Abstract
Chlorophyll breakdown is a visual phenomenon of leaf senescence and fruit ripening. It leads to the formation of colorless chlorophyll catabolites, a group of (chlorophyll-derived bilin-type) linear tetrapyrroles. Here, analysis and structure elucidation of the chlorophyll breakdown products in leaves of banana (Musa acuminata) is reported. In senescent leaves of this monocot all chlorophyll catabolites identified were hypermodified fluorescent chlorophyll catabolites (hmFCCs). Surprisingly, nonfluorescent chlorophyll catabolites (NCCs) were not found, the often abundant and apparently typical final chlorophyll breakdown products in senescent leaves. As a rule, FCCs exist only fleetingly, and they isomerize rapidly to NCCs in the senescent plant cell. Amazingly, in the leaves of banana plants, persistent hmFCCs were identified that accounted for about 80 % of the chlorophyll broken down, and yellow leaves of M. acuminata display a strong blue luminescence. The structures of eight hmFCCs from banana leaves were analyzed by spectroscopic means. The massive accumulation of the hmFCCs in banana leaves, and their functional group characteristics, indicate a chlorophyll breakdown path, the downstream transformations of which are entirely reprogrammed towards the generation of persistent and blue fluorescent FCCs. As expressed earlier in related studies, the present findings call for attention, as to still elusive biological roles of these linear tetrapyrroles.
Keywords: biosynthesis, chlorophyll, fluorescence, structures, tetrapyrroles
Dedicated to Professor Franz-Peter Montforts on the occasion of his 65th birthday
Introduction
Breakdown of chlorophyll, the very visible sign of leaf senescence[1] and fruit ripening,[2] still was a striking enigma about 25 years ago.[3] In the meantime, the structures of about two dozens of chlorophyll catabolites from higher plants have been elucidated[4–6] and several key enzymes have been identified, providing general insights into the basic processes involved in chlorophyll breakdown.[7, 8] Thus, the pathway of chlorophyll degradation in higher plants comprises early steps that take place in the chloroplasts[8] and lead to fluorescent chlorophyll catabolites (FCCs).[9] In the later stages of chlorophyll breakdown, which are associated with enzyme activities in the cytosol and with the vacuoles,[8] FCCs are converted into colorless and nonfluorescent degradation products.[10] The so called nonfluorescent chlorophyll catabolites (NCCs)[11] are the most prominent among the latter breakdown products. These colorless linear tetrapyrroles arise from FCCs by a spontaneous, acid-catalyzed isomerization[12, 13] and are essentially photo-inactive in day light.
The now known FCCs and NCCs from higher plants are bilin-type linear tetrapyrroles that reflect an oxygenolytic opening of the macrocycle of chlorophyll a at the northern α-meso-position, with retention of the meso carbon as a formyl group.[4, 5] These chlorophyll catabolites may be classified as formyloxobilins,[5, 10] as—except for their additional and characteristic substituted cyclopentanone ring—they display similar structural features as the heme-derived (dioxo)bilins.[14, 15] In most senescent leaves studied, NCCs have been found to accumulate.[4, 16] In addition, dioxobilin-type nonfluorescent chlorophyll catabolites (DNCCs) have been identified in some leaves,[8, 17] that is, linear tetrapyrroles that have lost their formyl group[17–19] (Figure 1). The natural dioxobilin-type chlorophyll catabolites resemble heme catabolites in higher plants remarkably closely.[18]
Figure 1.
Abbreviated structural outline of chlorophyll breakdown in senescent leaves and ripening fruits.[5] Chlorophylls (Chl a and b) are degraded to primary fluorescent chlorophyll catabolites (pFCC, or its C-1 epimer, epi-pFCC).[9, 24] FCCs with free propionic acid groups isomerize spontaneously by an acid-catalyzed reaction to the corresponding nonfluorescent chlorophyll catabolites (NCCs),[12] such as Hv-NCC-1.[4, 25] FCCs esterfied at the propionic acid group are persistent, such as Mc-FCC-56, a hypermodified FCC (hmFCC) in peels of ripe banana.[20–22] In an alternative path, dioxobilin-type nonfluorescent chlorophyll catabolites result from deformylation at ring B, such as Ap-DNCC from senescent leaves of Norway maple.[18, 26]
Peels of ripening bananas (Musa acuminata, Cavendish cultivar) contain a stunning variety of colorless chlorophyll catabolites, among them, blue luminescent hypermodified FCCs (hmFCCs).[20–22] These FCCs were found to be responsible for the surprising blue fluorescence of ripe(ning) bananas. hmFCCs feature complex ester functions at their propionyl substituent that inhibit the rapid FCC to NCC isomerization, and which therefore make hmFCCs persistent.[13, 21] In related exploratory studies, senescent leaves of banana plants were also investigated, and a structurally different hmFCC was found to accumulate, among other, still uncharacterized FCCs.[23] As reported here, we now have analyzed the major chlorophyll catabolites in such leaves in order to elucidate their structures and to help resolve the puzzle of their accumulation.
Results
Chlorophyll catabolites in senescent banana leaves: In freshly prepared extracts of yellow senescent leaves from bananas (Musa acuminata, Cavendish cultivar, short Ma) about a dozen of blue fluorescent fractions were detected by high performance liquid chromatography (HPLC), all of which were tentatively identified as fluorescent chlorophyll catabolites (FCCs) on the basis of their fluorescence and their absorbance characteristics.[11] Surprisingly, nonfluorescent chlorophyll catabolites (NCCs), the typically more abundant chlorophyll catabolites in leaves,[6] could not be detected in fresh extracts of yellow banana leaves (Figure 2 and the Experimental Section).
Figure 2.
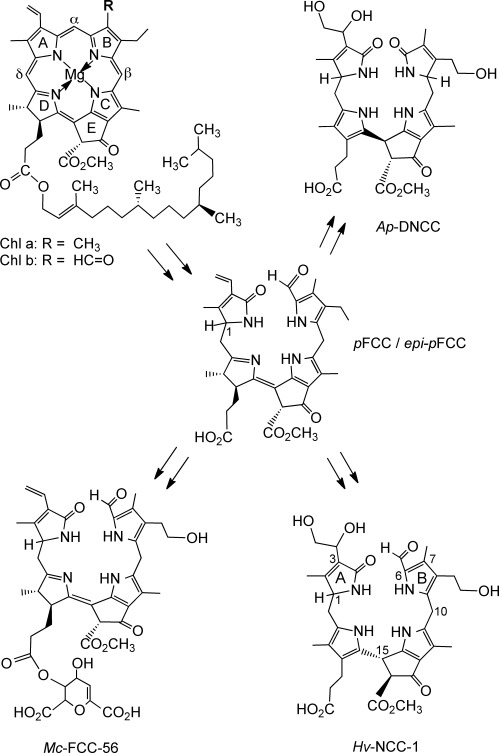
HPLC analysis of chlorophyll catabolites in an extract of yellow senescent banana leaves (Musa acuminata, Cavendish cultivar). The chromatogram was recorded with online detection of absorbance at 320 nm (lower trace) and fluorescence emission at 450 nm (upper trace, excitation at 350 nm). Fractions classified as fluorescent chlorophyll catabolites (FCCs) are highlighted and indexed according to the retention times (tR) observed under conditions of a standard analytical HPLC experiment.[20, 23] FCCs from banana leaves were thus named Ma-FCC-tR.
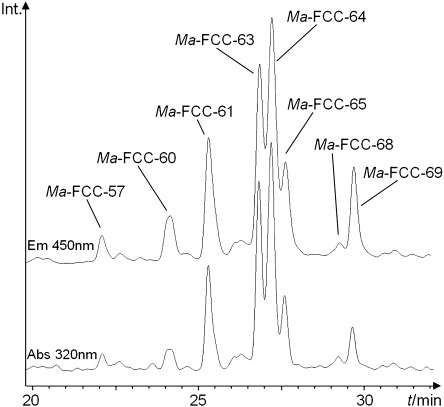
Quantification of tetrapyrroles in senescent banana leaves: In M. acuminata leaves, freshly harvested at different stages of senescence, the amounts of chlorophylls (a and b) and of fluorescent chlorophyll catabolites (FCCs) were determined quantitatively (Figure 3). For this purpose, green, greenish-yellow, yellow-greenish, shiny yellow and yellow-brownish areas were cut out from different banana leaves and extracted with methanol. Quantitative UV/Vis spectroscopic measurements of the filtered extracts of green leaves and analysis for their chlorophyll content[27] indicated the leaves to contain 61.3(±5.3) nmol cm−2 of chlorophyll a and b (n=3).
