Abstract
Transcription elongation consists of repetition of the nucleotide addition cycle: phosphodiester bond formation, translocation and binding of the next nucleotide. Inhibitor of multi-subunit RNA polymerase tagetitoxin (TGT) enigmatically slows down addition of nucleotides in a sequence-dependent manner, only at certain positions of the template. Here, we show that TGT neither affects chemistry of RNA synthesis nor induces backward translocation, nor competes with the nucleoside triphosphate (NTP) in the active center. Instead, TGT increases the stability of the pre-translocated state of elongation complex, thus slowing down addition of the following nucleotide. We show that the extent of inhibition directly depends on the intrinsic stability of the pre-translocated state. The dependence of translocation equilibrium on the transcribed sequence results in a wide distribution (∼1–103-fold) of inhibitory effects of TGT at different positions of the template, thus explaining sequence-specificity of TGT action. We provide biochemical evidence that, in pre-translocated state, TGT stabilizes folded conformation of the Trigger Loop, which inhibits forward and backward translocation of the complex. The results suggest that Trigger Loop folding in the pre-translocated state may serve to reduce backtracking of the elongation complex. Overall, we propose that translocation may be a limiting and highly regulated step of RNA synthesis.
INTRODUCTION
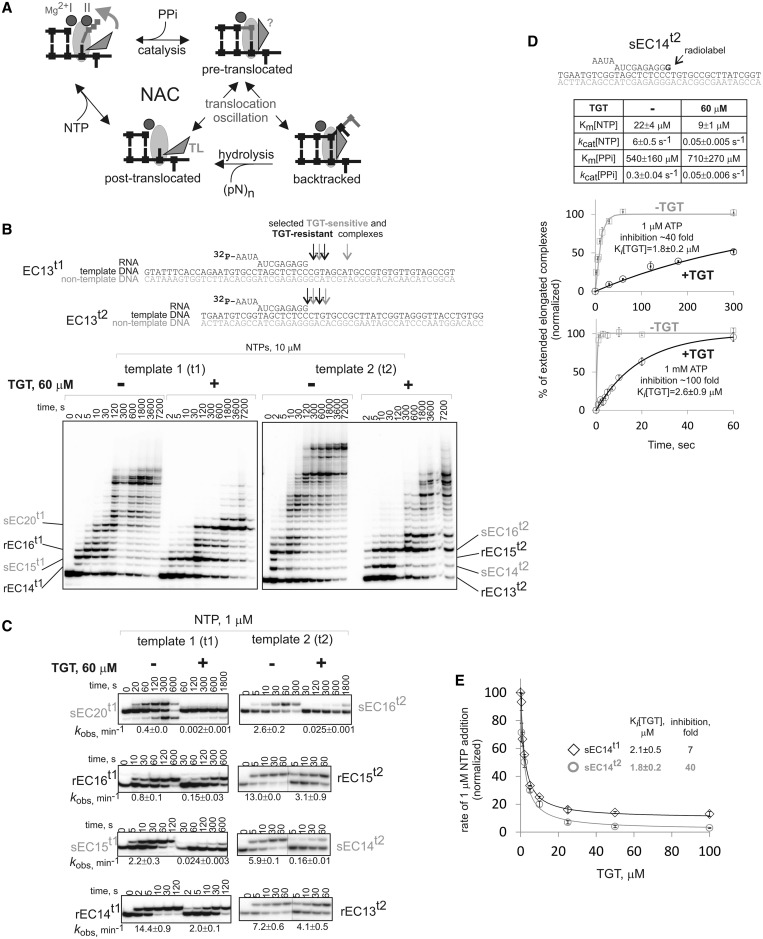
Bacterial RNA polymerase (RNAP), a central enzyme in gene expression, is a target for numerous inhibitors. RNAP inhibitor Tagetitoxin (TGT), produced by plant pathogen Pseudomonas syringae pv.tagetis (1), inhibits multi-subunit RNAPs from chloroplasts and bacteria (2) and eukaryotic pol III (3). TGT was proposed to disrupt the nucleotide addition cycle (NAC) (4–6). The NAC is the principal elemental step of RNAP functioning, which consists of several stages (Figure 1A). First, the incoming nucleoside triphosphate (NTP) substrate base pairs with the complementary template base in the i+1 site of the RNAP active center. On NTP binding, a flexible domain, the Trigger Loop (TL), folds and brings its catalytic residues (R1239 and H1242, Thermus aquaticus nomenclature), in the catalytically active configuration (7,8). R1239 and H1242 are thought to stabilize the transition state of the reaction of phosphodiester bond formation (9,10). Without transition state stabilization by the TL, the reaction is slowed down by four orders of magnitude (9,11). After the phosphodiester bond formation, the 3′-end of the transcript occupies the i+1 site of the active center (Figure 1A). To vacate the i+1 site for the next base of the template DNA strand and the next incoming NTP, translocation must take place. Translocation proceeds via molecular ratchet mechanism, which is powered by the Brownian motion. The oscillation between pre- and post-translocated states strongly depends on the sequence of the nucleic acids in the elongation complex (12–15). During translocation oscillation, RNAP may backtrack by one or more base pairs (Figure 1A). Backtracking by one base pair is a stable translocation state (16) coexisting with pre-translocated and post-translocated states (17). Backtracking on longer distances may become irreversible (18) and be detrimental to cell’s viability (19).
