] and is more effective as a “mito-protector” in sod2−/− mice [26]. Yet, lipophilicity alone does not determine effectiveness. For example, EUK-207 which, as discussed above, is more stable than EUK-189 and is also considerably less lipophilic (Table 1) [17, 70]. Both compounds showed equivalent potency and efficacy in an age-related cognitive impairment model [18, 21], and it was suggested that the decreased lipophilicity of EUK-207, relative to that of EUK-189, was balanced by its increased intracellular stability. Indeed, in cell culture studies, EUK-207 is more potent than EUK-189 in suppressing apoptosis [70], an intracellular injury, while having similar potency against extracellularly generated hydrogen peroxide (Doctrow et al., unpublished data), in a model in which catalase activity of a compound is related to its cytoprotective activity [5]. Attempts to increase the lipophilicity of cyclized salen manganese complexes have included EUK-207 analogs with aromatic bridge structures [20] or 5,5′-alkoxy substituents (Doctrow et al., unpublished). Such compounds were not appreciably more effective than EUK-207 in cellular models and, in some cases, were more toxic. The orally available “EUK-400 series” compounds were, for the most part, substantially more lipophilic than the salen Mn complexes to which they were compared. However, the most cytoprotective, least toxic Mn porphyrin, EUK-451, was the least lipophilic, with a negative logP value (Table 1), more similar to those of the salen Mn complexes than to the other “EUK-400 series” compounds [70]. While not conclusive, such observations do suggest that too much lipophilicity, besides making an agent difficult to formulate, could have toxicology implications. Overall, several factors such as lipophilicity, inherent stability, potential toxicity and, of course, in vivo efficacy must be weighed against one another in selecting a lead compound. The preponderance of data, in vitro as well as in vivo, indicates that EUK-207 exhibits a number of properties that justify its selection as a lead salen Mn complex.
Safety and tolerability
As with any class of therapeutic agent, the safety and tolerability of salen Mn complexes must be determined in the course of a normal drug development program. To date, salen Mn complexes have not undergone formal clinical testing. Salen Mn complexes, as noted above, are substantially less cytotoxic than Mn porphyrins such as the “EUK-400 series” compounds [70]. They are also well tolerated in chronic administration, for example, EUK-189 and EUK-207 were given to aged mice by continuous infusion for up to 6 months with no adverse effects noted [21]. The original prototype EUK-8 was given to mice by repeated injections (30 mg/kg ip, 3 times per wk) for 24 wks with no discernable impact on mortality, body weight or gross pathology assessment [16]. As discussed above [42, 70], EUK-189 and EUK-207 displayed no cytotoxicity under conditions where the “EUK-400 series” of Mn porphyrins did. Among a series of salen Mn complexes, those analogs that did exhibit toxicity in cell culture and in vivo included those that were less stable, apparently releasing toxic metabolites [5]. The renal injury model studies, however, do indicate that EUK-207 can cause localized reactions at high doses in irradiated skin, though this might not occur in other strains or species and other methods of delivery, even other formulations, might overcome this issue.
The role of the metal in a metal-ligand complex
The presence of a metal, even one such as Mn that is normally present in vivo, would be a potential toxicity concern for any agent, and would need to be addressed. Indeed, toxicity issues aside, the role of free metal, either as a contaminant [95], transferred by a metal-metal exchange or released via metabolism of the complexes, in the biological effects of any synthetic metal ligand complex is also an issue relevant to its mechanism(s) of action. In the case of salen Mn complexes, structure-activity relationship studies showed that Mn acetate salts had no catalase activity or cytoprotective properties, though they show SOD activity [5]. It is also of note that, among salen Mn complexes, more stable metal-ligand complexes show greater potency in cytoprotective assays, for example, EUK-207 is effective at lower doses than EUK-189 at preventing apoptosis, while the two compounds have similar catalytic properties [70] and essentially equivalent potency at protecting cells against extracellular hydrogen peroxide, where, unlike the apoptosis assay, intracellular stability of the salen Mn complex is not a factor (Doctrow et al., unpublished data). Other analytical controls, such as ESI-MS, chelator incubation studies [70, 95] and catalytic activity analysis of HPLC peaks corresponding to the intact metal-ligand complex can provide evidence that free metal contaminants are either not detectable, or are not responsible for biological properties of interest in vitro [70]. However, such control experiments cannot rule out the possibility, which exists for any therapeutically investigated metal-ligand complex, that the drug functions in part by delivering active metal to some site of action. Indeed, effective delivery of Mn could very well be a component of the in vivo mechanism of action of salen Mn complexes, Mn porphryins, and other biologically active metal ligand complexes. Though such complex mechanistic questions are difficult to address, future research in the field might help to illuminate them. More broadly, because of the interesting, potentially promising biological activities of metals as components of inorganic complexes, the field of “Metals in Medicine”, or medicinal inorganic chemistry, is of growing importance [96, 97]. At least three other classes of Mn-containing compounds have undergone clinical testing, either as diagnostic imaging agents [98] or experimental therapeutics [99, 100]. This should help to pave the way for developing new classes of Mn-based therapeutic agents such as salen Mn complexes.
