Introduction
Genetic exchange occurs via horizontal gene transfer in bacteria and archea or sexual reproduction in fungal and parasitic eukaryotic microbes. Sexual reproduction is universal, or nearly so, in eukaryotes. Until recently, most eukaryotic microbial pathogens were thought to be clonal and asexual due to the absence of a compatible partner or the lack of morphological or population genetic evidence for sexual reproduction [1]. However, many of these eukaryotic pathogens have been found recently to have extant cryptic sexual cycles (Figure 1). Sex enables microbial pathogens to reshuffle their genomes, increase genetic diversity, purge deleterious mutations, and produce infectious propagules.
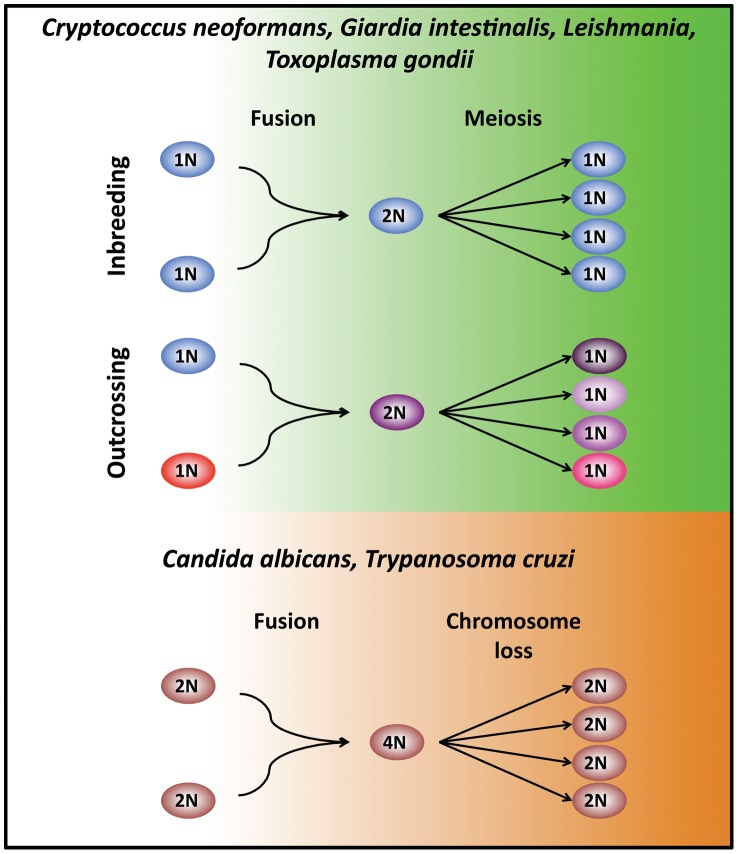
Figure 1. Modes of unisexual reproduction.
In haploid eukaryotes, cells (1N) of the same mating type can fuse or undergo endoreplication to generate a diploid intermediate (2N). DNA replication without cell division follows and meiosis produces four recombinant progeny (1N). Unisexual reproduction can also occur in 1) a clonal population, promoting inbreeding, or 2) between cells of the same mating type but of distinct genetic lineages to enable outcrossing. A similar unisexual cycle is observed in Candida and possibly also Trypanosoma, where diploid cells (2N) fuse to generate a tetraploid intermediate (4N). The resulting tetraploid cells (4N) return to the diploid state (2N) through either parasexual chromosome loss that occurs stochastically independent of meiosis (C. albicans, and possibly T. cruzi) or sexually via meiosis (T. brucei).
Unisexual Reproduction in Fungi
Sexual reproduction involving cells of opposite mating types or sexes comes with costs. Locating a compatible partner and undergoing mating and meiosis requires time and energy. In addition, sexual reproduction introduces genetic diversity, but in so doing rearranges well-adapted genomic configurations. In contrast, sexual reproduction involving cells of only one type (unisexual reproduction), via either mother-daughter cell-cell fusion or endoreplication, lowers the barrier to locating a compatible mating partner. Unisexual reproduction ameliorates the cost of losing a well-adapted phenotype in a particular niche while introducing more limited genetic diversity that may enhance the fitness of progeny in response to environmental changes, including drug treatments.
