Abstract
The endogenous opioid system, which alleviates physical pain, is also known to regulate social distress and reward in animal models. To test this hypothesis in humans (n = 18), we used a μ-opioid receptor (MOR) radiotracer to measure changes in MOR availability in vivo with positron emission tomography (PET) during social rejection (not being liked by others) and acceptance (being liked by others). Social rejection significantly activated the MOR system (i.e., reduced receptor availability relative to baseline) in the ventral striatum, amygdala, midline thalamus, and periaqueductal gray (PAG). This pattern of activation is consistent with the hypothesis that the endogenous opioids play a role in reducing the experience of social pain. Greater trait resiliency was positively correlated with MOR activation during rejection in the amygdala, PAG, and subgenual anterior cingulate cortex (sgACC), suggesting that MOR activation in these areas is protective or adaptive. In addition, MOR activation in the pregenual ACC was correlated with reduced negative affect during rejection. In contrast, social acceptance resulted in MOR activation in the amygdala and anterior insula, and MOR deactivation in the midline thalamus and sgACC. In the left ventral striatum, MOR activation during acceptance predicted a greater desire for social interaction, suggesting a role for the MOR system in social reward. The ventral striatum, amygdala, midline thalamus, PAG, anterior insula, and ACC are rich in MORs and comprise a pathway by which social cues may influence mood and motivation. MOR regulation of this pathway may preserve and promote emotional well-being in the social environment.
Keywords: opioid, PET, social, rejection, acceptance, depression, mu, stress
INTRODUCTION
Humans rely on acceptance into groups and intimate relationships for survival and emotional well-being. Threats to this need, such as social rejection (i.e., being excluded or not liked by others), can cause social withdrawal, impulsivity, substance abuse, and symptoms of anxiety and depression1–4. Research over the last decade has shown that social rejection and physical pain share similar neuronal pathways, leading to the theory of “social pain”5–9. This theory suggests that responses to social rejection are regulated by endogenous opioids and the μ-opioid receptor (MOR), which alleviates physical pain, but is also known to regulate social distress in several nonhuman species10–14.
A few studies suggest a role for the endogenous opioid system in ameliorating the effects of social rejection in humans. A central analgesic reduced brain fMRI blood-oxygenation-level dependent (BOLD) responses to social rejection15, and variations in the MOR gene were associated with BOLD sensitivity to social rejection16. However, regional changes in the MOR system in humans that may serve to reduce the experience of social rejection are not known. The MOR system also plays an important role in social reward in animal models17–20, however it is not known if the MOR system plays a similar role in humans. To examine regional changes in the MOR system during social rejection and acceptance, we measured changes in MOR availability in vivo using [11C]carfentanil, a ligand with high and selective affinity for MORs21, with positron emission tomography (PET).
MATERIALS AND METHODS
Subjects
Participants were 18 healthy volunteers aged 18–48 (13 female, 5 males; mean age ± SD, 32 ± 12 years) screened for active medical illness and psychiatric disorders using the SCID-IV non-patient version. No subjects were taking psychotropic medications, hormones, or hormonal contraception in the three months prior to study. Phase of menstrual cycle was not controlled for given that MOR binding potential (BP) in vivo is not influenced by phase in the menstrual cycle22. Study protocols were approved by the Institutional Review Board of the University of Michigan Medical School, and written informed consent was obtained.
Social Feedback Task
Several days prior to scanning, subjects completed an online personal profile that included age, major/occupation, a list of their interests, a short paragraph of their positive qualities, and a picture of themselves. Scores for the trait Ego Resiliency23 were also obtained at this time. Subjects selected at least 40 online profiles of preferred-sex individuals with whom they would be most interested in forming an intimate relationship, from a collection of 500 profiles of men and women. For each profile, subjects answered two questions (“Would I like this person?” and “Do I think this person would like me?”) on a 7-point Likert scale from “definitely no” to “definitely yes.” To increase feedback saliency, only profiles with the highest ratings for both questions were used. These strategies were chosen based on an fMRI study76 and another showing that social feedback from highly-rated, opposite-sex individuals (compared to low-rated or same-sex individuals) resulted in the greatest changes in brain activation24. Seventeen subjects identified themselves as heterosexual and rated only opposite sex profiles; one bisexual female chose to rate only female profiles. Nine subjects reported being single, 2 divorced, 5 in a relationship, and 2 married.
During the PET scan, subjects were presented with their highest-rated profiles along with feedback that they were not liked (Rejection), or liked (Acceptance) (Fig. 1a). Rejection and Acceptance blocks were 24 minutes each and contained 12 unique trials of equal length with varying levels of rejection/acceptance (7 trials “definitely no/yes”, 4 trials “very likely no/yes,” and 1 trial “likely no/yes”). Baseline scans were included to compare MOR BP during the same post-injection time frame (Fig. 1b). Baseline trials contained a similar visual presentation, with grayscale blocks in place of the pictures, and profile information and feedback presented as “N/A”. This task did not involve deception, but subjects were asked to imagine that the profiles and feedback were real (see Supplementary Methods). During each trial subjects reported on a 5-point Likert scale how much they felt sad, rejected, happy, and accepted (order randomized in each trial). Following each block, subjects were given a 4-item questionnaire measuring their current desire for social interaction (see Supplementary Methods). All behavioral responses were obtained using a five-button response box.
