Abstract
Radiation nephropathy and other normal tissue radiation injuries can be successfully mitigated, and also treated, by antagonists of the renin-angiotensin system (RAS). This implies a mechanistic role for that system in radiation nephropathy, yet no evidence exists to date of activation of the RAS by irradiation. RAS antagonists, including angiotensin converting enzyme inhibitors and angiotensin receptor blockers, are the standard of care in the treatment of subjects with other chronic progressive kidney diseases, in which they exert benefit by reducing both glomerular and tubulo-interstitial injury. These drugs are likely to act in a similar way to mitigate radiation nephropathy.
Keywords: radiation injuries, radiation nephropathy, renin-angiotensin system, angiotensins, captopril, mesangiolysis
INTRODUCTION
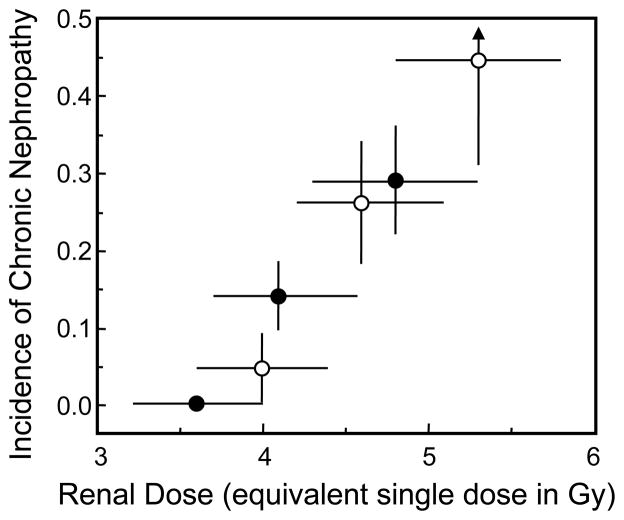
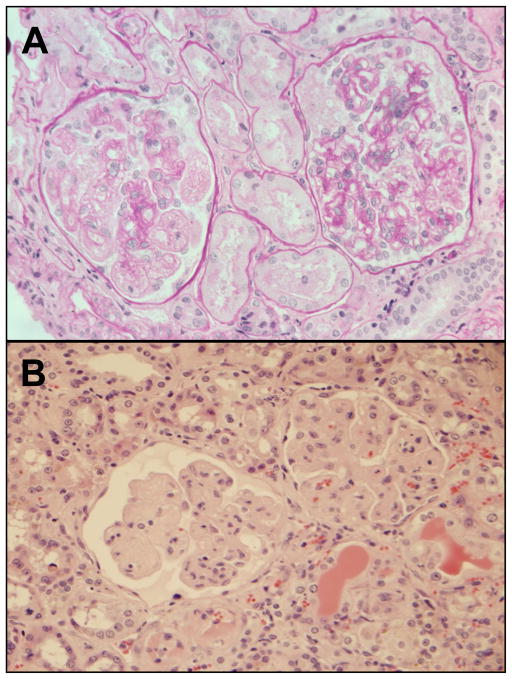
Radiation nephropathy occurs reliably after sufficient exposure of kidneys to ionizing radiation. Single-fraction total-body irradiation (TBI) of 10 Gy will cause radiation nephropathy in humans [1, 2] and in rats [2, 3] within six months after TBI. In both species, there is proteinuria, azotemia, and hypertension [2, 4]. Fifty years ago, radiation nephropathy was a known complication of radiation therapy for seminoma. In current medical practice, radiation nephropathy may occur after hematopoietic stem cell transplantation when TBI is used in the conditioning regimen, and after internal radionuclide cancer therapy [5]. Estimates from clinical data support the idea that single-fraction radiation doses as low as 4 Gy may lead to renal injury [6] (Fig. (1)). Thus there is the potential for radiation nephropathy after accidental or terrorist radiation exposures, particularly for partial body exposures for which death by hematopoietic marrow failure does not occur. In humans [1, 2] and in rats [2, 3], the physiological features of radiation-induced renal injury are similar (i.e., azotemia, hypertension, proteinuria), and the histology is similar, with glomerular mesangiolysis in both species (Fig. (2)). Treatment and mitigation of radiation nephropathy in humans and in rats can be achieved by use of antagonists of the renin-angiotensin system [7]. Together, these data point to mechanisms of injury and its mitigation that are shared between the two species. Therefore we have used the rat to test both the mechanism of radiation-induced renal injury, and the mechanism of its mitigation.
Fig. 1.
Dose–response curve for chronic nephropathy after TBI plus HSCT from the clinical reports of Lawton et al. [47] (●) and Miralbell et al. [48] (○). The “equivalent single dose” is derived from the actual fractionated low-dose-rate regimen by assuming an α/β of 1.5–4.0 Gy for the fractionation effect, and a dose-rate-reduction factor of 1.2–1.5 for the dose-rate effect. The vertical error bars are standard deviations; the horizontal error bars reflect the biological uncertainty in the “equivalent single dose” calculation. Reprinted with permission from Moulder and Cohen [6].
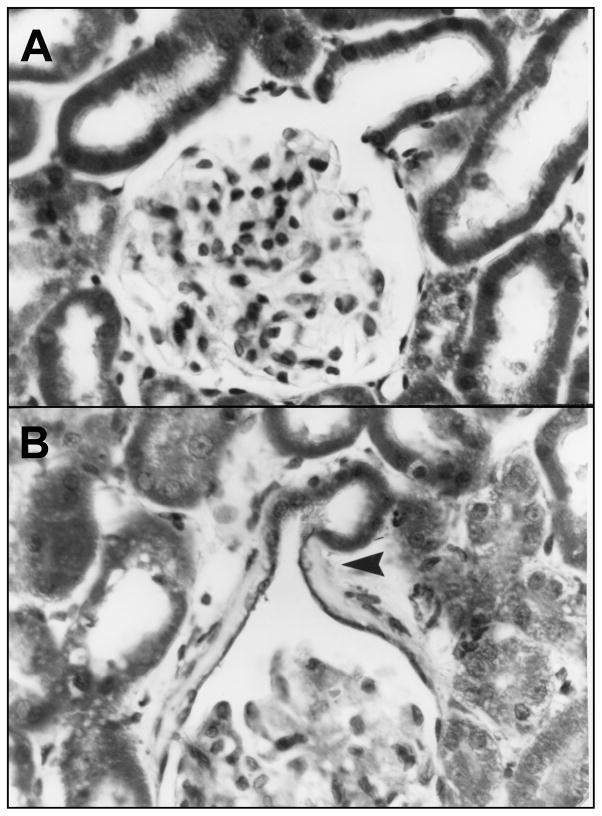
Fig. 2.
