Abstract
Aging affects voice production and is associated with reduced communicative ability and quality of life. Voice therapy is a critical component of treatment, but its effects on neuromuscular mechanisms are unknown. The ultrasonic vocalizations (USVs) of rats can be used to test the effects of aging and voice use on the laryngeal neuromuscular system. This study tested the hypothesis that age-related changes in the USVs of rats and laryngeal neuromuscular junctions can be reversed through vocal exercise. Young and old rats were trained for 8 weeks to increase their USVs and were compared with a no intervention group pre- and post-treatment. USV acoustics and aspects of neuromuscular junction (NMJ) morphology were measured in the thyroarytenoid muscle. Vocal training reduced or eliminated some age differences found in both USVs and NMJs. We conclude that vocal exercise may assist in mitigating age-related changes in voice characteristics and underlying neuromuscular adaptations.
Key Words: Voice, Larynx, Ultrasonic vocalization, Neuromuscular junction.
Voice problems (dysphonia) are common in elderly adults and have a significant negative impact on quality of life (1,2). Symptoms of age-related dysphonia include a breathy, weak vocal quality, and bowing of the vocal folds, indicating possible muscle atrophy within the larynx (3). An important intrinsic muscle of the larynx that is likely affected by age-related atrophy is the thyroarytenoid (TA) muscle. The TA muscle is active during laryngeal adduction to modulate airflow during expiratory tasks such as phonation, coughing, and laryngeal braking during exhalation of large tidal volumes, and it requires very rapid contraction speeds for voicing and airway protection (4,5).
Denervation-like changes in the senescent laryngeal neuromuscular system likely contribute to age-related dysphonia (6,7). This is evidenced at the neuromuscular junction (NMJ), which changes in size, complexity, density, and abundance in aging laryngeal muscles, including the TA muscle (8–10). Morphological and physiological adaptations in the aging NMJ, including a deterioration of the pre-post synaptic relationship (11), likely lead to decreased efficiency of synaptic transmission at the NMJ (12–14) and precede signs of atrophy in aging muscle fibers (15). NMJs of the TA muscle display different age-related morphological changes than limb muscles (16). Therefore, direct study of TA NMJ morphology will provide insight into how aging affects laryngeal muscles and how we can reduce or prevent the effects of aging on the laryngeal neuromuscular system.
Exercise in the limb musculature has been shown to reduce the effects of age on both muscle function (17–19) and NMJ morphology and physiology (20,21). Exercise programs have been developed for the aging voice, but there is little biological evidence to support the use of these interventions. The effects of vocal exercise on the underlying neuromuscular mechanisms of the larynx are unknown and difficult to study in humans. However, an animal model may prove useful in this regard. Understanding the mechanisms of exercise on the muscles of the upper airway is critical to developing treatments to prevent and/or reverse the effects of age-related voice disorders.
The ultrasonic vocalizations (USV) of rats have been used to study a variety of correlates between the brain and behavior, including the central mechanisms underlying laughter and joy (22) and vocal deficits related to Parkinson’s disease (23). Additionally, acoustic parameters of USVs, including bandwidth, peak intensity, and peak frequency, decrease in aging rats (24). Because the rat larynx has been implicated as the source of USVs (25–27), rat USVs also provide an opportunity to study peripheral neuromuscular changes in response to behavioral interventions.
The hypothesis of this study was that age-related decreases in bandwidth, peak intensity, and peak frequency of USVs and age-related changes in the size, complexity, and density of TA NMJs can be reversed through vocal exercise in the form of vocal training. This hypothesis was tested by comparing USV acoustics and NMJ morphology in the TA muscle in young adult and old rats that were vocally trained versus control groups of animals that were not trained.
Methods
Animals
The animal use protocol for this study was approved by the Animal Care and Use Committee of the University of Wisconsin–Madison School of Medicine and Public Health. Forty Fischer 344/Brown Norway male rats, 20 young adult (6 months old) and 20 old (29 months old), were obtained from the National Institute on Aging animal colony. At time of post-treatment analysis, young adult rats were 9 months old, representing mature young adulthood, and elderly rats were 32 months old, representing advanced senescence. Only male rats were used because the female estrus cycle influences USV production (28).
Rats were kept on a reversed light cycle. All investigator–rat interactions took place in a sound-isolated, darkened room dimly illuminated with red light. Rats remained in their home cages for all recordings, vocalization training sessions, and control condition interactions. Water and food were provided ad libitum. Rats were singly housed because socially isolated rats have a more robust response to methods that elicit vocalizations than socially housed rats (29).
Vocal Training
USVs were monitored and recorded using an ultrasonic microphone (UltraSoundGate CM16/CMPA, Avisoft Bioacoustics, Berlin, Germany) and USB recording interface (UltraSoundGate 116Hb, Avisoft Bioacoustics) connected to a Windows PC running Avisoft-RECORDER (Avisoft Bioacoustics). A real-time spectrogram was used to visually monitor USVs during recording and training sessions.
Baseline and post-training USV recordings from all rats were obtained using an existing procedure that reliably elicits vocalizations in male rats (30). In brief, a male rat was paired with a sexually receptive female rat in estrus. The female rat was removed when the male rat expressed interest in her, thereby eliciting a bout of vocalizations from the male rat. Each bout typically consisted of one to five vocalizations.
A total of 20 rats (10 young adult and 10 old) were randomly assigned to a vocal exercise group. The remaining 20 rats (10 young adult and 10 old) were assigned to a control group. Rats in the vocal exercise group were trained 5 days a week for 8 weeks to progressively increase their production of 50-kHz USVs. The elicitation technique described previously was paired with an operant conditioning paradigm in which male rats were rewarded with a food treat when they began vocalizing in response to the female rats. Initially, each individual bout of 50-kHz vocalizations was rewarded. In week 3 of the training, rewards were faded and given only after a string of vocalizations in rapid succession.
All rats in the vocal exercise group were trained to produce the same number of vocalizations in each session to equalize the exercise dose. The initial target of 50 vocalizations per session was determined by calculating the first quartile of the number of vocalizations produced during the baseline recordings. This provided a 2-week acclimation period for the operant conditioning. The target number was then increased weekly according to the following schedule: week 3 (75), week 4 (100), week 5 (150), week 6 (200), week 7 (250), and week 8 (300).
Rats in the control group were treated and housed identically to the vocal exercise group but did not receive vocal exercise. In lieu of the vocal exercise program, the control animals were given hand-fed food treats in their home cage 5 days a week to control for social effects of human contact. To ensure that this interaction did not elicit USVs, the control interaction was acoustically monitored once a week.