Figure 1.
Inhibition by TGT depends on the transcribed sequence. (A) Scheme of the NAC and translocation oscillation of the elongation complex between post-translocated, pre-translocated and backtracked states. The states of the TL (folded-vertical versus unfolded-horizontal) are shown based on biochemical and crystallographic data, except for pre-translocated state, conformation of the TL in which is not known. Note the reactions that are catalyzed in each translocation state. (B) Transcription in the absence or presence of TGT in elongation complexes of unrelated transcribed sequences (t1 and t2, shown above the gels; RNA was labeled at the 5′-end). Depicted are sECs and rECs analyzed in our study. (C) Kinetics of NTP incorporation in sECs and rECs in the presence or absence of TGT. Observed rate constants (kobs) are shown below gels (numbers that follow the ± sign are standard errors). Note that division of complexes into sECs and rECs is formal, and a broad distribution of TGT sensitivity is observed (see also Figure 2B). (D) Characteristics of TGT action analyzed in sEC14t2 (scheme at the top; radiolabel is in bold): inhibition of incorporation of 1 µM and 1 mM NTP in the absence or presence of TGT (error bars are standard deviations); Ki[TGT] in the presence of 1 µM and 1 mM NTP; affinity and rate constants for NTP incorporation and pyrophosphorolysis in the absence and presence of TGT (numbers that follow the ± sign are standard errors). (E) Inhibition constants for two complexes with different extent of TGT inhibition (error bars are standard deviations, numbers that follow the ± sign are standard errors).
Based on the structure of TGT with Thermus thermophilus RNAP holoenzyme (4), TGT was proposed to inhibit chemistry of phosphodiester bond formation by shifting catalytic Mg2+II and NTP phosphates in a position incompatible with catalysis (4). Later, molecular modeling suggested that TGT displaces the catalytic β′R1239 and β′H1242 of the TL and thus inhibits chemistry of phosphodiester bond formation (5). Another model suggested that TGT binds to the post-translocated complex, and by mimicking pyrophosphate molecule induces TL folding, which, in turn, forces a shift into the pre-translocated state (6). Importantly, however, all the models suggest uniform sequence-independent disruption of RNA synthesis and cannot explain the unique property of TGT to inhibit elongation only at certain registers (20).
Here, we show that TGT does not affect the chemistry of RNA synthesis or induces a shift into the pre-translocated state, but that, instead, it stabilizes the pre-translocated state of the elongation complex, thus inhibiting overall rate of elongation. The dependence of translocation equilibrium on the transcribed sequence results in a wide distribution in extent of inhibition by TGT among different elongation registers (from no to three orders of magnitude inhibition) and explains the enigmatic sequence-dependence of transcription inhibition by TGT. Our results also suggest that folding of the TL in the pre-translocated state is required for blocking of RNAP backtracking during transcription.
MATERIALS AND METHODS
Proteins and reagents
Wild type (WT) and mutant (β′R1239A and ΔTL) T. aquaticus RNAPs were described by us earlier (21). Oligonucleotides were from IDT. TGT was from Epicentre Technologies.
Transcription assays
All transcription experiments were done at 40°C in transcription buffer containing 40 mM KCl, 20 mM Tris–HCl (pH 6.8). Elongation complexes were assembled and immobilized exactly as described (21). RNA was labeled at the 5′-end using T4 PNK and γ[32P]ATP or at the 3′-end by incorporation of α[32P]NTP after complexes assembly as described (21). Stalled elongation complexes were obtained by walking the original EC13s to the desired position using subset of NTPs with subsequent removal of NTPs and Mg2+ by washing of immobilized complexes with transcription buffer. TGT was added before reactions for 1 min to 60 µM, unless otherwise specified. Reactions were started by addition of 10 mM MgCl2 with or without NTP(s) or PPi unless otherwise specified (concentrations specified in figures and figure legends). After incubation for times specified in figures or figure legends, reactions were stopped with formamide containing buffer.
Elongation complex EC27 was obtained on a template containing T7A1 promoter as described in (22), except synthesis of 11mer from promoter was performed at 60°C, synthesis of 20, 21 and 26mers—at 40°C, and incorporation of α[32P]UTP (labeling step) at position 27—at RT (temperature that does not allow efficient backtracking by T. aquaticus RNAP). After 1 min of incubation with α[32P]UTP, to half of the reaction, TGT was added, and reactions were transferred to 40°C to allow backtracking. Washing after labeling step was omitted. Reactions were incubated at 40°C for 1 h and stopped by addition of formamide containing buffer.