Light micrographs of radiation nephropathy. Panel A shows kidney biopsy histology from a subject with BMT nephropathy, 13 months after 14 Gy TBI in nine fractions. There is mesangial cell loss, and extreme widening of the subendothelial space, along with some interstitial scarring. Panel B shows kidney histology from a rat at 5 months after 10 Gy single-fraction TBI. There is a similar appearance to the human specimen, with mesangial cell loss, and extreme widening of the subendothelial space, along with interstitial scarring. Panel A is reproduced with permission of Oxford University Press, from Cohen [5], and panel B is reproduced with permission of Radiation Research from Sieber et al. [49].
In barrier-maintained WAG/RijCmcr rats, TBI followed by bone marrow transplant (BMT) from a syngeneic littermate causes renal injury as assessed by azotemia, hypertension or proteinuria (Fig (3)). The TBI can be single fraction or multi-fraction, with a 6-fraction regimen of 18.8 Gy producing injury that is roughly equivalent to that caused by a 10 Gy single dose [8]. The major cause of death in this model is renal failure that occurs from 4 to 6 months after TBI, but there is also liver and heart injury [9, 10].
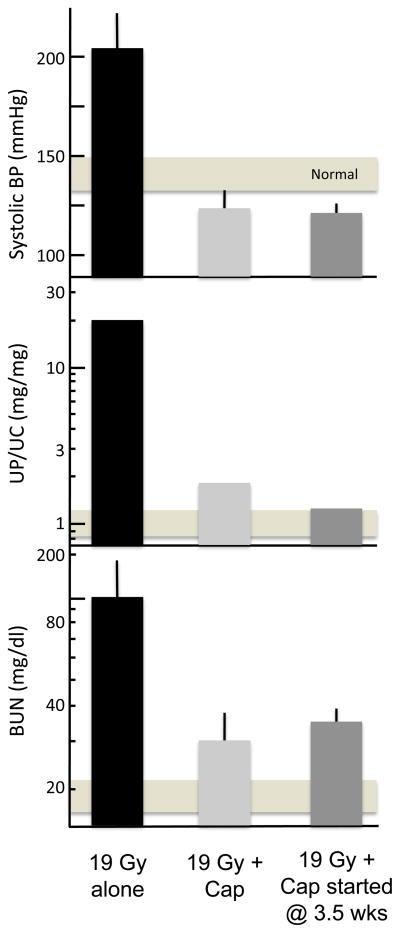
Fig. 3.
Effect of continuous captopril (Cap) therapy vs. captopril therapy started 3.5 weeks after BMT on renal function 26 weeks after fractionated TBI plus BMT in a rat model. The top panel shows blood pressure, the center panel shows proteinuria, and the bottom panel shows azotemia (as BUN). Values shown are geometric means with 95% confidence intervals. The horizontal bar shows the 95% confidence intervals for age-matched control rats. Adapted with permission of Bone Marrow Transplantation, from Moulder et al. [12].
MITIGATION OF RADIATION-INDUCED RENAL INJURY
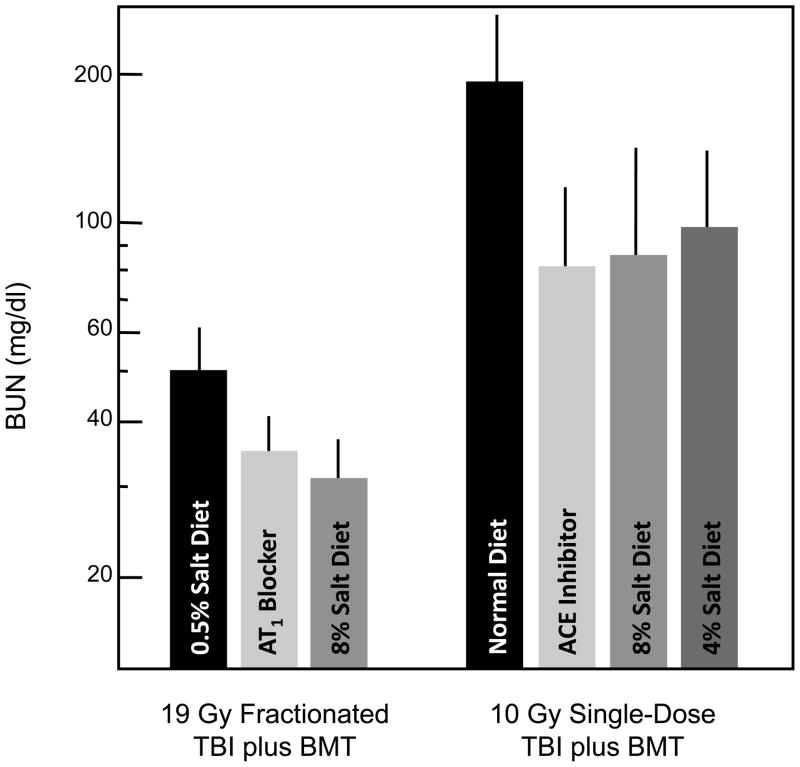
Mitigation is the use of an agent after the inciting injury, but before there is physiologic or pathologic evidence of that injury [11]. The angiotensin-converting enzyme (ACE) inhibitor captopril, at 500 mg/l in the drinking water, can mitigate radiation nephropathy caused by 18.8 Gy given in six fractions [12] (Fig. (3)). Even when drug therapy is not started until 3.5 weeks after TBI, captopril successfully mitigates radiation nephropathy [12] (Fig. (3)). We also found a significant benefit of captopril for mitigation of radiation nephropathy when it was used only from 4–10 weeks after TBI; however, short intervals of therapy before or after 4–10 weeks had considerably less benefit, suggesting that this interval after TBI was critical in the pathogenesis of radiation nephropathy [13]. We can also successfully mitigate radiation nephropathy in this model by use of the angiotensin II (AII) antagonist L-158,809, and by a high-salt (8% NaCl) diet (Fig. (4)). the effect of the high-salt diet is associated with suppression of blood and intra-renal AII levels [14].