Figure 3.
Quantification of chlorophylls and Ma-FCCs in green and in senescent banana leaves. The total amounts of chlorophylls a and b, and of FCCs are depicted as a function of the degree of senescence of the leaf samples (analysis of extracts from three samples with similar extent of yellowing). The chlorophyll content was calculated from UV/Vis spectra,[27] the amount of FCCs from FCC fractions isolated raw by semipreparative HPLC (data are normalized with respect to the chlorophyll content of green leaves).
Likewise, the overall amounts of FCCs were determined by analyzing the complete set of fractions containing FCCs by semipreparative HPLC and quantification of their FCC content by UV/Vis spectroscopy (see below and ref. [11]). Yellow senescent banana leaves were found to contain 47.2(±3.1) nmol cm−2 of FCCs (and still about 0.9(±0.5) nmol cm−2 of chlorophylls). These data indicated conversion of chlorophyll a and b to FCCs of at least 77 % and a total recovery of chlorophyll catabolites in apparently viable (shiny) yellow senescent leaves of bananas of about 80 %. In such yellow leaves the FCCs observed clearly accounted for the major part of the chlorophylls broken down during senescence, and NCCs were not detected.
Identification of major FCC fractions in extracts of senescent banana leaves: From the extracts of 60 g of yellow M. acuminata leaves the fractions of the most abundant four FCCs were separated by preparative and semipreparative HPLC and used for further spectroscopic characterization (see the Experimental Section). Cooling with liquid nitrogen during the extraction procedure was used to prevent eventual further reactions of the FCCs.[28] NCCs and yellow chlorophyll catabolites (YCCs)[29, 30] were not observed in these extracts.
The FCC fraction with a retention time of 25.6 min (under our conditions of the HPLC-experiment; Figure 2) was identified with the previously described Ma-FCC-61,[23] first by mass spectrometry (molecular formula C50H66N4O20) and then by one- and two-dimensional NMR spectroscopy studies. The new data confirmed the earlier deduced structure of this FCC as a 31,32-didehydro-82-hydroxy-132-(methoxycarbonyl)-173-[6′-α-galactopyranosyl-(1′→6′′)-β-galactopyranosyl-(1′′→1′′′)-glyceryl)]-1,4,5,10,17,18,20,22-octahydro-4,5-seco-(22H)-phytoporphyrin. From 60 g of the yellow banana leaves 2.35 mg (2.26 μmol) of Ma-FCC-61 were obtained as a dry white powder. The UV/Vis spectroscopic characteristics of Ma-FCC-61 were determined quantitatively, and were consistent with data for the related hmFCC, named Mc-FCC-56.[20]
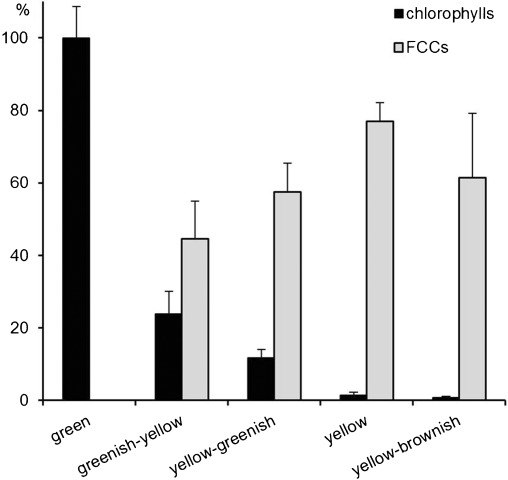
The two slightly less polar FCCs, Ma-FCC-63 and Ma-FCC-64, could be separated (in part) by preparative HPLC in MeOH/H2O (see the Experimental Section). The first separation was incomplete due to partial interconversion of these isomeric hmFCCs (see below). For the second, final purification step corresponding precautions were taken to prevent isomerization and possible transesterification (with methanol) by changing the solvent system to ACN/H2O and by running HPLC with shorter on-column times. The FCC containing fractions were directly frozen in liquid nitrogen, stored at −80 °C to avoid isomerization. The samples were lyophilized, to yield 0.79 mg (0.98 μmol) of analytical pure Ma-FCC-63, and 0.63 mg (0.77 μmol) of Ma-FCC-64, to be used for spectroscopic analyses. The UV/Vis spectra of the two FCCs as well as their CD spectra showed considerable similarities; these spectra were also comparable to those of Ma-FCC-69 (Figure 4).
Figure 4.
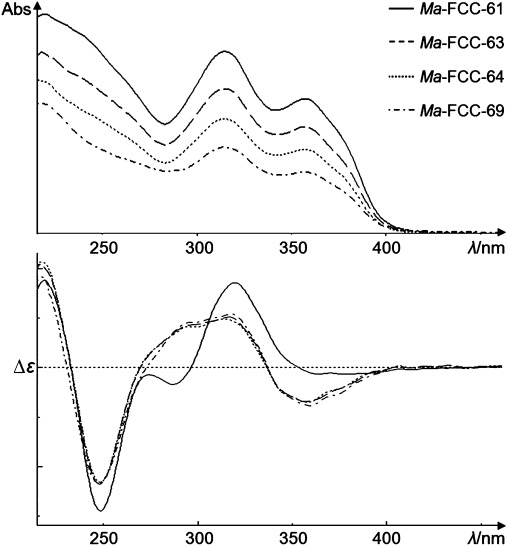
The four major FCCs in senescent banana leaves, Ma-FCC-61, Ma-FCC-63, Ma-FCC-64 and Ma-FCC-69, are hmFCCs and have similar UV/Vis spectra (top). The CD-spectra (bottom) of the glucosyl-carrying FCCs are similar, the digalactosyl-substituted Ma-FCC-61 deviates slightly; spectra of FCCs in MeOH (see the Experimental Section for details).
ESI-MS spectra of both, Ma-FCC-63 and Ma-FCC-64, indicated pseudo-molecular ions [M+H]+ at m/z 807.2, consistent with a (common) molecular formula of C41H50N4O13. Loss of up to three water molecules was observed at m/z 789.3, 771.3 and 753.3. A fragment at m/z 654.3 indicated loss of ring B. 1H NMR spectra (600 MHz) of Ma-FCC-63, as well as of Ma-FCC-64, in CD3CN/D2O 9:1 (v/v) at 10 °C showed each a set of the characteristic signals of the tetrapyrrole moiety, among them signals at low field due to a formyl and a vinyl group, three singlets and one doublet of four methyl groups at high field, as well as a sharp singlet of the methyl ester group at 3.65 ppm (Figure 5).
Figure 5.
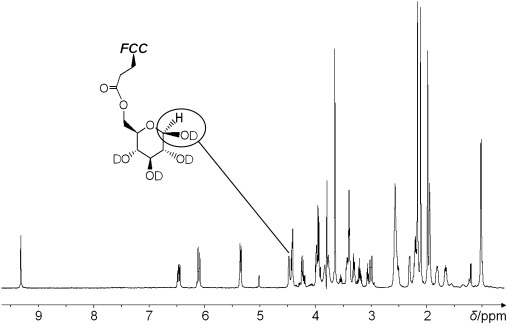
600 MHz 1H NMR spectrum of Ma-FCC-63 in CD3CN/D2O 9:1 (v/v) at 10 °C. The signal of the α-H-atom at the anomeric center is highlighted.