Products of all reactions were resolved by denaturing PAGE (8 M Urea), revealed by PhosphorImaging (GE Healthcare) and analyzed using ImageQuant software (GE Healthcare). Kinetics data were fitted to a single or double exponential equation and rates at different concentrations of NTP/Tgt/PPi to hyperbolic equation using non-linear regression procedure in SigmaPlot software (17,23).
RESULTS
TGT is a sequence-dependent inhibitor
As mentioned in the ‘Introduction’ section, TGT inhibits transcription in a sequence-specific manner (20). We decided to investigate this phenomenon in more detail. In our study, we used assembled elongation complexes formed with T. aquaticus RNAP (21). Complexes were assembled with fully complementary template and non-template strands and 13 nt long 5′-end labeled RNA (Figure 1B). First, we analyzed effect of TGT on transcription in the presence of all NTPs on two templates (t1 and t2) with unrelated transcribed sequences. As seen from Figure 1B, TGT (here and after 60 µM, TGT was used, unless otherwise specified) inhibited transcription only at a subset of positions on either of templates. We, however, observed no obvious sequence preference of inhibition (compare sequences in Figure 1B).
Distribution of TGT sensitivity among elongation complexes
We decided to compare the properties of elongation complexes that are either inhibited or not inhibited by TGT. We randomly chose several complexes from both templates that were or were not affected by TGT (Figure 1B). We isolated elongation complexes by walking RNAP to the corresponding positions and measured single NTP addition with or without TGT. Surprisingly, we observed a wide distribution of TGT sensitivity among the complexes, ranging from no inhibition to ∼200-fold inhibition (Figures 1C and 2B). For simplicity, the complexes may be formally divided in the two, TGT-resistant elongation complexes (rECs) (1.5- to 7-fold inhibition) and TGT-sensitive elongation complexes (sECs) (40- to 210-fold inhibition), groups.
Figure 2.
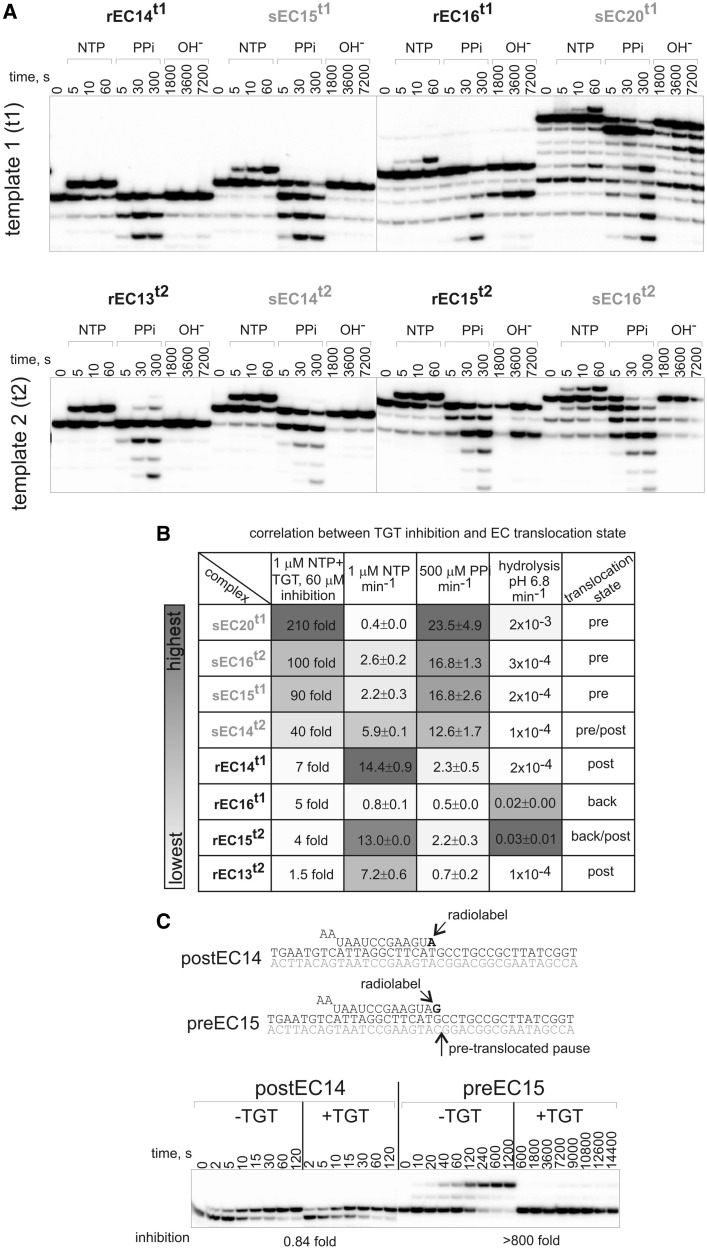
TGT targets the pre-translocated state of elongation complex. (A) Kinetics of NTP (1 µM) incorporation, pyrophosphorolysis (500 µM PPi) and phosphodiester bond hydrolysis in sECs and rECs. Representative gels are shown. (B) Summary of results on inhibition by TGT and the rates of reactions (kobs) in sECs and rECs (numbers that follow the ± sign are standard errors). Shades of gray in the ‘heat map’ reflect magnitude of effects: darkest gray corresponds to strongest inhibition of NTP addition by TGT or highest rates of reactions in the absence of TGT. Note the correlation between extent of inhibition of NTP addition by TGT and the rate of pyrophosphorolysis. The right column roughly shows the distribution between translocation states in elongation complexes, deduced from the rates of reactions. (C) Kinetics of NTP (1 µM) incorporation in the presence or absence of TGT in elongation complexes stabilized in the post-translocated (postEC14) and the pre-translocated (preEC15) states (12). Extent of inhibition by TGT is shown below gels.
