Abstract
This article will detail some of the issues that must be considered as institutional animal care and use committees (IACUCs) review the use of nonhuman primates (NHPs) in research. As large, intelligent, social, long-lived, and non-domesticated animals, monkeys are amongst the most challenging species used in biomedical research and the duties of the IACUC in relation to reviewing research use of these species can also be challenging. Issues of specific concern for review of NHP research protocols that are discussed in this article include scientific justification, reuse, social housing requirements, amelioration of distress, surgical procedures, and humane endpoints. Clear institutional policies and procedures as regards NHP in these areas are critical, and the discussion of these issues presented here can serve as a basis for the informed establishment of such policies and procedures.
Keywords: distress, IACUC, nonhuman primate, social housing, surgery
Introduction
Nonhuman primates (NHPs) have a unique position in biomedical research related to their close phylogenetic proximity to humans. This close proximity often serves as the basis for scientific justification of their use in research. However, it also creates significant challenges in ensuring that their physical and psychological needs are met. As large, intelligent, social, long-lived, and nondomesticated animals, monkeys are among the most challenging species used in biomedical research. This article will detail some of the issues that must be considered as institutional animal care and use committees (IACUCs) review the use of NHPs in research. The article will deal only with monkeys—not apes—and approaches this subject from the standpoint of US legal and regulatory requirements and policies. For a review of requirements and policies in other countries, see Bayne and Morris (2012).
Justification of the Choice of a Nonhuman Primate Species
In many cases, the question of scientific justification for using nonhuman primates (NHPs) will have been adequately addressed by the scientific review of a funding agency (e.g., the National Institutes of Health). However, there will remain cases where the institutional animal care and use committee (IACUC) is required to make decisions regarding justification without previous review by a scientific review committee. In those cases, the committee must seriously consider and understand the goals of the research.
Because of their high level of intelligence, NHPs are often perceived as requiring a more stringent justification for their research use. Use of species with the lowest degree of sentience is not a regulatory requirement; however, from the earliest elaborations on the concept of the Three Rs (replacement, reduction, and refinement), replacement was proposed to include replacement of more-sentient species with less -sentient species (Russell and Burch 1959; discussed in Bayne and Morris 2012). However, that replacement is only justified if the scientific goals of the study can be met with a less -sentient species. Recent publications, for example, have begun to question the validity of applying results from mouse models to humans (Seok et al. 2013) and the extent to which the use of a very limited number of model organisms constrains our understanding of basic biology by limiting the questions we ask and how we ask them (Bolker 2012). Although the investigator should justify why a less-sentient species was not selected, the IACUC must consider the justification of the choice of an NHP species in light of the goals of the study, neither automatically rejecting this choice because NHPs are more-sentient species nor automatically accepting this choice because NHPs are most closely related to humans. The IACUC may wish to draw upon expertise from outside the committee to answer questions regarding justification if the committee does not feel it has the requisite expertise.
Number of Animals and Reuse
Monkeys are long-lived, extremely valuable animals. Many studies that use NHPs are not terminal in nature, and it is relatively common for a monkey to be used in more than one study over the course of its life span. For example, an animal may be used in its early adulthood for one set of studies and for a different purpose once that animal is geriatric. Also, monkeys are often trained to perform behavioral tasks that make them especially valuable for future studies that require the same behavioral tasks to be performed. Although repeated use of a given animal is clearly not to be justified based upon reduced animal use or costs, the nature of studies with NHPs will often entail repeated use of subjects over many years.
Because of this type of use, the IACUC and the institutional animal care program may be required to consider the welfare of given monkeys in a broader sense (i.e., the lifetime welfare of that animal). Use in repeated studies over a long lifetime does not, in and of itself, indicate a welfare concern. However, it does mean that the IACUC may wish to consider previous uses of a given animal, factoring in the level or degree of interventions, as part of a set of criteria to determine whether continued use in future studies meets the institution's requirements for ensuring the welfare of that animal.
Distress
Definition of Distress
One of the main focuses of protocol reviews is to ensure that pain and distress are minimized. This reduction in pain and distress is reiterated throughout regulatory and welfare documents, including the Animal Welfare Act regulations (7 U.S.C. §§ 2131–2159 [2008]; 56 F.R. §§ 6495–6505 [1991]), the European Union's Directive (European Parliament 2010), and Guide for the Care and Use of Laboratory Animals (National Research Council [ NRC] 2011). Still, although distress is often discussed, the term is not always clearly defined. For example, although “distress” is used more than 30 times in the Animal Welfare Act (7 U.S.C. §§ 2131–2159 [2008]) regulations, it is never defined therein. In animal welfare literature and elsewhere, distress is often used interchangeably with “stress.” However, there are key distinctions between the two terms. Stress is typically considered to occur when there is “an actual or perceived perturbation that causes alterations to physiological homeostasis or psychological well being” (NRC 2008). Distress has been defined as an aversive state in which an animal fails to cope with stress (NRC 2011). In other words, when animals cannot cope with stress, it can lead to a state of distress. The NRC's Recognition and Alleviation of Distress in Laboratory Animals (2008) provides an excellent description of stress and distress in a captive environment.
Stressors aren't inherently negative or harmful. Some stressors can relieve boredom and help the animal learn to cope with various factors in their environment (Koolhaas et al. 2006). Even negative stressors do not necessarily lead to compromised well-being or distress. However, an animal's failure to cope with these stressors or stress that is chronic in nature can cause distress. As an example, temporarily removing a monkey from its social group is likely an acute stress for the individual but probably not a source of distress. Permanent removal from a social group, on the other hand, may cause distress. IACUC reviewers and principal investigators should understand the difference between stress and distress. Reducing sources of acute stress is always a goal of experimental refinement, whereas chronic stress and distress are more serious issues and should be approached with the same diligence as pain in research protocols (i.e., minimized or eliminated).
Distress is an internal state, and most NHPs are often stoic; therefore it can be difficult to tell when NHPs are experiencing distress. A common sign of distress in NHPs is a change in normal behavior patterns. Common examples of changes that may indicate distress include increased or decreased vocalizations, development of stereotypical or self-injurious behaviors, and a change in temperament (e.g., sudden onset of aggression in a normally friendly monkey). To identify changes in behavior, one must have a good understanding of what behavior is normal for the species as well as for the individual. This point underscores the importance of having a highly trained and attentive staff working with NHPs.
Assessing Distress
There are several factors to consider when assessing distress for an IACUC review. In general, procedures that may cause pain or distress to humans should be assumed to cause pain and distress in NHPs. However, there can be vast differences in how individuals respond to procedures. Factors such as species, age, sex, and even personality can affect how individuals respond to various stressors. Events or situations that are stressful to one individual might have no discernable effect on another. For example, separation from a social group is likely more stressful for a young monkey than an older monkey. Even short-term separations from mother can have both acute and long-term effects for infant macaques. Older macaques, on the other hand, do not typically show the same distress behaviors in response to temporary social separation. Shy or anxious individuals may become more distressed by certain procedures than exploratory or bold individuals. For example, in a study examining cognitive enrichment in chimpanzees, stress-sensitive individuals were more likely than stress-resistant subjects to become agitated by incorrect responses (Yamanashi and Matsuzawa 2010). Because of this individual variability, it is imperative staff are aware of normal behaviors for individual animals and are trained to identify potential signs of stress and distress in these animals.