Detailed information of the constitution of Ma-FCC-63 and Ma-FCC-64 was gained from multidimensional NMR spectroscopy (1H,1H COSY and ROESY spectra as well as 1H,13C HSQC and HMBC spectra).[31, 32] For both hmFCCs the signals of all 35 non-exchangeable protons and of 34 carbons of the core tetrapyrrole moiety could be assigned, establishing them to be functionalized FCCs (see the Experimental Section). Additional signals of seven hydrogen atoms were observed in the intermediate field region of the 1H NMR spectra and strong couplings in 1H,1H COSY and correlations to six carbons in 1H,13C HSQC indicated a hexo-pyranose unit in both FCCs. A 1H,13C HMBC correlation in the spectra of both FCCs between the carbonyl carbon at 173 and the 6′-hydrogen atoms of the sugar moiety identified the attachment site of the glucose unit to be the propionyl side chain. A doublet at 4.42 ppm (J=7.9 Hz) in the spectrum of Ma-FCC-63, and at 5.01 ppm (J=3.6 Hz) in the spectrum of Ma-FCC-64, were assigned to the H atom at C-1′ of the sugar moieties of these hmFCCs, suggesting them to represent two (slowly equilibrating) anomers.
The sugar moieties of Ma-FCC-63 and Ma-FCC-64 were further characterized as glucopyranose units, based on analysis of the values of the 1H,1H coupling constants within the two pyranose units, NOE correlations from 1H,1H ROESY spectra and of the 1H and 13C chemical shift data (Figure 6), and comparison with those of reference compounds.[33–36] Indeed, these two (slowly interconverting) FCCs were thus deduced to differ only by the stereochemistry at their anomeric centers. Accordingly, Ma-FCC-63 was identified as a 31,32-didehydro-82-hydroxy-132-(methoxycarbonyl)-173-(6′-β-glucopyranosyl)-1,4,5,10,17,18,20,22-octahydro-4,5-seco-(22H)-phytoporphyrin, and Ma-FCC-64 as the 173-(6′-α-glucopyranosyl) anomer.
Figure 6.
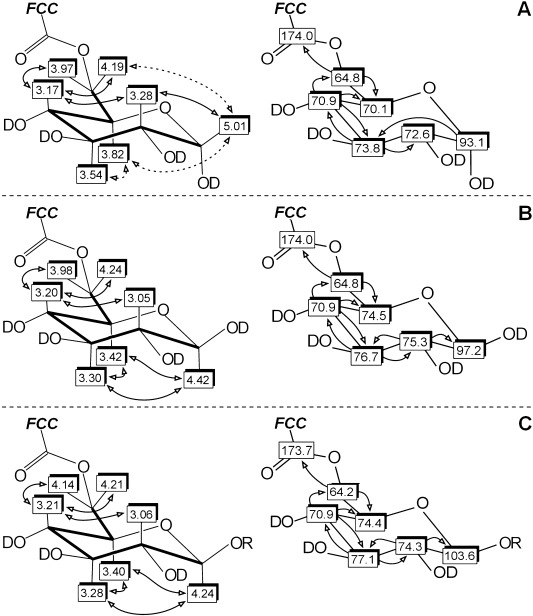
NMR analysis of the sugar ester moieties at the propionic acid side chain of: A) Ma-FCC-64, B) Ma-FCC-63, and C) Ma-FCC-69 (R=(3,4-dihydroxyphenyl)-ethyl group) based on 2D correlations (600 MHz, CD3CN/D2O 9:1 (v/v) or CD3CN (Ma-FCC-69), 10 °C). Left: Graphical representations of assigned 1H NMR signals and of homonuclear 1H,1H correlations: bold lines refer to COSY spectra, arrows to ROESY spectra. Right: Assigned signals of 13C atoms from heteronuclear 1H,13C correlations; shadowed boxes indicate 13C assignments from direct correlations (HSQC spectra), arrows point to 13C assignments from long-range couplings with H atoms, as seen in HMBC spectra.
Indeed, when samples of both isomers were stored in a 1:1 (v/v) mixture of methanol and water under argon atmosphere at room temperature in the dark, analysis by HPLC indicated complete equilibration to a 1:1 mixture after approximately 24 h in both cases (see Figure 7). Thus, the equilibration of the two FCCs occurred at a rate comparable to that of the free anomeric glycopyranoses in aqueous solution.[36]
Figure 7.
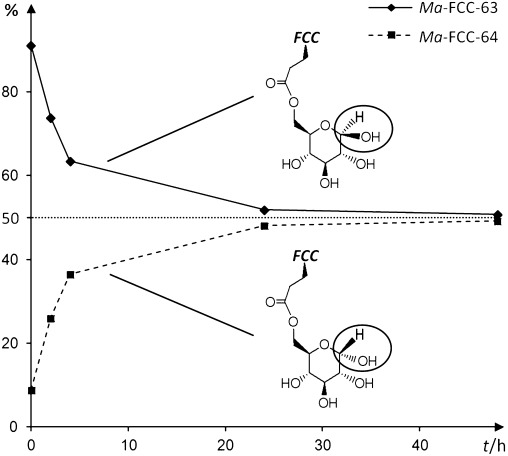
Anomerization of Ma-FCC-63 to Ma-FCC-64 in MeOH/H2O at room temperature under argon in the dark. The relative amounts of both FCCs were calculated from HPLC peak heights (detection at 320 nm).
The UV/Vis and CD spectra of the less polar FCC, named Ma-FCC-69, were similar to those of the more polar analogues (Figure 4). Its ESI-MS showed a signal for the pseudo-molecular ion, [M+H]+, at m/z 943.1, indicating a molecular formula of C49H58N4O15. A fragment at m/z 789.2 suggested the loss of a C8H10O3 unit (consistent with dihydroxyphenyl ethanol). Having in hand 0.55 mg (0.59 μmol) of analytical pure Ma-FCC-69, the constitution of Ma-FCC-69 could be determined by homo- and heteronuclear NMR spectroscopy experiments (Figure 8).
Figure 8.
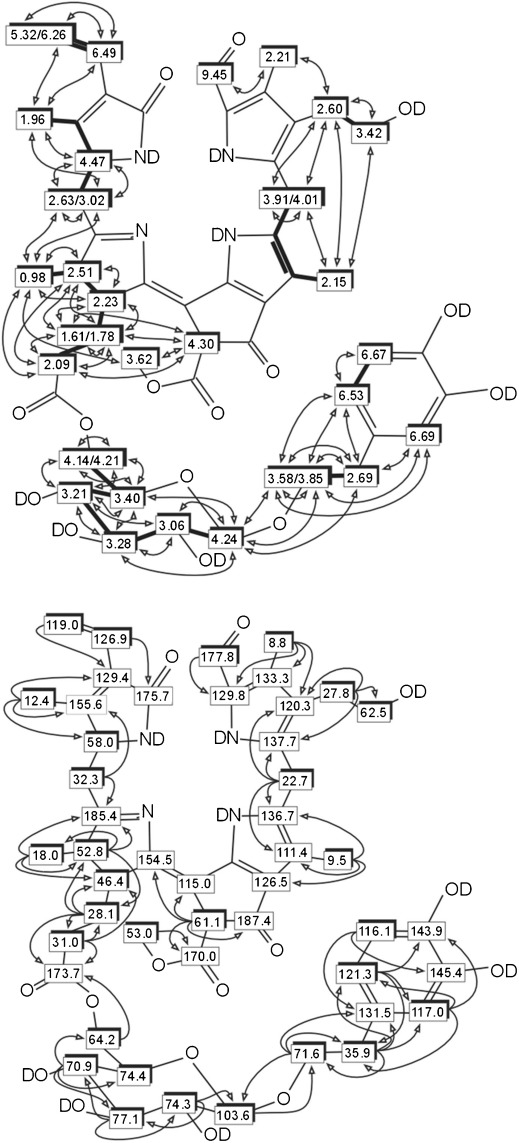
2D NMR derived molecular constitution of Ma-FCC-69. Top: graphical representations of homonuclear 1H,1H correlations: bold lines represent correlations from COSY, arrows represent correlations from ROESY. Bottom: heteronuclear 1H,13C correlations: shadowed boxes indicate assignments from direct correlations obtained from HSQC spectra, arrows indicate long-range couplings from HMBC spectra.