USV Analysis
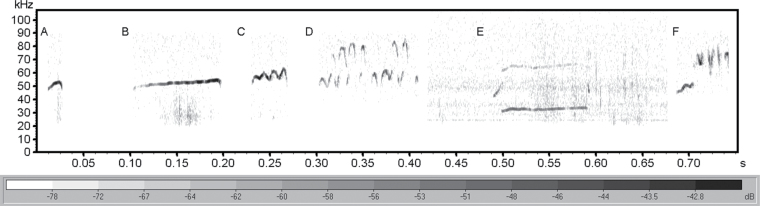
Baseline and post-training USVs were analyzed using SASLab Pro (Avisoft Bioacoustics) following an established protocol (30). After creating a spectrogram of the sound file, the beginning and end of each 50-kHz USV were automatically defined using an intensity threshold. Interfering noise and 22-kHz USVs were manually erased; then, each vocalization was classified by an experienced rater and labeled into one of the four categories (Figure 1) based on visual inspection of its frequency characteristics: (1) flat (steady frequency), (2) frequency modulated (FM) (regular frequency modulation with a sine wave–like appearance), (3) harmonic (fundamental frequency near 30kHz with a visible harmonic one octave above), or (4) step (abruptly bifurcated frequency components). These categories of 50-kHz USVs are well established, have been shown to respond differently to age and disease, and have strong inter- and intrarater reliability (24,31). The age and experimental groups of the recordings were masked during classifications.
Figure 1.
Spectrogram of representative USVs from the four classifications: (A) and (B) simple (short and long durations), (C) and (D) frequency modulated (FM) (small and large bandwidths), (E) harmonic, and (F) step. For acoustic analysis, FM, harmonic, and step USVs analyzed together as “complex.” USV = ultrasonic vocalization.
USVs identified as either FM, harmonic, or step were grouped together for statistical analyses because of their relative acoustical complexity (“complex USVs”), compared with the acoustically simple flat USVs (“simple USVs”). Acoustic parameters of all labeled vocalizations were then automatically measured using SASLab Pro. Overall measures of vocalization rate (per minute) and the percentage of complex USVs (%) were calculated. The following parameters were measured separately for simple and complex vocalizations: duration (ms), maximum amplitude (dB), and mean frequency (kHz). Additionally, for FM vocalizations, the frequency bandwidth (distance between maximum and minimum frequency expressed in kHz) and maximum frequency slope (the maximum frequency change between spectrogram time bins expressed in kHz/ms) were calculated.
NMJ Analysis
Within 48 hours of the post-training recordings, rats were euthanized via an IP injection overdose of Beuthanasia; the entire larynx was dissected, fixed for 1 hour in 4% formaldehyde solution in phosphate-buffered saline, cryoprotected overnight, then flash frozen, and stored at −80°C. Using a −20°C cryostat, 30-μm-thick transverse slices containing both the lateral and medial portions of the TA muscle were obtained from each larynx. Sections were mounted on slides, and the three primary structures of the NMJ (terminal Schwann cells, nerve terminal, and motor endplate) along with the innervating axon were labeled using immunohistochemistry (for detailed methods, please see Supplementary material).
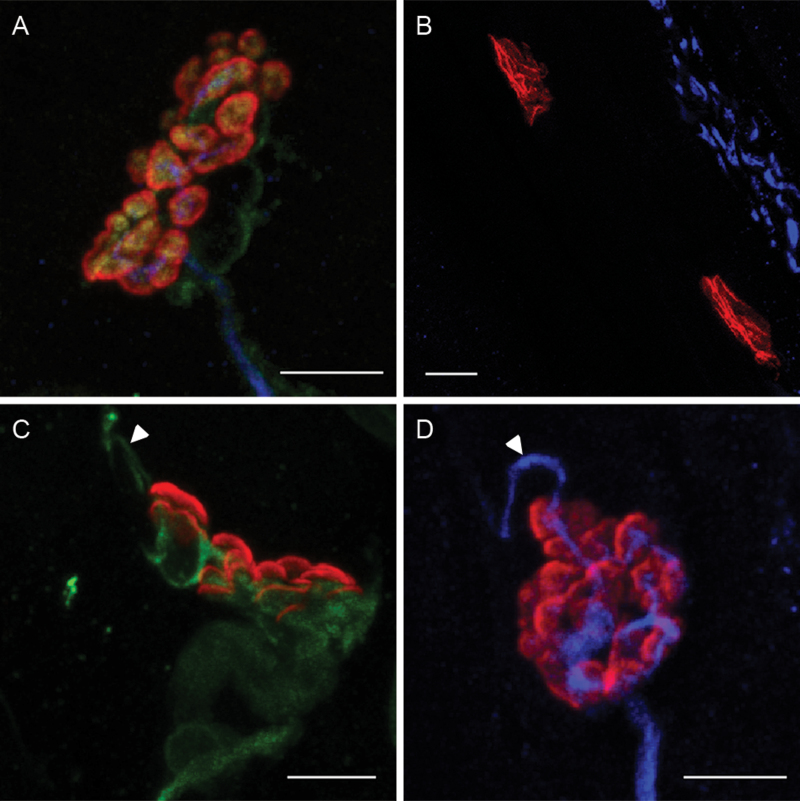
NMJs were imaged using a spectral confocal microscope (A1R, Nikon, Melville, NY) at the W.M. Keck Laboratory for Biological Imaging at the University of Wisconsin-Madison. NMJs were collected from both the left and right sides of at least three different laryngeal sections to account for possible intramuscle variability. A minimum of 18 NMJs from both the medial and lateral portions of the TA muscle were collected, for total of 36 NMJs for each animal. Using reference spectra collected from single-labeled laryngeal specimens, the 32-channel spectral images were linearly unmixed to create four-channel images, one channel for each fluorescent label. Unmixed images were pseudo colored as follows: red = motor endplate, green = Schwann cells, blue = axon, and yellow = nerve terminal membrane (Supplementary Figure 1).
NMJ morphology was assessed both qualitatively and quantitatively. All measurements were made in three dimensions as area measurements of 2D projections are less accurate than volumetric measurements of 3D reconstructions (11,32,33). Qualitative measures of the presence/absence of (1) axon withdrawal, (2) terminal Schwann cell projection(s), and (3) extrajunctional axon sprout(s) were made by examining both the four-color 3D reconstruction in Elements AR (Nikon) and each single-color channel in grayscale to avoid color bias. Axon withdrawal was assessed by the presence/absence of a stained axon in contact with the endplate. Terminal Schwann cell projections and extrajunctional axon sprouts were positively identified as a narrow, finger-like extension of staining beginning at and extending beyond the NMJ. Approximately 5% of the images were selected for inter-rater and intrarater reliability testing. Inter- and intrarater reliabilities (% agreement) were above 80% for all measures.
Images were quantitatively measured in ImageJ (34) using an automated measurement algorithm (11). Images were filtered using a 3 × 3 median filter, and the dynamic intensity range was normalized by linearly scaling the 12-bit images to 8-bit images. Image stacks were then converted to binary by applying an intensity threshold and further processed to remove small, isolated structures by applying a 3D median filter followed by a 3D erosion and dilation. Volumes (μm3) of the binary nerve terminal and motor endplate stacks were measured by multiplying the stained area on each slice by the stack spacing (0.3 μm).