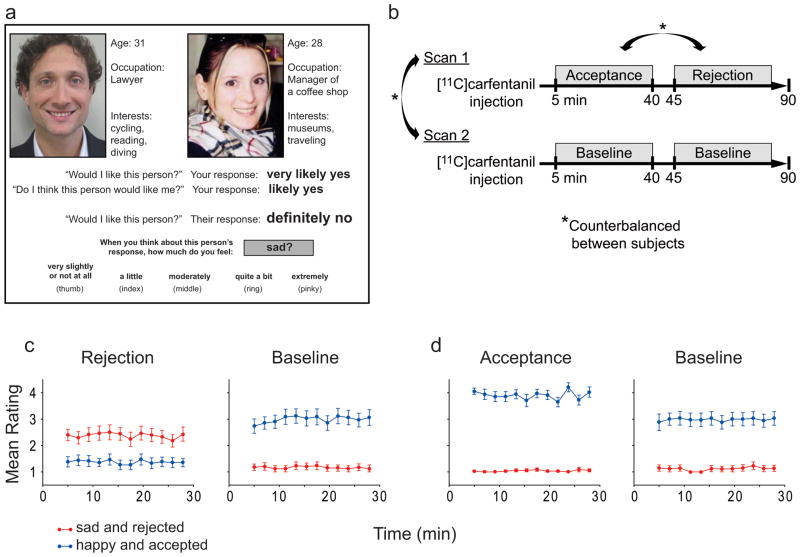
Figure 1.
Study design and behavioral results. (a) During the scan, the subject is presented with self-selected profiles (left) along with her own profile (right), viewed on a personal computer. The following information is presented in succession: the first line reminds the subject how much she liked this person, the second line reminds the subject that she believed this person would like her, the last line provides feedback that this person did not like her (Rejection shown here) or did like her (Acceptance). After each trial, subjects rate how they feel. (b) Each subject received an intravenous injection of [11C]-labeled carfentanil and completed two scans for examining Rejection and Acceptance blocks, compared with Baseline blocks from the same post-injection time frame. The order of scans 1 and 2, and Rejection and Acceptance, were counterbalanced between subjects using the Latin Squares design to control for potential order effects. (c) Subjects reported feeling more “sad and rejected” during the Rejection block, and (d) more “happy and accepted” during the Acceptance block, compared to matched Baseline blocks (mean ± s.e.m). Consent was obtained by DT Hsu to publish the likenesses in this image.
PET and Magnetic Resonance Imaging
Protocols for the acquisition and reconstruction for PET, and co-registration with structural MRIs are described in the Supplementary Methods. In brief, each subject completed two PET scans for comparing Rejection and Acceptance with Baseline blocks acquired during the same post-injection time frame (starting at either 5 or 45 minutes, Fig. 1b) as previously described for other paradigms25, 26. [11C]carfentanil, a ligand with high and selective affinity for MORs21 was synthesized at high specific activity (> 3000 Ci/mmol) and administered intravenously at the beginning of each scan. High resolution structural MRIs were co-registered with MOR binding maps and used for spatial normalization to standard space (Montreal Neurological Institute, Quebec, CA) (MNI).
Data Analysis
Within-subjects paired t-tests (two-tailed) were performed to compare mean subjective ratings between blocks. Affect ratings were categorized as “sad and rejected” (mean of the items “sad” and “rejected”), and “happy and accepted” (mean of “happy” and “accepted”).
MOR activation was defined as the reduction in MOR BP from Baseline to Rejection (or Acceptance) block. This difference represents processes such as competition between radiotracer and endogenous opioids, changes in the conformational state of the receptor after activation, or receptor internalization and trafficking, which are all related to endogenous neurotransmission26. The main contrasts of interest were modeled using Statistical Parametric Mapping v.8 (SPM8) (Wellcome Institute of Cognitive Neurology, London, UK). For each subtraction analysis, one- or two-sample t-values were calculated for each voxel using a pooled smoothed variance across pixels27.
A priori volumes of interest (VOIs) were created using MarsBaR region of interest toolbox (version 0.38) for SPM8 and included structures that are rich in MORs and respond to physical pain and/or social rejection8, 15, 16, 25. These included the ventral striatum (6 mm radius sphere centered at ±12, 13, −9 mm), amygdala (8 mm sphere at ±20, −2, −21 mm), anterior insula (6 mm sphere at ±43, 7, −2), midline thalamus (4 mm sphere at 0, −15, 6), periaqueductal gray (3 mm sphere at 0, −33, −11), and subgenual cingulate cortex (sgACC) (6 mm sphere at 0, 14, −8 mm). The dorsal anterior cingulate cortex (dACC) was constructed from the automated anatomical atlas28 using PickAtlas29, with a rostral-caudal boundary of y = 36, 016. A VOI in the pregenual anterior cingulate cortex (pgACC) (5 mm sphere at −3, 32, 2) was chosen from a previous study that showed peak MOR deactivation at this location during self-induced sadness26. VOIs were applied to subtraction images in standardized space and alpha levels were family-wise error (FWE) corrected. VOI data were also extracted with MarsBaR and correlated with the trait Ego Resiliency and behavioral changes.