Fig. 4.
Mitigation of radiation-induced azotemia (as BUN) 17 weeks after TBI plus BMT in a rat model. Data is shown as geometric means with 95% confidence intervals. One group of rats received 19 Gy fractionated TBI plus L-155809 (an AT1 receptor blocker) at 20 mg/l in their drinking water, or a high-salt (8% NaCl) or normal-salt (0.5% salt) diet; drug therapy started within 24 hours after TBI and continued for 10 weeks. A second group of rats received a single 10 Gy TBI dose and an ACE inhibitor (captopril) at 150 mg/l in their drinking water, or high-salt (4% or 8%) diets. Drug therapy started 7 days after TBI and continued for 9 weeks.
Mitigation in the context of radiological terrorism countermeasure development
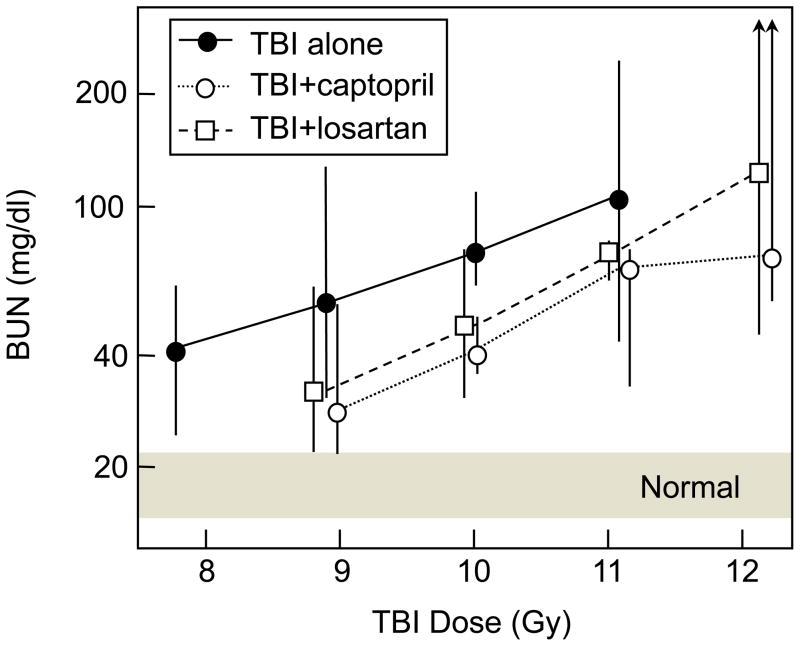
The 6-fraction TBI regimen was used because it resembled the schedules used for clinical BMT. Because of the recent interest in developing radiological terrorism countermeasures [11], we have now also tested captopril and an AII receptor blocker for mitigation of radiation nephropathy caused by single-fraction TBI. The primary surrogate endpoint evaluated was azotemia (BUN), because azotemia after irradiation is tightly correlated with the probability of eventual renal failure and with the severity of histopathological renal injury [15]. BUN was measured in all animals at 9–37 weeks after single dose TBI (7.8 to 13.3 Gy) plus BMT. At 17 weeks after TBI, BUN values (Fig. (5)) were available for all animals at doses up to 12.2 Gy, and the dose modification factors (DMFs) calculated by regression analysis were 1.25 (95% CI: 1.19–1.35) for captopril and 1.15 (1.10–1.24) for the AII type-1 blocker, losartan. Analysis after other follow-up times gave DMF that ranged from 1.19–1.32 for captopril and 1.18–1.27 for losartan. Captopril and losartan (Cozaar®, Merck) are both FDA-approved and would be readily available for emergency use. The doses of captopril and losartan used in these studies were chosen to match, on a mg/m2/day basis, those commonly used in humans for treatment of hypertension. For captopril, a typical adult human dose is 75–150 mg/day or 45–90 mg/m2/day; captopril in the drinking water at 150 mg/l delivers 70–90 mg/m2/day to a rat, or about 17 mg/kg/day. For losartan, a typical adult human dose is 50–100 mg/day or 30–60 mg/m2/day; losartan in the drinking water at 100 mg/l delivers 40–60 mg/m2/day to a rat, or about 11 mg/kg/day.
Fig. 5.
Mitigation of radiation-induced azotemia (as BUN) 17 weeks after TBI plus BMT in a rat model. Data is shown as medians and ranges (n = 5–6 per group) for rats receiving TBI alone (●), TBI plus captopril at 17 mg/kg/day (○), or TBI plus losartan at 11 mg/kg/day (□). Drug therapy started 10 days after TBI and continued for 26 weeks. Two rats each in the captopril and losartan groups at 12.2 Gy were euthanized prior to 17 weeks because of high BUN levels. At 12.2 Gy, where rats were euthanized prior to 17 weeks because of high BUN levels, the medians assume infinite BUN values for those rats.
We extended the previous studies of a high-salt diet to its use after single-fraction TBI. In this model, as for the multifraction TBI [14], a temporary increase of the dietary sodium, to either 4% or 8% dietary salt, exerted a substantial and significant long-term mitigating benefit (Fig. (4)). It is likely that the high-sodium diet suppressed the renin-angiotensin system activity in this model as it did in the multifraction model [14]. During the time of the high-salt diets, the blood pressure of the rats was elevated, not reduced as it was during the use of the RAS antagonism, yet the benefit of the temporary high-salt diets was as good as that of the RAS antagonists (Fig.(4)).