Two-dimensional measurements of NMJ morphology are affected by the angle at which the NMJ resides in the tissue relative to the microscope plane or angle of view (11). Therefore, before measuring synaptic overlap and motor endplate dispersion, each image stack was rotated to the angle that resulted in the maximum motor endplate area on a z-projection, simulating the en face orientation. Synaptic overlap was defined as the percentage of the total stained endplate area overlapped by the stained terminal area on a maximum z-projection. Motor endplate dispersion was calculated by measuring the area of a convex hull of the stained motor endplate area on a maximum z-projection of the rotated stack and then subtracting and dividing by the stained motor endplate area. Motor endplate fragmentation was a binary measure; an endplate was scored as fragmented if it contained two or more 3D objects as counted by the 3D Object Counter Image plugin (35).
Statistical Analyses
Each dependent variable was tested for equality of variances between combined age and experimental groups using Levene’s test. Based on variance equality, either the Student’s or Welch’s t test was used to test for differences between age groups in baseline USV measurements.
To analyze post-training differences in both USV and NMJ measurements, two-way analysis of variance (ANOVA) was used examining main effects of age and training as well as their interaction. In the case of unequal variances between groups, a log transformation was attempted. If heteroskedasticity persisted, the nonparametric Kruskal Wallis Test One-Way ANOVA by Ranks was used. Post hoc testing was completed using the Fisher’s protected least significant difference test or Wilcoxon’s rank sum test in the case of heteroskedasticity.
Effect sizes (Hedges’ unbiased d) with 50% and 95% confidence intervals were calculated for all USV and NMJ variables to compare differences between age groups within each experimental group (36).
For the variables with unequal variances, Levene’s test was used in pairwise testing of age and experimental group combinations to determine if age and/or training affected the variance between groups. It was hypothesized that old groups would have greater variability in measures of USV acoustics and NMJ morphology, whereas training would reduce variability.
The experimental unit was the individual rat. Therefore, multiple measures from each rat were averaged for each dependent variable to arrive at a single representative measure. This accounted for variance within each animal. All statistical analyses were completed using R (37–39). An alpha level of 0.05 was selected a priori for determining statistical significance.
Results
Ultrasonic Vocalizations
Baseline.
The duration of simple USVs in the control group was shorter than that in the trained group (F [1,36] = 4.36, p = .04) at baseline. Therefore, further analysis of the duration of simple USVs was not performed. All other USV acoustic measures were equal at baseline between the trained and control groups.
Young adult and old groups differed in the amplitude, frequency, and duration of their USVs at baseline (Table 1) (Figure 2). Maximum amplitude in the young adult group was greater than that in the old group for both simple (F [1,36] = 18.40, p = .02) and complex USVs (F [1,36] = 8.84, p = .03). The young adult group had a higher mean frequency of simple USVs than old (F [1,36] = 5.68, p = .02); the frequency of complex USVs was not significantly different between groups (F [1,36] = 2.96, p = .09). The complex USVs of the old group were significantly longer in duration than those of the young adult group (F [1,36] = 6.32, p = .02). There were no baseline age differences in vocalization rate, the percentage of complex USVs, FM bandwidth, or maximum FM frequency slope.
Table 1.
Main Effects of Age and Training on Ultrasonic Vocalizations
| Baseline | After 8 Weeks | |||||
|---|---|---|---|---|---|---|
| Young | Old | Young | Old | Control | Trained | |
| Vocalization rate (per minute) | 35.5±5.1 | 31.4±3.5 | 36.6±3.7 | 31.6±4.9* | 23.8±3.4 | 44.4±4.0* |
| Percentage complex (%) | 65.6±2.2 | 70.0±2.8 | 67.7±2.0 | 61.7±3.0 | 62.8±2.7 | 66.6±2.4 |
| Duration simple (ms) | 16.6±1.2 | 18.6±1.4 | 19.1±1.3 | 19.5±1.4 | 17.8±1.3 | 20.8±1.4 |
| Duration complex (ms) | 29.6±0.9 | 32.9±0.9† | 28.4±0.8 | 37.9±1.7† | 31.4±1.3 | 34.9±2.0 |
| Maximum amplitude simple (dB) | −40.0±1.1 | −43.7±1.0† | −44.5±0.5 | −47.5±0.5† | −46.5±0.7 | −45.6±0.6 |
| Maximum amplitude complex (dB) | −35.9±0.9 | −38.9±1.0† | −40.0±0.6 | −42.3±0.5† | −41.3±0.7 | −41.0±0.6 |
| Mean frequency simple (kHz) | 54.2±0.6 | 51.8±0.8† | 58.9±0.7 | 57.6±0.9 | 59.3±0.7 | 57.3±0.9 |
| Mean frequency complex (kHz) | 53.5±0.6 | 52.0±0.6 | 60.0±0.8 | 56.7±0.8† | 57.9±0.8 | 58.8±1.0 |
| Bandwidth FM (kHz) | 18.3±0.6 | 18.9±0.9 | 18.1±0.8 | 16.9±0.9 | 16.4±0.8 | 18.6±0.8 |
| Maximum FM frequency slope (kHz/ms) | 12.3±0.6 | 12.0±0.8 | 10.6±0.6 | 9.8±0.6 | 9.8±0.6 | 10.7±0.6 |
Notes: Values are means ± SE; n = 40 (10 per age/experimental group combination). FM = frequency modulation.
*Significant interaction (p ≤ .05) between age and training.
†Significant difference (p ≤ .05) from young group.
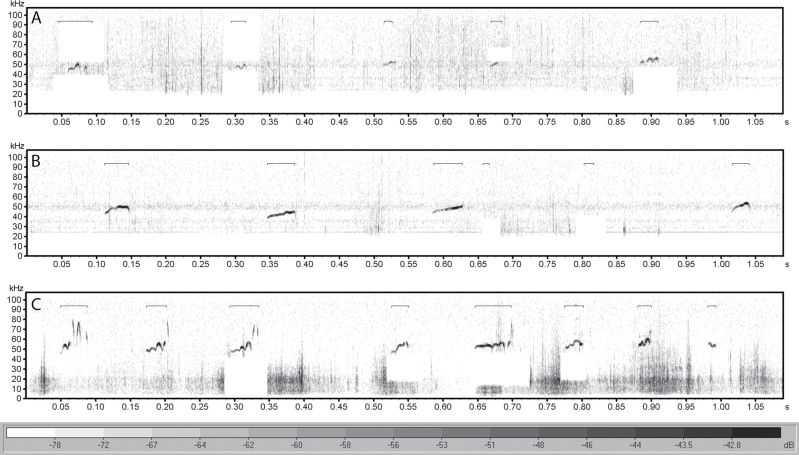
Figure 2.
Representative USVs recorded post-training demonstrating the smaller amplitude found in USVs from (A) an old control rat, compared with USVs from both (B) an old trained rat, and (C) a young control rat. USV = ultrasonic vocalization.
Post-training.