V. Conclusion
Synthetic SOD/catalase mimetics, particularly the salen Mn complexes exemplified by EUK-189 and EUK-207, mitigate radiation injury to normal tissues, including lung, kidney, skin and oral mucosa. These findings indicate that these agents could have potential value as adjunct therapies following radiation therapy. Future development of salen Mn complexes should focus on optimizing their methods of delivery and increasing an understanding of their mechanisms of action and potential side effects.
Acknowledgments
Development of the salen Mn complexes, including EUK-207, was funded in part by GM57770 (SRD). Renal, cutaneous, and pulmonary studies and further compound development were supported by AI067734 (JEM), including a pilot grant (RAR) to study EUK-400 compounds. Some pulmonary studies were also supported by AI81294 (MM). The mucositis study was performed by Biomodels, LLC. (Wellesley, MA) under the direction of STS, with funding by Eukarion, Inc. The mitochondrial injury studies were supported by CA72156 (PJT).
Contributor Information
RA Rosenthal, Pulmonary Center, Department of Medicine, Boston University School of Medicine, Boston, MA.
B. Fish, Department of Radiation Oncology, Medical College of Wisconsin, Milwaukee, WI
R.P. Hill, Princess Margaret Hospital, University of Toronto, Toronto, Ont
KD Huffman, Pulmonary Center, Department of Medicine, Boston University School of Medicine, Boston, MA.
Z. Lazarova, Department of Dermatology, Medical College of Wisconsin, Milwaukee, WI
J Mahmood, Princess Margaret Hospital, University of Toronto, Toronto, Ont.
M Medhora, Department of Radiation Oncology, Medical College of Wisconsin, Milwaukee, WI.
R Molthen, Department of Pulmonary Medicine, Medical College of Wisconsin, Milwaukee.
J.E. Moulder, Department of Radiation Oncology, Medical College of Wisconsin, Milwaukee, WI
ST Sonis, Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine; Divisions of Oral Medicine and Dentistry, Brigham and Women’s Hospital and the Dana-Farber Cancer Institute, Boston, MA.
P.J. Tofilon, Radiation Oncology Branch, National Cancer Center, National Institutes of Health, Bethesda, MD
S.R. Doctrow, Pulmonary Center, Department of Medicine, Boston University School of Medicine, Boston, MA
References
- 1.Movsas B, Vikram B, Hauer-Jensen M, Moulder JE, Basch E, Brown SL, Kachnic LA, Dicker AP, Coleman CN, Okunieff P. Decreasing the Adverse Effects of Cancer Therapy: National Cancer Institute Guidance for the Clinical Development of Radiation Injury Mitigators. Clin Cancer Res. 17(2):222–228. doi: 10.1158/1078-0432.CCR-10-1402. [DOI] [PubMed] [Google Scholar]
- 2.Greenberger JS, Epperly MW. Radioprotective antioxidant gene therapy: potential mechanisms of action. Gene Therapy and Molec Biol. 2004;8:31–44. [Google Scholar]
- 3.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80(Spec No 1):S23–31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 4.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16(2):130–43. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 5.Doctrow SR, Huffman K, Marcus CB, Tocco G, Malfroy E, Adinolfi CA, Kruk H, Baker K, Lazarowych N, Mascarenhas J, Malfroy B. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. J Med Chem. 2002;45(20):4549–58. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 6.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. 4. Oxford University Press; Oxford: 2007. [Google Scholar]