RESULTS
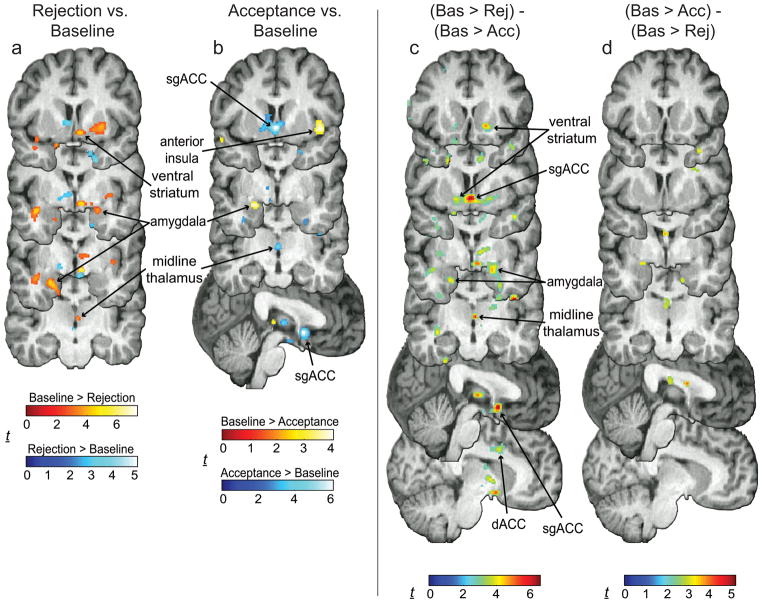
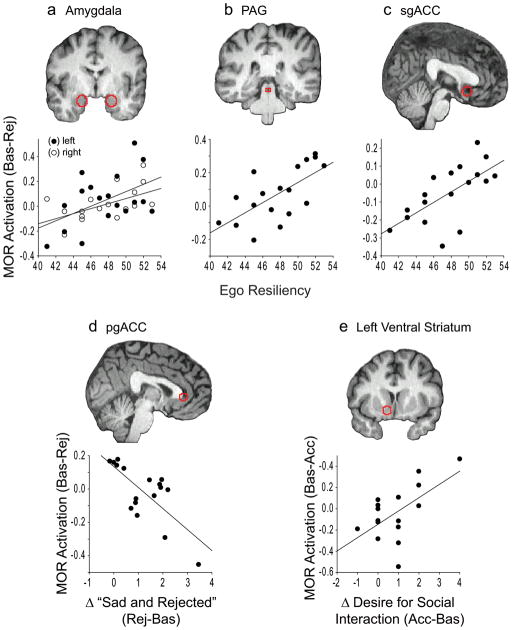
During Rejection compared to matched Baseline block, subjects reported feeling more “sad and rejected” (t16 = 5.11, p < 0.001) and less “happy and accepted” (t16 = 6.03, p < 0.001, Fig. 1c). Significant MOR activation was found in the left and right amygdala, right ventral striatum, midline thalamus, and PAG (Fig. 2a, Table 1). No significant MOR deactivations were found. The trait Ego Resiliency was positively correlated with MOR activation during Rejection in the amygdala (left, r = 0.48, p = 0.04; right, r = 0.54, p = 0.02), PAG (r = 0.66, p = 0.003), and sgACC (r = 0.65, p = 0.003) (Fig. 3a–c). Increased ratings for “sad and rejected” was negatively correlated with MOR activation in the pgACC (r = −0.73, p < 0.001, Fig. 3d). Subjects reported a decreased desire for social interaction following Rejection compared to Baseline (t16 = 2.14, p = 0.048), however this change was not significantly correlated with MOR activation in the VOIs. During Acceptance compared to matched Baseline block, subjects reported feeling more “happy and accepted” (t16 = 3.71, p = 0.002), with no change in “sad and rejected” (t16 = 0.87, p = 0.40) (Fig. 1d). Significant MOR activation was found in the right anterior insula and left amygdala (Fig 2b, Table 1), whereas significant deactivation was found in the midline thalamus and sgACC (Fig. 2b, Table 1). Neither Ego Resiliency nor scores for “happy and accepted” were significantly correlated with MOR activation in the VOIs. Subjects reported an increased desire for social interaction following Acceptance compared to Baseline (t15 = 2.91, p = 0.01), and this change was positively correlated with MOR activation in the left ventral striatum (r = 0.60, p = 0.01, Fig. 3e).
Figure 2.
Changes in MOR BP. (a) MOR activation during Rejection is shown in red-yellow, MOR deactivation in shades of blue. Greater activation was found in the right ventral striatum, bilateral amygdala, and midline thalamus. (b) Greater activation during Acceptance was found in the anterior insula and left amygdala, whereas greater deactivation was found in the midline thalamus and sgACC. (c) Greater activation during Rejection compared to Acceptance blocks was found in the right ventral striatum, bilateral amygdala, midline thalamus, sgACC and dACC. (d) Relatively little activation was found for the opposite contrast. For all images, contrast t maps are rendered onto a template brain in MNI space. Display threshold: p < 0.01, whole-brain uncorrected.
Table 1.
Changes in MOR BP in VOIs. Locations of peaks are shown in x, y, z coordinates (mm) in MNI space.
| VOI | Baseline-Rejection | Rejection-Baseline | Baseline-Acceptance | Acceptance-Baseline | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Peak | t | Peak | t | Peak | t | Peak | t | |
|
| ||||||||
| Ventral Striatum (R) | 16, 12, −6 | 3.90* | --- | --- | --- | --- | --- | --- |
| Amygdala (L) | −26, −4, −23 | 4.53** | --- | --- | −22, −3, −17 | 3.91* | --- | --- |
| Amygdala (R) | 23, 2, −17 | 3.62* | --- | --- | --- | --- | --- | --- |
| Midline Thalamus | 3, −18, 6 | 3.68** | --- | --- | --- | --- | 0, −12, 4 | 3.83** |
| PAG | 0, −33, −12 | 2.30* | --- | --- | --- | --- | --- | --- |
| Anterior Insula (R) | --- | --- | --- | --- | 44, 8, −6 | 3.91* | --- | --- |
| SgACC | --- | --- | --- | --- | --- | --- | 0, 9, −6 | 6.09*** |
p < 0.05,
p < 0.01,
p < 0.001, FWE-corrected within VOI. Dashes indicate no clusters detected at a threshold of p < 0.05. Significant changes in MOR BP were not found in the left ventral striatum, left anterior insula, dACC, or pgACC. L, left; R, right
Figure 3.