Neither extent of inhibition nor inhibition constant (Ki[TGT]) was affected by NTP concentration (Figure 1D). TGT slightly decreased Km[NTP] (Figure 1D) consistent with the proposed TGT-induced folding of the TL (5), which may stabilize NTP bound in the active center. The differences in inhibition among elongation complexes were not due to the different affinities of complexes to TGT because Ki[Tgt] for ECs with different TGT-sensitivities were similar (Figure 1E) and substantially lower than TGT concentration (60 µM) used in the experiments. These results suggest that TGT uniformly binds to the RNAP active center but inhibits NTP addition depending on some intrinsic properties of the elongation complex.
TGT acts only on the pre-translocated state of the elongation complex
The aforementioned results suggest that the selective action of TGT is determined by the variations in the NAC properties among elongation complexes. The transcribed sequence can affect NAC by influencing translocation equilibrium (12–15).Therefore, we hypothesized that TGT may act differently on complexes with different translocation states.
To test this hypothesis, we analyzed translocation states of sECs and rECs by measuring the rates of NTP addition, pyrophosphorolysis and phosphodiester bond hydrolysis catalyzed by RNAP exclusively from the post-translocated, pre-translocated and backtracked states, respectively (Figure 1A). Comparison of the rates of these reactions can give information about relative stabilization of elongation complex in each particular translocation state (12).
The results revealed a distribution of translocation equilibriums among eight complexes chosen earlier in the text (Figure 2A andB). Strikingly, however, we observed a clear correlation between TGT sensitivity of a complex and the rate of pyrophosphorolysis in it (‘heat map’ in Figure 2B). The differences in the rates of pyrophosphorolysis were not due to the variation in affinity of ECs to PPi, as Km[PPi] was similar for different complexes (544 ± 160 µM for sEC14t2and 513 ± 40 µM for rEC15t2; not shown). Thus, faster pyrophosphorolysis reflects stronger stabilization of the complex in the pre-translocated state. Consistently, the rates of NTP addition and phosphodiester bond hydrolysis were relatively slower in complexes with faster pyrophosphorolysis (Figure 2B). Therefore, correlation between TGT sensitivity and the rate of pyrophosphorolysis suggests that TGT manifests its action only in the pre-translocated state of the elongation complex, and its action depends on the intrinsic translocation equilibrium of the complex. The latter may explain the sequence dependence of TGT action.
The aforementioned results predict that NTP addition in an arbitrary complex stabilized in the pre-translocated state would be inhibited by TGT, whereas an arbitrary post-translocated complex would be resistant to TGT. To test this prediction, we used two complexes characterized by us earlier, stabilized in the pre- (preEC15) and in the post-translocated state (postEC14) (12). As can be seen from Figure 2C, in full agreement with the aforemetnioned prediction, TGT slowed down NTP addition by preEC15 >800-fold, whereas no inhibition was observed with postEC14. Importantly, the aforementioned results indicate that TGT does not inhibit RNA extension in post-translocated complexes.