It is also important to ascertain whether procedures can affect individuals other than the one undergoing the procedure. IACUC members should take these indirect sources of distress into account when reviewing protocols. As an example, protocols involving infant NHPs often require frequent separation of the infant and its mother. In this example, IACUCs and others typically assess the amount of distress to the infant. However, recurrent infant removals are likely both emotionally and physiologically stressful for the mother as well (particularly if she needs to be sedated for the separation). Further, removing an infant from a mother often results in agitation among other individuals in the room. Therefore, protocols should evaluate and address the potential distress not only to the subject but also to other individuals. In these situations, there may be need for post approval monitoring.
In addition, some projects may result in ongoing, residual distress. As an example, studies of addiction often involve the ingestion of alcohol or other addictive drugs. These drugs may not cause a great deal of distress to subjects while they are on the study; however, sudden cessation of an addictive substance may result in severe withdrawal symptoms identical to those observed in humans. Further, the long-term physiologic damage addictive drugs are known to cause may impair an animal's future health and well-being. These sorts of protocols should address how distress will be alleviated even if it occurs after the project is “finished.”
Another factor primary investigators and IACUCs should evaluate is the potential additive effects of various procedures. Because NHPs are so valuable as experimental subjects, they are often assigned to serial studies (as detailed above). Even within one study, they often receive more than one procedure. Procedures may be approved by the IACUC at various times (through modifications). Although an individual procedure may appear benign and not be expected to cause a great deal of distress to a subject, the additive effect of multiple, disparate procedures may result in significant animal distress. To date, there are no clear rules about how many procedures are “too many” within a protocol or within the lifetime of a research animal or how the level or degree of any given intervention should figure into these assessments. This information would be a great refinement to research protocols and would help IACUCs ensure that distress is minimized.
How to Alleviate Distress
There are ethical as well as scientific reasons for minimizing distress. Reducing pain and distress for laboratory animals is the main tenet of “refinement,” one of the Three Rs (Russell and Burch 1959). The Three Rs are widely recognized as guiding principles for the ethical use of animals in research and are implicit in the review of protocols. From a scientific perspective, distress and the stress underlying it can negatively affect the physiologic and psychologic well-being of subjects. Stress can alter many physiologic parameters, which can, in turn, negatively impact scientific outcomes. Therefore, it is important for protocol reviewers to be aware of procedures that can cause stress, both directly and indirectly, as well as some practices that might help mitigate distress.
There are several ways to minimize distress for laboratory primates. IACUC reviewers should be aware of these and encourage their use.
Refine Procedures within the Protocol
First and foremost, principal investigators should search for alternatives to procedures known to cause pain and distress. Information about such refinements to technologies or procedures can be obtained through literature searches, scientific meetings, or consultation with experts. The continuance of distressful procedures when less-distressful alternatives have been developed and are available that will meet the scientific objectives should not be allowed. An example of a procedural refinement is replacing traditional open laparotomies with laparoscopic surgeries. Laparoscopic surgeries are less invasive, less painful, and result in faster recovery than traditional laparotomies. Another example is the development of subcutaneous min pumps that deliver a constant drug/hormone dose without subjecting animals to frequent injections. IACUC reviewers should have confidence that the principal investigator has investigated all alternatives.
In many cases, performing different procedures at the same time can be a refinement, particularly when doing so reduces the number of times an animal must be handled or sedated. Scientific procedures requiring sedation can also be paired with husbandry or clinical procedures such as tuberculosis testing or physical exams to reduce the number of times animals are sedated. Ketamine hydrogen chloride and other similar sedatives are often thought to be innocuous; however, they can have residual effects. Ketamine use has been correlated with increased cortisol levels and decreased appetite after recovery in macaques (Crockett et al. 2000). Therefore, efforts to reduce the frequency of its use should be considered.
Training
The use of positive reinforcement training (PRT) to train animals to cooperate with various research or husbandry procedures can help reduce stress associated with these procedures. For this reason, the incorporation of PRT into a protocol is a significant refinement and should be used when possible. PRT is a type of operant conditioning in which the subject is presented with a stimulus (e.g., a verbal command), responds by performing a specific behavior (e.g., present a body part for injection), and is provided with positive reinforcement (e.g., food treat). Several species of NHPs have been trained to accept venipuncture, to remain stationary for injection, or to move to a new location in their cage using PRT techniques.
There are many benefits associated with PRT. It desensitizes animals to potentially stressful stimuli, such as injections (Schapiro et al. 2005), thereby reducing fear and anxiety related to these procedures. In addition, by allowing individuals to cooperate with the procedures (i.e., they can choose whether they want to participate), PRT provides subjects with a sense of control over their environment (Laule et al. 2003). Studies have shown that PRT can promote overall well-being and reduce stress and distress. It is also a way of communicating to the animal what will happen, thereby instilling predictability.
Habituation and desensitization are techniques of PRT that can be used to help reduce stress associated with procedures. In habituation, animals are exposed to the stressful stimulus (e.g., presence of a needle) in an effort to “get used to it.” Desensitization is a more active process in which the aversive stimulus (e.g., being touched with a needle) is paired with a positive reward (i.e., treat). Through this pairing, procedures become less stressful. The animals also have some control, in that their response to the stimulus determines the outcome. For example, NHPs can be desensitized to the squeeze-back mechanism of the cage by having the trainer slowly move the cage forward and provide a treat to the animal for remaining calm. If the animal does not remain calm, then the squeeze-back is returned. These processes can reduce distress for the animals. The importance of this kind of training has been highlighted by its recent inclusion in the 8th edition of the Guide to the Care and Use of Laboratory Animals (NRC 2011).
It is important to recognize that not every animal will habituate to every situation. For example, although most animals readily habituate to procedures such as wearing a jacket (for tether situations), sitting in a primate chair, or wearing an activity collar, there are some animals that never adapt. IACUC reviewers should expect principal investigators to include information about how they will determine if an animal is not habituating to a procedure and what they will do in that situation. Examples of behaviors that may indicate animals are not habituating to a procedure include agitation, distress vocalizations, and self-injurious or other self-directed behaviors.
Socialization
Most NHPs are social, and thus being housed without a conspecific can be a significant source of distress. Therefore, as discussed in the subsequent section, reviewers should pay close attention to how animals will be housed during the protocol. Social housing can help reduce stress and help animals better cope with stressful stimuli. For example, marmosets relocated to a novel room with a conspecific showed fewer physiologic effects than those moved by themselves (Smith and French 1997). For this reason, strategies that maximize social housing with conspecifics are also significant protocol refinements that should be considered. However, there may also be costs associated with social housing if animals need to be removed and reintroduced frequently for procedures. Because frequent disruptions is stressful to the entire group, subjects in protocols requiring many procedures may be better off pair housed than group housed. The reviewers must weigh the costs and benefits of various housing options.
When social housing with conspecifics is not an option, socialization with a trusted caregiver may be used to help reduce distress. Such positive interactions, which can be achieved through provision of treats or PRT, can reduce abnormal behavior and other indices of distress in a variety of NHP species. Such interactions are often best if performed by one or two caregivers.
Enrichment
Predictability and control over the environment are important factors in the transition between stress and distress. Animals that can predict when a stress is likely to occur or control the duration of the stressors show fewer signs of distress than others (NRC 2008). One way to provide animals with a sense of control is to provide them with the ability to make choices for themselves. This can be accomplished, at least to a degree, by the provision of environmental enrichment.