The signals of the 35 non-exchangeable protons of the tetrapyrrole core structure could be assigned in the same way as described above (see also the Experimental Section).[27] The 600 MHz 1H NMR spectrum of Ma-FCC-69 in CD3CN showed the signals of seven additional protons in the intermediate field range, consistent with a hexopyranosyl group. The heteronuclear correlation between the 6′-methylene protons of the sugar unit and the carbonyl carbon of the propionate side chain (in an 1H,13C HMBC spectrum) indicated an ester linkage, that is, similar to that in Ma-FCC-63. The sugar moiety was identified as a β-glycopyranosyl unit by analysis of the coupling pattern in 1H,1H COSY and ROESY NMR spectra, as well as by correlations to six carbons in the 1H,13C HSQC and HMBC spectra. 1H,1H coupling constants together with 1H and 13C shifts in comparison to reference compounds provided further support for a glucopyranosyl moiety.[34] Three additional signals in the 1H NMR spectrum showed 1H,1H ROESY correlations to the hydrogen at the anomeric center, supported by a heteronuclear correlation between the 1′-hydrogen of the pyranose and carbon 1′′ of the (unknown) aglycon moiety. The latter was identified as a 2-(3,4-dihydroxyphenyl)-ethyl group, and Ma-FCC-69 was thus a 31,32-didehydro-82-hydroxy-132-(methoxycarbonyl)-173-[6′-β-glucopyranosyl-(1′→1′′)-(2-[3,4-dihydroxyphenyl]-ethyl)]-1,4,5,10,17,18,20,22-octahydro-4,5-seco-(22H)-phytoporphyrin. Interestingly, a chlorophyll catabolite with the same molecular constitution is already known as an FCC in senescent leaves of the Peace Lily, Spathiphyllum wallisii (Sw).[37]
Comparison of Ma-FCC-69 with Sw-FCC-62, an hmFCC from senescent leaves of the Peace Lily: Ma-FCC-69 was deduced to have the same molecular constitution as Sw-FCC-62, isolated from senescent leaves of the tropical evergreen S. wallisii.[37] To test the eventual identity of the two hmFCCs, their elution properties were compared in HPLC experiments. Solutions of Ma-FCC-69 and Sw-FCC-62 in MeOH/H2O were separately analyzed by HPLC, and as a 1:1 mixture of both in a co-injection experiment (see Figure 9). Ma-FCC-69 was found to elute later in a reversed-phase system than Sw-FCC-62, and the two FCCs were shown to be non-identical. Their stereochemical difference was assigned to the configuration of the 1-position, which was indicated to be of the epi-type in catabolites from bananas, since it is of the normal-type in the leaves from the Peace Lily.[37]
Figure 9.
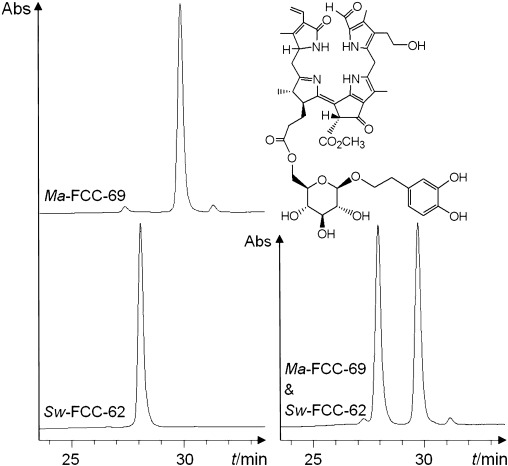
Separate analysis of Ma-FCC-69 and Sw-FCC-62 by HPLC (UV/Vis detection at 320 nm). Co-injection of a 1:1 mixture (right). HPLC analysis shows the two isomeric hmFCCs to differ.
Selected spectroscopic data of minor FCC fractions in extracts of senescent banana leaves: Four additional, minor FCC fractions were analyzed by HPLC and mass spectrometry only (see the Experimental Section). Three of them, tentatively named Ma-FCC-60, Ma-FCC-65 and Ma-FCC-68, showed [M+H]+ pseudo-molecular ion peaks at m/z 807.2, and therefore, are suggested to be isomers of Ma-FCC-63 and Ma-FCC-64. The ESI-MS spectrum of the more polar catabolite, Ma-FCC-57, was consistent with a molecular formula of C50H66N4O20, that is, as found for Ma-FCC-61.
Discussion
Nonfluorescent chlorophyll catabolites (NCCs) are a typical result of chlorophyll breakdown in senescent leaves.[5, 8, 38] A recent study with apples and pears also revealed the formation of NCCs in ripening fruit. These NCCs, furthermore, were the same as those in leaves of the corresponding fruit trees.[39] This suggested the existence of a common path of chlorophyll breakdown in leaf senescence and fruit ripening, that led to NCCs.[5, 39] The discovery of the striking accumulation of blue fluorescent chlorophyll catabolites (FCCs) in the peels of ripening bananas (Musa acuminata, Cavendish cultivar)[20, 21] stimulated us to also study the nature of the corresponding catabolites in senescent (yellow) banana leaves. A fluorescent chlorophyll catabolite was identified in banana leaves, which was non-identical to those from the banana fruit.[23]
In senescent M. acuminata leaves FCCs accumulate, and NCCs are not found: Indeed, as was shown here, the structures of the leaf hmFCCs differ characteristically from those in the peels of banana fruit, a result, apparently, from processes that occur at the stage of the FCCs. In senescent banana leaves, hypermodified FCCs (hmFCCs) accumulate and their amounts come up to as much as about 80 % of the degraded chlorophylls. Thus, hmFCCs are, by far, the major product from chlorophyll breakdown, and they induce senescent M. acuminata leaves to fluoresce blue (Figure 10). Remarkably, the formation of NCCs appears to be completely inhibited due to efficient esterification of FCCs, and formation of the persistent hmFCCs.
Figure 10.
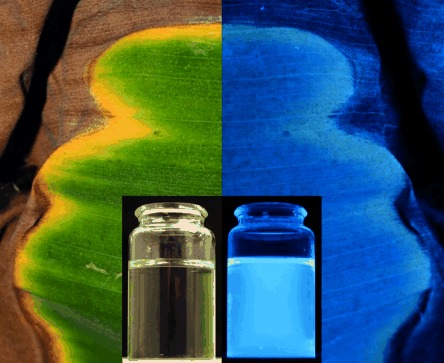
Photographs of a banana leaf with green and yellow sections, taken with a mounted camera, as well as of an FCC containing solution (insets), under day light (left) and UV light (at 366 nm, right).
The banana plant (M. acuminata) belongs to the Zingiberales, an order of monocotyledons that includes bananas, gingers, and their relatives.[40] In this (tropical) evergreen chlorophyll breakdown may have a major purpose other than (is suspected) in deciduous plants. This may be reflected by the striking accumulation of hmFCCs in senescent banana leaves, and their structural variety. A somewhat related situation was found recently in the senescent leaves of another tropical evergreen, the Peace Lily (S. wallisii), in which a persistent hmFCC accumulated (called Sw-FCC-62) besides lesser amounts of NCCs.[23, 37] In both of these monocotyls a particular hmFCC has now been identified (Sw-FCC-62 and Ma-FCC-69), in which the glucosyl moiety carries a dihydroxyphenylethyl aglycon. As was shown here, the hmFCCs from the two evergreens had the same molecular constitution, and still were non-identical. A stereochemical difference was indicated that could be assigned to the C-1 position. This stereodivergence is due to the action of two lines of (RCC) reductases during chlorophyll breakdown.[41] RCC reductase in banana leaves is thus of type-2, providing the epi-series of colorless chlorophyll catabolites, as likewise found in banana fruit.[28]
M. acuminata leaf FCCs are esterified with two types of hexopyranoses: In an exploratory earlier study of senescent (yellow) leaves of the banana plant an hmFCC was characterized, named Ma-FCC-61.[23] The persistent Ma-FCC-61 was esterified with a 6-α-galactopyranosyl-(1→6)-β-galactopyranosyl-(1→1)-glycerol moiety. In the present investigation, the structures of three slightly less polar and similarly abundant hmFCCs were determined, Ma-FCC-63, Ma-FCC-64 and Ma-FCC-69 (Figures 6 and 8), and they were compared with the structure of Ma-FCC-61 (Figure 11). In contrast to Ma-FCC-61, these three slightly less polar hmFCCs are all esterified by a glucopyranosyl group, attached at the critical propionate with its primary 6′-OH-group. Ma-FCC-63 and Ma-FCC-64 both contain a terminal glucopyranosyl moiety and undergo quick anomerization in aqueous solutions (Figure 7). Ma-FCC-69 carries a 3,4-dihydroxyphenyl-ethyl-aglycon at its glucopyranosyl ester moiety. In a formal sense, Ma-FCC-69 may arise from Ma-FCC-63/Ma-FCC-64 by covalent attachment of the (3,4-dihydroxyphenyl)-ethyl group. Therefore, Ma-FCC-63 (and its anomer Ma-FCC-64) can represent either a (remnant of a) potential precursor or a product of a partial degradation of Ma-FCC-69. Although the functional and biosynthetic interrelationship between these three Ma-FCCs is not clear at this stage, they are glucopyranosyl esters, indicated to represent a group separate from Ma-FCC-61, a digalactosyl derivative.