TGT inhibits backwards translocation
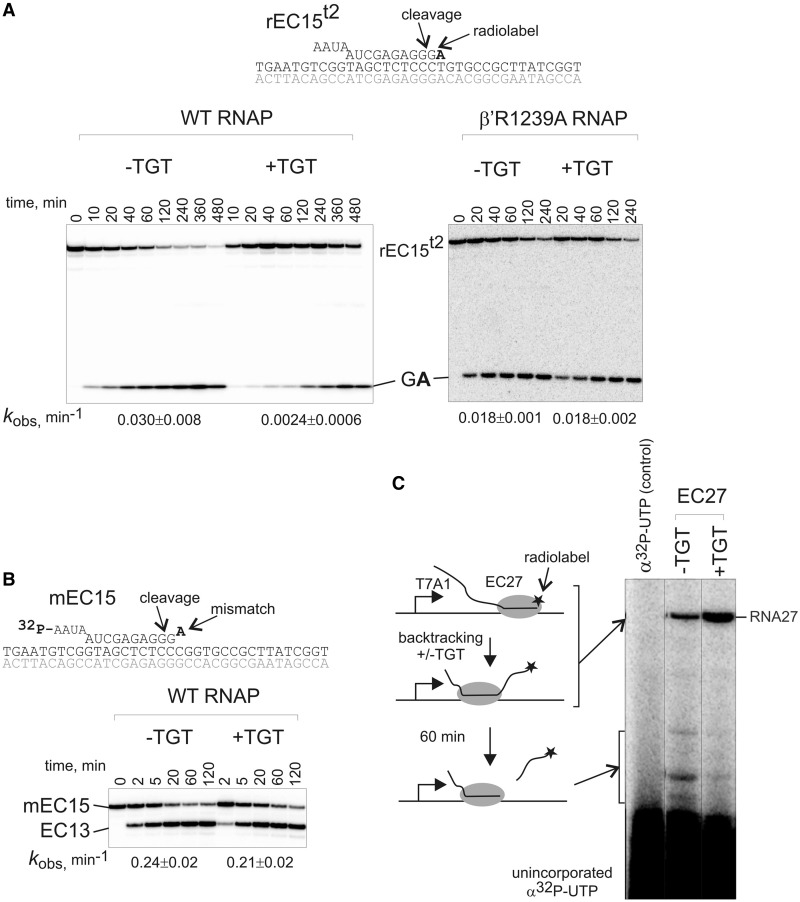
The correlation between the extent of inhibition of NTP addition by TGT and the stability of the pre-translocated state of the elongation complex suggests that TGT may further stabilize the intrinsic pre-translocated state and thus inhibit forward translocation required for RNA synthesis. We hypothesized that if TGT stabilizes the pre-translocated state of the elongation complex, it should inhibit shift into the backtracked state, which coexists in equilibrium with the pre- and post-translocated states (12,16,17). To test that, we used rEC15t2 prone to backtracking by 1 bp (17) (Figure 3A). To analyze the effect of TGT on backtracking of rEC15t2, we measured the rate of RNA hydrolysis, which proceeds exclusively from backtracked state (12,17). As can be seen from Figure 3A, TGT inhibited RNA hydrolysis in rEC15t2. This effect is achieved solely due to inhibition of backward translocation rather than chemistry of the reaction because TGT does not affect RNA cleavage in the stably backtracked elongation complex [Figure 3B; mEC15t2; with 3′-end AMP of the RNA mismatched with the template DNA (23)].
Figure 3.
TGT inhibits backtracking by acting through the TL. (A) Kinetics of second phosphodiester bond hydrolysis in rEC15t2 by WT RNAP and mutant β′R1239A RNAP in the presence or absence of TGT. Rate constants are shown below the gels (numbers that follow the ± sign are standard errors). (B) Kinetics of second phosphodiester bond hydrolysis in mEC15 (scheme above gel; RNA was labeled at the 5′-end) by WT RNAP in the presence or absence of TGT. Note that the 3′-end NMP of the RNA (bold) is mismatched with the template strand, thus stabilizing complex in the backtracked state [which results in faster hydrolysis than in rEC15t2 in panel (A)]. Rate constants are shown below the gels (numbers that follow the ± sign are standard errors). (C) TGT inhibits deep backtracking. The scheme of the experiment is shown to the left of the gel. RNA in EC27, which was obtained by walking from T7A1 promoter, was labeled at the 3′-end (asterisk) at RT to prevent backtracking and then transferred to 40°C for 1 h in the presence or absence of TGT. Backtracking of EC27 was monitored by RNA hydrolysis (the major cleavage band corresponds to the 16 nt long cleavage product). Radioactive NTP was not removed from the reaction resulting in the smear at the bottom of the gel.
Backtracking by further than one base pair may result in the formation of an arrested complex. We tested whether TGT can prevent backtracking on longer distances. We used a well-characterized backtracking-prone elongation complex EC27 obtained by transcription from T7A1 promoter, which backtracks by up to 18 base pairs (18). RNA was labeled at the 3′-end by incorporation of α[32P]UTP, and the complexes were allowed to fall into arrest in the presence or absence of TGT. Falling into arrest can be detected by the appearance of long products of RNA hydrolysis. As can be seen from Figure 3C, TGT inhibited backtracking of EC27.
Overall, our results suggest that TGT inhibits both forward and backward translocation of RNAP by stabilizing the pre-translocated state.
TGT stabilizes pre-translocated state via the TL
Earlier modeling proposed that TGT stabilizes the folded conformation of the TL (5,6). This proposition, however, was supported by equivocal experimental evidence (24). Analysis of inhibition of backtracking by TGT may provide further support to this model; only fully folded TL would be able to block disengagement of the 3′-end of RNA during backtracking. We analyzed whether inhibition of backtracking by TGT proceeds via the TL or whether TGT can inhibit backtracking on its own. One of the determinants of TGT action, but not TGT binding to RNAP (4), is arginine β′R1239 of the TL (5). We, therefore, constructed mutant RNAP, which had β′R1239 substituted for alanine (β′R1239A RNAP) and tested whether TGT inhibits backtracking by this RNAP. As seen from Figure 3A, TGT did not inhibit phosphodiester bond hydrolysis by β′R1239 RNAP in rEC15t2. Given that neither β′R1239 (21) nor TGT (earlier in the text) influences the chemistry of phosphodiester bond hydrolysis, the result suggests that backtracking by WT RNAP was blocked by the folded TL, which was stabilized in this conformation by TGT. We therefore conclude that TGT stabilizes the pre-translocated state by stabilizing the TL in the folded state.