Extracted data from VOIs (red outlines) correlated with trait resiliency and state changes. MOR activation during Rejection correlated with Ego Resiliency in the (a) amygdala (b) PAG, and (c) sgACC, suggesting that high-resilient individuals are more capable of MOR activation in these regions during rejection. (d) Increased ratings for “sad and rejected” were negatively correlated with MOR activation in the pgACC (i.e., subjects who felt less “sad and rejected” had greater MOR activation during Rejection). (e) During Acceptance, increased ratings in the desire for social interaction were positively correlated with MOR activation in the left ventral striatum.
Further analysis showed that Rejection induced significantly greater activation than Acceptance in the right ventral striatum, bilateral amygdala, midline thalamus, and sgACC (Fig. 2c, Table 2). MOR activation was not significantly greater during Acceptance compared to Rejection in any VOIs (Fig. 2d). In several structures, MOR activation during Rejection was positively correlated with MOR activation during Acceptance. Such correlations were found in the ventral striatum (left, r = 0.85, p < 0.001; right, r = 0.54, p = 0.02), midline thalamus (r = 0.77, p < 0.001), anterior insula (left, r = 0.79, p < 0.001; right, r = 0.62, p = 0.006), and dACC (left, r = 0.86, p < 0.001; right, r = 0.92, p < 0.001), but not in the amygdala, PAG, pgACC, or sgACC.
Table 2.
Changes in MOR activation in VOIs during Rejection greater than Acceptance. Locations of peaks are shown in x, y, z coordinates (mm) in MNI space.
| VOI | (Bas-Rej) − (Bas-Acc) | |
|---|---|---|
|
| ||
| Peak | t | |
|
| ||
| Ventral Striatum (L) | −14, 8, −9 | 3.71* |
| Ventral Striatum (R) | 16, 14, −6 | 4.73** |
| Amygdala (L) | −20, −4, −29 | 5.90** |
| Amygdala (R) | 20, 2, −17 | 4.72* |
| Midline Thalamus | 2, −15, 6 | 5.33*** |
| SgACC | 0, 8, −8 | 6.14*** |
p < 0.05,
p < 0.01,
p < 0.001, FWE-corrected within VOI. Significant differences were not found in the PAG, anterior insula, dACC, or pgACC. For the opposite contrast, (Bas-Acc) – (Bas-Rej), no significant clusters in any VOIs were detected. L, left; R, right
In a follow up analysis, Ego Resiliency and behavioral measures during Rejection or Acceptance compared to Baseline blocks were not significantly different between subjects who reported being single/divorced (n = 11) vs. in a relationship/married (n = 7) (two-sample t-tests, p > 0.10). During Rejection, MOR activations (Table 1) were not significantly different between those who were single/divorced vs. in a relationship/married (p’s > 0.36). During Acceptance, only MOR activation (Table 1) in the right anterior insula was greater in subjects who reported to be in a relationship/married compared to those who were single/divorced (t16 = 2.15, p = 0.05).
DISCUSSION
This is the first study to show regional changes in the human MOR system in response to social rejection and acceptance. Social rejection produced greater overall MOR activation compared to acceptance. Higher trait resiliency predicted a greater magnitude of activation in the amygdala, PAG, and sgACC, suggesting that MOR activation during rejection in these areas is protective or adaptive. During acceptance, MOR activation in the left ventral striatum was positively correlated with an increased desire for social interaction. As established in animal models, all of these structures have high levels of MORs30–34, and comprise a pathway by which social stimuli may influence mood and motivation35–38.
In support of the view that MOR activation is protective or adaptive, a greater predisposition for resiliency predicted a greater magnitude of MOR activation during rejection in the amygdala, PAG, and sgACC (Fig. 3a–c). MOR activation in the amygdala is associated with analgesia39 and reducing norepinephrine release40, potentially to regulate emotional responses to arousing stimuli41. Similarly, the PAG is a pivotal site for coordinating visceral and behavioral responses to pain and other stressors42, and MOR-mediated signaling in the PAG attenuates pain and distress behaviors43–45. Consistent with these roles for the amygdala and PAG, one study showed that scores from daily feelings of social rejection correlated with fMRI BOLD signal in these structures during a social exclusion task46. BOLD signal in sgACC, an area strongly linked to sadness and major depressive disorder47, 48, has also been shown to increase during social exclusion49, 50, and predicts increases in depressive symptoms51. Consistent with these data, the present study found that during rejection, high-resilient individuals are more capable of MOR activation in the amygdala, PAG, and sgACC, potentially serving a protective function by reducing rejection-induced neuronal activity in these regions.
During rejection, MOR activation was significantly increased in the right ventral striatum in the area of the nucleus accumbens (Fig. 2a, Table 1). Although opioid activity in the nucleus accumbens is well known for its role in reward52, 53, it also plays a role in reducing physical pain25, 54, 55, and may therefore play a similar role during social rejection. MOR activation during rejection was also significantly increased in the midline thalamus, which has among the highest levels of MOR BP in humans56 and displays the greatest MOR activation following different types of acute pain25, 57. Animal studies show that the highest thalamic density of MORs is found in the paraventricular nucleus34, a midline thalamic nucleus consistently and strongly activated following a wide variety stressors, and involved in regulating the effects of repeated stress58, 59. MOR-mediated signaling inhibits thalamic neurons60 and the paraventricular nucleus is specifically connected to structures involved in regulating stress responses, mood, and motivation, including the nucleus accumbens, amygdala, PAG, anterior insula, and sgACC36–38. Thus, MOR activation in the midline thalamus may serve to coordinate the responses of multiple structures during social rejection.