Cryptococcus neoformans is a basidiomycetous pathogenic yeast found in both the environment and infected hosts. Cryptococcus has a bipolar mating system with two mating types, a and α [2], and a well-defined sexual cycle involving cells of opposite mating type that fuse and undergo a dimorphic yeast-hyphal transition. At the hyphal tips, basidia form where meiosis occurs followed by multiple rounds of mitosis, producing long spore chains [3], [4]. Spores are infectious propagules that are inhaled by the host [5], [6].
Although C. neoformans has a defined a-α sexual cycle, the α mating type predominates in environmental and clinical isolates, and in many niches the population is exclusively α, dramatically limiting opportunities for a-α sexual reproduction [7]. Recent studies have shown that C. neoformans undergoes unisexual reproduction that involves an alternative dimorphic transition to hyphal growth, production of basidia, meiosis, and sporulation [5], [6], [8]. This commonly occurs with α strains but has also been reported to occur with some strains of the a mating type [3], [9], [10]. The pathway is controlled by multiple genetic loci as a quantitative trait, of which the MATα locus allele provides the major contribution [10]. Diploidization occurs during unisexual reproduction: either early to produce a diploid yeast that then produces a diploid monokaryotic hyphae, or late in which a haploid yeast produces a haploid monokaryotic hyphae in which karyogamy is delayed until the basidia form (similar to a-α sexual reproduction) [10], [11].
Meiotic recombination occurs at a similar frequency in spores produced by either opposite sexual or unisexual reproduction [8]. Moreover, the key meiotic genes SPO11 and DMC1 (which induce and repair DNA DSBs that provoke meiotic recombination) are both dispensable for hyphal and basidia development, but critical for sporulation during unisexual reproduction: the dmc1 and spo11 mutants produce fewer spores, often in only two instead of four chains, and their germination frequency is severely impaired resulting in only 2% or 3.7% the wild-type level of viable spore production in these meiotic mutants [8], [12]. Thus, unisexual reproduction is a complete sexual cycle involving meiotic production of spores.
While the unisexual cycle has been directly observed only under laboratory conditions, mounting population genetic evidence supports that unisexual cycles occur in nature in both clonal and genetically divergent populations leading to recombination in exclusively α populations and also resulting in diploid intermediates or products with αADα or αAAα genotypes [13]–[19]. Moreover, recent studies have shown that unisexual reproduction between genetically identical cells generates phenotypic and genotypic diversity, including SNPs, chromosomal translocations, and aneuploidy, which may enhance competitive fitness in different environments [20]. Interestingly, population genetic analyses also implicate unisexual reproduction in the origins of the strains responsible for the outbreak caused by the sibling species Cryptococcus gattii on Vancouver Island and in the Pacific Northwest. Unisex may also be producing the spores causing the outbreak given that the lineages responsible are all of the α mating type [15].
These studies illustrate how unisexual reproduction may 1) admix genetic diversity to facilitate adaptive selection and 2) serve as a mutagen to generate genetic diversity de novo in otherwise clonal populations. In the latter case, the intermediate diploid state could also serve as a capacitor for evolution, allowing the accumulation of multiple recessive mutations whose combination may be advantageous when released into the haploid state, as has been observed in A. nidulans [21].
Candida albicans, the most common human fungal pathogen, was until recently thought to be strictly asexual. An extant heterothallic parasexual cycle has been defined involving diploid a/a and α/α cells that undergo cell-cell fusion and nuclear fusion, yielding a tetraploid intermediate [22]–[24]. Surprisingly, meiosis has not yet been observed in C. albicans, and the tetraploid returns to the diploid state through stochastic parasexual chromosome loss, which generates considerable aneuploidy, as well as infrequent Spo11-dependent recombination [25]. Unexpectedly, a/a cells can express both the a and α pheromone genes in response to nutrient limitation, which led to the discovery of a unisexual cycle in C. albicans [26]. Mutation of Bar1, a protease that cleaves α-factor to regulate autocrine and paracrine signaling, enables an a-a unisexual cycle [27]. This homothallic parasexual cycle is also induced in ménage à trois matings in which a third mating type partner in limited abundance donates pheromone to promote a-a or α-α mating [27]. Remarkably, pheromones from other species such as Candida dubliniensis or Candida parapsilosis can induce unisexual reproduction of C. albicans [28].