Figure 11.
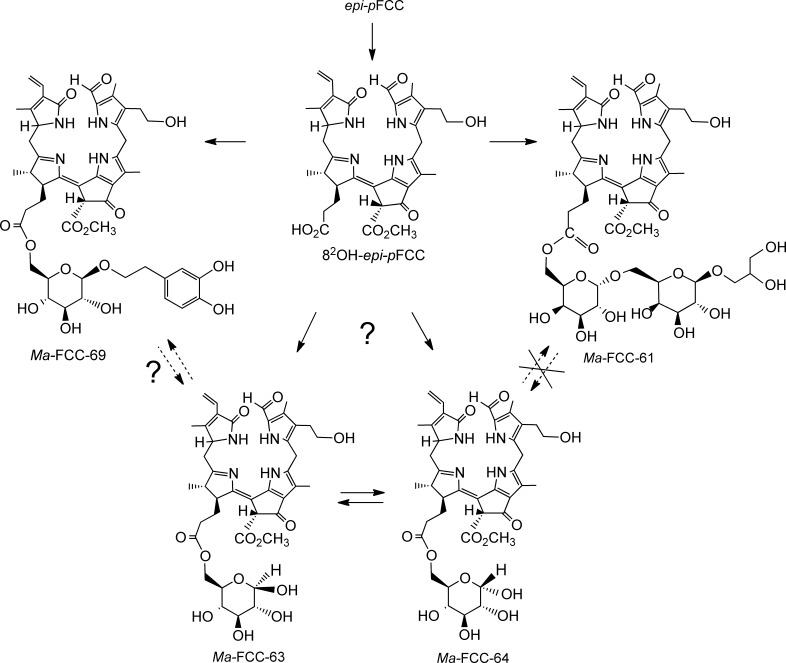
Structural outline of the proposed later part of chlorophyll degradation in senescent leaves of bananas, beginning with epi-pFCC, the C1 epimer of the primary FCC (pFCC).[2, 9, 13] Hydroxylation at the ethyl side chain of ring B leads to the secondary FCC (82-OH-epi-pFCC), which is proposed to be the common precursor of all downstream catabolites in banana leaves. Modifications forming complex propionate esters stop the common path and lead to hypermodified fluorescent chlorophyll catabolites (hmFCCs), such as Ma-FCC-61 and Ma-FCC-69. Possible cleavage at the anomeric center of the glucopyranosyl moiety of Ma-FCC-69 could give monoglycosylated Ma-FCC-63, which the other way around could be a potential precursor of Ma-FCC-69. As shown, isomerization of Ma-FCC-63 to its anomer Ma-FCC-64 and vice versa occurs in protic solutions.
The remarkable 6-α-galactopyranosyl-(1→6)-β-galactopyranosyl-(1→1)-glycerol moiety found in Ma-FCC-61 from senescent M. acuminata leaves relates chlorophyll catabolites with the ubiquitous membrane components of the thylakoids and elsewhere in plant leaves, which carry digalactosyl diacylglycerides as their polar head.[42] Ma-FCC-61 may thus be a building block for further assembly of more complex, so far unidentified tetrapyrrolic (bilin-type) pigments, or it could represent an adventitious cleavage product, carrying the polar remains of a membrane component.[42, 43] Increased lipophilic character (and membrane affinity) of hmFCCs in the banana leaves could be relevant for binding to cell membranes, and the intra- and intercellular transport through them.[44] In this context, the structure of the light-harvesting porphyrinoids (chlorophylls c) from the marine photoautotroph Emiliania huxleyi (a coccolithophore) is of interest, in which lipophilic digalactosyl diacylglyceride esters replace the phytol ester of the chlorophylls (apparently in a functional way).[43] Indeed, extracts of senescent banana leaves do contain several very minor fractions that have been tentatively identified as FCCs. These presumed FCCs are less polar even than Ma-FCC-69, and will be the subject of further studies to characterize their structures.
The strikingly different dihydroxyphenylethyl aglycon in Ma-FCC-69 appears to be among the typical constituents of low-molecular-weight natural products isolated from dicotyls, and suggested to be useful as taxonomic markers for this class of higher plants.[34] It was found in hmFCCs from two distantly related tropical evergreens, encouraging the consideration of physiological roles in these monocots. Clearly, at present, the role of such phenylethyl glycosides for the further fate and possible use of hmFCCs in the plants is unknown.
Chlorophyll breakdown in M. acuminata leaves is reprogrammed by efficient esterification of FCCs: Esterification at their propionyl group appears to be a general feature of the FCCs detected in senescent leaves of M. acuminata. When carrying a free propionic acid function, FCCs are typically only fleetingly existent, and are programmed to undergo isomerization to NCCs.[12, 13] In contrast, esterification of the propionyl side chain generates persistent hmFCCs, and provides the chemical basis for the accumulation of these hmFCCs. Thus, it stalls chlorophyll breakdown at the stage of FCCs, and inhibits the further isomerization of the latter to the NCCs. The observed esterification may be rationalized as a catabolic intervention[20] and purposeful reprogramming towards the biosynthesis of the persistent hmFCCs.
Selective attachment of β-glucopyranosyl units via their anomeric center to the terminal oxygen of the hydroxyl-ethyl side chain at their 8-position is a typical feature of a variety of NCCs (X in Figure 12) and of some hmFCCs, as well.[21] Indeed, the glycosidation observed in NCCs is (currently) presumed to already occur at the stage of the corresponding FCC precursors in the cytosol, where it would be catalyzed by still unidentified glycosidases.[8, 45] This type of glycosidation is reminiscent of the glucuronidation of bilirubine (in mammals),[15] and has also been rationalized on the basis of the hypothetical requirements for the transport of FCCs into the vacuoles (where they isomerize to the corresponding NCCs). In contrast, esterification of FCCs by a gluco- or galactopyranosyl group at the critical propionate with the primary 6′-OH-group of the sugar units, as found here, provides sugar esters that may have (different) basic physiological roles: the sugar esters stabilize hmFCCs against their acid induced isomerization to NCCs (as also achieved, similarly, by a daucic acid residue[20]), and they simultaneously provide linkers for attachment of further groups. Thus, (at least) two further types of hypothetical cytosolic enzymes are suggested, which wait for identification.
Figure 12.
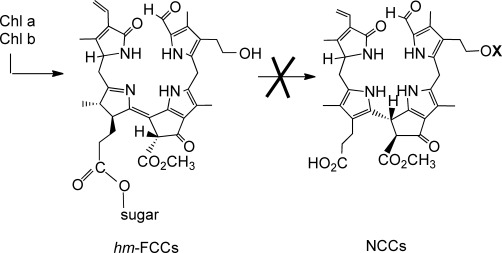
In banana leaves chlorophylls (Chl a and b) are degraded and esterified with sugars to hypermodified FCCs (hmFCCs), which are not degraded further to the nonfluorescent NCCs (X=H, β-glucopyranosyl, etc.).