MITIGATION OF HUMAN RADIATION NEPHROPATHY
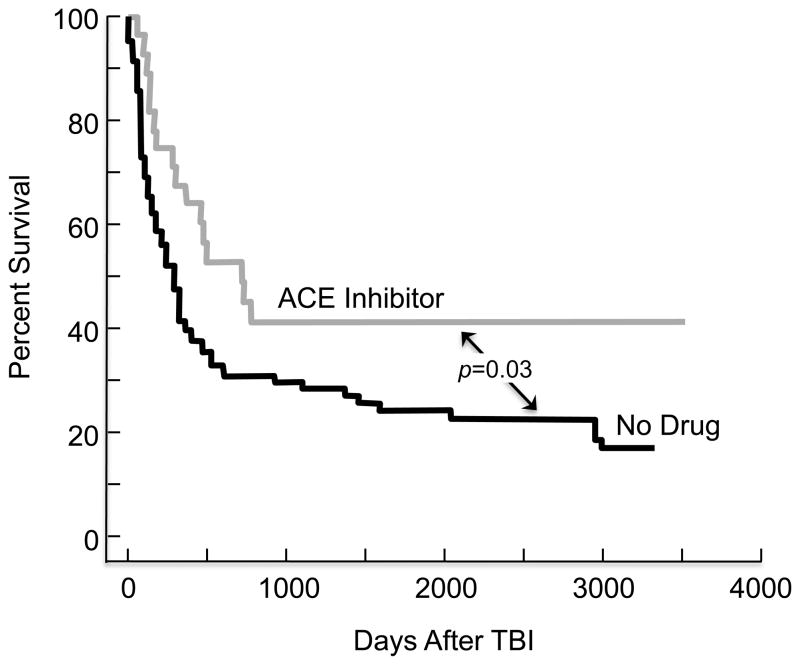
When preceded by TBI, hematopoietic stem cell transplantation (HSCT) can result in chronic kidney disease that is a congener of radiation nephropathy [16]. Captopril, compared to placebo, reduced the frequency of this complication in a single-center study [17]. Extended analysis of this study has shown a significant benefit of captopril on patient survival (Fig. (6)). The benefit of captopril in this clinical study was not as impressive as it has been for the irradiated laboratory rat. That is probably because radiation is not the only cause of chronic kidney disease after HSCT, and captopril does not mitigate all forms of renal injury. Nonetheless, the benefit of captopril appears to be shared between rats and human; this supports the notion that radiation nephropathy is similar between these two species, has a similar mechanism, and is mitigated in a similar fashion.
Fig. 6.
Actuarial patient survival of subjects who underwent TBI-based BMT at the Medical College of Wisconsin, as part of a randomized trial of captopril versus placebo. Those who were on captopril (gray line) had a significantly better long term survival, p=0.03, compared to those on either placebo or those who did not participate in the study (black line). This figure includes data from Cohen et al. [17].
MECHANISMS OF INJURY: THE RENIN-ANGIOTENSIN SYSTEM
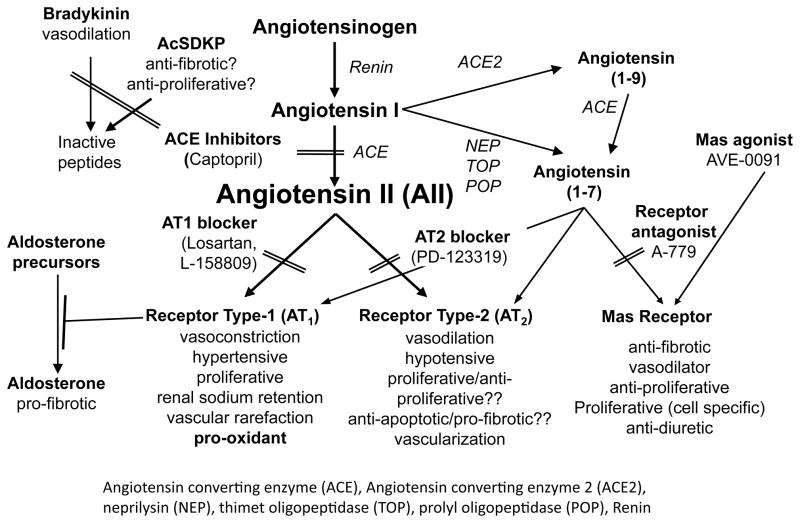
The RAS is a multi-organ system of enzymes and their peptide substrates (Fig. (7)). It functions in an endocrine and paracrine fashion. Thus, renin from the kidneys cleaves angiotensinogen from the liver into angiotensin I that is in turn cleaved to AII by lung-derived ACE. Yet all of these components are also found within the kidneys, and intrarenal activation of the RAS has been much investigated in human and experimental disease [18]. Because of the beneficial effect of ACE inhibition and AII receptor blockade in radiation nephropathy, and in other radiation injuries including lung [19] and brain [20], we tested whether the RAS was activated in radiation nephropathy. We found only scant evidence for this; serum renin activity and protein were not elevated, and neither serum nor intrarenal AII were elevated after TBI [4]. In addition, renal cell membrane AII receptor binding did not differ between irradiated and normal rats [21]. In a single study, we did find some increase in intrarenal angiotensinogen in rats after 17 Gy fractionated TBI [22], but these data are hard to reconcile with the data showing no increase in intrarenal AII.
Fig. 7.
The renin-angiotensin system.
Despite the lack of evidence for radiation-induced activation of the RAS, suppression of the RAS does however appear to be beneficial, as shown by the studies of increased dietary sodium (Fig. (4)). The benefit of RAS suppression suggests that its normal activity is deleterious in the irradiated subject, and/or that a countervailing system has reduced activity after irradiation, so that lowering the activity of the RAS would be needed to reduce injury. That countervailing system could involve nitric oxide (NO). There may be a deficit of NO in radiation nephropathy, but our data to-date suggest that it is a late occurrence, after the pathophysiologic changes have taken place, rather than an early one [23].
The RAS has components beyond its central core of renin and AII (Fig. (7)). These peripheral components include aldosterone, angiotensin(1–7), N-acetyl-seryl-aspartyl-lysyl-proline (AcSDKP), and bradykinin. Each of these has been implicated in the mechanism of some types of renal injury [24–28], so it is possible that these peripheral components could be important effectors in radiation nephropathy.