All rats produced the target number of vocalizations in each session although the time it took for an individual rat to produce the target was variable. The mean session duration throughout the 8 weeks of training was 4.8 minutes (SD = 3.1 minutes). Post-training age effects were different from baseline age effects. At baseline, frequency of complex USVs in the old group was lower but not significantly different than the young group. However, this difference increased and was significant post-training (F [1,36] = 8.47, p = .006). The opposite was observed in the mean frequency of simple USVs; the baseline difference between age groups was not present post-training (F [1,36] = 1.33, p = .26). The longer duration of complex USVs in the old group observed at baseline was also present post-training (F [1,36] = 29.63, p < .001).
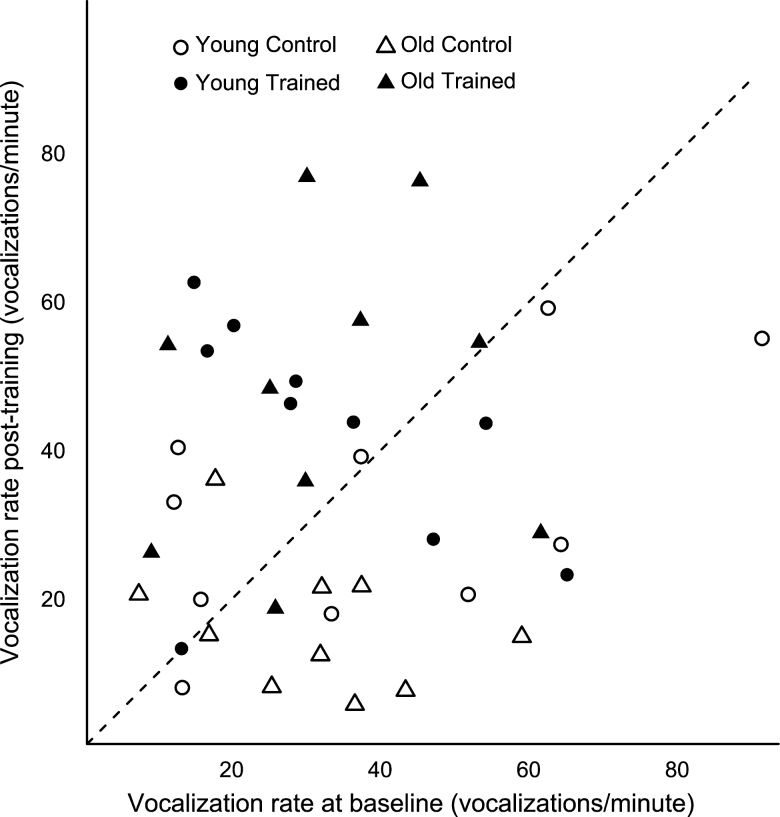
There was a significant interaction between training and age on vocalization rate (F [1,36] = 4.53, p = .04). The old trained group had a higher vocalization rate than the old control group (p = .0001), and the young adult control group had a higher vocalization rate than the old control group (p = .03). Comparing baseline and post-training call rates showed that 14/20 of the trained rats increased their vocalization rate from baseline, whereas the opposite was seen in the control rats; 14/20 of the control rats decreased their vocalization rate after 8 weeks of no training (Figure 3).
Figure 3.
Comparison of vocalization rate pre/postintervention. Symbols above the 45° dashed line indicate an increased rate after 8 weeks relative to baseline.
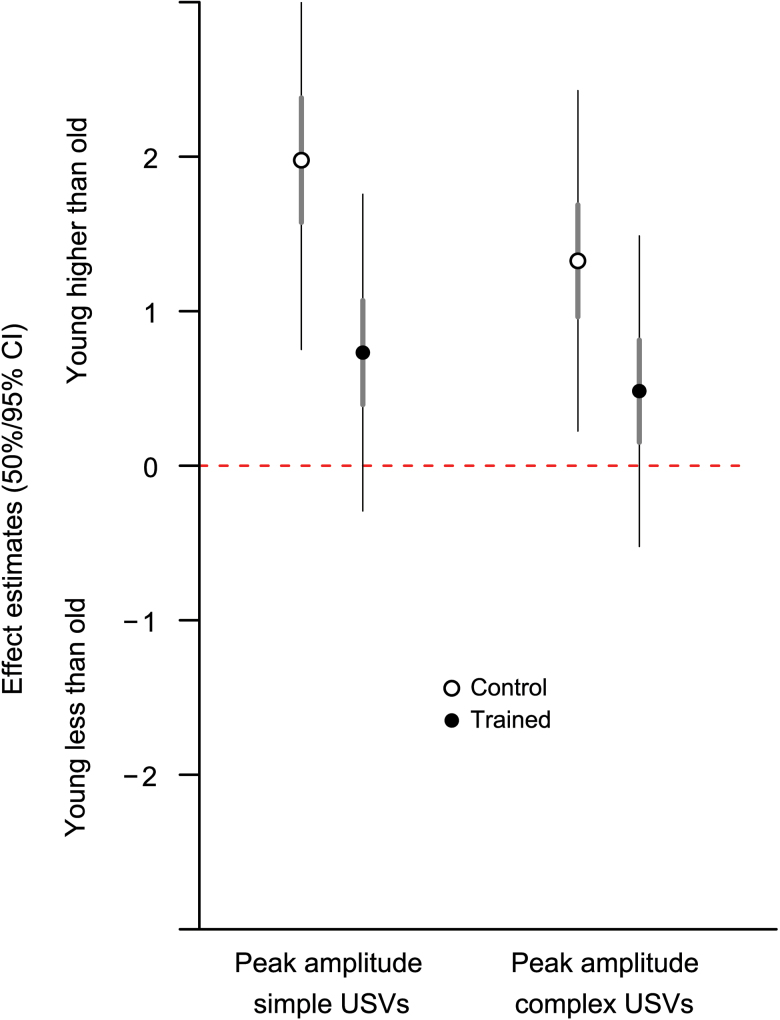
Training reduced the amplitude difference between old and young adult groups seen at baseline. Although there was not a significant interaction between age and training (F [1,36] = 2.83, p = .10, simple; F [1,36] = 1.87, p = .18, complex), examination of effect sizes showed the post-training difference in amplitude was significant only between young adult and old in the control group (95% confidence interval not overlapping zero) and not in the trained group (Figure 4). Training did not affect USV duration or frequency although there were age effects that differed from those observed at baseline (discussed previously). There were no training effects in the percentage of complex USVs, FM bandwidth, or maximum FM frequency slope.
Figure 4.
Effect sizes with confidence intervals of comparisons between young and old demonstrating that the young adult control group had higher ultrasonic vocalization amplitudes than old in control group only (open circle = control group, filled circle = trained group).
Neuromuscular Junction
A total of 1,185 images containing 1,655 NMJs were collected. Results for all tests of age and training for the lateral and medial TA muscle portions are summarized in Tables 2 and 3.
Table 2.