- 7.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80(4):251–9. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 8.Jiang J, Stoyanovsky DA, Belikova NA, Tyurina YY, Zhao Q, Tungekar MA, Kapralova V, Huang Z, Mintz AH, Greenberger JS, Kagan VE. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172(6):706–17. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kouvaris JR, Kouloulias VE, Vlahos LJ. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist. 2007;12(6):738–47. doi: 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- 10.Grdina DJ, Murley JS, Kataoka Y, Baker KL, Kunnavakkam R, Coleman MC, Spitz DR. Amifostine induces antioxidant enzymatic activities in normal tissues and a transplantable tumor that can affect radiation response. Int J Radiat Oncol Biol Phys. 2009;73(3):886–96. doi: 10.1016/j.ijrobp.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS, Hauer-Jensen M, Hill RP, Kolesnick RN, Macvittie TJ, Marks C, McBride WH, Metting N, Pellmar T, Purucker M, Robbins ME, Schiestl RH, Seed TM, Tomaszewski JE, Travis EL, Wallner PE, Wolpert M, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Radiat Res; Report of an NCI Workshop; December 3–4, 2003; 2004. pp. 711–28. [DOI] [PubMed] [Google Scholar]
- 12.Sharpe MA, Ollosson R, Stewart VC, Clark JB. Oxidation of nitric oxide by oxomanganese salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem J. 2002;366:97–107. doi: 10.1042/BJ20020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rong Y, Doctrow SR, Tocco G, Baudry M. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc Natl Acad Sci U S A. 1999;96(17):9897–902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pong K, Doctrow SR, Baudry M. Prevention of 1-methyl-4-phenylpyridinium- and 6-hydroxydopamine-induced nitration of tyrosine hydroxylase and neurotoxicity by EUK-134, a superoxide dismutase and catalase mimetic, in cultured dopaminergic neurons. Brain Res. 2000;881(2):182–9. doi: 10.1016/s0006-8993(00)02841-9. [DOI] [PubMed] [Google Scholar]
- 15.Doctrow SR, Huffman K, Marcus CB, Musleh W, Bruce A, Baudry M, Malfroy B. In: Antioxidants in Disease Mechanisms and Therapeutic Strategies. Sies H, editor. Academic Press; New York: 1997. pp. 247–270. [Google Scholar]
- 16.Doctrow SR, Adinolfi C, Baudry M, Huffman K, Malfroy B, Marcus CB, Melov S, Pong K, Rong Y, Smart J, Tocco G. In: Oxidative Stress and Aging: Advances in Basic Science, Diagnostics, and Intervention. Rodriguez H, Cutler R, editors. World Scientific Publishing Company; Singapore, London, NJ: 2003. pp. 1324–1342. [Google Scholar]
- 17.Doctrow SR, Baudry M, Huffman K, Malfroy B, Melov S. In: Medicinal Inorganic Chemistry. Sessler SRDJL, McMurry TJ, Lippard SJ, editors. American Chemical Society and Oxford University Press; New York: 2005. pp. 319–347. [Google Scholar]
- 18.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, Baudry M. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A. 2003;100(14):8526–31. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung C, Rong Y, Doctrow S, Baudry M, Malfroy B, Xu Z. Synthetic superoxide dismutase/catalase mimetics reduce oxidative stress and prolong survival in a mouse amyotrophic lateral sclerosis model. Neurosci Lett. 2001;304(3):157–60. doi: 10.1016/s0304-3940(01)01784-0. [DOI] [PubMed] [Google Scholar]
- 20.Malfroy-Camine B, Doctrow SR. U.S. Patent Number 7,122,537. Cyclic salen-manganese compounds: reactive oxygen species scavengers useful as antioxidants in the treatment and prevention of diseases. 2006
- 21.Clausen A, Doctrow S, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging (online 2008) 2010;31:425–33. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajagopalan MS, Stone B, Rwigema JC, Salimi U, Epperly MW, Goff J, Franicola D, Dixon T, Cao S, Zhang X, Buchholz BM, Bauer AJ, Choi S, Bakkenist C, Wang H, Greenberger JS. Intraesophageal manganese superoxide dismutase-plasmid liposomes ameliorates novel total-body and thoracic radiation sensitivity of NOS1−/− mice. Radiat Res. 2010;174(3):297–312. doi: 10.1667/RR2019.