Similar to C. neoformans, where unisexual reproduction can introduce genomic changes in a clonal population [20], the aneuploidy-prone parasexual cycle also induces a range of novel genotypes in C. albicans that may provide selective advantages in novel environments. Although aneuploidy can be deleterious, in fungi aneuploidy can also confer drug resistance to antifungal therapy and promote pathogen survival in the host [29]–[31]. Moreover, parasexual reproduction in C. albicans is linked to a morphogenic change from white to opaque cells. Opaque cells are specialized in mating, while white cells are highly virulent. Although white cells are infertile, pheromone from rare opaque cells stimulates adhesion and biofilm formation of white cells [32]. Thus, roles of unisexual development may extend beyond mating and genetic diversity with the capacity to induce virulence attributes in hostile environments.
There are other pathogenic fungi once thought to be asexual that we now appreciate may be cryptically sexual or unisexual. Recent studies on the AIDS-associated pathogen Penicillium marneffei provide genetic evidence of a sexual cycle, and the presence of a clonal population with limited recombination rates suggests selfing may also occur in this fungus [33]. Unisexual reproduction is so far uncommon in the fungal kingdom, but many different species undergo other forms of homothallic sexual cycles. Some fungi harbor both mating type locus alleles or idiomorphs in their genome, which can be linked or unlinked, while others can switch mating type (Saccharomyces cerevisiae and Schizosaccharomyces pombe and related yeasts). Others have a single mating type locus and complete a sexual cycle in the absence of an opposite–mating type partner. Others species, such as N. africana, N. galapagosensis, N. dodgei, and N. lineolata, may undergo unisexual reproduction given that their populations appear to harbor one MAT locus idiomorph yet they are sexual [34]–[38]. Furthermore, the obligate intracellular microsporidian fungal pathogen Encephalitozoon cuniculi contains two HMG domains similar to the MAT locus of zygomycetes, although an extant sexual cycle has not been observed [39]. Recent studies have revealed low levels of heterozygosity in four E. cuniculi strains, indicative of a diploid nuclear state that may reflect a cryptic unisexual cycle in this “asexual” fungus [40].
Unisexual Reproduction in Parasites
Among a broader group of microbial pathogens, the protozoan parasites also harbor extant sexual cycles that in some cases are now known to be cryptic or unisexual. Although these pathogens exhibit interesting and unusual sexual cycles, essentially nothing is as yet known about how sexes or mating types are established.
Until recently the intestinal parasite Giardia intestinalis was thought to be asexual; however, population genetics studies revealed evidence of genetic exchange, and the genome harbors a suite of meiotic genes [41], [42]. This diplomonad parasite is binucleate, and the two nuclei of a single isolate can fuse to exchange genetic information, followed by homologous recombination that is possibly directed by meiotic gene homologs [43]. The cues that trigger cell-cell fusion in the population remain to be explored. However, high levels of genetic exchange in the population provide evidence that outcrossing is likely occurring.
In the pathogen Leishmania, which is highly clonal and was therefore thought to be asexual, recent studies have revealed that an extant sexual cycle occurs in the sand fly vector [44]–[46]. Both outcrossing and selfing have been observed in Plasmodium species, in which a single isolate can differentiate and produce fertile male and female gametes that undergo sexual reproduction. In Trypanosoma, diploids may fuse to create an intermediate tetraploid that may undergo random chromosome loss through a parasexual cycle similar to C. albicans [47]. In Toxoplasma gondii, sexual outcrossing and subsequent self-mating generated highly virulent T. gondii clones that were responsible for a toxoplasmosis outbreak in Brazil and other global locales [48]. Unisexual reproduction preserved a well-adapted genomic configuration and generated abundant spores fueling this outbreak.
Other Examples
Unisexual reproduction has emerged as an adaptive mechanism in eukaryotic microbes, but also extends beyond unicellular organisms. In plants, self-pollination is surprisingly common. Transitions from cross-pollination to self-pollination are frequently observed; for example, the model plant Arabidopsis thaliana reproduces almost exclusively through self-pollination (∼99%) yet retains the ability to outcross at a low frequency (∼1%). Such transitions are thought to enable provincial species with restricted niches to emerge as successfully dispersed cosmopolitan species. In Bdelloid rotifers, males, an extant sexual cycle, and meiosis all appear to be absent, and these organisms are only known to reproduce via parthenogenesis [49]. More than 80 unisexual species of fishes, insects, reptiles, and amphibians also reproduce via parthenogenesis. Facultative parthenogenesis, where sexual species resort to reproduction in the absence of a compatible partner, is more widespread than obligate parthenogenesis. In vertebrates and mammals, even facultative parthenogenesis is very rare due to genomic imprinting. However, some isolated female sharks have given birth to live young (all daughters) in the absence of a male partner [50]. Facultative selfing has been documented not only in sharks but also in komodo dragons [51], and some species of domesticated birds. Moreover, genetic manipulation of the imprinting mechanisms in female mice resulted in healthy, live offspring produced via parthenogenesis under laboratory conditions [52]. Both unisexual reproduction and parthenogenesis can mitigate some of the costs associated with sex, but a common consequence is a reduced level of genetic exchange or diversity.