Main Effects of Age and Training on Neuromuscular Junctions in the Lateral Thyroarytenoid Muscle
| Young | Old | Control | Trained | |
|---|---|---|---|---|
| Presynaptic remodelling | ||||
| Axon withdrawal (%) | 39.9±4.6 | 40.9±5.2 | 37.9±4.2 | 43.0±5.5 |
| Axon sprouts (%)* | 2.3±0.8 | 9.0±2.3† | 4.4±1.7 | 6.9±2.0 |
| Terminal Schwann cell processes (%) | 14.3±2.5 | 19.9±3.7 | 17.6±3.2 | 16.7±3.3 |
| Size and pre/postrelationship | ||||
| Nerve terminal volume (µm3) | 76.6±8.9 | 74.4±9.5 | 63.4±7.3 | 87.6±10.0 |
| Motor endplate volume (µm3) | 224.4±8.5 | 247.9±8.4 | 230.1±11.1 | 242.3±5.6 |
| Overlap (%) | 33.9±4.3 | 34.3±4.7 | 33.1±4.1 | 35.1±4.9 |
| Endplate stability | ||||
| Dispersion (%)‡ | 10.9±0.5 | 13.5±0.7§ | 12.4±0.9 | 11.9±0.4§ |
| Fragmented endplates (%)‡ | 11.6±1.4 | 28.7±2.5† | 20.7±3.2 | 19.6±2.4 |
Notes: Values are means ± SE; n = 40 (20 per group).
*Nonparametric testing completed due to heteroskedasticity.
†Significant difference (p ≤ .05) from young group.
‡Log transformed to achieve homoskedasticity; values shown untransformed.
§Significant interaction (p ≤ .05) between age and training.
Table 3.
Main Effects of Age and Training on Neuromuscular Junctions in the Medial Thyroarytenoid Muscle
| Young | Old | Control | Trained | |
|---|---|---|---|---|
| Presynaptic remodelling | ||||
| Axon withdrawal (%)* | 18.5±3.6 | 15.2±3.5 | 15.6±3.8 | 18.1±3.3 |
| Axon sprouts (%) | 2.7±0.8 | 5.2±1.6 | 4.7±1.3 | 3.2±1.2 |
| Terminal Schwann cell processes (%) | 8.6±1.5 | 17.8±2.8† | 15.9±2.8 | 10.5±1.9 |
| Size and pre/postrelationship | ||||
| Nerve terminal volume (µm3) | 88.8±10.0 | 127.5±9.1† | 104.0±8.9 | 112.3±11.9 |
| Motor endplate volume (µm3) | 101.9±4.0 | 116.3±4.4† | 105.5±4.9 | 112.7±4.0 |
| Overlap (%) | 64.8±4.9 | 71.1±4.2 | 70.1±4.5 | 65.8±4.6 |
| Endplate stability | ||||
| Dispersion (%) | 8.5±0.5 | 12.0±0.7† | 10.6±0.7 | 9.9±0.7 |
| Fragmented endplates (%)‡ | 19.9±2.1 | 34.2±2.6† | 25.2±2.1 | 28.9±3.4 |
Notes: Values are means ± SE; n = 40 (20 per group).
*Nonparametric testing completed due to heteroskedasticity.
†Significant difference (p ≤ .05) from young group.
‡Log transformed to achieve homoskedasticity; values shown untransformed.
Effects of age.
Presynaptic remodeling was greater in the old group than that in the young adult group, as reflected by axon sprout and Schwann cell process percentage (Figure 5). In the lateral TA muscle, the old group had a significantly higher percentage of axon sprouts than the young adult by approximately 7% (W = 122, p = .02). In the medial TA muscle, the old group had double the percentage of terminal Schwann cell processes found in young adult (F [1,36] = 8.94, p = .005). No significant effects of age were found in the percentage of axon withdrawal.
Figure 5.
Maximum z-projections of confocal image stacks showing examples of (A) an NMJ with no qualitative signs of presynaptic remodeling from an old trained rat, (B) axon withdrawal from two motor endplates from a young trained rat (note axon bundle on top right but no axon extending to either motor endplate), (C) a Schwann cell projection (arrowhead) from an NMJ from an old trained rat, and (D) an axon sprout (arrowhead) from an NMJ from an old control rat (scale bars = 5 µm) (red = motor endplate, green = Schwann cells, blue = axon, and yellow = nerve terminal).
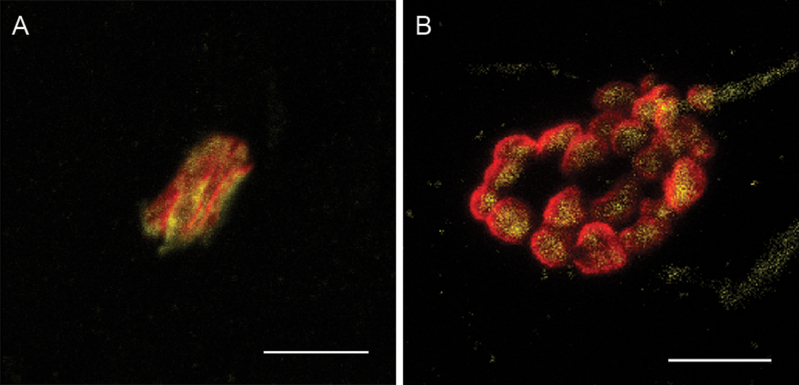
There was a significant main effect of age on the volume of both nerve terminals and motor endplates in the medial TA muscle; the old group had a larger nerve terminal volume (F [1,36] = 7.90, p = .008) and motor endplate volume (F [1,36] = 5.77, p = .02) than the young adult group (Figure 6). A similar trend was seen in the motor endplate volume in the lateral TA muscle (F [1,36] = 3.91, p = .06). Aging had no significant effect on synaptic overlap in either the lateral or medial TA muscle.
Figure 6.
Micrographs of motor endplates (red) and nerve terminals (yellow) from rats in (A) the young trained group and (B) the old control group, demonstrating the larger volume in both the motor endplate and nerve terminal in the old group compared with the young group, as well as the increased dispersion and fragmentation in the old control group (scale bars = 5 μm).
The old group showed greater motor endplate instability than the young adult group, as reflected in measures of dispersion and fragmented motor endplates. The old group had higher motor endplate dispersion than the young adult group in the medial TA muscle (F [1,36] = 17.23, p = .0002). In the lateral TA muscle, there was a significant interaction between age and training (discussed later) (F [1,36] = 4.64, p = .04). A main effect of age was found in the percentage of fragmented motor endplates; in the lateral TA muscle, the old group had 17% more fragmented motor endplates than young adult (F [1,36] = 10.81, p = .002), and 14% more in the medial TA muscle (F [1,36] = 10.80, p = .002).
Effects of training.