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epperly MW, Epstein CJ, Travis EL, Greenberger JS. Decreased pulmonary radiation resistance of manganese superoxide dismutase (MnSOD)-deficient mice is corrected by human manganese superoxide dismutase-Plasmid/Liposome (SOD2-PL) intratracheal gene therapy. Radiat Res. 2000;154(4):365–74. doi: 10.1667/0033-7587(2000)154[0365:dprrom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Aykin-Burns N, Slane BG, Liu AT, Owens KM, O’Malley MS, Smith BJ, Domann FE, Spitz DR. Sensitivity to Low-Dose/Low-LET Ionizing Radiation in Mammalian Cells Harboring Mutations in Succinate Dehydrogenase Subunit C is Governed by Mitochondria-Derived Reactive Oxygen Species. Radiat Res. 2011;175(2):150–8. doi: 10.1667/rr2220.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinerfeld D, Traini MD, Weinberger RP, Cochran B, Doctrow SR, Harry J, Melov S. Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J Neurochem. 2004;88(3):657–67. doi: 10.1046/j.1471-4159.2003.02195.x. [DOI] [PubMed] [Google Scholar]
- 26.Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J Neurosci. 2001;21(21):8348–53. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith RA, Murphy MP. Animal and human studies with the mitochondria-targeted antioxidant MitoQ. Ann N Y Acad Sci. 2010;1201:96–103. doi: 10.1111/j.1749-6632.2010.05627.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang HJ, Doctrow SR, Xu L, Oberley LW, Beecher B, Morrison J, Oberley TD, Kregel KC. Redox modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress. Faseb J. 2004;18(13):1547–9. doi: 10.1096/fj.04-1629fje. [DOI] [PubMed] [Google Scholar]
- 29.Peng J, Stevenson FF, Doctrow SR, Andersen JK. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. J Biol Chem. 2005;280(32):29194–8. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HJ, Drake VJ, Xu L, Xie L, Oberley LW, Kregel KC. Heat-induced liver injury in old rats is associated with exaggerated oxidative stress and altered transcription factor activation. FASEB J. 2003;17:2293–2295. doi: 10.1096/fj.03-0139fje. [DOI] [PubMed] [Google Scholar]
- 31.Tocco G, Illigens BM, Malfroy B, Benichou G. Prolongation of alloskin graft survival by catalytic scavengers of reactive oxygen species. Cell Immunol. 2006;241(2):59–65. doi: 10.1016/j.cellimm.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Luciani A, Villella VR, Vasaturo A, Giardino I, Raia V, Pettoello-Mantovani M, D’Apolito M, Guido S, Leal T, Quaratino S, Maiuri L. SUMOylation of tissue transglutaminase as link between oxidative stress and inflammation. J Immunol. 2009;183(4):2775–84. doi: 10.4049/jimmunol.0900993. [DOI] [PubMed] [Google Scholar]
- 33.Luciani A, Villella VR, Vasaturo A, Giardino I, Pettoello-Mantovani M, Guido S, Cexus ON, Peake N, Londei M, Quaratino S, Maiuri L. Lysosomal accumulation of gliadin p31–43 peptide induces oxidative stress and tissue transglutaminase-mediated PPARgamma downregulation in intestinal epithelial cells and coeliac mucosa. Gut. 2010;59(3):311–9. doi: 10.1136/gut.2009.183608. [DOI] [PubMed] [Google Scholar]
- 34.Schultheiss TE, Stephens LC. Invited review: permanent radiation myelopathy. Br J Radiol. 1992;65(777):737–53. doi: 10.1259/0007-1285-65-777-737. [DOI] [PubMed] [Google Scholar]
- 35.Valk PE, Dillon WP. Radiation injury of the brain. AJNR Am J Neuroradiol. 1991;12(1):45–62. [PMC free article] [PubMed] [Google Scholar]
- 36.Schultheiss TE. Cerebral radiation necrosis: regarding Schultheiss et al IJROBP 31:1093–1112;1995. Int J Radiat Oncol Biol Phys. 1995;32(4):1269. doi: 10.1016/0360-3016(95)98067-i. [DOI] [PubMed] [Google Scholar]
- 37.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153(4):357–70. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery DL. Astrocytes: form, functions, and roles in disease. Vet Pathol. 1994;31(2):145–67. doi: 10.1177/030098589403100201. [DOI] [PubMed] [Google Scholar]
- 39.Wilson JX. Antioxidant defense of the brain: a role for astrocytes. Can J Physiol Pharmacol. 1997;75(10–11):1149–63. [PubMed] [Google Scholar]