The question of physiological roles of chlorophyll catabolites in higher plants: Chlorophyll breakdown in higher plants may be considered, first of all, to be a detoxification process, helpful, indirectly, in permitting the remobilization of nitrogen from chlorophyll-binding proteins to proceed during senescence.[8, 46] Indeed, a physiological function of the ubiquitous chlorophyll catabolites is still unknown. This is striking, as it contrasts the important and well-known physiological functions in plants and in algae of the structurally related heme catabolites (e.g., of biliverdin and the phycobilins), as well as those of biliverdin and bilirubin in animals.[14, 15, 47, 48]
The colorless NCCs exhibit the properties of remarkable antioxidants, which may be of particular relevance in senescent leaves, as well as in ripening fruit.[5] Possibly, the related (but less well studied) FCCs could have similar beneficial properties that may help to extend the viability of senescent or ripened plant tissue.[24] Indeed, in senescent M. acuminata leaves chlorophyll breakdown appears to be reprogrammed to furnish the persistent blue luminescent hmFCCs. Clearly, it deviates from the pathway towards colorless and photoinactive tetrapyrroles, such as NCCs (and the related DNCCs), typical of senescent leaves of higher plants.[5, 10] The extraordinary biosynthetic esterifications of FCCs in yellow banana leaves (and fruit) provides uniquely structured hmFCCs, which suggests the possible existence of endogenous physiological roles of FCCs, other than those of NCCs. Specific hmFCCs are indicated, for example, to accumulate selectively in the senescent tissue surrounding dark necrotic parts of banana peels.[21, 22]
FCCs absorb UV light very effectively and may represent, for example, a type of sun screen against UV light for the plant.[10] They also emit blue light, that is, they act as optical brighteners to the human eye.[20] In a related sense, the hmFCCs are natural endogenous fluorescence signals that may be useful as noninvasive, molecular tools for biochemical investigations for studying cellular (senescence) processes in plants.[21, 23] Indeed, in bright yellow bananas,[20, 28] and even more so, in senescent banana leaves, persistent hmFCCs accumulate,[23] and are the molecular origin of their blue luminescence. In a broader sense, the fascinating colors that appear in fall leaves and in ripening fruit, due to the degradation of chlorophyll, stimulate further considerations with respect to the biological[49] and ecological[50, 51] relevance of such color changes. In a few exceptional cases, degreening leaves are, indeed, known to develop strong luminescence, one example being the fall leaves of Ginkgo biloba.[52] The luminescence of the senescent leaves of this tree is mainly caused by an unsaturated alkaloid.[52]
Clearly, the bright colors of fruit are primarily seen as signals for frugivorous animals, helping to attract these for the purpose of increasing the local distribution of the seeds.[53, 54] Fruits and plants in tropical regions furnish indispensable feed for domestic animals.[55] This underscores the relevance of chlorophyll breakdown in providing suitable visual signals to mark ripening fruits. The blue luminescence observed in bananas has also been rationalized on this basis.[20] Possibly, similar arguments may also apply to the leaves of fruit-bearing plants. “Fruit flagging” could be an additional optical signal of fruit-bearing plants, relayed by the help of colorful and possibly luminescent leaves in the surrounding of ripe fruit.[56] The development of bright and distinct colors of fruit and of leaves, even in the UV- and blue-light regions,[57] as well as the complementary capacity for their perception by insects, birds and other animals may contribute significantly to the means of communication between plants and animals.[58, 59] Often, but not always, this type of communication is beneficial for both sides: as recently reported, insect eating plants make use of a still uncharacterized blue luminescent compound to attract insects into their deadly fly traps.[60]
Conclusion
We describe here the identification and structure elucidation of a group of FCCs in extracts of senescent Musa acuminata leaves. These leaves accumulate hypermodified FCCs (hmFCCs) massively, and luminesce blue, when excited by UV light, due to these chlorophyll catabolites. The persistent hmFCCs may be useful as natural endogenous luminescent signals of cell death that may open up access to new, noninvasive observations of cellular processes in leaves and fruit. In contrast to the situation in other known senescent leaves, the Ma-FCCs are not further processed in the banana leaves to NCCs or related nonfluorescent tetrapyrroles. This discovery indicates chlorophyll breakdown in banana leaves to be completely reprogrammed. It provides further examples to contrast the view,[24, 49] that chlorophylls are degraded in senescent leaves by a general and common pathway to NCCs, which were presumed earlier to be the typical end products of chlorophyll breakdown in leaves. Ma-FCCs represent new variants of unique and linear tetrapyrroles. The exploration of their possible physiological functions could lead to a fundamental expansion of our views on why chlorophyll is broken down in higher plants.
Experimental Section
Materials
Plant material: Yellow-greenish senescent leaves of bananas (Musa acuminata, Cavendish cultivar) for standard analysis and preparative extractions were harvested at the plantation Malpais Trece (Garachico, Island of Tenerife, Spain) and transported at ambient temperature to Innsbruck, where they were frozen in liquid N2 and stored at −80 °C, until they were analyzed. Leaves at different stages of senescence for determination of chlorophyll content and total amount of FCCs were collected from plants grown at the University of Innsbruck (Center for Chemistry and Biomedicine, Institute of Organic Chemistry) and picked directly before use. The batches were shown to contain the same catabolites, but in slightly different distributions.
Chemicals: Commercially available solvents (reagent grade) were redistilled and in the case of dichloromethane (CH2Cl2) filtered over Alox before use for extractions. HPLC grade methanol (MeOH) was from VWR (West Chester, USA); acetonitrile (ACN) from Sigma–Aldrich (St. Louis, USA), ultrapure water (18 MΩ cm−1) from a Millipore apparatus. The 1 and 5 g SepPak C18 cartridges were from Waters Associates (Milford, USA).
Methods
Analytical and semipreparative HPLC: Shimadzu HPLC system was used with manual sampler, DGU-20A5 online degasser, LC-20AD pump, CBM-20A system controller, SPD-M20A diode array detector, Jasco FP-920 fluorescence detector, a Rheodyne injection valve with 20 μL (analytical HPLC) or 200 μL (semipreparative HPLC) loop. Data were collected and processed with Shimadzu LC Solution. Phenomenex Hyperclone ODS 5 μm 250×4.6 mm i.d. column connected to a Phenomenex ODS 4×3 mm i.d. precolumn used at room temperature. Flow rate 0.5 mL min−1 (for analytical HPLC and semipreparative determination of FCC content in leaves) or 0.8 mL min−1 (for semipreparative isolation of main FCCs); solvent system for analytical HPLC and semipreparative determination of FCC content in leaves: solvent A: water; solvent B: MeOH; analytical and co-injection experiments, solvent composition A/B (v/v): 0–5 min: 60:40; 5–38 min: 60:40 to 20:80; 38–45 min: 20:80 to 0:100; 45–55 min: 0:100; 55–60 min: 0:100 to 60:40; determination of FCC content in leaves: 0–3 min: 55:45; 3–13 min: 55:45 to 48:52; 13–15 min: 48:52 to 25:75; 15–25 min: 25:75 to 15:85; 25–26 min: 15:85 to 0:100; 26–35 min: 0:100; 35–40 min: 0:100 to 55:45; isomerization experiments: 0–3 min: 52:48; 3–36 min: 52:48 to 28:72; 36–40 min: 28:72 to 0:100; 40–46 min: 0:100; 46–50 min: 0:100 to 52/48.
Preparative HPLC: Hewlett Packard Series 1100 HPLC system was used with manual sampler, G1322A online degasser, Agilent G1311A quatpump, G1315A diode array detector, Agilent G1321A fluorescence detector, a Rheodyne injection valve with 1 mL loop. Data were collected and processed with Agilent ChemStation. Phenomenex Hyperclone ODS 5 μm 250×21.2 mm i.d. column connected to a ODS precolumn used at room temperature. Flow rate 5 mL min−1; solvent system: solvent A: water, solvent B: MeOH; solvent composition A/B (v/v): 0–10 min: 65:35; 10–190 min: 65:35 to 30:70; 190–195 min: 30:70 to 0:100; 195–210 min: 0:100; 210–220 min: 0:100 to 65:35.