The pattern of MOR activation in the amygdala, thalamus, and ventral striatum during social rejection was similar to that during physical pain25, 61, supporting the hypothesis that responses to social rejection and physical pain are regulated by overlapping neuronal pathways5–9. In contrast, a previous study showed overall MOR deactivation when healthy adults made themselves feel sad by focusing on a sad autobiographical event, selected and rehearsed prior to scanning26. In this study, 10 of the 14 subjects recalled the death of a loved one, 3 recalled romantic breakups, and 1 a recent argument with a friend. Thus, differences in MOR activation between the present study and the induced sadness study might be explained by different mechanisms associated with unrehearsed emotional responses during social feedback vs. rehearsed bereavement. Since the pattern of MOR activation during social rejection is similar to that of physical pain25, 61, it is possible that naturalistic coping with an external stressor results in overall MOR activation, compared to permissive, internally-generated sadness which results in MOR deactivation26. Despite these differences, MOR activation in the pgACC was negatively correlated with increased ratings of negative affect during both social rejection (Fig. 3d) and induced sadness26, suggesting a common role for MOR activation in the pgACC in dampening negative affect regardless of how the emotion was elicited.
A few fMRI studies also suggest that external cues vs. internally generated cues may account for some of the differences observed in MOR activation during social feedback vs. induced sadness26. In two fMRI studies where subjects were asked to view a photo of a romantic ex-partner and relive the rejection experience (i.e., a combination of external and internal cues), increased BOLD activation was found in the anterior insula and ACC8, 62, whereas recalling sad thoughts about a recent romantic breakup (i.e., internal cues only) resulted in deactivation in areas including the insula and ACC63. Thus, the pattern of activation found in the former fMRI studies8, 62 is more consistent to the present study (all using external or a combination of external/internal cues), whereas the latter fMRI study is consistent to the MOR study using induced sadness26 (all using internal cues). However, combining the interpretation of results from MOR PET studies with fMRI studies is tentative since the relationship between MOR activation and BOLD signal is not known.
The MOR system also plays a role in mediating social reward. In humans, variations in the MOR gene were shown to be associated with social hedonic capacity64, and a large body of animal work shows that MOR-mediated signaling plays an important role in social reward17–20. The present study showed increased MOR activation in the amygdala and anterior insula during acceptance (Fig. 2b, Table 1), consistent with increased opioid activation observed in the amygdala during an amusing video clip65, and in the anterior insula following amphetamine administration66. Interestingly, MOR activation in the right insula was also greater in those who were in a relationship or married compared to those who were single or divorced. It is intriguing that those in relationships have greater MOR activation during social acceptance, suggesting that being in a social pair bond may promote a more responsive MOR system. However given the limited number of subjects per group (n = 7 and 11), this hypothesis needs to be confirmed in a larger sample size.
MOR activation in the left ventral striatum in the region of the nucleus accumbens was positively correlated with an increased desire for social interaction. This finding is consistent with a recent report showing that in adolescent rats, MORs but not delta- or kappa-opioid receptors in the nucleus accumbens mediate social play behavior20. The present study also found significant MOR deactivation during acceptance in the midline thalamus and sgACC (Fig 2b, Table 1). In the thalamus, MOR deactivation during acceptance may serve to “permit” the positive effects of acceptance. Consistent with this hypothesis, a previous study in rats showed that a MOR agonist injected into the medial thalamus raised the threshold for both pain and positive reinforcement67. This hypothesis may also be particularly important for the sgACC, which is associated with anhedonia in major depressive disorder48, 68.
MOR activation during rejection showed positive correlations with MOR activation during acceptance in several areas, suggesting that the MOR system responds to both types of stimuli. This is consistent with animal studies showing that endogenous opioid release reduces distress during social separation, and facilitates positive emotions during play10–14, 17–20. The present study further adds to this model by showing regional specificity in MOR activation during rejection and acceptance. Differences in the magnitude of MOR activation were found in specific areas during rejection compared to acceptance (Fig 2, Tables 1 and 2). Furthermore, the areas where MOR activation correlations were not found (i.e., amygdala, PAG, pgACC, sgACC) were also the only areas that correlated with resiliency and negative affect during rejection (Fig. 3a–d), suggesting that MOR activation in these structures were specific to rejection. While it is also possible that the overall greater MOR activation during rejection was due to greater saliency of rejection compared to acceptance, the magnitude of affective change between the two conditions were not different (Fig. 1c, d), and subjects reported that both conditions felt equally similar to real-life experiences (see Supplementary Methods). Future studies will need to establish a causal relationship between MOR activity and subjective feelings by pharmacologically manipulating the MOR system and measuring changes in regional MOR BP and affect69, 70.
The present study found that MOR activation in the dACC was greater during rejection than during acceptance (Figs. 2c). Although this activation did not reach statistical significance within the large dACC VOI, the location of the peak was similar to that in fMRI studies of social rejection5, 8, suggesting that MOR activation plays a role in regulating dACC activity during rejection. Surprisingly, MOR activation in anterior insula, which is activated in fMRI studies of rejection9, was significant during acceptance but not rejection. This may suggest that MOR activation in the anterior insula, which may be involved in both negative and positive emotions71, is more sensitive to social acceptance than rejection. This is consistent with one fMRI study showing that the anterior insula is activated while being liked by others24. Future studies will need to investigate the relationship between BOLD and MOR activity in response to social rejection and acceptance.