- 40.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–86. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 41.Brown DR. Neurons depend on astrocytes in a coculture system for protection from glutamate toxicity. Mol Cell Neurosci. 1999;13(5):379–89. doi: 10.1006/mcne.1999.0751. [DOI] [PubMed] [Google Scholar]
- 42.Vorotnikova E, Rosenthal RA, Tries M, Doctrow SR, Braunhut SJ. Novel synthetic SOD/catalase mimetics mitigate capillary endothelial cell apoptosis. Radiation Res. 2010;173:748–59. doi: 10.1667/RR1948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pong K, Doctrow SR, Huffman K, Adinolfi CA, Baudry M. Attenuation of staurosporine-induced apoptosis, oxidative stress, and mitochondrial dysfunction by synthetic superoxide dismutase and catalase mimetics, in cultured cortical neurons. Exp Neurol. 2001;171(1):84–97. doi: 10.1006/exnr.2001.7747. [DOI] [PubMed] [Google Scholar]
- 44.Otterson MF, Nie L, Link BJ, Schmidt JL, Rafiee P. Protective effect of EUK-207 on irradiated human intestinal microvascular endothelial cells (HIMEC) Abstracts, Experimental Biology. 2011 [Google Scholar]
- 45.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10(10):1713–65. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 46.Marks LB, Yu X, Vujaskovic Z, Small WJ, Folz R, Anscher MS. Radiation-induced lung injury. Seminars in Radiation Oncology. 2003;13:333–45. doi: 10.1016/S1053-4296(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 47.Fleckenstein K, Zgonjanin L, Chen L, Rabbani Z, Jackson IL, Thrasher B, Kirkpatrick J, Foster WM, Vujaskovic Z. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys. 2007;68(1):196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finkelstein JN, Johnston CJ, Baggs R, Rubin P. Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994;28:621–31. doi: 10.1016/0360-3016(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 49.Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006;79(2):231–8. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan V, Doctrow S, Singh VK, Whitnall MH. Evaluation of EUK-189, a synthetic superoxide dismutase/catalase mimetic as a radiation countermeasure. Immunopharmacol Immunotoxicol. 2008;30(2):271–90. doi: 10.1080/08923970801925331. [DOI] [PubMed] [Google Scholar]
- 51.Limoli CL, Giedzinski E, Baure J, Doctrow SR, Rola R, Fike JR. Using superoxide dismutase/catalase mimetics to manipulate the redox environment of neural precursor cells. Radiat Prot Dosimetry. 2006;122(1–4):228–36. doi: 10.1093/rpd/ncl458. [DOI] [PubMed] [Google Scholar]
- 52.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow S, Hill R. Mitigation of radiation-induced lung injury by genestein and EUK-207. Int J Rad Biol. 2011 doi: 10.3109/09553002.2011.583315. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh SN, Wu Q, Mader M, Fish BL, Moulder JE, Jacobs ER, Medhora M, Molthen RC. Vascular injury after whole thoracic x-ray irradiation in the rat. Int J Radiat Oncol Biol Phys. 2009;74(1):192–9. doi: 10.1016/j.ijrobp.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang R, Ghosh SN, Zhu D, North PE, Fish BL, Morrow NV, Lowry T, Nanchal R, Jacobs ER, Moulder JE, Medhora M. Structural and functional alterations in the rat lung following whole thoracic irradiation with moderate doses: injury and recovery. Int J Radiat Biol. 2008;84(6):487–97. doi: 10.1080/09553000802078396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, Jacobs ER, Medhora M. Renin-Angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. 2009;75(5):1528–36. doi: 10.1016/j.ijrobp.2009.07.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao F, Ghosh SN, Kma L, Wu Q, Molthen RC, Fish BL, Moulder JE, Doctrow SR, Medhora M. Angiotensin converting enzyme (ACE) inhibitors in combination with EUK-207 for mitigation of radiation pneumonitis. Abstracts, 56th Annual Meeting of the Radiation Research Society; 2010. PS1.50. [Google Scholar]