TGT blocks pyrophosphate entry to the active center
Stabilization of the pre-translocated state of the elongation complex by TGT suggests that TGT would increase the rate of pyrophosphorolysis. We however observed inhibition of pyrophosphorolysis by the antibiotic (Figure 1D). Inhibition was not due to competition of TGT and PPi in the active center, as Km[PPi] was similar in the absence or presence of TGT (Figure 1D). The chemistry of the reaction is also unlikely to be inhibited, given that chemistry of the direct reversal of pyrophosphorolysis, NTP addition, is not inhibited by the antibiotic (earlier in the text).
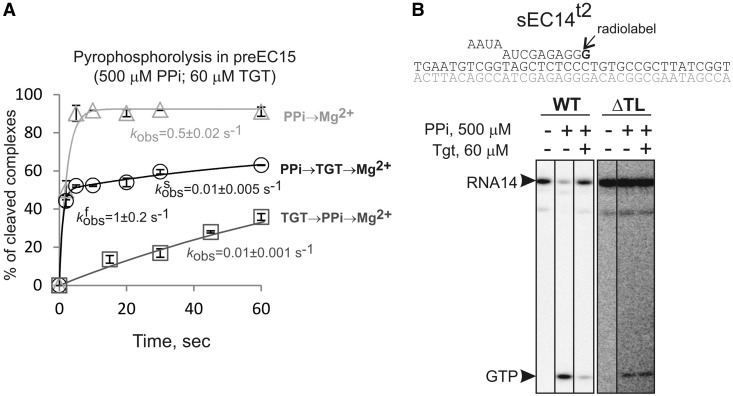
We argued that by stabilizing the TL in the folded conformation, TGT added before PPi may block the entry of PPi to the active center and thus inhibit pyrophosphorolysis. To test this hypothesis, 500 µM (∼Km[PPi]) PPi was added to the complexes before TGT to allow PPi binding before TL folding. The reaction was started by addition of Mg2+. Indeed, we observed that part of the complexes undergone pyrophosphorolysis as quickly as in the absence of TGT (Figure 4A). Pyrophosphorolysis in the rest of the complexes, however, was slowed down to the same extent as when TGT is added before PPi (Figure 4A). Consistently with the hypothesis that TGT blocks pyrophosphorolysis via the TL, TGT was not able to inhibit pyrophosphorolysis by RNAP lacking the TL, ΔTL RNAP (Figure 4B). The results therefore support the idea that, in the pre-translocated state, TGT stabilizes the TL in folded conformation.
Figure 4.
TGT/TL blocks PPi entry to the active center. (A) Kinetics of pyrophosphorolysis in preEC15 (error bars are standard deviation). PPi (500 µM) was added without TGT (light gray triangles), after TGT (dark gray squares) or before TGT (black circles). Reactions were started with Mg2+. Data for ‘light gray’ and ‘dark gray’ curves were fitting well in single-exponential equation. The ‘black’ kinetics clearly had two phases and was fitted into double exponential equation. The observed rate constants are shown next to plots (numbers that follow the ± sign are standard errors). For ‘black’ plot, constants for both slow and fast, fractions are shown. (B) TGT (60 µM) inhibits pyrophosphorolysis (500 µM) by wild-type RNAP (10 s), but not by RNAP lacking entire TL, ΔTL (2 h).
DISCUSSION
The principal finding of the study is that TGT inhibits translocation of RNAP by stabilizing the pre-translocated state of the elongation complex. Its action therefore strongly depends on the intrinsic translocation equilibrium of the elongation complex, explaining the sequence dependence of inhibition. For example, assume a mainly pre-translocated elongation complex with translocation distribution 10%post:90%pre. Assume also that TGT stabilizes the pre-translocated state by 1.36 kcal/mol. Such stabilization would result in a further shift of the distribution to 1%post:99%pre, which, in turn, would lead to ∼10-fold inhibition of NTP addition. In contrast, the same stabilization by 1.36 kcal/mol of the pre-translocated state in elongation complex with distribution 90%post:10%pre would change the distribution to 50%post:50%pre, and thus would inhibit NTP addition by only ∼2-fold. Therefore, NTP addition in complexes, whose translocation equilibrium is shifted toward the post-translocated state, is largely resistant to the TGT because their inherently unstable pre-translocated state can only weekly be stabilized by TGT. The more translocation equilibrium of the elongation complex is shifted toward the pre-translocated state, the stronger this state is stabilized by TGT. Therefore, TGT cannot actively shift the complex into the pre-translocated state, as was suggested earlier (6). The uniform shift of all elongation complexes into the pre-translocated state proposed earlier (6) would also be not consistent with the sequence specificity of inhibition of transcription by TGT. At present, we cannot distinguish whether TGT influences the real rates of isomerization between pre- and post-translocated states, which is yet to be determined.