Although enrichment per se may not be part of the protocol review itself, it should be addressed in situations in which distress is possible. For example, protocols that require single housing of NHPs should discuss additional enrichment that will be provided, including items such as mirrors, foraging puzzles, or increased interaction with caregivers. Enrichment can also help individuals cope with procedures that may cause stress. For example, providing monkeys with play time in an activity cage can help reduce anxiety toward restraint in a primate chair (McGuffey et al. 2002). When protocols include procedures for which distress is expected, the IACUC should consider requesting the provision of enrichment to help alleviate the distress.
Define Humane Endpoints
It is important for principal investigators to clearly establish humane endpoints, not only for pain but also for distress (see Endpoint Determination). Such endpoints for distress should address how to recognize when a distress is unable to be alleviated. For animals that develop behavioral problems such as self-injurious behavior, factors that may be put into place include removal from the study, pharmacologic intervention, or euthanasia.
IACUC Determination of Distress
Ultimately, it is the responsibility of the IACUC to decide the level of tolerable distress for each protocol. This decision is likely to be based on several factors, including the number of animals involved, the impact on human and/or animal health, and the duration and extent of the stressor.
Social Housing Issues
Background
Primates in the wild live in a variety of social groupings ranging from family units consisting of a mated pair and their young (e.g., marmosets), to one-male “harem” groups consisting of an adult male, several females, and their young (e.g., hamadryas baboons), to multi-male, multi-female troops consisting of several adult males, adult females, and their young (e.g., rhesus macaques (Napier and Napier 1994). Regardless of the species, NHPs spend much of the day interacting with members of their social group.
Current guidelines regarding the housing of laboratory NHPs reflects the importance of primate sociality by addressing their social needs in captivity. The 8th edition of the Guide for the Care and Use of Laboratory Animals (NRC 2011) emphasizes that social contact is essential to primate well-being and that facilities should address the social needs of NHPs. “Single housing of social animals should be the exception and justified based on experimental requirements or veterinary-related concerns about animal well-being” (NRC 2011, p. 64). Similarly, the Animal Welfare Act (7 U.S.C. §§ 2131–2159 [2008]) requires that research facilities develop a plan for environmental enrichment “adequate to promote the psychological well-being of nonhuman primates” (7 U.S.C. §§ 2131–2159 [2008], section 3.81), which includes their social needs. This view that NHPs have social needs is reflected in the literature and is based in large part on the extent of their social interactions in the wild (Novak and Suomi 1991; Reinhardt et al. 1995).
However, not all captive NHPs can be housed in social groups. There may be scientific justifications for an exemption to social housing (i.e., an investigator may demonstrate that a study requires single housing to maintain scientific validity). Alternatively, some individual monkeys may be debilitated, aggressive, or incompatible and cannot safely be housed with conspecifics. Sick animals may need to be separated to prevent disease transmission. Such non scientific exemptions may be made by the attending veterinarian or the IACUC of a research facility and must be reviewed every 30 days unless the exemption is made permanent (Animal Welfare Act, 7 U.S.C. §§ 2131–2159 [2008], section 3.81). Lack of appropriate caging is not a valid reason for an exemption. When animals are singly housed for a valid experimental, clinical, or behavioral reason, single housing should be limited to the minimum length of time required, and the need for single housing should be reviewed by the IACUC and veterinarian on a regular basis. In addition, visual, auditory, olfactory, and tactile contact with compatible conspecifics should be provided along with supplementary enrichment and positive human interaction (NRC 2011).
Housing Options
There are many questions to answer when determining the most appropriate type of housing for NHPs. Examples of these questions include: What are the research requirements? How often/how easily does the animal need to be accessed? How compatible are the individuals? What are the health implications of the housing choice? How long will the animal need to remain in this type of housing? In general, laboratory housing of NHPs can be divided into three main categories: single housing, pair housing, and group housing. Within these main categories, there are many additional options. For example, intermittent contact may be used as an alternative to continuous contact. This may include separating groups overnight when fewer personnel are available to monitor behavior, separating a pair when feeding special diets or collecting samples such as urine or feces, or socially housing the animals during some phases of the study but not others. Alternatively, if the research design allows, perhaps some (compatible) subjects could be paired, whereas other (incompatible) subjects could remain separate. Although these are only a few examples of decisions that need to be considered, they demonstrate the complexity of housing choices and their potential impact on the animals’ behavior and well-being. Therefore, investigators need to include behaviorists and veterinarians in this aspect of the study design to determine the best housing situation to accommodate both the needs of the animals and the needs of the research.
Single Housing
Single housing consists of one animal using a single space. The benefits of single housing may include easy access to the animal, reduction in disease transmission, and elimination of wounding from fights. Single housing also makes sample collection, such as the collection of urine or feces, easier. However, these benefits also come at a cost. For example, the immune responses of singly housed animals were shown to differ from those housed socially, which may have an impact on immunologic research (Schapiro et al. 2000). In addition, because primates are social animals, when they are singly housed, they exhibit a reduced repertoire of species-typical behavior and may lack coping strategies for dealing with environmental stressors;these effects are best defined in macaques (Novak and Suomi 1991). Singly housed macaques are also at a greater risk of exhibiting abnormal behavior and self-inflicted wounding (Lutz et al. 2003).The detrimental effect of single housing is most noticeable in infant monkeys. Those reared in isolation develop a syndrome that includes crouching, self-clasping, self-sucking, and rocking, particularly when stressed (Harlow and Harlow 1962; Mason and Sponholz 1963), and the greater the early social deprivation, the more likely they exhibit fearful and withdrawn behavior (Sackett 1967). Although the rearing of infants individually is not used as a standard practice, the housing of older animals in individual cages is not uncommon in laboratory facilities. As with infants, housing adults individually also comes at a behavioral cost. Single housing at an early age or for an extended period of time has been shown to increase the likelihood of behaviors such as motor stereotypy, eye poking, hair pulling, self-grasping, self-biting, and self-injurious behavior (Lutz et al. 2003; Vandeleest et al. 2011), and the proportion of the first four years spent in single housing has been shown to be positively associated with abnormal behavior (Bellanca and Crockett 2002). Because of such costs to the animal, the decision to use this form of housing needs to be carefully weighed.
Protected- or grooming-contact caging is a form of single housing that allows for some physical contact through widely spaced bars that separate the animals. The monkeys can physically interact, but they cannot enter each other's cage. The benefit of this form of housing is that it allows for individualized feeding and sample collection (e.g., the collection of urine and feces) while also allowing for an increased amount of social contact (e.g., grooming), natural behavior, and individual choice or control (Crockett et al. 1997), although it does not mimic the effects of true social housing in all circumstances or in all species (Baker, Crockett, et al. 2012). Protected-contact caging may more closely mimic social housing in some species (e.g., long tailed macaques) than in others (e.g., rhesus macaques) (Baker, Crockett, et al. 2012 ; Lee et al. 2012). Grooming-contact pairs appear to be most compatible if one of the pair is female. For example, in one study, 100% of female/female and male/female longtailed macaque pairs were successful, whereas 89% of male/male pairs were compatible (Crockett et al. 1997).