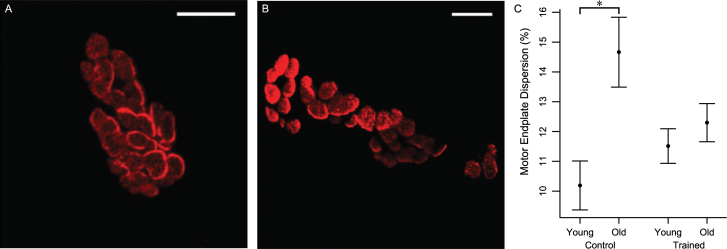
There was a significant interaction in the lateral TA muscle between age and training that showed the effect of age on dispersion was limited to the control group (Figure 7). The old group had a higher dispersion than the young adult group within the control group (p = .0007) but not within the trained group (p = .5). There was no effect of training on dispersion in the medial TA muscle. No significant effects of training were found in the remaining measures of NMJ morphology.
Figure 7.
Maximum z-projection images demonstrating the difference in dispersion between motor endplates from (A) an old rat in the trained group and (B) an old rat in the control group. In image B, note the greater unstained black space between the red-stained acetylcholine receptor clusters compared with image A. Also note the increased fragmentation of the motor endplate from the old control rat (scale bars = 5 µm). (C) In the lateral thyroarytenoid muscle, there was a significant interaction of age and training on dispersion; within the control group, the old group had a higher dispersion than the young adult group. There was no difference between age groups within the trained group. Data are shown as mean and standard error.
Differences in variance.
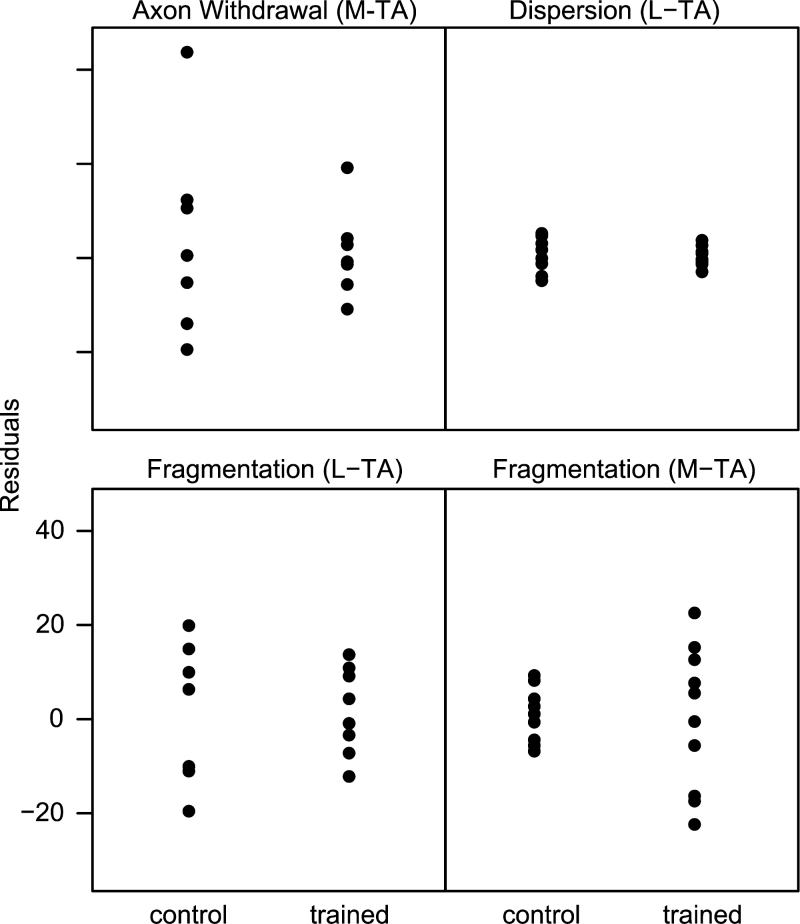
Differences in variance between the old trained and old control groups accounted for four of the five variables that did not pass Levene’s test of equal variance (Figure 8). In the lateral TA muscle, the old control group had higher variability than the old trained group in dispersion (p = .02) and percentage of fragmented endplates (p = .03). Similarly in the medial TA muscle, the old trained group had higher variability in percentage of axon withdrawal (p = .023). An opposite effect of training was seen in the percentage of fragmented endplates in the medial TA muscle, with the old trained group having higher variability than the old control group (p = .0002). The heteroskedasticity in the remaining variable, percentage of axon sprouts in the lateral TA muscle, was due to an effect of age, with higher variability in the old group than the young adult group (trained and control groups combined) (p = .0003).
Figure 8.
Plot of residuals showing training significantly decreases variability in the old group (range of residuals in old trained is less than old control) in all plotted variables except fragmentation in the medial TA, which shows the opposite effect (L-TA = lateral TA; M-TA = medial TA). TA = thyroarytenoid.
Discussion
The hypothesis of this research was that vocal training would reverse age-related changes in USVs and laryngeal NMJs of senescent rats. This hypothesis was tested by examining rat USV acoustics and NMJ morphology in the lateral and medial TA muscle. Results showed that (1) age-related differences were found in both USV acoustics and NMJ morphology and (2) some of these differences were mitigated by vocal training. Accordingly, the results of this study supported the hypothesis.
Effects of Age
The effect of age on USVs was evidenced by smaller amplitude, higher frequency, and longer duration of USVs in the old group than that in the young adult group. In TA NMJs, aging was associated with increased presynaptic remodeling, larger volumes of the pre- and postsynaptic components, and increased motor endplate instability. Therefore, aging was associated with both acoustic changes in USVs and morphological changes of the NMJ.
These age-related differences in USVs are consistent with other studies on acoustic changes in senescent rat vocalizations (24,40). Basken and colleagues (24) hypothesized increased duration of USVs with advanced age may have been an attempt to compensate for reduced intensity. It may also indicate a deficit in the fine motor control needed to begin and end USVs. Loss of fine motor control may result from an increase in the size of the motor unit seen with aging (41). Indeed, acoustic changes in vocalizations from old rats have been associated with a decrease in the number of primary motoneurons in the nucleus ambiguus, suggesting motor unit remodeling with age in the TA muscle (24). This interpretation is supported by EMG findings from the human TA muscles that show longer motor unit durations in older participants (42). The signs of presynaptic remodeling of the NMJ seen in the old group in this study are likely another sign of this age-related motor unit remodeling. Age-related changes at the NMJ may be a primary cause of motor unit remodeling (43). The age-related changes in the NMJ are similar to an earlier study of aging NMJs in the rat TA muscle that found age-related increases in the number of terminal Schwann cell processes and qualitative signs of receptor cluster degradation (8). Therefore, aging is associated with changes in NMJ morphology in the rat TA muscle.
Effects of Training
The effect of training on USVs was evident by the higher post-training vocalization rate in the trained group than the control group. Additionally, the trained group had no age-related difference in USV amplitude, whereas the baseline age effect of lower USV amplitude in old persisted in the control group. Vocal training also mitigated an effect of aging on the NMJ by reducing motor endplate dispersion in the old trained group. Therefore, vocal training changed vocal behavior by increasing vocalization rate and reduced age-related effects on USV acoustics and neuromuscular mechanisms in the TA muscle, a muscle used in USV production.