The aforementioned considerations suggest that TGT stabilizes a natural structural intermediate of the elongation complex that occurs during translocation oscillation. TGT was recently proposed to act through stabilization of the folded conformation of the flexible domain of the active center, the TL (5,6). Our results on blocking the entry for PPi and inhibition of backtracking by TGT support the idea that TGT acts on the pre-translocated state by stabilizing folded state of the TL. This, in turn, suggests that folding of the TL on its own may influence translocation oscillation of the elongation complex by stabilizing the pre-translocated state. Stabilization of the pre-translocated state by the folded TL may have the following important implication during transcription. From the pre-translocated state, RNAP may backtrack, leading to formation of arrested complexes, harmful for the cell (19). Our results suggest that folding of the TL may decrease the probability of backtracking by stabilizing the pre-translocated state. Notably, during transcription, TGT increases probability of RNA extension in complexes that are prone to backtracking (see, for example, rEC16t1 and rEC15t2 in Figure 1B). In this case, stabilization of the pre-translocated state by TGT increases chances of the complex to shift into the post-translocated state before it backtracks, the effect of TGT that dominates on these complexes over its inhibitory ability. Given that the TL is also able to fold in the backtracked state (21), it is possible that the TL acts to prevent backtracking at every single base pair step of backwards movement of RNAP. It however remains unclear whether TL folding is an obligatory event on shift of elongation complex into the pre-translocated state. The translocation equilibrium is determined by the intrinsic signals of the nucleic acids and can only be modulated to some extent by TGT and possibly the TL.
Our results show that, while binding in the active center, TGT does not compete with NTP in the active center. Consistently, affinity to PPi was also unchanged in the presence of TGT. These observations contradict to the theoretical model, in which TGT mimics PPi molecule when bound in the active center (6). However, our conclusions that TGT and NTP or PPi can coexist in the active center are further supported by findings that TGT inhibits chemistry of neither phosphodiester bond formation nor pyrophosphorolysis.
Translocation pausing was predicted theoretically (14) and was recently observed experimentally (12). Notably, in relatively low NTPs concentrations, pauses sensitive to TGT are observed frequently (Figure 1B) and may also exist, but cannot be detected by biochemical techniques, owing to short life-time, in high NTPs. Though it remains to be determined how frequent the translocation pauses are at physiological NTPs concentrations, the result may indicate that translocation may be a rate-limiting step of elongation and, thus, be a target for transcription regulation. Earlier, it was also shown that, during initiation of transcription, TGT does not inhibit synthesis of the first dinucleotide (which is consistent with our results that TGT does not affect chemistry of the reaction) but inhibits synthesis of longer abortive products (25). Taken together with our results, it suggests that translocation of RNAP during abortive initiation may determine the rate of transcription initiation and/or promoter escape.
FUNDING
Royal Society University Research Fellowship (to Y.Y.); UK Biotechnology and Biological Sciences Research Council and the European Research Council [ERC-2007-StG 202994-MTP to N.Z.]. Funding for open access charge: UK Biotechnology and Biological Sciences Research Council and the European Research Council [ERC-2007-StG 202994-MTP].
Conflict of interest statement. None declared.
ACKNOWLEDGEMENTS
The authors thank Dmitry G. Vassylyev for critical reading of the manuscript.
REFERENCES
- 1.Mitchell RE, Durbin RD. Tagetitoxin, a toxin produced by Pseudomonas-syringae pv tagetis: purification and partial characterization. Physiol. Plant Pathol. 1981;18:157–168. [Google Scholar]
- 2.Mathews DE, Durbin RD. Tagetitoxin inhibits RNA synthesis directed by RNA polymerases from chloroplasts and Escherichia coli. J. Biol. Chem. 1990;265:493–498. [PubMed] [Google Scholar]
- 3.Steinberg TH, Mathews DE, Durbin RD, Burgess RR. Tagetitoxin: a new inhibitor of eukaryotic transcription by RNA polymerase III. J. Biol. Chem. 1990;265:499–505. [PubMed] [Google Scholar]