Spectroscopy: UV/Vis: λmax [nm] (log ε or εrel), Hitachi U-3000 spectrophotometer, solvents: MeOH (for Ma-FCC-61, Ma-FCC-63, Ma-FCC-64, Ma-FCC-69) or MeOH/H2O about 1:1 (v/v; HPLC elution mix, for other FCCs); concentrations of FCCs were calculated by using the extinction coefficient of Ma-FCC-61 at 317 nm (log ε=4.36). CD: λmin/max [nm] (Δε), Jasco J715, solvent: MeOH. NMR spectroscopy: δ [ppm], J [Hz], Bruker UltraShield Avance II+600 MHz; 1H,1H homonuclear (COSY, ROESY, TOCSY) and 1H,13C heteronuclear (HSQC, HMBC) experiments;[31, 32] 10 °C; residual solvent peaks (CD2HOD: δH=3.31 ppm, δC=49.00 ppm; CD2HCN: δH=1.94 ppm, δC=1.32 ppm) were used as internal reference[61] signals are classified as singlet (s), doublet (d), doubled doublet (dd), double doubled doublet (ddd), triplet (t) and multiplet (m); apparent=app.; broad=br. ESI-MS:[62] m/z (rel. abundance; type of ion); Finnigan LCQ Classic, ESI source; positive ion mode, spray voltage 4.5 kV, solvent MeOH/H2O 1:1 (v/v), signals due to isotopomers and their relative intensities are shown for base peaks and [M+H]+ pseudo-molecular ions.
Analysis of chlorophyll catabolites in senescent leaves by analytical HPLC: Yellow senescent banana leaves (200 mg wet weight, M. acuminata, Cavendish cultivar) were frozen in liquid N2 and pulverized frozen in a mortar. After extraction with cold MeOH (400 μL) and centrifugation of the resulting suspension for 3 min at 12 700g, the supernatant was diluted 2:1 (v/v) with water and centrifuged (3 min, 12 700g) once more. The resulting yellow extract was injected into the analytical HPLC system by using absorbance and fluorescence detection (Figure 2).
Quantification of tetrapyrroles in banana leaves (Figure 3): From freshly harvested banana leaves three samples each (leaf areas between 9 and 26 cm2) at five different senescence stages were cut out (green, greenish-yellow, yellow-greenish, yellow, yellow-brownish; n=3; 15 samples in total). Each sample was frozen in liquid N2, pulverized in a mortar with sea sand and extracted with MeOH. The slurry was centrifuged for 2 min at 12 700g, the supernatant was removed and the residue was ground and extracted a second time. The procedure was repeated four to five times (until the solid residue was colorless).
Determination of chlorophyll content in banana leaves: The obtained methanolic extracts were combined and diluted with MeOH to 10.0 mL (or to 20.0 mL) in a volumetric flask. From UV/Vis spectroscopic analysis of these fractions the concentration of chlorophyll was calculated first,[27] and from this the amount of chlorophyll per cm2 leaf section.
Determination of the total amount of FCCs in banana leaves: An aliquot (7.0 mL) of each obtained methanolic fractions from the chlorophyll content measurements was diluted 1:4 (v/v) with H2O and (if necessary after centrifugation for 2 min at 12 700g) applied to a preconditioned 1 g SepPak cartridge. After being washed with H2O (15 mL) the FCC containing fraction was eluted with MeOH (5 mL). The solvents were removed by using a rotary evaporator and the remaining residue was dissolved in 500 μL MeOH/H2O 1:1 (v/v). An aliquot (200 μL) of the solution was separated by semipreparative HPLC, all FCC containing fractions were collected. The unified solutions of each run were diluted with MeOH to 5.0 mL in a volumetric flask and analyzed by UV/Vis spectroscopy. From the absorbance at 317 nm the total concentration of FCCs was calculated.
Isolation of chlorophyll catabolites: Yellow-greenish senescent banana leaves (60 g wet weight; Musa acuminata, Cavendish cultivar) were frozen in liquid N2, mixed with sea sand (15 g) and ground to a fine powder. To the collected fine powder ice-cooled CH2Cl2 (100 mL) were added and the cold slurry was filtered through a Buchner funnel. Extraction with CH2Cl2 was repeated four times with 80 mL each to remove chlorophyll and unpolar carotinoides. Afterwards ice-cooled MeOH (100 mL) was added to the plant material and the cold slurry was filtered once more. This procedure was repeated four times with MeOH (50 mL each). The collected methanolic extracts were concentrated to 50 mL at reduced pressure by using a rotary evaporator. After being diluted with H2O (200 mL) the yellow clear solution was applied to a preconditioned 5 g SepPak cartridge and rinsed with MeOH/H2O 1:3 (v/v; 80 mL). The FCC-containing fraction was eluted with MeOH (30 mL), dried under reduced pressure and redissolved in MeOH/H2O 2:1 (v/v; 3 mL). Further purification by three preparative HPLC runs gave crude samples of the described FCCs. The fractions of Ma-FCC-61, Ma-FCC-63, Ma-FCC-64 and Ma-FCC-69 of all three runs were unified, reduced to dryness by using a rotary evaporator, redissolved in MeOH/H2O 2:1 (v/v) and repurified by semipreparative HPLC. Solvent A: water, solvent B: ACN; solvent composition A/B (v/v). Isolation of Ma-FCC-61: 0–2 min: 75:25; 2–20 min: 75:25 to 72:28; 20–22 min: 72:28 to 0:100; 22–28 min: 0:100; 28–30 min: 0:100–75:25. Isolation of Ma-FCC-63 and Ma-FCC-64: 0–2 min: 73:27; 2–20 min: 73:27 to 70:30; 20–22 min: 70:30 to 0:100; 22–28 min: 0:100; 28–30 min: 0:100 to 73:27. Isolation of Ma-FCC-69: 0–2 min: 70:30; 2–20 min: 70:30 to 68:32; 20–22 min: 68:32 to 0:100; 22–28 min: 0:100; 28–30 min: 0:100 to 70:30. The solvents of the collected analytical pure samples were evaporated in vacuo to give 2.35 mg (2.26 μmol) of Ma-FCC-61, 0.79 mg (0.98 μmol) of Ma-FCC-63, 0.63 mg (0.77 μmol) of Ma-FCC-64 and 0.55 mg (0.59 μmol) of Ma-FCC-69. The dried samples were used for spectroscopic analyses and all further experiments.
Spectroanalytical data
Ma-FCC-61: UV/Vis (c=3.08×10−5 m) λmax (log ε)=382 (sh, 4.02), 359 (4.23), 317 (4.36), 251 nm (sh, 4.36); CD (c=3.08×10−5 m)=377 (−0.4), 320 (4.3), 287 (−0.9), 274 (−0.4), 249 (−7.3), 219 (4.4); 1H NMR (CD3OD): δ=1.11 (d, J=7.3 Hz, H3C(181)), 1.78 (m, HAC(171)), 1.88 (m, HBC(171)), 2.06 (s, H3C(21)), 2.19 (s, H3C(121)), 2.23 (s, H3C(71)), 2.35 (m, H2C(172)), 2.36 (m, HC(17)), 2.44 (dd, J=8.9/18.5 Hz, HAC(20)), 2.64 (m, H2C(81)), 2.65 (m, HC(18)), 3.05 (dd, J=3.9/18.4 Hz, HBC(20)), 3.36 (dd, J=5.0/10.0 Hz, HAC(1′′′)), 3.41 (dd, J=6.0/11.5 Hz, HAC(3′′′)), 3.44 (m, HC(3′′)), 3.45 (m, HAC(82)), 3.49 (m, HC(2′′)), 3.52 (dd, J=4.7/11.5 Hz, HBC(3′′′)), 3.56 (m, HBC(82)), 3.59 (m, HAC(6′′)), 3.64 (m, HC(5′c)), 3.65 (m, HBC(1′′′)), 3.68 (m, HC(2′′′)), 3.71 (s, H3C(135)), 3.78 (dd, J=3.7/10.1 Hz, HC(2′)), 3.82 (m, HC(3′)), 3.84 (m, HBC(6′′)), 3.88 (br d, J∼3.3 Hz, HC(4′′)), 3.91 (br d, J∼3.0 Hz, HC(4′)), 3.92 (d, J=7.6 Hz, HC(1′′)), 4.01 (m, HAC(6′)), 4.02 (br s, H2C(10)), 4.11 (dd, J∼2.9/9.0 Hz, HC(5′)), 4.29 (dd, J=3.0/11.3 Hz, HBC(6′)), 4.42 (s, HC(132)), 4.74 (dd, J=3.9/8.8 Hz, HC(1)), 4.85 (d, J=3.7 Hz, HC(1′)), 5.40 (dd, J=2.3/11.7 Hz, HAC(32)), 6.20 (dd, J=2.2/17.7 Hz, HBC(32)), 6.52 (dd, J=11.6/17.7 Hz, HC(31)), 9.36 ppm (s, HC(5)); ESI-MS m/z (%): 1083.3 (28), 1082.4 (58), 1081.4 (100, [M+K]+); 1065.5 (92, [M+Na]+); 1045.3 (7), 1044.3 (19), 1043.3 (32, C50H67N4O20+, [M+H]+); 1027.5 (1, [M−C3H9O3+2 K]+); 951.2 (3, [M−C3H8O3+H]+); 933.3 (1, [M−C3H10O4+H]+); 789.2 (7, [M−C9H18O8+H]+); 771.4 (1, [M−C9H20O9+H]+); 753.4 (1, [M−C9H22O10+H]+); 636.3 (1, [M−C17H29NO10+H]+).