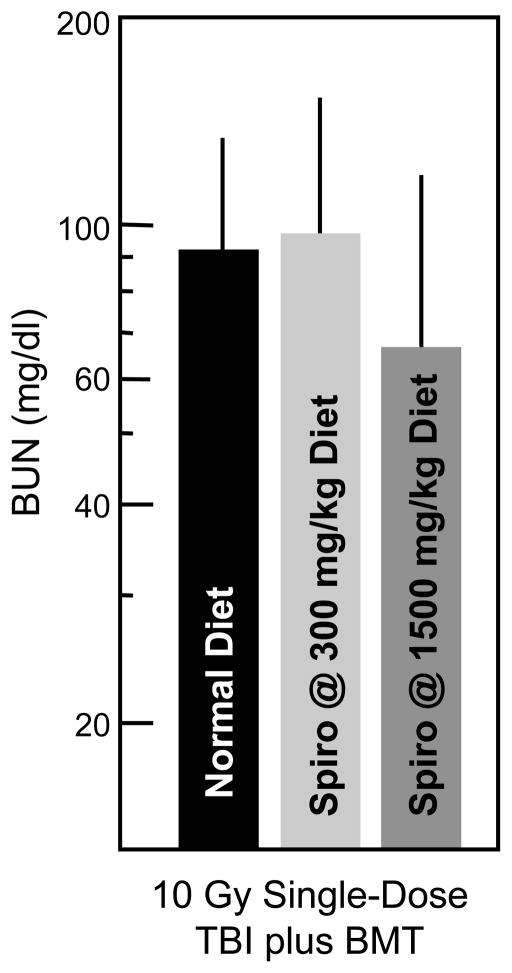
To assess the role of aldosterone, we tested the benefit of mitigation by a specific aldosterone antagonist, spironolactone. This substance antagonizes aldosterone at its receptor. Spironolactone was first used to attempt to mitigate radiation nephropathy at a dose of 300 mg/kg in the diet; it was ineffective, as shown in Fig. (8), Based on data from the remnant kidney model, in which spironolactone was used at a dose of 1500 mg/kg [25], we used this higher dose and again found it to be ineffective as a mitigation of radiation injury (Fig. (8)).
Fig. 8.
Effect of spironolactone (Spiro) on radiation-induced azotemia (as BUN). Data is shown as geometric means with 95% confidence intervals. Rats received 10 Gy single-dose TBI (plus BMT) plus spironolactone at a dose of 300 or 1500 mg/kg in the diet.
Subsequent studies of the adrenal axis and its hormones have shown no elevation in plasma aldosterone levels at either 8 or 70 days after 10 Gy single-dose TBI (Cohen et al, unpublished observation1). The remnant kidney model is different, in that there is significant elevation of the plasma aldosterone in that model. Thus, it is perhaps not surprising that spironolactone would be beneficial in the remnant kidney model of chronic renal failure [25], whereas we did not find a benefit in radiation nephropathy. Nonetheless, Jaggi et al. [29] have found a benefit of spironolactone in murine radiation nephropathy caused by an internal radionuclide emitter. Thus, the role of aldosterone in radiation nephropathy may warrant further study.
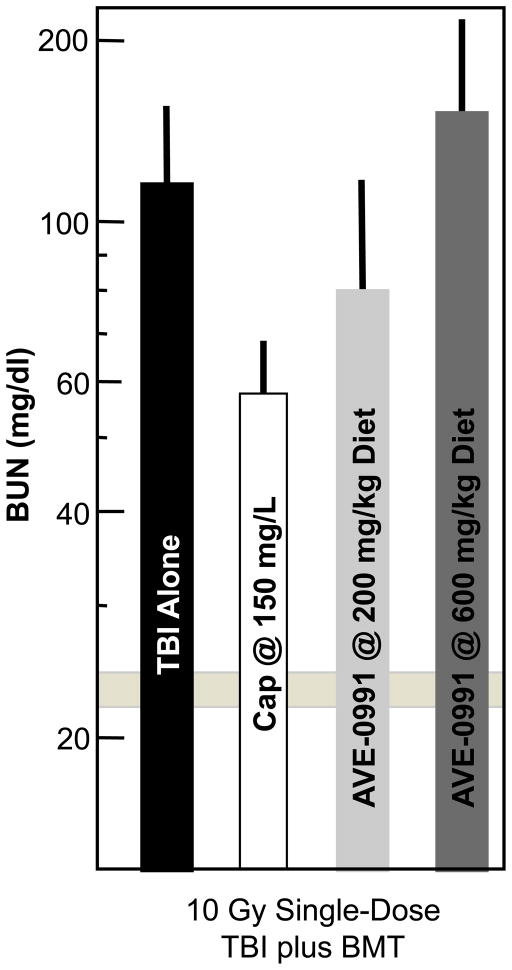
To test the role of angiotensin(1–7) and its cognate receptor Mas (Fig. (7)), we tested the effect of a Mas receptor antagonist (A779) and angiotensin(1–7) on radiation nephropathy. In separate studies a Mas receptor agonist (AVE0991) was tested. This was predicated on the reported actions of angiotensin(1–7) as being generally opposite to that of AII [30]. Neither AVE0991 (Fig. (9)) nor A779 (600 μg/kg/day for 3 through 10 weeks after irradiation, Fish et al,, unpublished observation2) had a significant effect on the evolution of kidney function after 10 Gy single-dose TBI. Angiotensin(1–7) at 600 μg/kg/day for 3 through 10 weeks after 10 Gy TBI gave slight, but non-significant, mitigation of radiation nephropathy (Fish et al,, unpublished observation2). Thus, the beneficial actions of ACE inhibitors and AII antagonists in this model do not appear to depend on the Mas receptor. The only other published long-term study of the angiotensin(1–7) pathway showed an adverse, not beneficial effect, of angiotensin(1–7) infusion on experimental diabetic nephropathy [31].
Fig. 9.
Effect of the Mas receptor agonist AVE0991 on radiation-induced azotemia (as BUN) at 17 weeks after 10 Gy single-dose TBI (plus BMT) in a rat model. Drugs were started 3–6 hours after TBI and continued for the duration of the study. Data is shown as geometric means with 95% confidence intervals. The horizontal bar shows the 95% confidence intervals for age-matched normal rats.
To test the role of AcSDKP, rats were given 18.8 Gy fractionated TBI (plus BMT) and randomized to AcSDKP by subcutaneous minipump at 700 μg/kg/day, captopril 500 mg/l in the drinking water, or no drug therapy; both drugs were given at 3 through 10 weeks after TBI. This AcSDKP dose raised the serum AcSDKP concentrations to levels similar to those seen in the rats on captopril, but use of AcSDKP had no mitigating effect on radiation nephropathy (Moulder et al., unpublished observation3). This result runs counter to the notion that AcSDKP plays an important role in the beneficial effect of captopril.
We have sought to test the role of bradykinin, but have been unable to do so, as there are no analogues or antagonists suitable for long-term use. Brown Norway rats with a mutation that putatively leads to low serum bradykinin levels have been used to test whether captopril retains its mitigating benefit. But the serum of these rats appears to have bradykinin concentrations in the normal range, which put into question their utility to test the bradykinin pathway.