Pair Housing
Pair housing is defined as two animals occupying the same space. Because social interactions are a key component of primate natural history, social housing, such as pair housing, is considered to be the most effective form of enrichment for non-group-housed laboratory macaques (Lutz and Novak 2005). Unlike single housing, pair housing allows for a greater expression of species-typical behavior such as grooming and social play (Crockett et al. 1994; Eaton et al. 1994; Schapiro and Bloomsmith 1994). Because social stimulation can be variable and unpredictable, it is less likely to result in habituation than other inanimate forms of enrichment. For example, after more than 1 year together, paired rhesus macaques spent more time with each other than with an inanimate object, demonstrating a lack of habituation (Reinhardt 1990). Pair housing has also been reported to reduce abnormal behavior in captive macaques (Baker, Bloomsmith, et al. 2012; Eaton et al. 1994; Reinhardt 1999; Weed et al. 2003), and a compatible social partner may serve as a buffer and aid in stress reduction, although the results for stress reduction are variable (Baker, Bloomsmith, et al. 2012; Doyle et al. 2008; Gilbert and Baker 2011). Additional studies reported that paired juvenile rhesus macaques required less clinical treatment and therapy than did singlyor group-housed animals (Schapiro and Bushong 1994), and there was no difference in health measures or weight between paired and singly housed adult female rhesus macaques (Eaton et al. 1994). With respect to research, pairing did not have an impact on biomedical implants such as cranial and eye implants or on controlled access to water (Roberts and Platt 2005). Pair housing is also an option for studies using operant behavior testing, but housing may affect some test parameters, which should be assessed during experimental design (Hotchkiss and Paule 2003).
As with single housing, the benefits of pairing can come at a cost. Severe wounding and lacerations are a risk (Abney et al. 2011; Crockett et al. 1994). Not all pairs are successful, but some pairings are predicted to have better outcomes than others. For example, female pairs tend to be more successful than male pairs (86–100% vs. 51–92% success) (Abney et al. 2011; Crockett et al. 1994; Eaton et al. 1994; Reinhardt 2002), and juvenile pairs tend to be more successful than adult pairs (100% vs. 51–92%) (Abney et al. 2011; Reinhardt 2002). When pairs were deemed incompatible, it was often because of persistent fighting, wounding, or food monopolization (Baker, Bloomsmith, et al. 2012).Because of the potential for wounding, one needs to weigh the costs and benefits for each pair. For example, young animals that would be housed together for an extended period of time would benefit greatly from pairing. However, if adult male macaques are to be paired for a brief period of time (e.g., less than a month), the benefits of pairing may not outweigh the risk of wounding and physical harm.
Although small New World monkeys, such as squirrel monkeys and marmosets, are also social in nature, their reaction to pair housing attempts may be quite different from that observed in macaques. For example, as opposed to macaques or baboons that naturally live in large multi-male, multi-female groups, marmosets normally live in groups with only one reproductive male and female, with the remainder of the group made up of that pair's offspring. Marmosets of both sexes are highly territorial and extremely aggressive toward unfamiliar individuals of the same sex. The only published studies purporting to successfully pair house unrelated marmosets of the same sex (Majolo et al. 2003) used adolescents as one member of the pair. However, this strategy requires acting against the best welfare interest of the adolescent who is best left in its own natal group. Therefore, pair-housing strategies for marmosets often include housing same-sexed siblings together or housing females with vasectomized males. When marmosets are housed singly with visual, auditory, and olfactory contact with other animals, abnormal behavior is exceedingly rare.
Pairing of monkeys that have previously not been socially housed is a complex process that requires a thoughtful plan and individuals knowledgeable about the species and the individual animals involved to be successful. The IACUC should be cognizant of the complexities of this process when assessing housing plans for NHPs. The IACUC should also be cognizant of the wide range of species-specific social factors that will affect decisions regarding housing of different monkey species.
Group Housing
Group housing is defined as a social group of three or more individuals occupying the same space. Depending on the facilities available and on management needs, groups can range in size from very small (e.g., 3) to very large (e.g., in the hundreds). Types of group housing can also vary by group composition (e.g., family or juvenile group) and location (e.g., indoors vs. outdoors). The methods used for housing groups are constrained by species differences, housing facilities, and research needs. For example, small species such as marmosets that live in family groups can be housed indoors in a smaller space, whereas medium-sized harem groups of macaque monkeys (one male, multiple females) would require more space, either indoors or out, and large troops of macaques or baboons containing as many as 100 or more individuals would be housed in large, outdoor corrals. The procedures used for forming groups vary by species, numbers, and housing conditions. They can range from gradually building up a group in stages (Westergaard et al. 1999), to adding members through births (Bernstein 1991), to the simultaneous introduction of unfamiliar individuals all at once (Bernstein 1991; Westergaard et al. 1999).
Housing NHPs in social groups most closely replicates the social interactions the animals experience in the wild, further promoting species-typical behaviors and psychologic well-being. For example, group-housed animals spend more time social grooming, locomoting, and exploring (Kessel and Brent 2001; Schapiro et al. 1996; Spring et al. 1997) and less time exhibiting abnormal behavior (Bayne et al. 1992; Kessel and Brent 2001; Schapiro et al. 1996; Spring et al. 1997) than those singly housed. Not surprisingly, the benefits of social housing also greatly outweigh the benefits of inanimate enrichment (Schapiro et al. 1996; Spring et al. 1997). However, with these benefits also come costs. One potential cost of group housing is that the animals are less accessible when housed in larger social groups. This issue can be overcome with proper cage design and/or animal training. For example, animals housed in groups can be trained to enter smaller enclosures, transfer boxes, or chutes for separation and individual access (Schlabritz-Loutsevitch et al. 2004), and implanted microchips can be used to identify individuals accessing items such as food from a pellet dispenser (Wilson et al. 2008). Another cost of social housing is intragroup aggression. Severe outbreaks of aggression can occur in groups, resulting in injury or even death (Judge et al. 1994; Samuels and Henrickson 1983). In large breeding groups of macaques, the aggression can occur when a dominant matriline is overthrown (Oates-O'Brien et al. 2010). However, procedures can be put into place to reduce the impact of aggression. For example, in severe situations, the individual, group, or matriline involved can be removed (Judge et al. 1994). Adding an adult male to a group of females significantly reduces the extent of female/female aggression, and in cases where pregnancy is not desired, a vasectomized male may be used (Erwin 1977). Alternatively, using an enclosure that contains visual barriers, allowing for visual or social separation, helps to reduce wounding in groups (Westergaard et al. 1999). Ongoing behavioral monitoring is essential for managing social groups and for prompt intervention when aggression is apparent.
Conclusions
Given the social nature of NHPs, social housing is an essential factor to consider in promoting their psychologic well-being. There is little doubt that social housing with compatible partners is a preferred housing condition, but it can come at a cost, such as the risk of injury. However, single housing comes with its own risks, including behavioral problems and reduced well-being. Therefore, careful consideration needs to be made regarding how NHPs are to be housed for laboratory research. Decisions regarding the animal's housing are based on a number of factors, including the research needs of the investigator and the health and social needs of the animal. All relevant individuals— such as the principal investigator, the veterinarian, and the behaviorist— need to be included in this decision-making process to make well-informed choices. Housing options can vary greatly with respect to the facility design, species, and research needs. Ideally, the environment chosen will benefit both the animals and the research program. In cases where the IACUC grants exemptions from social housing, there should be clear policies regarding how those decisions are made, how the adequacy of the environment is defined, and how often the housing situation must be re assessed.
Surgery
Surgical procedures are common components of animal protocols in biomedical research. Detailed reviews of the proposed surgical procedures and evaluation of the qualifications of the personnel responsible for conducting the surgical procedures are the responsibilities of the IACUC. Key questions to consider when reviewing surgical procedures within an IACUC protocol include the following:
Is the procedure described a survival or nonsurvival surgery? Is it major or minor surgery?
What is the proposed frequency for which the surgeries will be performed, if applicable?
Is the procedure described performed routinely at your institution, or is it experimental?