- 57.Molthen RC, Wu Q, Fish BL, Jacobs ER, Moulder JE, Doctrow SR, Medhora M. Mitigation of radiation-induced pneumonitis and pulmonary vascular injury using combined therapy: angiotensin converting enzyme inhibitors and superoxide dismutase (SOD) mimetics. Abstracts, 56th Annual Meeting of the Radiation Research Society; 2010. PS1.28. [Google Scholar]
- 58.Molteni A, Moulder JE, Cohen EF, Ward WF, Fish BL, Taylor JM, Wolfe LF, Brizio-Molteni L, Veno P. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol. 2000;76(4):523–32. doi: 10.1080/095530000138538. [DOI] [PubMed] [Google Scholar]
- 59.Williams JP, Hill RP, Haston C, Johnston C, Miller J, Zimmerman C, Hernady E, Reed C, Finkelstein JN. A combined therapeutic approach to pulmonary mitigation following a radiological event. Abstract, 56th Annual Meeting of the Radiation Res. Society; 2010. PS1.61. [Google Scholar]
- 60.Cohen E, Moulder J. In: Cancer and the Kidney. Cohen E, editor. Oxford University Press; Oxford: 2011. pp. 193–204. [Google Scholar]
- 61.Moulder JE, Fish BL, Cohen EP. ACE inhibitors and AII receptor antagonists in the treatment and prevention of bone marrow transplant nephropathy. Curr Pharm Des. 2003;9(9):737–49. doi: 10.2174/1381612033455422. [DOI] [PubMed] [Google Scholar]
- 62.Cohen EP, Fish BL, Moulder JE. Mitigation of radiation injuries via suppression of the renin-angiotensin system: emphasis on radiation nephropathy. Curr Drug Targets. 2010;11(11):1423–9. doi: 10.2174/1389450111009011423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cohen EP, Fish BL, Irving AA, Rajapurkar MM, Shah SV, Moulder JE. Radiation Nephropathy is not Mitigated by Antagonists of Oxidative Stress. Radiat Res. 2009;172(2):260–4. doi: 10.1667/RR1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Doctrow SR, Damphousse CA, Fish B, Huffman K, Jourdan M, Lazorova Z, Moulder J, Rosenthal RA. Synthetic superoxide dismutase/catalase mimetics to mitigate radiation-induced normal tissue damage. Rad. Res. 55th Annual Meeting; 2009. Abstract S1403:26. [Google Scholar]
- 65.Lenarczyk M, Cohen EP, Fish BL, Irving AA, Sharma M, Driscoll CD, Moulder JE. Chronic oxidative stress as a mechanism for radiation nephropathy. Radiat Res. 2009;171(2):164–72. doi: 10.1667/RR1454.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen EP, Irving AA, Drobyski WR, Klein JP, Passweg J, Talano JA, Juckett MB, Moulder JE. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2008;70(5):1546–51. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sonis ST, Peterson RL, Edwards LJ, Lucey CA, Wang L, Mason L, Login G, Ymamkawa M, Moses G, Bouchard P, Hayes LL, Bedrosian C, Dorner AJ. Defining mechanisms of action of interleukin-11 on the progression of radiation-induced oral mucositis in hamsters. Oral Oncol. 2000;36(4):373–81. doi: 10.1016/s1368-8375(00)00012-9. [DOI] [PubMed] [Google Scholar]
- 68.Sonis ST, Lindquist L, Vugt AV, Stewart AA, Stam K, Qu GY, Iwata KK, Haley JD. Prevention of chemotherapy-induced ulcerative mucositis by TGF-beta. Cancer Research. 1994;54:1135–38. [PubMed] [Google Scholar]
- 69.Murphy CK, Fey EG, Watkins BA, Wong V, Rothstein D, Sonis ST. Efficacy of superoxide dismutase mimetic M40403 in attenuating radiation-induced oral mucositis in hamsters. Clin Cancer Res. 2008;14(13):4292–7. doi: 10.1158/1078-0432.CCR-07-4669. [DOI] [PubMed] [Google Scholar]
- 70.Rosenthal RA, Huffman KD, Fisette LW, Damphousse CA, Callaway WB, Malfroy B, Doctrow SR. Orally available Mn porphyrins with superoxide dismutase and catalase activities. J Biol Inorg Chem. 2009;14(6):979–91. doi: 10.1007/s00775-009-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarther H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 72.Roy S, Khanna S, Nallu K, Hunt TK, Sen CK. Dermal wound healing is subject to redox control. Mol Ther. 2006;13(1):211–20. doi: 10.1016/j.ymthe.2005.07.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Muller K, Meineke V. Radiation-induced alterations in cytokine production by skin cells. Exp Hematol. 2007;35(4 Suppl 1):96–104. doi: 10.1016/j.exphem.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 74.De Loecker W, Van der Schueren E, Stas ML, Doms D. The effects of x-irradiation on collagen metabolism in rat skin. Int J Radiat Biol Relat Stud Phys Chem Med. 1976;29(4):351–8. doi: 10.1080/09553007614550401. [DOI] [PubMed] [Google Scholar]