Conclusions
We now appreciate that eukaryotic microbial pathogens, including fungi and parasites, are not clonal and asexual, but rather have extant sexual cycles that are cryptic, parasexual, or even unisexual. Two of the three most common systemic human fungal pathogens (Candida and Cryptococcus) have retained extant sexual and parasexual cycles involving both bisexual and unisexual reproduction, which may provide a broader range of adaptive evolutionary strategies. Similar paradigms have now emerged for several eukaryotic parasites, suggesting this may be a general mode of adaptation for microbial pathogens, enabling them to preserve well-adapted genotypes. Given that sex is ubiquitous throughout the eukaryotic tree of life and yet we are confronted with a panoply of diverse mechanisms via which mating type (or sex) is specified and mating partners (or gametes) are distinguished and recognized, one possibility is that unisexual reproduction was the original ancestral form of sexual reproduction to which mating types and sexes were added later. If so, the finding that extant unisexual reproduction occurs in both fungal and parasitic pathogens may reflect a return to a more ancestral mode of reproduction rather than the emergence of an entirely new process promoting genetic change and exchange.
Acknowledgments
We thank Cecelia Shertz Wall and Sujal Phadke for critical reading and comments on the manuscript.
Funding Statement
The work in JH's lab is supported by NIH R37 AI39115 grant from NIH/NIAID. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tibayrenc M, Ayala FJ (2012) Reproductive clonality of pathogens: a perspective on pathogenic viruses, bacteria, fungi, and parasitic protozoa. Proc Natl Acad Sci U S A 109: E3305–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lengeler KB, Fox DS, Fraser JA, Allen A, Forrester K, et al. (2002) Mating-type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot Cell 1: 704–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hull CM, Heitman J (2002) Genetics of Cryptococcus neoformans . Annu Rev Genet 36: 557–615. [DOI] [PubMed] [Google Scholar]
- 4. Kwon-Chung KJ (1976) Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans . Mycologia 68: 821–833. [PubMed] [Google Scholar]
- 5. Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM (2009) Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans . Infect Immun 77: 3491–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR, Heitman J (2009) Spores as infectious propagules of Cryptococcus neoformans . Infect Immun 77: 4345–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kwon-Chung KJ, Bennett JE (1978) Distribution of α and a mating types of Cryptococcus neoformans among natural and clinical isolates. Am J Epidemiol 108: 337–340. [DOI] [PubMed] [Google Scholar]
- 8. Lin X, Hull CM, Heitman J (2005) Sexual reproduction between partners of the same mating type in Cryptococcus neoformans . Nature 434: 1017–1021. [DOI] [PubMed] [Google Scholar]
- 9. Tscharke RL, Lazera M, Chang YC, Wickes BL, Kwon-Chung KJ (2003) Haploid fruiting in Cryptococcus neoformans is not mating type α-specific. Fungal Genet Biol 39: 230–237. [DOI] [PubMed] [Google Scholar]
- 10. Lin X, Huang JC, Mitchell TG, Heitman J (2006) Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATα allele enhances filamentation. PLoS Genet 2: e187 doi:10.1371/journal.pgen.0020187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee SC, Heitman J (2012) Function of Cryptococcus neoformans KAR7 (SEC66) in karyogamy during unisexual and opposite-sex mating. Eukaryot Cell 11: 783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feretzaki M, Heitman J (2013) Genetic circuits that govern bisexual and unisexual reproduction in Cryptococcus neoformans . PLoS Genet 9: e1003688 doi:10.1371/journal.pgen.1003688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin X, Litvintseva AP, Nielsen K, Patel S, Floyd A, et al. (2007) αADα hybrids of Cryptococcus neoformans: evidence of same-sex mating in nature and hybrid fitness. PLoS Genet 3: e186 doi:10.1371/journal.pgen.0030186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin X, Patel S, Litvintseva AP, Floyd A, Mitchell TG, et al. (2009) Diploids in the Cryptococcus neoformans serotype A population homozygous for the α mating type originate via unisexual mating. PLoS Pathog 5: e1000283 doi:10.1371/journal.ppat.1000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, et al. (2005) Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437: 1360–1364. [DOI] [PubMed] [Google Scholar]