MECHANISMS OF MITIGATION
Mesangiolysis
The glomerular lesion of mesangiolysis is specifically described by Antignac et al. [1] in her 1989 report of chronic renal failure after TBI-based hematopoietic stem cell transplantation, and we have found it in all of our cases of BMT nephropathy. This lesion is a rarefaction of the glomerular mesangium [32], as shown on Fig. (2), and it is seen in thrombotic microangiopathies (i.e., hemolytic uremic syndrome) such as is often described in radiation nephropathy [16]. Thrombotic microangiopathies appear to depend on endothelial injury, and endothelial injury is a clear-cut early feature of radiation nephropathy [33, 34]. Experimental NO deficiency, as caused by knockout of the endothelial NO synthase in mice, also causes mesangiolysis [35]. It is possible that the NO deficiency that occurs in radiation nephropathy [23] could lead to unopposed effects of normal levels of AII, thus explaining the beneficial effects of its blockade. A similar imbalance of normal levels of AII and deficient ones for NO was invoked by Verhagen et al. [36] in a NO-deficient model of renal injury.
Glomerular and Tubulo-interstitial Injury
Although the characteristic feature of radiation nephropathy is the glomerular change of mesangiolysis, there is also tubulo-interstitial injury. That could occur from direct radiation injury to tubular epithelium, or secondary to the proteinuria that results from the glomerular injury. Antagonists of the RAS would be expected to exert benefit by mitigating glomerular injury and thus reducing intraglomerular pressure [37], leading to reduction in proteinuria and consequently reduction in tubulo-interstitial damage from that proteinuria [38]. In this regard, the early part of the tubule, at the place where glomerulus drains into the tubule, is apt to have the most injury from proteins or cytokines emanating from the injured glomerulus (Fig. (10)). The scarring that takes place at the glomerulo-tubular junction, as shown in Fig. (10), can clearly explain the known close relation of interstitial scarring to the glomerular filtration rate. Progression of the glomerulotubular neck stenosis can lead to closure of the tubule at that point, leading to an atubular glomerulus that no longer adds its filtrate to the urine for excretion. We have shown that the ACE inhibitor captopril has a beneficial histological effect in the treatment of established radiation nephropathy, and that it prevents the disconnection of the glomerulus from its own draining tubule [39]. This shows clearly how reduction of injury at the glomerulo-tubular junction could prevent progressive renal failure. We have previously shown histological evidence for the benefit of captopril on glomerular and tubular injury when used for either mitigation or treatment of radiation nephropathy [15, 40]. This combined effect, reduction of both glomerular and tubular injury, is the accepted mechanism for the benefit of RAS antagonists in human chronic kidney disease in general [41]. Sebekova et al. [42] showed reversal of mesangiolysis by an ACE inhibitor in a renal injury model in obese Zucker rats. It is thus possible that in the radiation model, mitigation of mesangiolysis is a primary mechanism of action of ACE inhibitors, with a subsequent downstream benefit to reduce the tubulointerstitial injury and scarring. These drugs, ACE inhibitors and AII receptor blockers, do have important anti-hypertensive effects, which are relevant in the treatment of established kidney disease, from radiation or other causes. But it is unlikely that anti-hypertensive effects would be relevant to mitigation of radiation nephropathy, as these drugs show benefit when used before the start of radiation induced-hypertension [13, 43].
Fig. 10.
A normal glomerulus and its draining tubule (panel A) and one from a rat that underwent 18 Gy TBI in six fractions (panel B). The irradiated specimen shows periglomerular scarring that is accentuated at the glomerular-tubular junction (arrowhead), with narrowing of that junction, or neck, at that point. This neck stenosis can lead to complete closure and the formation of an atubular, non-filtering glomerulus. That evolution is stopped by captopril treatment. Adapted with permission of the Journal of Pathology, from Cohen et al. [39].
Oxidative Stress
The immediate radiolysis of water, and the resulting indirect injury to DNA, is the accepted mechanism for the initial X-ray induced injury to normal tissues. In kidney, external beam irradiation leads to damage to all cell types – glomerular, tubular, and vascular. Internal radionuclide injury may have a different intraorgan distribution, depending on tubular cell uptake and the type of particulate emission. Both modalities could cause immediate oxidative injury. It has been proposed that longer-term, chronic oxidative stress may be a mechanism of normal tissue radiation injury [44]. Moreover, the RAS could play a role by AII-mediated stimulation of NADPH oxidase, with production of reactive oxygen species as a result [45]. However, we have not found evidence for chronic oxidative stress in radiation nephropathy [8]. Moreover, agents with anti-oxidant activity were found to be ineffective in mitigating radiation nephropathy [46]; of which one, apocynin, is said to directly antagonize NADPH oxidase. In sum, the role of chronic oxidative stress in radiation nephropathy remains unproven, as is the putative role of AII.
CONCLUSIONS
Radiation nephropathy is a known complication of cancer therapy, and has been reproduced in laboratory animals [6]. It can be mitigated by antagonists of the renal angiotensin system, even though there is no evidence for activation of this system by irradiation. The benefits of renin-angiotensin system antagonism in radiation nephropathy are also seen in other kidney diseases, and are likely to depend on mitigation of both glomerular and tubulo-interstitial injury.
Acknowledgments
The able and constant assistance of Marylou Mäder is gratefully acknowledged. Dr. X. Allen Li performed the dosimetry. Yvonne Morauski helped to prepare the manuscript. These studies were done with the support of a contract from the National Institutes of Health, NIH/NIAID U19AI067734 (John Moulder, P.I.).
Footnotes
Cohen EP, Raff H, Bruder ED, Jankowski BM. Adrenal axis response to total body irradiation. 55th Annual Meeting of the Radiation Research Society, Savannah, October 2009.
Fish BL, Moulder JE, Mäder M, Schock A, Cohen EP. Ang(1–7): A mechanism for the efficacy of ACE inhibitors in the mitigation of radiation nephropathy? 55th Annual Meeting of the Radiation Research Society, Savannah, 2009.
Moulder JE, Fish BL, Irving AA, Mäder ML, Cohen EP. Exploring mechanisms for the efficacy of ACE inhibitors and AII blockers in radiation nephropathy. 13th International Congress of Radiation Research, San Francisco, 2007.