- 75.Rudolph R, Vande Berg J, Schneider JA, Fisher JC, Poolman WL. Slowed growth of cultured fibroblasts from human radiation wounds. Plast Reconstr Surg. 1988;82(4):669–77. doi: 10.1097/00006534-198810000-00019. [DOI] [PubMed] [Google Scholar]
- 76.Goldschmidt H, Sherwin WK. Reactions to ionizing radiation. J Am Acad Dermatol. 1980;3(6):551–79. doi: 10.1016/s0190-9622(80)80067-3. [DOI] [PubMed] [Google Scholar]
- 77.Gorodetsky R, Mou XD, Fisher DR, Taylor JM, Withers HR. Radiation effect in mouse skin: dose fractionation and wound healing. Int J Radiat Oncol Biol Phys. 1990;18(5):1077–81. doi: 10.1016/0360-3016(90)90443-n. [DOI] [PubMed] [Google Scholar]
- 78.Jourdan MM, Olasz EB, Moulder JE, Fish BL, Mader M, Schock A, Morrow N, Semenenko V, Doctrow SR, Lazarova Z. Mitigation of combined radiation and skin wound injury by SOD/catalase mimetic EUK-207. Rad. Res. 55th Annual Meeting; 2009. Abstract PS1.35:64. [Google Scholar]
- 79.Lopez A, Wu X, Olasz EB, Lazar J, Sells R, Fish BL, Mader M, Schock AM, Althouse BJ, Moulder JE, Lazarova Z. Gene Expression Profiling in Irradiated Skin. Radiat Res Society Abstracts. 2010:PS1. [Google Scholar]
- 80.Jourdan M, Lopez A, Olasz E, Moulder J, Fish B, Mäder M, Schock A, Althouse B, Doctrow S, Lazarova Z. Effect of superoxide dismutase (SOD)/catalase mimetic EUK-207 on the radiation-induced skin injury. Journal of Investigative Dermatology. 2010;(Suppl 1s):S2. [Google Scholar]
- 81.Kumar S, Kolozsvary A, Kohl R, Lu M, Brown S, Kim JH. Radiation-induced skin injury in the animal model of scleroderma: implications for post-radiotherapy fibrosis. Radiat Oncol. 2008;3:40–47. doi: 10.1186/1748-717X-3-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sen CK, Roy S. Redox signals in wound healing. Biochim Biophys Acta. 2008;1780(11):1348–61. doi: 10.1016/j.bbagen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo JD, Wang YY, Fu WL, Wu J, Chen AF. Gene therapy of endothelial nitric oxide synthase and manganese superoxide dismutase restores delayed wound healing in type 1 diabetic mice. Circulation. 2004;110(16):2484–93. doi: 10.1161/01.CIR.0000137969.87365.05. [DOI] [PubMed] [Google Scholar]
- 84.Demianenko IA, Vasilieva TV, Domnina LV, Dugina VB, Egorov MV, Ivanova OY, Ilinskaya OP, Pletjushkina OY, Popova EN, Sakharov IY, Fedorov AV, Chernyak BV. Novel mitochondria-targeted antioxidants, “Skulachev-ion” derivatives, accelerate dermal wound healing in animals. Biochemistry (Mosc) 2010;75(3):274–80. doi: 10.1134/s000629791003003x. [DOI] [PubMed] [Google Scholar]
- 85.Meunier B, Cosledan F. Orally bioavailable low molecular weight metalloporphyrins. Eukarion, Inc: World Intellectual Property Organization; 2005. (PCT application number 20060241095) [Google Scholar]
- 86.Liang LP, Huang J, Fulton R, Day BJ, Patel M. An orally active catalytic metalloporphyrin protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in vivo. J Neurosci. 2007;27(16):4326–33. doi: 10.1523/JNEUROSCI.0019-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trova MP, Gauuan PJ, Pechulis AD, Bubb SM, Bocckino SB, Crapo JD, Day BJ. Superoxide dismutase mimetics. Part 2: synthesis and structure-activity relationship of glyoxylate- and glyoxamide-derived metalloporphyrins. Bioorg Med Chem. 2003;11(13):2695–707. doi: 10.1016/s0968-0896(03)00272-4. [DOI] [PubMed] [Google Scholar]