Who will be performing the surgery? Are they trained and qualified to perform this procedure?
What are the details of preoperative assessment, animal preparation, anesthesia, analgesia, and postoperative care?
Survival or Nonsurvival, Major or Minor
Careful review of the detailed description of the proposed surgical procedure provided by the principal investigator will help IACUC members determine if the proposed procedure is categorized as a survival or nonsurvival surgery and if it is major or minor surgery. In survival surgery, animals are recovered and awaken from anesthesia, whereas in nonsurvival surgery, animals are euthanized and not recovered from anesthesia. For survival surgery, aseptic surgical technique is practiced under sterile conditions in dedicated surgical facilities. For nonsurvival surgery, aseptic surgical technique is often relaxed, and the use of sterile gloves, drapes, and instruments are not required. Additionally, animal anesthetic recovery and postoperative analgesia are not applicable in the case of nonsurvival surgery. However, preoperative animal preparation, proper anesthesia delivery and record-keeping, and appropriate peri-operative analgesia practices of nonsurvival surgery should be held to the same standards as those of survival surgery.
The distinction between major and minor surgical procedures has ethical and regulatory implications. Factors often used to defined surgery as major include penetration or exposure of a body cavity and production of substantial impairment of physical or physiologic functions; minor surgery is situations that do not meet these standards. From a regulatory standpoint, the Animal Welfare Act (7 U.S.C. §§ 2131–2159 [2008]) regulations prohibit multiple major survival surgeries on a single animal unless scientifically justified by the principal investigator and specifically approved by the IACUC (9 C.F.R. §§ 2.31[1985]). Multiple major survival surgical procedures can be justified if they are interrelated components of a research project and/or if they will conserve scarce animal resources. Cost savings alone is not sufficient justification for performing multiple major survival surgeries on a single animal. If an investigator requests multiple major survival surgeries in separate unrelated research protocols, the institutional o fficial must submit a request to the US Department of Agriculture/Animal and Plant Health Inspection Service and receive special approval. Major survival surgeries are more invasive than minor surgeries and therefore constitute a significant cost to the animal subject in terms of recovery time and the potential for associated pain and suffering. The IACUC should pay special attention to animal well-being through continued evaluation of outcomes in protocols involving any major survival surgery.
Frequency of Surgery
When reviewing surgical procedures within an IACUC protocol, it is important that reviewers assess each aspect (e.g., surgical description, anesthesia, and analgesia) of each procedure as well as the cumulative effect of multiple procedures on the animal subjects. When multiple different surgical procedures are proposed, it is very helpful for the investigator to present a summary table that lists each procedure, the total number of times each procedure is to be performed, and the time interval between successive procedures (frequency), when applicable (Figure 1).
Figure 1:
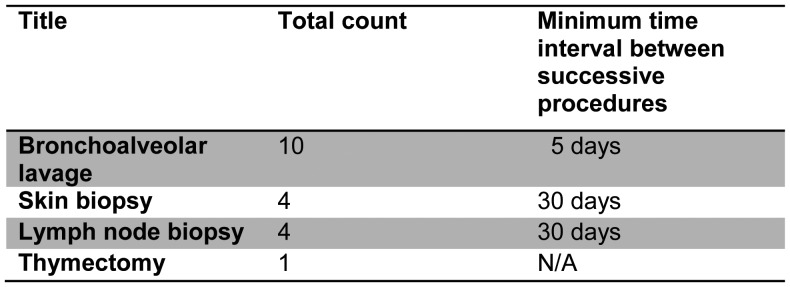
Example summary of surgical procedures
The assessment of the cumulative effect of multiple procedures on subjects during the course of a protocol is made easier when investigators provide a timeline of surgical events (Figure 2). The order of surgical procedures and recovery periods between surgical procedures should be carefully considered to ensure the proposed timeline allows adequate incision healing and anesthesia recovery. Surgical events often require separation of an individual NHP from its social group to allow for postoperative drug administration and incision healing. Frequent social isolation can be disruptive and stressful to NHPs. For this reason, it is often preferable to perform multiple surgical procedures together during a single anesthetic event, assuming the subject is healthy and total anesthesia time is not excessive. This practice of “stacking” procedures reduces overall surgery and anesthesia time. The risks associated with increased time under anesthesia necessary to perform multiple procedures must be weighed against the risks and discomfort associated with multiple anesthetic events, repetitive postoperative drug regimens, increased animal handling and distress, and reduced recovery time between procedures that may occur when procedures are not stacked. When stacking procedures, stratification of procedures into groups that have similar risk for infection is necessary. For example, a clean-contaminated procedure (e.g., colonoscopy) should not be conducted in a serial fashion with sterile procedures (e.g., lymph node biopsy or laparoscopy).
Figure 2:
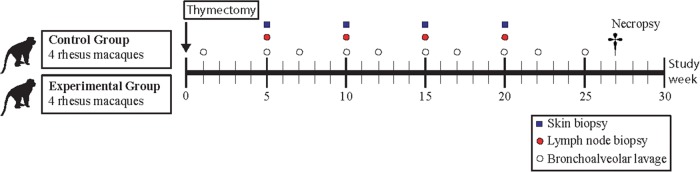
Example timeline of surgical procedures
Experimental Surgical Procedures
Some of the surgical procedures proposed in research with NHPs are non standard or experimental. It is important the IACUC performs conscientious, ongoing review as these procedures are developed. One strategy the IACUC may employ for novel procedures is the approval of a pilot study in which the procedure is performed on a small group (n = 1– 3 animals). Upon completion, the principal investigator is then required to report the outcomes of the pilot study. The IACUC reviews these outcomes and may then decide to approve, deny, or request modification and additional pilot data. This strategy will help to protect an institution against a situation in which multiple procedures are performed in rapid succession that results in unintended or harmful outcomes in multiple animals. It is the job of the IACUC to foresee risks and impose an incremental approach, especially when outcomes are unknown, to help prevent the needless multiplication of harmful outcomes.
Personnel Qualifications
The IACUC protocol should explicitly identify the personnel responsible for performing the surgical procedure as well as their qualifications, training, and experience. The IACUC and the a ttending veterinarian are responsible for determining whether the training and experience of the personnel are adequate for the procedure described. This may become difficult when, for example, an individual has extensive training and procedural competence in human surgery but little experience in NHP species. In this example, additional training may be tailored to include interspecies variations in anatomy, physiology, pharmacology, and postoperative care (NRC 2011).
Surgery Details
Preoperative Considerations
Preoperative assessment of surgical and anesthetic risks include review of each animal's medical history, including weight, age, sex, blood work parameters that may be relevant to the surgical procedure, and any complications that may have occurred during previous anesthetic or surgical events. Presurgical fasting mitigates the serious risk associated with vomiting and pulmonary aspiration of stomach contents during anesthesia. Fasting times should be appropriate for the age and species of the animal subjects.
Anesthesia
Anesthesia (a state of unconsciousness and insusceptibility to pain produced by administration of anesthetic agents) and analgesia (the relief of pain) should be considered together as both contribute to balanced anesthesia. The objectives of modern, balanced anesthesia are to minimize pain and reduce the potential for adverse effects associated with anesthetic and analgesic agents by administering a mixture of smaller doses of two or more agents. This strategy summates the advantages of the individual components of the mixture while being safer than a large dose of a single agent. Additionally, the dose of anesthetic agents and the likelihood of their adverse effects on patients may be significantly reduced by the use of analgesics before painful stimuli. Some examples of analgesics are opioids (such as hydromorphone, buprenorphine, and fentanyl); nonsteroidal anti-inflammatory drugs (such as carprofen, meloxicam, and acetaminophen) ; local anesthetics (such as lidocaine and bupivacaine); and N-methyl d-aspartatereceptor antagonists (such as ketamine). Tranquilizers and anxiolytics (such as midazolam) may provide muscle relaxation and smooth the transition from wakefulness to anesthesia. The veterinary staff is an excellent resource for determining the appropriate selection of anesthetics and analgesics for a proposed surgical procedure.