Another effect of training was decreased variability in measures of NMJ morphology in the old trained group relative to the old control group. This suggests that the effect of training may have been stronger on certain individual animals than that on group means. Studying the same NMJ in vivo over time in a single animal may provide a more accurate picture of the effect of training. Repeated in vivo imaging has been used with more superficial muscles (44), but the challenges of difficulty with accessibility and small size have not yet been overcome in the larynx.
These changes in NMJ morphology may have physiological and functional consequences related to voice. Reliable synaptic transmission at the NMJ depends on precise spatial arrangement of the synapse and is necessary for consistent and dependable muscle contraction (45). Age-related disruption of the synaptic structure as found in this study, including expansion of both pre- and postsynaptic components and fragmentation and dispersion of the motor endplate, likely affects reliability of synaptic transmission at the NMJ (46–48). Physiological study of the aging TA muscle has shown decreased muscle contraction capability and a higher likelihood for synaptic failure in old rats (10). The decrease in motor endplate dispersion associated with vocal training may serve to improve synaptic transmission and, therefore, increase muscle strength and improve muscle function as evidenced by increased USV amplitude in the trained group of old rats.
Factors Influencing USV Acoustics
The NMJ is only part of a cluster of central and peripheral mechanisms that are likely affected by exercise and aging. For example, changes in laryngeal tissue composition and biomechanics in humans have been implicated as causes for acoustic changes in the senescent voice (49,50); the impact of age on laryngeal biomechanics in the rat, however, is unknown. Additionally, USVs are produced with an egressive airflow, and, therefore, age-related changes in breathing and pulmonary function likely affect USVs. Respiratory mechanisms for speech in humans are altered with advanced age (51,52). Changes in rat pulmonary function with aging have been identified (53) although subglottal pressure during USV production has only been studied in young rats (26). Finally, the paradigm used to elicit and train USVs relied on the male rats’ interest in a female rat. Therefore, age-related decline in mating behavior may have affected age-related differences in USVs (54). Despite the multitude of possible factors contributing to changes of USVs with age and exercise, the results from this study provide the first evidence for a relationship between USVs and NMJs.
Clinical Implications
The results of this research have demonstrated that behavioral vocal training affects neuromuscular plasticity in a senescent muscle of vocalization within a rat model. There are clinical implications to these findings because the training methods employed are a model for those used with elderly human patients in voice therapy, which are also behavioral in nature. Because skeletal muscles in aged rats have many properties in common with those of aging human skeletal muscles (55), the signs of neuromuscular plasticity with vocal training reported here may represent part of a larger group of mechanisms underlying treatment-related vocal modifications in aged patients. However, this is the first study to demonstrate an effect of vocal training on age-related USV deficits coincident with neuromuscular plasticity. Future studies are needed to strengthen the translation of these findings to a human population. Diagnosing dysphonia in humans includes measures of the social and communicative impacts of the vocal deficits. Therefore, examining the social impact of age-related USV deficits in rats will further support this as a translational model of age-related dysphonia. Furthermore, establishing that the rescue of vocal deficits with training also has an effect on vocal communication-dependent behavior in other animals will strengthen the ecological validity of this line of work. The results presented here suggest that alterations in NMJ morphology may be part of the neuromuscular adaptations occurring with vocal training in aging populations receiving voice therapy.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
Funding
This work was supported by the National Institute on Deafness and Other Communication Disorders at the National Institutes of Health (R01 DC008149, R01 DC005935, T32 DC009401, and P30 DC010754).
Acknowledgments
The authors thank Drs. Susan Thibeault, Nathan Welham, and Gary Weismer for their advice and guidance and Kelsey Beach and Allison Schaser for their technical support. They also thank Lance Rodenkirch and the W.M. Keck Laboratory for Biological Imaging for assistance with the confocal microscopy.
References
- 1. Roy N, Stemple J, Merrill RM, Thomas L. Epidemiology of voice disorders in the elderly: preliminary findings. Laryngoscope. 2007;117:628–633 [DOI] [PubMed] [Google Scholar]
- 2. Verdonck-de Leeuw IM, Mahieu HF. Vocal aging and the impact on daily life: a longitudinal study. J Voice. 2004;18:193–202 [DOI] [PubMed] [Google Scholar]
- 3. Kendall K. Presbyphonia: a review. Curr Opin Otolaryngol Head Neck Surg. 2007;15:137–140 [DOI] [PubMed] [Google Scholar]
- 4. Hiroto I, Hirano M, Toyozumi Y, Shin T. Electromyographic investigation of the intrinsic laryngeal muscles related to speech sounds. Ann Otol Rhinol Laryngol. 1967;76:861–872 [DOI] [PubMed] [Google Scholar]
- 5. Kuna ST, Insalaco G, Woodson GE. Thyroarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol. 1988;65:1332–1339 [DOI] [PubMed] [Google Scholar]
- 6. Mortelliti AJ, Malmgren LT, Gacek RR. Ultrastructural changes with age in the human superior laryngeal nerve. Arch Otolaryngol Head Neck Surg. 1990;116:1062–1069 [DOI] [PubMed] [Google Scholar]
- 7. Périé S, St Guily JL, Callard P, Sebille A. Innervation of adult human laryngeal muscle fibers. J Neurol Sci. 1997;149:81–86 [DOI] [PubMed] [Google Scholar]
- 8. Connor NP, Suzuki T, Lee K, Sewall GK, Heisey DM. Neuromuscular junction changes in aged rat thyroarytenoid muscle. Ann Otol Rhinol Laryngol. 2002;111(7 Pt 1):579–586 [DOI] [PubMed] [Google Scholar]
- 9. Gambino DR, Malmgren LT, Gacek RR. Age-related changes in the neuromuscular junctions in the human posterior cricoarytenoid muscles: a quantitative study. Laryngoscope. 1990;100:262–268 [DOI] [PubMed] [Google Scholar]
- 10. McMullen CA, Andrade FH. Functional and morphological evidence of age-related denervation in rat laryngeal muscles. J Gerontol A Biol Sci Med Sci. 2009;64:435–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson AM, Connor NP. Effects of electrical stimulation on neuromuscular junction morphology in the aging rat tongue. Muscle Nerve. 2011;43:203–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elkerdany MK, Fahim MA. Age changes in neuromuscular junctions of masseter muscle. Anat Rec. 1993;237:291–295 [DOI] [PubMed] [Google Scholar]