There is no conflict of interest.
References
- 1.Antignac C, Gubler MC, Leverger G, Broyer M, Habib R. Delayed renal failure with extensive mesangiolysis following bone marrow transplantation. Kidney Int. 1989;35:1336–44. doi: 10.1038/ki.1989.132. [DOI] [PubMed] [Google Scholar]
- 2.Cohen EP, Robbins MEC. Radiation nephropathy. Semin Nephrol. 2003;23:486–99. doi: 10.1016/s0270-9295(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 3.Moulder JE, Fish BL, Abrams RA. Renal toxicity following total body irradiation and syngeneic bone marrow transplantation. Transplantation. 1987;43:589–92. [PubMed] [Google Scholar]
- 4.Cohen EP, Fish BL, Moulder JE. The renin-angiotensin system in experimental radiation nephropathy. J Lab Clin Med. 2002;139:251–7. doi: 10.1067/mlc.2002.122279. [DOI] [PubMed] [Google Scholar]
- 5.Cohen EP. Radiation nephropathy. In: Davison AM, Cameron JS, Grünfeld JP, Ponticelli C, Ritz E, Winearls CG, et al., editors. Oxford Textbook of Clinical Nephrology. Oxford: Oxford University Press; 2005. pp. 1091–4. [Google Scholar]
- 6.Moulder JE, Cohen EP. Radiation-induced multi-organ involvement and failure: the contribution of radiation effects on the renal system. Br J Radiol Suppl. 2005;27:82–8. [Google Scholar]
- 7.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Sem Rad Onc. 2007;17:141–8. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Lenarczyk M, Cohen EP, Fish BL, Irving AA, Sharma M, Driscoll CD, et al. Chronic oxidative stress as a mechanism for radiation nephropathy. Radiat Res. 2009;171:164–72. doi: 10.1667/RR1454.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker JE, Fish BL, Su J, Haworth ST, Strande JL, Komorowski RA, et al. 10 Gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int J Radiat Biol. doi: 10.3109/09553000903264473. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moulder JE, Fish BL, Holcenberg JS, Sun GX. Hepatic function and drug pharmacokinetics after total body irradiation plus bone marrow transplant. Int J Radiat Oncol Biol Phys. 1990;19:1389–96. doi: 10.1016/0360-3016(90)90349-o. [DOI] [PubMed] [Google Scholar]
- 11.Coleman CN, Blakely WF, Fike JR, MacVittie TJ, Metting NF, Mitchell JB, et al. Molecular and cellular biology of moderate-dose (1–10 Gy) radiation and potential mechanisms of radiation protection: Report of a workshop at Bethesda, Maryland, December 17–18, 2001. Radiat Res. 2003;159:812–34. doi: 10.1667/rr3021. [DOI] [PubMed] [Google Scholar]
- 12.Moulder JE, Fish BL, Cohen EP. Noncontinuous use of angiotensin converting enzyme inhibitors in the treatment of experimental bone marrow transplant nephropathy. Bone Marrow Transplant. 1997;19:729–36. doi: 10.1038/sj.bmt.1700732. [DOI] [PubMed] [Google Scholar]
- 13.Cohen EP, Fish BL, Moulder JE. Successful brief captopril treatment in radiation nephropathy. J Lab Clin Med. 1997;129:536–47. doi: 10.1016/s0022-2143(97)90008-1. [DOI] [PubMed] [Google Scholar]
- 14.Moulder JE, Fish BL, Cohen EP. Dietary sodium modification and experimental radiation nephropathy. Int J Radiat Biol. 2002;79:903–11. doi: 10.1080/09553000210155897. [DOI] [PubMed] [Google Scholar]
- 15.Cohen EP, Molteni A, Hill P, Fish BL, Ward WF, Moulder JE, et al. Captopril preserves function and ultrastructure in experimental radiation nephropathy. Lab Invest. 1996;75:349–60. [PubMed] [Google Scholar]
- 16.Cohen EP, Lawton CA, Moulder JE. Bone marrow transplant nephropathy: Radiation nephritis revisited. Nephron. 1995;70:217–22. doi: 10.1159/000188587. [DOI] [PubMed] [Google Scholar]
- 17.Cohen EP, Irving AA, Drobyski WR, Klein JP, Passweg J, Talano J, et al. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2008;70:1546–51. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: From physiology to the pathobiology of hypertension and kidney disease. Pharm Rev. 2007;59:251–87. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh SN, Zhang R, Fish BL, Semenenko VA, Li XA, Moulder JE, et al. Renin-angiotensin system suppression mitigates experimental radiation pneumonitis. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2009.07.1743. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryu S, Kolozsvary A, Jenrow KA, Brown SL, Kim JH. Mitigation of radiation-induced optic neuropathy in rats by ACE inhibitor ramipril: importance of ramipril dose and treatment time. J Neuro-Oncol. 2007;82:119–24. doi: 10.1007/s11060-006-9256-4. [DOI] [PubMed] [Google Scholar]
- 21.Cohen EP, Fish BL, Sharma M, Li XA, Moulder JE. Role of the angiotensin II type-2 receptor in radiation nephropathy. Trans Res. 2007;150:106–15. doi: 10.1016/j.trsl.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobori H, Ozawa Y, Suzaki Y, Prieto-Carrasquero MC, Nishiyama A, Shoji T, et al. Intratubular angiotensinogen in hypertension and kidney diseases. Amer J Hyperten. 2006;19:541–50. doi: 10.1016/j.amjhyper.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen EP, Fish BL, Moulder JE. The role of nitric oxide in radiation nephropathy. Arch Physiol Biochem. 1996;104:200–6. doi: 10.1076/apab.104.2.200.12880. [DOI] [PubMed] [Google Scholar]
- 24.Wenzel U. Aldosterone and progression of renal disease. Curr Opin Nephrol Hypertens. 2008;17:44–50. doi: 10.1097/MNH.0b013e3282f29028. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu MH, Coimbra TM, de Araujo M, Menezes LF, Seguro AC. N-acetylcysteine attenuates the progression of chronic renal failure. Kidney Int. 2005;68:2208–17. doi: 10.1111/j.1523-1755.2005.00677.x. [DOI] [PubMed] [Google Scholar]