Anesthesia is traditionally divided into three phases: premedication, induction, and maintenance. Premedications are intended to reduce patient anxiety, sedate, provide analgesia, reduce anesthetic requirements, and facilitate induction of general anesthesia. The induction phase involves the rapid transition to unconsciousness. Induction agents are preferably administered intravenously to abbreviate the excitatory stage of anesthesia induction, thus reducing related complications. Maintenance agents prolong anesthesia for the duration of the surgery and allow for quick recovery. Maintenance is typically achieved by providing a carefully controlled mixture of oxygen and a volatile anesthetic agent that is continuously inhaled by the patient. Maintenance can also be achieved with constant rate infusion of some injectable anesthetic drugs (such as propofol).
NHPs provide many unique challenges with regard to animal handling and occupational safety. Although it would be ideal to premedicate NHPs with a tranquilizer followed by intravenous induction of anesthesia as described above, this approach would require handling and restraint of unpredictable and potentially aggressive animals for intravenous injection. Because of the serious occupational safety risks involved, this approach is generally not feasible (see Occupational Safety). For this reason, there is heavy reliance on dissociative anesthetics (e.g., ketamine) administered intramuscularly to induce anesthesia in NHPs. Respiratory drive, airway reflexes, ventilation, and blood pressure are wellmaintained with dissociative agents relative to other anesthetic agents, making them reasonably safe in the hands of experienced personnel. Anesthesia should be tailored for the species, surgical procedure, and experience of the anesthetist.
For very brief, minor procedures, an induction agent may provide adequate, safe anesthesia when combined with a local block and a systemic analgesic agent. Repeated, serial injections of an induction agent should not be approved as a suitable alternative to general maintenance anesthesia for procedures that cannot be accomplished with a single dose of an induction agent.
There are always risks associated with anesthesia. Careful consideration of the surgical procedure, possible complications associated with the procedure, and factors such as age, obesity, and health status of the patient helps to mitigate these risks. An anesthetic protocol should take all of these factors into account. A resuscitation plan, rescue drugs, and emergency equipment should be readily available wherever anesthesia is conducted. In the experience of one author (Hobbs), the vast majority of anesthetic complications and deaths could be prevented by the simple practices of establishing venous access by intravenous catheter and establishing an open airway by endotracheal intubation. These are easy, fast, and inexpensive precautions that should be strongly encouraged or required for every surgical procedure. For a detailed discussion of anesthesia in NHPs, see Murphy and colleagues (2012).
Physiologic Monitoring
Physiologic monitoring provides information to assess the adequacy of anesthesia as well as the intraoperative well-being of the animal during the surgical procedure. Respiratory rate and pattern, pulse rate, mucous membrane color, capillary refill time, jaw tone, and palpebral reflex are essential parameters that may be easily obtained by trained personnel with no monitoring equipment. The addition of pulse oximetry allows continuous monitoring of pulse and oxygen saturation of hemoglobin. For brief anesthetic events, trained personnel with a pulse oximeter may provide adequate physiologic monitoring.
For longer anesthetic events, the risks to the patient increase. Common complications include cardiopulmonary compromise, ventilation abnormalities, hypothermia, and hypotension. It is prudent to monitor additional physiologic parameters such as core body temperature, electrocardiogram, blood pressure (indirect blood pressure cuff or direct arterial line), and end tidal carbon dioxide. These additional monitoring tools should not take the place of direct, hands-on patient assessment. Trends observed in these parameters are often predictive of anesthetic emergencies well in advance of physiologic crisis. For this reason, an anesthesia record that includes these parameters should be kept at regular intervals (usually every 10–15 minutes) so that trends may be identified and corrective actions taken. Consistent physiologic monitoring should continue until the animal has recovered from anesthesia. The assignment of a dedicated anesthetist for surgical procedures ensures continuous and conscientious monitoring of these patient parameters, enabling early recognition of abnormal trends that may predict physiologic instability or crisis.
Postoperative Care
Postoperative analgesia typically consists of opioids, nonsteroidal anti-inflammatory drugs, or both. In a research setting, the objective is to minimize pain and distress as much as possible without compromising the scientific integrity of the research. However, the IACUC should not approve a protocol that omits the use of appropriate analgesics for postoperative pain. The prospective prediction of pain and discomfort that a given surgical procedure will cause may be obtained through veterinary consultation. Proposed dosing intervals for each analgesic drug should be checked against the published duration of the drug's analgesic effect. Non pharmacological methods of alleviating distress postoperatively may be included in the management of individual cases but should never be considered a substitute for pharmacologic control of pain. Supplemental heat, extra cage padding, dimmed lighting, and the provision of highly palatable food and liquids are common means of moderating postoperative distress.
A specific plan for the evaluation of pain and discomfort for animal subjects undergoing surgery must be included in the IACUC protocol. Postoperative observation and the determination of animal pain or distress require a good working knowledge of (1) the normal behavior and appearance of the individual animal being assessed and (2) the signs of pain or distress exhibited by the NHP species. Many institutions have incorporated pain scoring systems which assist in the identification of specific symptoms that, when observed, trigger an immediate request for veterinary support. The IACUC should carefully evaluate the qualifications of personnel responsible for postoperative assessments as well as the validity of any pain scoring systems they may use. In addition to the specific assessments being used, the time intervals during which recovery will be assessed must be specified in the protocol and subsequently documented in the surgical records. For a detailed discussion of analgesia in NHPs, see Murphy and colleagues (2012).
Endpoint Determination
It is ideal when scientific aims and objectives of a study can be accomplished without adverse effects, pain, or distress to the animals involved. However, this is not always possible, and the IACUC must carefully evaluate humane endpoints. Endpoints are criteria used to end experimental studies earlier (than intended) to avoid or terminate unrelieved pain and/or distress (National Institutes of Health, Office of Laboratory Animal Welfare [NIH-OLAW] 2002b). Consideration must be given to the scientific requirements of the study, expected adverse effects the research animals may experience, the likely time course and progression of those adverse effects, and the earliest predictive indicators of present or impending adverse effects. The effective use of endpoints requires that properly trained and qualified individuals perform both general and study-specific observations of the animals at appropriate time intervals. The assessment criteria and required response must be clearly defined in each IACUC protocol. Ideally, humane endpoints are sought that can be used to end studies as close to the onset of pain or distress as is possible while meeting the justified scientific needs of the study. The attending veterinarian must contribute to establishment of endpoints as well as aid in their interpretation when questions arise.
As stated previously, it is important for principal investigators to clearly establish humane endpoints, not only for pain but also for distress. Such endpoints for distress should address how to recognize when a distress is unable to be alleviated. For animals that develop behavioral problems such as self-injurious behavior, factors that may be put into place include removal from the study, pharmacologic intervention, or euthanasia.