- 16. Bui T, Lin X, Malik R, Heitman J, Carter D (2008) Isolates of Cryptococcus neoformans from infected animals reveal genetic exchange in unisexual, alpha mating type populations. Eukaryot Cell 7: 1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saul N, Krockenberger M, Carter D (2008) Evidence of recombination in mixed-mating-type and alpha-only populations of Cryptococcus gattii sourced from single eucalyptus tree hollows. Eukaryot Cell 7: 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiremath SS, Chowdhary A, Kowshik T, Randhawa HS, Sun S, et al. (2008) Long-distance dispersal and recombination in environmental populations of Cryptococcus neoformans var. grubii from India. Microbiology 154: 1513–1524. [DOI] [PubMed] [Google Scholar]
- 19. Chowdhary A, Hiremath SS, Sun S, Kowshik T, Randhawa HS, et al. (2011) Genetic differentiation, recombination and clonal expansion in environmental populations of Cryptococcus gattii in India. Environ Microbiol 13: 1875–1888. [DOI] [PubMed] [Google Scholar]
- 20. Ni M, Feretzaki M, Li W, Floyd-Averette A, Mieczkowski P, et al. (2013) Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans . PLoS Biol 11: e1001653 doi:10.1371/journal.pbio.1001653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schoustra SE, Debets AJM, Slakhorst M, Hoekstra RF (2007) Mitotic recombination accelerates adaptation in the fungus Aspergillus nidulans . PLoS Genet 3: e68 doi:10.1371/journal.pgen.0030068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hull CM, Raisner RM, Johnson AD (2000) Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289: 307–310. [DOI] [PubMed] [Google Scholar]
- 23. Magee BB, Magee PT (2000) Induction of mating in Candida albicans by construction of MTLa and MTLalpha strains. Science 289: 310–313. [DOI] [PubMed] [Google Scholar]
- 24. Miller MG, Johnson AD (2002) White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110: 293–302. [DOI] [PubMed] [Google Scholar]
- 25. Forche A, Alby K, Schaefer D, Johnson AD, Berman J, et al. (2008) The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol 6: e110 doi:10.1371/journal.pbio.0060110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bennett RJ, Johnson AD (2006) The role of nutrient regulation and the Gpa2 protein in the mating pheromone response of C. albicans . Mol Microbiol 62: 100–119. [DOI] [PubMed] [Google Scholar]
- 27. Alby K, Schaefer D, Bennett RJ (2009) Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans . Nature 460: 890–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alby K, Bennett RJ (2011) Interspecies pheromone signaling promotes biofilm formation and same-sex mating in Candida albicans . Proc Natl Acad Sci U S A 108: 2510–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Selmecki A, Forche A, Berman J (2006) Aneuploidy and isochromosome formation in drug-resistant Candida albicans . Science 313: 367–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Selmecki AM, Dulmage K, Cowen LE, Anderson JB, Berman J (2009) Acquisition of aneuploidy provides increased fitness during the evolution of antifungal drug resistance. PLoS Genet 5: e1000705 doi:10.1371/journal.pgen.1000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sionov E, Lee H, Chang YC, Kwon-Chung KJ (2010) Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog 6: e1000848 doi:10.1371/journal.ppat.1000848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Daniels KJ, Srikantha T, Lockhart SR, Pujol C, Soll DR (2006) Opaque cells signal white cells to form biofilms in Candida albicans . EMBO J 25: 2240–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Henk DA, Shahar-Golan R, Devi KR, Boyce KJ, Zhan N, et al. (2012) Clonality despite sex: the evolution of host-associated sexual neighborhoods in the pathogenic fungus Penicillium marneffei . PLoS Pathog 8: e1002851 doi:10.1371/journal.ppat.1002851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahoney DP, Huang LH, Backus MP (1969) New homothallic Neurosporas from tropical soils. Mycologia 61: 264–272. [PubMed] [Google Scholar]