This study demonstrates that social rejection and acceptance activate the MOR system in neuronal pathways regulating mood and motivation. The pattern of MOR activation during social rejection was similar to that previously found during sustained physical pain, suggesting an overlapping role for the MOR system in regulating both social rejection and physical pain. In addition, resiliency traits and subjective experiences were associated with MOR responses to rejection. On the other hand, social acceptance resulted in weaker MOR activation, and activation in the nucleus accumbens was significantly correlated with an increased desire for social interaction. Thus, the MOR system may play a dual role in reducing social distress and mediating social reward, as have been shown in animal studies. This study provides a first step towards understanding the neurochemical regulation of positive and negative social cues in humans, suggesting a potential mechanism for rejection sensitivity and social anhedonia in major depressive, social anxiety, substance use, eating, body dysmorphic, and borderline personality disorders72–75.
Supplementary Material
Acknowledgments
Sources of support:
This research was supported by National Institute of Health grants K01 MH085035 (D.T.H.), K23 MH074459 (S.A.L.), R01 DA022520 and R01 DA027494 (J.K.Z.), a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (D.T.H.), pilot grants from the Michigan Institute for Clinical & Health Research (D.T.H.), a Rachel Upjohn Clinical Scholars Award (D.T.H.), and the Phil F. Jenkins Foundation (J.K.Z.).
Footnotes
Supplementary information is available at Molecular Psychiatry’s website.
Conflict of Interest: Dr. Koeppe is a consultant for Avid Corp., Merck, and Johnson & Johnson; Dr. Mickey received salary support from St. Jude Medical for research unrelated to this manuscript; Dr. Langenecker received compensation for consulting to CogState Limited unrelated to this manuscript. All other authors declare no conflict of interest.
References
- 1.Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. J Pers Soc Psychol. 2005;88:589–604. doi: 10.1037/0022-3514.88.4.589. [DOI] [PubMed] [Google Scholar]
- 2.Williams KD. Ostracism. Annu Rev Psychol. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- 3.Mallott MA, Maner JK, DeWall N, Schmidt NB. Compensatory deficits following rejection: the role of social anxiety in disrupting affiliative behavior. Depress Anxiety. 2009;26:438–446. doi: 10.1002/da.20555. [DOI] [PubMed] [Google Scholar]
- 4.Slavich GM, O’Donovan A, Epel ES, Kemeny ME. Black sheep get the blues: a psychobiological model of social rejection and depression. Neurosci Biobehav Rev. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 6.Ehnvall A, Mitchell PB, Hadzi-Pavlovic D, Malhi GS, Parker G. Pain during depression and relationship to rejection sensitivity. Acta Psychiatr Scand. 2009;119:375–382. doi: 10.1111/j.1600-0447.2008.01316.x. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald G, Jensen-Campbell LA, editors. Social Pain: Neuropsychology and Health Implications of Loss and Exclusion. 1. American Psychological Association (APA); 2011. p. 264. [Google Scholar]
- 8.Kross E, Berman MG, Mischel W, Smith EE, Wager TD. Social rejection shares somatosensory representations with physical pain. Proc Natl Acad Sci USA. 2011;108:6270–6275. doi: 10.1073/pnas.1102693108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- 10.Panksepp J, Herman B, Conner R, Bishop P, Scott JP. The biology of social attachments: opiates alleviate separation distress. Biol Psychiatry. 1978;13:607–618. [PubMed] [Google Scholar]
- 11.Panksepp J, Herman BH, Vilberg T, Bishop P, DeEskinazi FG. Endogenous opioids and social behavior. Neurosci Biobehav Rev. 1980;4:473–487. doi: 10.1016/0149-7634(80)90036-6. [DOI] [PubMed] [Google Scholar]
- 12.Herman BH, Panksepp J. Ascending endorphin inhibition of distress vocalization. Science. 1981;211:1060–1062. doi: 10.1126/science.7466377. [DOI] [PubMed] [Google Scholar]
- 13.Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- 14.Kalin NH, Shelton SE, Lynn DE. Opiate systems in mother and infant primates coordinate intimate contact during reunion. Psychoneuroendocrinology. 1995;20:735–742. doi: 10.1016/0306-4530(95)00023-2. [DOI] [PubMed] [Google Scholar]
- 15.Dewall CN, Macdonald G, Webster GD, Masten CL, Baumeister RF, Powell C, et al. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21:931–937. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- 16.Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proc Natl Acad Sci USA. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siegel MA, Jensen RA, Panksepp J. The prolonged effects of naloxone on play behavior and feeding in the rat. Behav Neural Biol. 1985;44:509–514. doi: 10.1016/s0163-1047(85)91024-6. [DOI] [PubMed] [Google Scholar]
- 18.Vanderschuren LJ, Niesink RJ, Spruijt BM, Van Ree JM. Mu- and kappa-opioid receptor-mediated opioid effects on social play in juvenile rats. Eur J Pharmacol. 1995;276:257–266. doi: 10.1016/0014-2999(95)00040-r. [DOI] [PubMed] [Google Scholar]