Occupational Safety
Public Health Service policy places responsibility for ensuring a safe working environment for personnel involved in the animal care and use program with the institution (NIH-OLAW 2002a). Several factors make working with NHPs in a research setting potentially dangerous. NHPs are not domesticated animals, and they are often aggressive. Consequently, personnel working with NHPs can suffer severe scratches and bites. NHPs also pose multiple serious zoonotic risks. Macaques, for example, carry Cercopithecine herpesvirus 1, which can cause fatal encephalitis in humans. Additionally, the research environment often includes needles, heavy equipment, anesthetic gases, biological agents, and radioactive elements. For these reasons, occupational safety is a critical concern when working with NHPs. The IACUC should consider the safety of personnel when evaluating experimental design and proposed activities.
Many separate institutional components, including animal care, environmental health and safety, occupational health, radiation safety, and institutional biosafety, are involved in ensuring occupational safety. A natural point of convergence for these functionally distinct institutional elements is the IACUC. For this reason, the IACUC should be familiar with the responsibilities of the various safety committees and organizations at their institution. The institution should ensure that the functions of the committees are coordinated. The NRC (1997) publication Occupational Health and Safety in the Care and Use of Research Animals covers a wide variety of occupational health and safety issues and is a valuable resource for IACUC members.
Conclusions
As large, intelligent, social, long-lived, and non domesticated animals, monkeys are among the most challenging species used in biomedical research, and the duties of the IACUC in relation to reviewing research use of these species can also be challenging. Clear institutional policies and procedures regarding scientific justification, re use, social housing requirements, amelioration of distress, surgical procedures, and humane endpoints are critical. The discussion of these issues presented here can serve as a basis for the informed establishment of such policies and procedures.
Acknowledgments
The professional expertise used to prepare this manuscript was developed at and supported by NIH grants P51OD011133 to Texas Biomedical Research Institute/Southwest National Primate Research Center (Tardif and Lutz) and P51OD011092 to the Oregon Health Sciences University/Oregon National Primate Research Center (Coleman and Hobbs).
References
- Abney DM, Poor LL, Reuther KJ. Socialization of adult male cynomolgus macaques: benefits vs. costs. Am J Primatol. 2011;73 41. [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, Schoof V, Maloney M. Benefits of pair housing are consistent across a diverse population of rhesus macaques. Appl Anim Behav Sci. 2012;137:148–156. doi: 10.1016/j.applanim.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Crockett CM, Lee GH, Oettinger BC, Schoof V, Thom JP. Pair housing for female longtailed and rhesus macaques in the laboratory: Behavior in protected contact versus full contact. J Appl Anim Welf Sci. 2012;15:126–143. doi: 10.1080/10888705.2012.658330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayne K, Dexter S, Suomi S. A preliminary survey of the incidence of abnormal behavior in rhesus monkeys (Macaca mulatta) relative to housing condition. Lab Anim Sci. 1992;21:38–46. [Google Scholar]
- Bayne K, Morris TH. Laws, regulations and policies relating to the care and use of nonhuman primates in biomedical research. In: Abee CR, Mansfield K, Tardif S, Morris T, editors. Nonhuman Primates in Biomedical Research. Vol. 1. London: Elsevier, p; 2012. pp. 35–56. [Google Scholar]
- Bellanca RU, Crockett CM. Factors predicting increased incidence of abnormal behavior in male pigtailed macaques. Am J Primatol. 2002;58:57–69. doi: 10.1002/ajp.10052. [DOI] [PubMed] [Google Scholar]
- Bernstein IS. Social housing of monkeys and apes: Group formations. Lab Anim Sci. 1991;41:329–333. [PubMed] [Google Scholar]
- Bolker J. There's more to life than rats and flies. Nature. 2012;491:31–33. doi: 10.1038/491031a. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Bellanca RU, Bowers CL, Bowden DM. Grooming-contact bars provide social contact for individually caged laboratory macaques. Contemp Top Lab Anim Sci. 1997;36:53–60. [PubMed] [Google Scholar]
- Crockett CM, Bowers CL, Bowden DM, Sackett GP. Sex differences in compatibility of pair-housed adult longtailed macaques. Am J Primatol. 1994;32:73–94. doi: 10.1002/ajp.1350320202. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Shimoji M, Bowden DM. Behavior, appetite, and urinary cortisol responses by adult female pigtailed macaques to cage size, cage level, room change, and ketamine sedation. Am J Primatol. 2000;52:63–80. doi: 10.1002/1098-2345(200010)52:2<63::AID-AJP1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Doyle LA, Baker KC, Cox LD. Physiological and behavioral effects of social introduction on adult male rhesus macaques. Am J Primatol. 2008;70:542–550. doi: 10.1002/ajp.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton GG, Kelley ST, Axthelm MK, Iliff-Sizemore SA, Shiigi SM. Psychological well-being in paired adult female rhesus (Macaca mulatta) Am J Primatol. 1994;33:89–99. doi: 10.1002/ajp.1350330204. [DOI] [PubMed] [Google Scholar]
- Erwin J. Factors influencing aggressive behavior and risk of trauma in the pigtail macaque (Macaca nemestrina) Lab Anim Sci. 1977;27:541–547. [PubMed] [Google Scholar]
- European Parliament. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. 2010 [Google Scholar]
- Gilbert MH, Baker KC. Social buffering in adult male rhesus macaques (Macaca mulatta): Effects of stressful events in single vs. pair housing. J Med Primatol. 2011;40:71–78. doi: 10.1111/j.1600-0684.2010.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. The effect of rearing conditions on behavior. Bull Menninger Clin. 1962;26:213–224. [PubMed] [Google Scholar]
- Hotchkiss CE, Paule MG. Effect of pair-housing on operant behavior task performance by rhesus monkeys. Contemp Top Lab Anim Sci. 2003;42:38–41. [PubMed] [Google Scholar]
- Judge PG, deWaal FBM, Paul KS, Gordon TP. Removal of a trauma-inflicting alpha matriline from a group of rhesus macaques to control severe wounding. Lab Anim Sci. 1994;44:344–350. [PubMed] [Google Scholar]
- Kessel A, Brent L. The rehabilitation of captive baboons. J Med Primatol. 2001;30:71–80. doi: 10.1034/j.1600-0684.2001.300201.x. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, Buwalda B. Stress and adaptation. Toward ecologically relevant animal models. Curr Dir Psychol Sci. 2006;15:109–112. [Google Scholar]
- Laule GE, Bloomsmith MA, Schapiro SJ. The use of positive reinforcement training techniques to enhance the care, management, and welfare of primates in the laboratory. J Appl Anim Welf Sci. 2003;6:163–173. doi: 10.1207/S15327604JAWS0603_02. [DOI] [PubMed] [Google Scholar]
- Lee GH, Thom JP, Chu KL, Crockett CM. Comparing the relative benefits of grooming-contact and full-contact pairing for laboratory-housed adult female Macaca fascicularis. Appl Anim Behav Sci. 2012;137:157–165. doi: 10.1016/j.applanim.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. Am J Primatol. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Novak MA. Environmental enrichment for nonhuman primates: Theory and application. ILAR J. 2005;46:178–191. doi: 10.1093/ilar.46.2.178. [DOI] [PubMed] [Google Scholar]