- 26.Omata M, Taniguchi H, Koya D, Kanasaki K, Sho R, Kato Y, et al. N-acetyl-seryl-aspartyl-lysyl-proline ameliorates the progression of renal dysfunction and fibrosis in WKY rats with established anti-glomerular basement membrane nephritis. J Amer Soc Nephrol. 2006;17:674–85. doi: 10.1681/ASN.2005040385. [DOI] [PubMed] [Google Scholar]
- 27.Peng H, Carretero OA, Liao TD, Peterson EL, Rhaleb NE. Role of N-acetyl-seryl-aspartyl-lysyl-proline in the antifibrotic and anti-inflammatory effects of the angiotensin-converting enzyme inhibitor captopril in hypertension. Hypertension. 2007;49:695–703. doi: 10.1161/01.HYP.0000258406.66954.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doggrell SA. Bradykinin B2 receptors as a target in diabetic nephropathy. Current Opin Invest Drugs. 2006;7:251–5. [PubMed] [Google Scholar]
- 29.Jaggi JS, Seshan SV, McDevitt MR, Sgouros G, Hyjek E, Scheinberg DA. Mitigation of radiation nephropathy after internal α-particle irradiation of kidneys. Int J Radiat Oncol Biol Phys. 2006;64:1503–12. doi: 10.1016/j.ijrobp.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 30.Santos RAS, Ferreira AJ, Silva ACE. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exper Physiol. 2008;93:519–27. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 31.Shao Y, He M, Zhou L, Yao T, Huang Y, Lu LM. Chronic angiotensin (1-7) injection accelerates STZ-induced diabetic renal injury. Acta Pharm Sinica. 2008;29:829–37. doi: 10.1111/j.1745-7254.2008.00812.x. [DOI] [PubMed] [Google Scholar]
- 32.Morita T, Churg J. Mesangiolysis. Kidney Int. 1983;24:1–9. doi: 10.1038/ki.1983.119. [DOI] [PubMed] [Google Scholar]
- 33.Glatstein E, Fajardo LF, Brown JM. Radiation injury in the mouse kidney -- Sequential light microscopic study. Int J Radiat Oncol Biol Phys. 1977;2:933–43. doi: 10.1016/0360-3016(77)90191-2. [DOI] [PubMed] [Google Scholar]
- 34.Robbins MEC, Jaenke RS, Bywaters T, Golding SJ, Rezvani M, Whitehouse E, et al. Sequential evaluation of radiation-induced glomerular ultrastructural changes in the pig kidney. Radiat Res. 1993;135:351–64. [PubMed] [Google Scholar]
- 35.Nakayama T, Sato W, Kosugi T, Zhang L, Campbell-Thompson M, Yoshimura A, et al. Endothelial injury due to eNOS deficiency accelerates the progression of chronic renal disease in the mouse. Am J Physiol Renal Physiol. 2009;296:F317–F27. doi: 10.1152/ajprenal.90450.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verhagen AMG, Braam B, Boer P, Gröne JJ, Koomans HA, Joles JA. Losartan-sensitive renal damage caused by chronic NOS inhibition does not involve renal angiotensin II concentrations. Kidney Int. 1999;56:222–31. doi: 10.1046/j.1523-1755.1999.00542.x. [DOI] [PubMed] [Google Scholar]
- 37.Meyer TW, Anderson S, Rennke HG, Brenner BM. Reversing glomerular hypertension stabilizes established glomerular injury. Kidney Int. 1987;31:752–9. doi: 10.1038/ki.1987.62. [DOI] [PubMed] [Google Scholar]
- 38.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–96. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen EP, Regner K, Fish BL, Moulder JE. Stenotic glomerulotubular necks in radiation nephropathy. J Pathol. 2000;190:484–8. doi: 10.1002/(SICI)1096-9896(200003)190:4<484::AID-PATH529>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 40.Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ACE inhibitors. Int J Radiat Oncol Biol Phys. 1993;27:93–9. doi: 10.1016/0360-3016(93)90425-u. [DOI] [PubMed] [Google Scholar]
- 41.Brewster UC, Perazella MA. The renin-angiotensin-aldosterone system and the kidney: effects on kidney disease. Am J Med. 2004;116:263–72. doi: 10.1016/j.amjmed.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 42.Sebekova K, Lill M, Boor P, Heidland A, Amann K. Functional and partial morphological regression of established renal injury in the obese zucker rat by blockade of the renin-angiotensin system. Am J Nehrol. 2009;29:164–70. doi: 10.1159/000151771. [DOI] [PubMed] [Google Scholar]
- 43.Moulder JE, Fish BL, Cohen EP. Brief pharmacologic intervention in experimental radiation nephropathy. Radiat Res. 1998;150:535–41. [PubMed] [Google Scholar]
- 44.Zhao W, Diz DI, Robbins ME. Oxidative damage pathways in relation to normal tissue injury. Br J Radiol. 2007;80:S23–S31. doi: 10.1259/bjr/18237646. [DOI] [PubMed] [Google Scholar]
- 45.Sachse A, Wolf G. Angiotensin II-Induced reactive oxygen species and the kidney. J Amer Soc Nephrol. 2007;18:2439–46. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 46.Cohen EP, Fish BL, Irving AA, Rajapurkar MM, Shah SV, Moulder JE. Radiation nephropathy is not mitigated by antagonists of oxidative stress. Radiat Res. 2009;172:260–4. doi: 10.1667/RR1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lawton CA, Cohen EP, Murray KJ, Derus SW, Moulder JE. Long-term results of selective renal shielding in patients undergoing total body irradiation in preparation for bone marrow transplantation. Bone Marrow Transplant. 1997;12:1069–74. doi: 10.1038/sj.bmt.1701022. [DOI] [PubMed] [Google Scholar]
- 48.Miralbell R, Bieri S, Mermillod B, Helg C, Sancho G, Pastoors B, et al. Renal toxicity after allogeneic bone marrow transplantation: The combined effects of total-body irradiation and graft-versus-host disease. J Clin Oncol. 1996;14:579–85. doi: 10.1200/JCO.1996.14.2.579. [DOI] [PubMed] [Google Scholar]
- 49.Sieber F, Muir SA, Cohen EP, North PE, Fish BL, Irving AA, et al. High-dose selenium for the mitigation of radiation injury: a pilot study in a rat model. Radiat Res. 2009;171:368–73. doi: 10.1667/0033-7587-171.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]