- 35. Nygren K, Strandberg R, Wallberg A, Nabholz B, Gustafsson T, et al. (2011) A comprehensive phylogeny of Neurospora reveals a link between reproductive mode and molecular evolution in fungi. Mol Phylogenet Evol 59: 649–663. [DOI] [PubMed] [Google Scholar]
- 36. Glass NL, Vollmer SJ, Staben C, Grotelueschen J, Metzenberg RL, et al. (1988) DNAs of the two mating-type alleles of Neurospora crassa are highly dissimilar. Science 241: 570–573. [DOI] [PubMed] [Google Scholar]
- 37. Glass NL, Smith ML (1994) Structure and function of a mating-type gene from the homothallic species Neurospora africana . Mol Gen Genet 244: 401–409. [DOI] [PubMed] [Google Scholar]
- 38. Arnaise S, Zickler D, Glass NL (1993) Heterologous expression of mating-type genes in filamentous fungi. Proc Natl Acad Sci U S A 90: 6616–6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee SC, Corradi N, Doan S, Dietrich FS, Keeling PJ, et al. (2010) Evolution of the sex-related locus and genomic features shared in microsporidia and fungi. PLoS ONE 5: e10539 doi:10.1371/journal.pone.0010539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Selman M, Sak B, Kvac M, Farinelli L, Weiss LM, et al. (2013) Extremely reduced levels of heterozygosity in the vertebrate pathogen Encephalitozoon cuniculi . Eukaryot Cell 12: 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cooper MA, Adam RD, Worobey M, Sterling CR (2007) Population genetics provides evidence for recombination in Giardia . Curr Biol 17: 1984–1988. [DOI] [PubMed] [Google Scholar]
- 42. Ramesh MA, Malik SB, Logsdon JM Jr (2005) A phylogenomic inventory of meiotic genes; evidence for sex in Giardia and an early eukaryotic origin of meiosis. Curr Biol 15: 185–191. [DOI] [PubMed] [Google Scholar]
- 43. Poxleitner MK, Carpenter ML, Mancuso JJ, Wang CJ, Dawson SC, et al. (2008) Evidence for karyogamy and exchange of genetic material in the binucleate intestinal parasite Giardia intestinalis . Science 319: 1530–1533. [DOI] [PubMed] [Google Scholar]
- 44. Akopyants NS, Kimblin N, Secundino N, Patrick R, Peters N, et al. (2009) Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector. Science 324: 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rougeron V, Banuls AL, Carme B, Simon S, Couppie P, et al. (2011) Reproductive strategies and population structure in Leishmania: substantial amount of sex in Leishmania Viannia guyanensis . Mol Ecol 20: 3116–3127. [DOI] [PubMed] [Google Scholar]
- 46. Inbar E, Akopyants NS, Charmoy M, Romano A, Lawyer P, et al. (2013) The mating competence of geographically diverse Leishmania major strains in their natural and unnatural sand fly vectors. PLoS Genet 9: e1003672 doi:10.1371/journal.pgen.1003672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaunt MW, Yeo M, Frame IA, Stothard JR, Carrasco HJ, et al. (2003) Mechanism of genetic exchange in American trypanosomes. Nature 421: 936–939. [DOI] [PubMed] [Google Scholar]
- 48. Wendte JM, Miller MA, Lambourn DM, Magargal SL, Jessup DA, et al. (2010) Self-mating in the definitive host potentiates clonal outbreaks of the apicomplexan parasites Sarcocystis neurona and Toxoplasma gondii . PLoS Genet 6: e1001261 doi:10.1371/journal.pgen.1001261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mark Welch DB, Meselson M (2000) Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288: 1211–1215. [DOI] [PubMed] [Google Scholar]
- 50. Feldheim KA, Chapman DD, Sweet D, Fitzpatrick S, Prodohl PA, et al. (2010) Shark virgin birth produces multiple, viable offspring. J Hered 101: 374–377. [DOI] [PubMed] [Google Scholar]
- 51. Watts PC, Buley KR, Sanderson S, Boardman W, Ciofi C, et al. (2006) Parthenogenesis in Komodo dragons. Nature 444: 1021–1022. [DOI] [PubMed] [Google Scholar]
- 52. Kono T, Obata Y, Wu Q, Niwa K, Ono Y, et al. (2004) Birth of parthenogenetic mice that can develop to adulthood. Nature 428: 860–864. [DOI] [PubMed] [Google Scholar]