- Mason WA, Sponholz RR. Behavior of rhesus monkeys raised in isolation. J Psych Res. 1963;1:299–306. doi: 10.1016/0022-3956(63)90005-0. [DOI] [PubMed] [Google Scholar]
- Majolo B, Buchanan-Smith HM, Morris K. Factors affecting the successful pairing of unfamiliar common marmoset (Callithrix jacchus) females: The preliminary results. Anim Welf. 2003;12:327–337. [Google Scholar]
- McGuffey LH, McCully CL, Bernacky BJ, Blaney SM. Incorporation of an enrichment program into a study protocol involving long-term restraint in macaques. Lab Anim. 2002;31:37–39. doi: 10.1038/5000198. [DOI] [PubMed] [Google Scholar]
- Murphy KL, Baxter MG, Flecknell PA. Anesthesia and analgesia in nonhuman primates. In: Abee CR, Mansfield K, Tardif S, Morris T, editors. Nonhuman Primates in Biomedical Research. Vol. 1. London: Elsevier, p; 2012. pp. 403–436. [Google Scholar]
- Napier JR, Napier PH. Cambridge MA: MIT Press; 1994. The Natural History of the Primates. [Google Scholar]
- [NIH-OLAW] National Institutes of Health, Office of Laboratory Animal Welfare. 2nd ed. Bethesda MD: National Institutes of Health; 2002a. Institutional Animal Care and Use Committee Guidebook. [Google Scholar]
- NIH-OLAW. Bethesda MD: National Institutes of Health; 2002b. Public Health Service Policy on Humane Care and Use of Laboratory Animals. [Google Scholar]
- [NRC] National Research Council. 8th ed. Washington: National Academies Press; 2011. Guide for the Care and Use of Laboratory Animals. [PubMed] [Google Scholar]
- NRC. Washington: National Academy Press; 1997. Occupational Health and Safety in the Care and Use of Research Animals. [Google Scholar]
- NRC. Washington: National Academic Press; 2008. Recognition and Alleviation of Distress in Laboratory Animals. [PubMed] [Google Scholar]
- Novak MA, Suomi SJ. Social interaction in nonhuman primates: an underlying theme for primate research. Lab Anim Sci. 1991;41:308–314. [PubMed] [Google Scholar]
- Oates-O'Brien RS, Farver TB, Anderson-Vicino KC, McCowan B, Lerche NW. Predictors of matrilineal overthrows in large captive breeding groups of rhesus macaques (Macaca mulatta) JAALAS. 2010;49:196–201. [PMC free article] [PubMed] [Google Scholar]
- Reinhardt V. Time budget of caged rhesus monkeys exposed to a companion, a PVC perch, and a piece of wood for an extended time. Am J Primatol. 1990;20:51–56. doi: 10.1002/ajp.1350200108. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Pair-housing overcomes self-biting behavior in macaques. Lab Primate Newsletter. 1999;38:4–5. [Google Scholar]
- Reinhardt V. Addressing the social needs of macaques used for research. Lab Primate Newsletter. 2002;41:7–10. [Google Scholar]
- Reinhardt V, Liss C, Stevens C. Social housing of previously single-caged macaques: What are the options and the risks? Anim Welf. 1995;4:307–328. [Google Scholar]
- Roberts SJ, Platt ML. Effects of isosexual pair-housing on biomedical implants and study participation in male macaques. Contemp Top Lab Anim Sci. 2005;44:13–18. [PubMed] [Google Scholar]
- Russell WMS, Burch RL. London: Methuen; 1959. The Principles of Humane Experimental Technique. [Google Scholar]
- Sackett GP. Some persistent effects of different rearing conditions on preadult social behavior of monkeys. J Comp Physiol Psych. 1967;64:363–365. doi: 10.1037/h0088031. [DOI] [PubMed] [Google Scholar]
- Samuels A, Henrickson RV. Outbreak of severe aggression in captive Macaca mulatta. Am J Primatol. 1983;5:277–281. doi: 10.1002/ajp.1350050314. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA. Behavioral effects of enrichment on pair-housed juvenile rhesus monkeys. Am J Primatol. 1994;32:159–170. doi: 10.1002/ajp.1350320302. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Suarez SA, Porter LM. Effects of social and inanimate enrichment on the behavior of yearling rhesus monkeys. Am J Primatol. 1996;40:247–260. doi: 10.1002/(SICI)1098-2345(1996)40:3<247::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bushong D. Effects of enrichment on veterinary treatment of laboratory rhesus macaques (Macaca mulatta) Anim Welf. 1994;3:25–36. [Google Scholar]
- Schapiro SJ, Nehete PN, Perlman JE, Sastry KJ. A comparison of cell-mediated immune responses in rhesus macaques housed singly, in pairs, or in groups. Appl Anim Behav Sci. 2000;68:67–84. doi: 10.1016/s0168-1591(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Perlman JE, Thiele E, Lambeth S. Training nonhuman primates to perform behaviors useful in biomedical research. Lab Anim (NY) 2005;34:37–42. doi: 10.1038/laban0505-37. [DOI] [PubMed] [Google Scholar]
- Schlabritz-Loutsevitch NE, Howell K, Rice K, Glover EJ, Nevill CH, Jenkins SL, Cummins LB, Frost PA, McDonald TJ, Nathanielsz PW. Development of a system for individual feeding of baboons maintained in an outdoor group social environment. J Med Primatol. 2004;33:117–126. doi: 10.1111/j.1600-0684.2004.00067.x. [DOI] [PubMed] [Google Scholar]
- Seok J., Warren HS, Cuenca AG, Mindrinos MN, Baker HV, Xu W, Richards DR, McDonald-Smith GP, Gao H, Hennessy L, Finnerty CC, López CM, Honari S, Moore EE, Minei JP, Cuschieri J, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Jeschke MG, Klein MB, Gamelli RL, Gibran NS, Brownstein BH, Miller-Graziano C, Calvano SE, Mason PH, Cobb JP, Rhame LG, Lowry SF, Maier RV, Moldawer LL, Herndon DN, Davis RW, Xiao W, Tompkins RG. Genomic responses in mouse models poorly mimic human inflammatory diseases. PNAS. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TE, French JA. Social and reproductive condition modulates urinary cortisol excretion in black tufted-ear marmosets (Callithrix kuhli) Am J Primatol. 1997;42:253–268. doi: 10.1002/(SICI)1098-2345(1997)42:4<253::AID-AJP1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Spring SE, Clifford JO, Tomko DL. Effect of environmental enrichment devices on behaviors of single- and group-housed squirrel monkeys (Saimiri sciureus) Contemp Top Anim Sci. 1997;36:72–75. [PubMed] [Google Scholar]
- Vandeleest JJ, McCowan B, Capitanio JP. Early rearing interacts with temperament and housing to influence the risk for motor stereotypy in rhesus monkeys (Macaca mulatta) Appl Anim Sci Behav. 2011;132:81–89. doi: 10.1016/j.applanim.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed JL, Wagner PO, Byrum R, Parrish S, Knezevich M, Powell DA. Treatment of persistent self-injurious behavior in rhesus monkeys through socialization: A preliminary report. Contemp Top Anim Sci. 2003;42:21–23. [PubMed] [Google Scholar]
- Westergaard GC, Izard MK, Drake JH, Suomi SJ, Higley JD. Rhesus macaque (Macaca mulatta) group formation and housing: Wounding and reproduction in a specific pathogen free (SPF) colony. Am J Primatol. 1999;49:339–347. doi: 10.1002/(SICI)1098-2345(199912)49:4<339::AID-AJP4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: Social status effects on caloric consumption. Physiol Behav. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanashi Y, Matsuzawa T. Emotional consequences when chimpanzees (Pan troglodytes) face challenges: Individual differences in self-directed behaviours during cognitive tasks. Anim Welf. 2010;19:25–30. [Google Scholar]