- 88.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, Vujaskovic Z. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67(2):573–80. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, Vujaskovic Z. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48(8):1034–43. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Declercq L, Sente I, Hellemans L, Corstjens H, Maes D. Use of the synthetic superoxide dismutase/catalase mimetic EUK-134 to compensate for seasonal antioxidant deficiency by reducing pre-existing lipid peroxides at the human skin surface. Int J Cosmet Sci. 2004;26(5):255–63. doi: 10.1111/j.1467-2494.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 91.Gonzalez PK, Doctrow SR, Malfroy B, Fink MP. Role of oxidant stress and iron delocalization in acidosis-induced intestinal epithelial hyperpermeability. Shock. 1997;8(2):108–14. doi: 10.1097/00024382-199708000-00008. [DOI] [PubMed] [Google Scholar]
- 92.Bruce AJ, Malfroy B, Baudry M. Beta-amyloid toxicity in organotypic hippocampal cultures: protection by EUK-8, a synthetic catalytic free radical scavenger. Proc Natl Acad Sci (USA) 1996;93:2312–2316. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robert A, Loock B, Momenteau M, Meunier B. Catalase modeling with metalloporphyrin complexes having an oxygen ligand in a proximal position. Comparison with complexes containing a proximal nitrogen. Inorg Chem. 1991;30:706–711. [Google Scholar]
- 94.Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59(3):527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 95.Reboucas JS, Spasojevic I, Batinic-Haberle I. Pure MnTBAP is not a superoxide dismutase mimic in aqueous systems: a case of structure-activity relationship as a watchdog mechanism in experimental therapeutics and biology. Free Radic Biol Med. 2007;43(Suppl 1):S26. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 96.Dabrowiak J. Metals in Medicine. Wiley; NY: 2010. [Google Scholar]
- 97.Sessler JL, Doctrow SR, McMurry TJ, Lippard SJ. Medicinal Inorganic Chemistry. American Chemical Society; NY: 2005. [Google Scholar]
- 98.Seale MK, Catalano OA, Saini S, Hahn PF, Sahani DV. Hepatobiliary-specific MR contrast agents: role in imaging the liver and biliary tree. Radiographics. 2009;29(6):1725–48. doi: 10.1148/rg.296095515. [DOI] [PubMed] [Google Scholar]
- 99.Orrell RW. AEOL-10150 (Aeolus) Curr Opin Investig Drugs. 2006;7(1):70–80. [PubMed] [Google Scholar]
- 100.Salvemini D, Riley DP. Nonpeptidyl mimetics of superoxide dismutase in clinical therapies for diseases. Cell Mol Life Sci. 2000;57(11):1489–92. doi: 10.1007/PL00000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noel F, Ijichi A, Chen JJ, Gumin GJ, Tofilon PJ. X-ray-mediated reduction in basic fibroblast growth factor expression in primary rat astrocyte cultures. Radiat Res. 1997;147(4):484–9. [PubMed] [Google Scholar]
- 102.Gong B, Chen Q, Almasan A. Ionizing radiation stimulates mitochondrial gene expression and activity. Radiat Res. 1998;150(5):505–12. [PubMed] [Google Scholar]
- 103.Poot M, Zhang YZ, Kramer JA, Wells KS, Jones LJ, Hanzel DK, Lugade AG, Singer VL, Haugland RP. Analysis of mitochondrial morphology and function with novel fixable fluorescent stains. J Histochem Cytochem. 1996;44(12):1363–72. doi: 10.1177/44.12.8985128. [DOI] [PubMed] [Google Scholar]
- 104.Galante YM, Hatefi Y. Resolution of complex I and isolation of NADH dehydrogenase and an iron--sulfur protein. Methods Enzymol. 1978;53:15–21. doi: 10.1016/s0076-6879(78)53007-3. [DOI] [PubMed] [Google Scholar]
- 105.Hatefi Y, Stiggall DL. Preparation and properties of succinate: ubiquinone oxidoreductase (complex II) Methods Enzymol. 1978;53:21–7. doi: 10.1016/s0076-6879(78)53008-5. [DOI] [PubMed] [Google Scholar]
- 106.Ragan CI, Hatefi Y. Isolation of the iron-sulfur-containing polypeptides of NADH: oxidoreductase ubiquinone. Methods Enzymol. 1986;126:360–9. doi: 10.1016/s0076-6879(86)26036-x. [DOI] [PubMed] [Google Scholar]