- 19.Trezza V, Baarendse PJJ, Vanderschuren LJMJ. The pleasures of play: pharmacological insights into social reward mechanisms. Trends Pharmacol Sci. 2010;31:463–469. doi: 10.1016/j.tips.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trezza V, Damsteegt R, Achterberg EJM, Vanderschuren LJMJ. Nucleus accumbens μ-opioid receptors mediate social reward. J Neurosci. 2011;31:6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titeler M, Lyon RA, Kuhar MJ, Frost JF, Dannals RF, Leonhardt S, et al. Mu opiate receptors are selectively labelled by [3H]carfentanil in human and rat brain. Eur J Pharmacol. 1989;167:221–228. doi: 10.1016/0014-2999(89)90582-7. [DOI] [PubMed] [Google Scholar]
- 22.Smith YR, Zubieta JK, del Carmen MG, Dannals RF, Ravert HT, Zacur HA, et al. Brain opioid receptor measurements by positron emission tomography in normal cycling women: relationship to luteinizing hormone pulsatility and gonadal steroid hormones. J Clin Endocrinol Metab. 1998;83:4498–4505. doi: 10.1210/jcem.83.12.5351. [DOI] [PubMed] [Google Scholar]
- 23.Block J. The challenge of response sets: unconfounding meaning, acquiesence, and social desirability in the MMPI. Appleton-Century-Crofts; 1965. p. 168. [Google Scholar]
- 24.Davey CG, Allen NB, Harrison BJ, Dwyer DB, Yücel M. Being liked activates primary reward and midline self-related brain regions. Hum Brain Mapp. 2010;31:660–668. doi: 10.1002/hbm.20895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 26.Zubieta J-K, Ketter TA, Bueller JA, Xu Y, Kilbourn MR, Young EA, et al. Regulation of human affective responses by anterior cingulate and limbic mu-opioid neurotransmission. Arch Gen Psychiatry. 2003;60:1145–1153. doi: 10.1001/archpsyc.60.11.1145. [DOI] [PubMed] [Google Scholar]
- 27.Worsley KJ, Evans AC, Marrett S, Neelin P. A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab. 1992;12:900–918. doi: 10.1038/jcbfm.1992.127. [DOI] [PubMed] [Google Scholar]
- 28.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 29.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 30.Delfs JM, Kong H, Mestek A, Chen Y, Yu L, Reisine T, et al. Expression of mu opioid receptor mRNA in rat brain: an in situ hybridization study at the single cell level. J Comp Neurol. 1994;345:46–68. doi: 10.1002/cne.903450104. [DOI] [PubMed] [Google Scholar]
- 31.Mansour A, Fox CA, Thompson RC, Akil H, Watson SJ. mu-Opioid receptor mRNA expression in the rat CNS: comparison to mu-receptor binding. Brain Res. 1994;643:245–265. doi: 10.1016/0006-8993(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 32.Mansour A, Fox CA, Burke S, Akil H, Watson SJ. Immunohistochemical localization of the cloned mu opioid receptor in the rat CNS. J Chem Neuroanat. 1995;8:283–305. doi: 10.1016/0891-0618(95)00055-c. [DOI] [PubMed] [Google Scholar]
- 33.Minami M, Onogi T, Toya T, Katao Y, Hosoi Y, Maekawa K, et al. Molecular cloning and in situ hybridization histochemistry for rat mu-opioid receptor. Neurosci Res. 1994;18:315–322. doi: 10.1016/0168-0102(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 34.Ding YQ, Kaneko T, Nomura S, Mizuno N. Immunohistochemical localization of mu-opioid receptors in the central nervous system of the rat. J Comp Neurol. 1996;367:375–402. doi: 10.1002/(SICI)1096-9861(19960408)367:3<375::AID-CNE5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Panksepp J. Neuroscience. Feeling the pain of social loss. Science. 2003;302:237–239. doi: 10.1126/science.1091062. [DOI] [PubMed] [Google Scholar]
- 36.Li S, Kirouac GJ. Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol. 2008;506:263–287. doi: 10.1002/cne.21502. [DOI] [PubMed] [Google Scholar]
- 37.Hsu DT, Price JL. Paraventricular thalamic nucleus: subcortical connections and innervation by serotonin, orexin, and corticotropin-releasing hormone in macaque monkeys. J Comp Neurol. 2009;512:825–848. doi: 10.1002/cne.21934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, Kirouac GJ. Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct. 2012;217:257–273. doi: 10.1007/s00429-011-0360-7. [DOI] [PubMed] [Google Scholar]
- 39.Helmstetter FJ, Bellgowan PS, Poore LH. Microinfusion of mu but not delta or kappa opioid agonists into the basolateral amygdala results in inhibition of the tail flick reflex in pentobarbital-anesthetized rats. J Pharmacol Exp Ther. 1995;275:381–388. [PubMed] [Google Scholar]
- 40.Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- 41.McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 42.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 43.Miczek KA, Thompson ML, Shuster L. Naloxone injections into the periaqueductal grey area and arcuate nucleus block analgesia in defeated mice. Psychopharmacology (Berl) 1985;87:39–42. doi: 10.1007/BF00431775. [DOI] [PubMed] [Google Scholar]
- 44.Vivian JA, Miczek KA. Interactions between social stress and morphine in the periaqueductal gray: effects on affective vocal and reflexive pain responses in rats. Psychopharmacology (Berl) 1999;146:153–161. doi: 10.1007/s002130051101. [DOI] [PubMed] [Google Scholar]
- 45.Shaikh MB, Lu CL, Siegel A. Affective defense behavior elicited from the feline midbrain periqueductal gray is regulated by mu and delta opioid receptors. Brain Res. 1991;557:344–348. doi: 10.1016/0006-8993(91)90158-r. [DOI] [PubMed] [Google Scholar]
- 46.Eisenberger NI, Gable SL, Lieberman MD. Functional magnetic resonance imaging responses relate to differences in real-world social experience. Emotion. 2007;7:745–754. doi: 10.1037/1528-3542.7.4.745. [DOI] [PubMed] [Google Scholar]