- 4.Vassylyev DG, Svetlov V, Vassylyeva MN, Perederina A, Igarashi N, Matsugaki N, Wakatsuki S, Artsimovitch I. Structural basis for transcription inhibition by tagetitoxin. Nat. Struct. Mol. Biol. 2005;12:1086–1093. doi: 10.1038/nsmb1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artsimovitch I, Svetlov V, Nemetski SM, Epshtein V, Cardozo T, Nudler E. Tagetitoxin inhibits RNA polymerase through trapping of the trigger loop. J. Biol. Chem. 2011;286:40395–40400. doi: 10.1074/jbc.M111.300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malinen AM, Turtola M, Parthiban M, Vainonen L, Johnson MS, Belogurov GA. Active site opening and closure control translocation of multisubunit RNA polymerase. Nucleic Acids Res. 2012;40:7442–7451. doi: 10.1093/nar/gks383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vassylyev DG, Vassylyeva MN, Zhang J, Palangat M, Artsimovitch I, Landick R. Structural basis for substrate loading in bacterial RNA polymerase. Nature. 2007;448:163–168. doi: 10.1038/nature05931. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Bushnell DA, Westover KD, Kaplan CD, Kornberg RD. Structural basis of transcription: role of the trigger loop in substrate specificity and catalysis. Cell. 2006;127:941–954. doi: 10.1016/j.cell.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuzenkova Y, Bochkareva A, Tadigotla VR, Roghanian M, Zorov S, Severinov K, Zenkin N. Stepwise mechanism for transcription fidelity. BMC Biol. 2010;8:54. doi: 10.1186/1741-7007-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang X, Wang D, Weiss DR, Bushnell DA, Kornberg RD, Levitt M. RNA polymerase II trigger loop residues stabilize and position the incoming nucleotide triphosphate in transcription. Proc. Natl Acad. Sci. USA. 2010;107:15745–15750. doi: 10.1073/pnas.1009898107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Temiakov D, Zenkin N, Vassylyeva MN, Perederina A, Tahirov TH, Kashkina E, Savkina M, Zorov S, Nikiforov V, Igarashi N, et al. Structural basis of transcription inhibition by antibiotic streptolydigin. Mol. Cell. 2005;19:655–666. doi: 10.1016/j.molcel.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Bochkareva A, Yuzenkova Y, Tadigotla VR, Zenkin N. Factor-independent transcription pausing caused by recognition of the RNA-DNA hybrid sequence. EMBO J. 2012;31:630–639. doi: 10.1038/emboj.2011.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tadigotla VR, Maoiléidigh DO, Sengupta AM, Epshtein V, Ebright RH, Nudler E, Ruckenstein AE. Thermodynamic and kinetic modeling of transcriptional pausing. Proc. Natl Acad. Sci. USA. 2006;103:4439–4444. doi: 10.1073/pnas.0600508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bai L, Shundrovsky A, Wang MD. Sequence-dependent kinetic model for transcription elongation by RNA polymerase. J. Mol. Biol. 2004;344:335–349. doi: 10.1016/j.jmb.2004.08.107. [DOI] [PubMed] [Google Scholar]
- 15.Hein PP, Palangat M, Landick R. RNA transcript 3′-proximal sequence affects translocation bias of RNA polymerase. Biochemistry. 2011;50:7002–7014. doi: 10.1021/bi200437q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D, Bushnell DA, Huang X, Westover KD, Levitt M, Kornberg RD. Structural basis of transcription: backtracked RNA polymerase II at 3.4 angstrom resolution. Science. 2009;324:1203–1206. doi: 10.1126/science.1168729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roghanian M, Yuzenkova Y, Zenkin N. Controlled interplay between trigger loop and Gre factor in the RNA polymerase active centre. Nucleic Acids Res. 2011;39:4352–4359. doi: 10.1093/nar/gkq1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komissarova N, Kashlev M. Transcriptional arrest: Escherichia coli RNA polymerase translocates backward, leaving the 3′ end of the RNA intact and extruded. Proc. Natl Acad. Sci. USA. 1997;94:1755–1760. doi: 10.1073/pnas.94.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutta D, Shatalin K, Epshtein V, Gottesman ME, Nudler E. Linking RNA polymerase backtracking to genome instability in E. coli. Cell. 2011;146:533–543. doi: 10.1016/j.cell.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinberg TH, Burgess RR. Tagetitoxin inhibition of RNA polymerase III transcription results from enhanced pausing at discrete sites and is template-dependent. J. Biol. Chem. 1992;267:20204–20211. [PubMed] [Google Scholar]
- 21.Yuzenkova Y, Zenkin N. Central role of the RNA polymerase trigger loop in intrinsic RNA hydrolysis. Proc. Natl Acad. Sci. USA. 2010;107:10878–10883. doi: 10.1073/pnas.0914424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zenkin N, Naryshkina T, Kuznedelov K, Severinov K. The mechanism of DNA replication primer synthesis by RNA polymerase. Nature. 2006;439:617–620. doi: 10.1038/nature04337. [DOI] [PubMed] [Google Scholar]
- 23.Zenkin N, Yuzenkova Y, Severinov K. Transcript-assisted transcriptional proofreading. Science. 2006;313:518–520. doi: 10.1126/science.1127422. [DOI] [PubMed] [Google Scholar]
- 24.Klyuyev S, Vassylyev DG. The binding site and mechanism of the RNA polymerase inhibitor tagetitoxin: an issue open to debate. Transcription. 2012;3:46–50. doi: 10.4161/trns.19468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathews DE, Durbin RD. Mechanistic aspects of tagetitoxin inhibition of RNA polymerase from Escherichia coli. Biochemistry. 1994;33:11987–11992. doi: 10.1021/bi00205a038. [DOI] [PubMed] [Google Scholar]