- 13. Fahim MA, Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol. 1982;11:641–656 [DOI] [PubMed] [Google Scholar]
- 14. Rosenheimer JL. Factors affecting denervation-like changes at the neuromuscular junction during aging. Int J Dev Neurosci. 1990;8:643–654 [DOI] [PubMed] [Google Scholar]
- 15. Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas LB, Harrison AL, Stemple JC. Aging thyroarytenoid and limb skeletal muscle: lessons in contrast. J Voice. 2008;22:430–450 [DOI] [PubMed] [Google Scholar]
- 17. Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775 [DOI] [PubMed] [Google Scholar]
- 18. Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988;64:1038–1044 [DOI] [PubMed] [Google Scholar]
- 19. Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol. 2001;115:359–372 [DOI] [PubMed] [Google Scholar]
- 20. Alshuaib WB, Fahim MA. Effect of exercise on physiological age-related change at mouse neuromuscular junctions. Neurobiol Aging. 1990;11:555–561 [DOI] [PubMed] [Google Scholar]
- 21. Andonian MH, Fahim MA. Endurance exercise alters the morphology of fast- and slow-twitch rat neuromuscular junctions. Int J Sports Med. 1988;9:218–223 [DOI] [PubMed] [Google Scholar]
- 22. Panksepp J. Neuroevolutionary sources of laughter and social joy: modeling primal human laughter in laboratory rats. Behav Brain Res. 2007;182:231–244 [DOI] [PubMed] [Google Scholar]
- 23. Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behav Brain Res. 2007;182:284–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Basken JN, Connor NP, Ciucci MR. Effect of aging on ultrasonic vocalizations and laryngeal sensorimotor neurons in rats. Exp Brain Res. 2012;219:351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson AM, Ciucci MR, Russell JA, Hammer MJ, Connor NP. Ultrasonic output from the excised rat larynx. J Acoust Soc Am. 2010;128:EL75–EL79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riede T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. J Neurophysiol. 2011;106:2580–2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts LH. Evidence for the laryngeal source of ultrasonic and audible cries of rodents. J Zool. 1975;175:243–257 [Google Scholar]
- 28. Matochik JA, White NR, Barfield RJ. Variations in scent marking and ultrasonic vocalizations by Long-Evans rats across the estrous cycle. Physiol Behav. 1992;51:783–786 [DOI] [PubMed] [Google Scholar]
- 29. Panksepp J, Burgdorf J. 50-kHz chirping (laughter?) in response to conditioned and unconditioned tickle-induced reward in rats: effects of social housing and genetic variables. Behav Brain Res. 2000;115:25–38 [DOI] [PubMed] [Google Scholar]
- 30. Johnson AM, Doll EJ, Grant LM, Ringel L, Shier JN, Ciucci MR. Targeted training of ultrasonic vocalizations in aged and Parkinsonian rats. J Visual Exp: JoVE. 2011;54:e2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ciucci MR, Ahrens AM, Ma ST, et al. Reduction of dopamine synaptic activity: degradation of 50-kHz ultrasonic vocalization in rats. Behav Neurosci. 2009;123:328–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prakash YS, Smithson KG, Sieck GC. Measurements of motoneuron somal volumes using laser confocal microscopy: comparisons with shape-based stereological estimations. Neuroimage. 1993;1:95–107 [DOI] [PubMed] [Google Scholar]
- 33. Wang S, Kawabuchi M, Zhou CJ, Hirata K, Tan H, Kuraoka A. The spatiotemporal characterization of endplate reoccupation, with special reference to the superposition patterns of the presynaptic elements and the postsynaptic receptor regions during muscle reinnervation. J Peripher Nerv Syst. 2004;9:144–157 [DOI] [PubMed] [Google Scholar]
- 34. Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophoton Int. 2004;11:36–42 [Google Scholar]
- 35. Bolte S, Cordelières FP. A guided tour into subcellular colocalization analysis in light microscopy. J Microsc. 2006;224(Pt 3):213–232 [DOI] [PubMed] [Google Scholar]
- 36. Nakagawa S, Cuthill IC. Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev Camb Philos Soc. 2007;82:591–605 [DOI] [PubMed] [Google Scholar]
- 37. Morales M. sciplot: Scientific Graphing Functions for Factorial Designs. 2010 [Google Scholar]
- 38. R Development Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011 [Google Scholar]
- 39. Sarkar D. Lattice: Multivariate Data Visualization with R. New York: Springer; 2008 [Google Scholar]
- 40. Peterson JR, Watts CR, Morris JA, Shelton JM, Cooper BG. Laryngeal aging and acoustic changes in male rat ultrasonic vocalizations. Dev Psychobiol. 2012. July 20. 10.1002/dev.21072 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 41. Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol. 1995;45:397–458 [DOI] [PubMed] [Google Scholar]
- 42. Takeda N, Thomas GR, Ludlow CL. Aging effects on motor units in the human thyroarytenoid muscle. Laryngoscope. 2000;110:1018–1025 [DOI] [PubMed] [Google Scholar]
- 43. Santo Neto H, Marques MJ. Estimation of the number and size of motor units in intrinsic laryngeal muscles using morphometric methods. Clin Anat. 2008;21:301–306 [DOI] [PubMed] [Google Scholar]
- 44. Balice-Gordon RJ. In vivo approaches to neuromuscular structure and function. Methods Cell Biol. 1997;52:323–348 [DOI] [PubMed] [Google Scholar]
- 45. Slater CR. Structural determinants of the reliability of synaptic transmission at the vertebrate neuromuscular junction. J Neurocytol. 2003;32:505–522 [DOI] [PubMed] [Google Scholar]
- 46. Balice-Gordon RJ. Age-related changes in neuromuscular innervation. Muscle Nerve Suppl. 1997;5:S83–S87 [DOI] [PubMed] [Google Scholar]
- 47. Smith DO. Reduced capabilities of synaptic transmission in aged rats. Exp Neurol. 1979;66:650–666 [DOI] [PubMed] [Google Scholar]
- 48. Smith DO. Acetylcholine storage, release and leakage at the neuromuscular junction of mature adult and aged rats. J Physiol. 1984;347:161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hammond TH, Gray SD, Butler JE. Age- and gender-related collagen distribution in human vocal folds. Ann Otol Rhinol Laryngol. 2000;109(10 Pt 1):913–920 [DOI] [PubMed] [Google Scholar]
- 50. Hirano M, Kurita S, Sakaguchi S. Ageing of the vibratory tissue of human vocal folds. Acta Otolaryngol. 1989;107:428–433 [DOI] [PubMed] [Google Scholar]
- 51. Hoit JD, Hixon TJ. Age and speech breathing. J Speech Hear Res. 1987;30:351–366 [DOI] [PubMed] [Google Scholar]
- 52. Huber JE, Spruill J., 3rd Age-related changes to speech breathing with increased vocal loudness. J Speech Lang Hear Res. 2008;51:651–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johanson WG, Jr, Pierce AK. Lung structure and function with age in normal rats and rats with papain emphysema. J Clin Invest. 1973;52:2921–2927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McGinnis MY, Yu WH. Age-related changes in androgen receptor levels in cranial nerve nuclei of male rats. Brain Res Bull. 1995;36:581–585 [DOI] [PubMed] [Google Scholar]
- 55. Cartee GD. What insights into age-related changes in skeletal muscle are provided by animal models? J Gerontol A Biol Sci Med Sci. 1995;50:137–141 [DOI] [PubMed] [Google Scholar]