- 47.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 48.Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 49.Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Mayes LC, et al. Dissociable brain mechanisms for processing social exclusion and rule violation. Neuroimage. 2011;54:2462–2471. doi: 10.1016/j.neuroimage.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Dev Psychopathol. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelley AE, Bakshi VP, Haber SN, Steininger TL, Will MJ, Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- 53.Peciña S. Opioid reward ‘liking’ and ‘wanting’ in the nucleus accumbens. Physiol Behav. 2008;94:675–680. doi: 10.1016/j.physbeh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt BL, Tambeli CH, Barletta J, Luo L, Green P, Levine JD, et al. Altered nucleus accumbens circuitry mediates pain-induced antinociception in morphine-tolerant rats. J Neurosci. 2002;22:6773–6780. doi: 10.1523/JNEUROSCI.22-15-06773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gear RW, Aley KO, Levine JD. Pain-Induced Analgesia Mediated by Mesolimbic Reward Circuits. J Neurosci. 1999;19:7175–7181. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sprenger T, Berthele A, Platzer S, Boecker H, Tölle TR. What to learn from in vivo opioidergic brain imaging? Eur J Pain. 2005;9:117–121. doi: 10.1016/j.ejpain.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Bencherif B, Fuchs PN, Sheth R, Dannals RF, Campbell JN, Frost JJ. Pain activation of human supraspinal opioid pathways as demonstrated by [11C]-carfentanil and positron emission tomography (PET) Pain. 2002;99:589–598. doi: 10.1016/S0304-3959(02)00266-X. [DOI] [PubMed] [Google Scholar]
- 58.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 59.Bhatnagar S. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol. 2002;14:403–410. doi: 10.1046/j.0007-1331.2002.00792.x. [DOI] [PubMed] [Google Scholar]
- 60.Brunton J, Charpak S. mu-Opioid peptides inhibit thalamic neurons. J Neurosci. 1998;18:1671–1678. doi: 10.1523/JNEUROSCI.18-05-01671.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zubieta J-K, Bueller JA, Jackson LR, Scott DJ, Xu Y, Koeppe RA, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fisher HE, Brown LL, Aron A, Strong G, Mashek D. Reward, addiction, and emotion regulation systems associated with rejection in love. J Neurophysiol. 2010;104:51–60. doi: 10.1152/jn.00784.2009. [DOI] [PubMed] [Google Scholar]
- 63.Najib A, Lorberbaum JP, Kose S, Bohning DE, George MS. Regional brain activity in women grieving a romantic relationship breakup. Am J Psychiatry. 2004;161:2245–2256. doi: 10.1176/appi.ajp.161.12.2245. [DOI] [PubMed] [Google Scholar]
- 64.Troisi A, Frazzetto G, Carola V, Di Lorenzo G, Coviello M, D’Amato FR, et al. Social hedonic capacity is associated with the A118G polymorphism of the mu-opioid receptor gene (OPRM1) in adult healthy volunteers and psychiatric patients. Soc Neurosci. 2011;6:88–97. doi: 10.1080/17470919.2010.482786. [DOI] [PubMed] [Google Scholar]
- 65.Koepp MJ, Hammers A, Lawrence AD, Asselin MC, Grasby PM, Bench CJ. Evidence for endogenous opioid release in the amygdala during positive emotion. NeuroImage. 2009;44:252–256. doi: 10.1016/j.neuroimage.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 66.Colasanti A, Searle GE, Long CJ, Hill SP, Reiley RR, Quelch D, et al. Endogenous Opioid Release in the Human Brain Reward System Induced by Acute Amphetamine Administration. Biological Psychiatry. 2012;72:371–377. doi: 10.1016/j.biopsych.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 67.Carr KD, Bak TH. Medial thalamic injection of opioid agonists: μ-agonist increases while κ-agonist decreases stimulus thresholds for pain and reward. Brain Research. 1988;441:173–184. doi: 10.1016/0006-8993(88)91396-0. [DOI] [PubMed] [Google Scholar]
- 68.Pizzagalli DA, Oakes TR, Fox AS, Chung MK, Larson CL, Abercrombie HC, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9:325, 393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- 69.Greenwald MK, Johanson C-E, Moody DE, Woods JH, Kilbourn MR, Koeppe RA, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28:2000–2009. doi: 10.1038/sj.npp.1300251. [DOI] [PubMed] [Google Scholar]
- 70.Weerts EM, Wand GS, Kuwabara H, Xu X, Frost JJ, Wong DF, et al. Association of smoking with μ-opioid receptor availability before and during naltrexone blockade in alcohol-dependent subjects. Addict Biol. 2012 doi: 10.1111/adb.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Craig ADB. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 72.Stafford L. Interpersonal rejection sensitivity: toward exploration of a construct. Issues Ment Health Nurs. 2007;28:359–372. doi: 10.1080/01612840701244250. [DOI] [PubMed] [Google Scholar]
- 73.Levinson CA, Rodebaugh TL. Social anxiety and eating disorder comorbidity: the role of negative social evaluation fears. Eat Behav. 2012;13:27–35. doi: 10.1016/j.eatbeh.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneier FR, Martin LY, Liebowitz MR, Gorman JM, Fyer AJ. Alcohol abuse in social phobia. Journal of Anxiety Disorders. 1989;3:15–23. [Google Scholar]
- 75.Fang A, Asnaani A, Gutner C, Cook C, Wilhelm S, Hofmann SG. Rejection sensitivity mediates the relationship between social anxiety and body dysmorphic concerns. J Anxiety Disord. 2011;25:946–949. doi: 10.1016/j.janxdis.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nat Neurosci. 2006;9:1007–1008. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.