Ma-FCC-63: UV/Vis (c=2.5×10−5 m) λmax (εrel)=382 (sh, 0.45), 358 (0.74), 317 (1.00), 248 nm (sh, 1.03); CD (c=2.5×10−5 m)=357 (−1.8), 317 (2.6), 294 (sh, 2.1), 272 (sh, 0.3), 249 (−5.9), 218 (5.1); 1H NMR (CD3CN/D2O 9:1, v/v): δ=1.01 (d, J=7.2 Hz, H3C(181)), 1.65 (m, HAC(171)), 1.80 (m, HBC(171)), 1.97 (s, H3C(21)), 2.10 (s, H3C(121)), 2.16 (s, H3C(71)), 2.19 (m, H2C(172)), 2.30 (m, HC(17)), 2.51 (dd, J=7.2/18.3 Hz, HAC(20)), 2.56 (m, H2C(81) and HC(18)), 3.00 (dd, J=3.8/18.3 Hz, HBC(20)), 3.05 (app. t, J∼8.2/8.8 Hz, HC(2′)), 3.20 (app. t, J∼9.3/9.5 Hz, HC(4′)), 3.30 (app. t, J=8.5/9.2 Hz, HC(3′)), 3.39 (t, J=6.6 Hz, H2C(82)), 3.42 (m, HC(5′)), 3.65 (s, H3C(135)), 3.93/3.96 (AB system, J=16.3 Hz, H2C(10)), 3.98 (dd, J=6.9/11.9 Hz, HAC(6′)), 4.24 (dd, J∼1.5/11.8 Hz, HBC(6′)), 4.41 (s, HC(132)), 4.42 (d, J=7.9 Hz, HC(1′)), 4.47 (dd, J=4.0/7.1 Hz, HC(1)), 5.35 (dd, J∼1.6/11.7 Hz, HAC(32)), 6.10 (dd, J∼1.5/17.8 Hz, HBC(32)), 6.46 (dd, J=11.7/17.7 Hz, HC(31)), 9.31 ppm (s, HC(5)); 13C NMR (CD3CN/D2O 9:1, v/v): δ=8.9 (71), 9.5 (121), 12.7 (21), 18.1 (181), 22.9 (10), 27.5 (81), 28.0 (171), 31.3 (172), 33.7 (20), 47.1 (17), 52.2 (18), 53.6 (135), 57.9 (1), 61.3 (132), 62.4 (82), 64.8 (6′), 70.9 (4′), 74.5 (5′), 75.3 (2′), 76.7 (3′), 97.2 (1′), 111.6 (12), 113.9 (15), 119.6 (32), 120.1 (8), 125.7 (13), 127.0 (31), 128.1 (3), 128.6 (6), 134.0 (7), 135.8 (11), 137.1 (9), 152.8 (16), 155.9 (2), 169.7 (133), 174.0 (173), 174.4 (4), 178.5 (5), 186.0 (19), 188.3 ppm (131); ESI-MS m/z (%): 861.0 (5); 845.3 (92, [M+K]+); 831.3 (15), 830.4 (44), 829.4 (100, [M+Na]+); 809.2 (6), 808.3 (17), 807.2 (32, C41H51N4O13+, [M+H]+); 789.3 (10, [M−H2O+H]+); 771.3 (3, [M−2 H2O+H]+); 654.3 (3, [M−C8H11NO2+H]+).
Ma-FCC-64: UV/Vis (c=1.9×10−5 m) λmax (εrel)=382 (sh, 0.44), 360 (0.73), 317 (1.00), 252 nm (sh, 0.98); CD (c=1.9×10−5 m)=358 (−1.8), 315 (2.5), 294 (sh, 2.0), 271 (sh, 0.2), 248 (−5.9), 218 nm (5.3); 1H NMR (CD3CN/D2O 9:1, v/v): δ=1.02 (d, J=7.2 Hz, H3C(181)), 1.67 (m, HAC(171)), 1.80 (m, HBC(171)), 1.98 (s, H3C(21)), 2.11 (s, H3C(121)), 2.16 (s, H3C(71)), 2.20 (m, H2C(172)), 2.31 (m, HC(17)), 2.52 (dd, J=7.3/18.3 Hz, HAC(20)), 2.57 (m, H2C(81) and HC(18)), 3.01 (dd, J=3.7/18.2 Hz, HBC(20)), 3.17 (app. t, J∼9.3/9.7 Hz, HC(4′)), 3.28 (dd, J∼3.6/9.7 Hz, HC(2′)), 3.39 (t, J=6.6 Hz, H2C(82)), 3.54 (superimposed by water signal, HC(3′)), 3.65 (s, H3C(135)), 3.82 (ddd, J∼1.7/5.9/9.8 Hz, HC(5′)), 3.93/3.96 (AB system, J=16.5 Hz, H2C(10)), 3.97 (m, HAC(6′)), 4.19 (dd, J∼1.6/11.9 Hz, HBC(6′)), 4.40 (s, HC(132)), 4.48 (dd, J=3.8/7.1 Hz, HC(1)), 5.01 (d, J=3.6 Hz, HC(1′)), 5.35 (dd, J∼1.7/11.6 Hz, HAC(32)), 6.13 (dd, J∼1.6/17.7 Hz, HBC(32)), 6.47 (dd, J=11.7/17.7 Hz, HC(31)), 9.33 ppm (s, HC(5)); 13C NMR (CD3CN/D2O 9:1, v/v): δ=8.9 (71), 9.4 (121), 12.7 (21), 18.0 (181), 22.9 (10), 27.4 (81), 28.0 (171), 31.1 (172), 33.6 (20), 47.1 (17), 52.1 (18), 53.5 (135), 57.8 (1), 61.1 (132), 62.3 (82), 64.8 (6′), 70.1 (5′), 70.9 (4′), 72.6 (2′), 73.8 (3′), 93.1 (1′), 111.6 (12), 114.3 (15), 119.5 (32), 120.1 (8), 125.9 (13), 126.9 (31), 128.3 (3), 128.9 (6), 133.9 (7), 135.9 (11), 137.1 (9), 152.8 (16), 155.9 (2), 169.8 (133), 174.0 (173), 174.5 (4), 178.4 (5), 186.0 (19), 187.9 ppm (131); ESI-MS m/z (%): 847.3 (18), 846.3 (46), 845.3 (100, [M+K]+); 829.4 (98, [M+Na]+); 809.2 (7), 808.3 (25), 807.3 (40, C41H51N4O13+, [M+H]+); 789.3 (19, [M−H2O+H]+); 771.3 (5, [M−2 H2O+H]+); 753.3 (5, [M−3 H2O+H]+); 654.3 (4, [M−C8H11NO2+H]+).
Ma-FCC-69: UV/Vis (c=1.5×10−5 m) λmax (εrel)=383 (sh, 0.42), 360 (0.71), 317 (1.00), 253 nm (sh, 0.97); CD (c=1.5×10−5 m)=360 (−2.0), 318 (2.7), 294 (sh, 2.3), 272 (sh, −0.1), 248 (−5.9), 216 (4.6); 1H NMR (CD3CN): δ=0.98 (d, J=7.3 Hz, H3C(181)), 1.61 (m, HAC(171)), 1.78 (m, HBC(171)), 1.96 (s, H3C(21)), 2.09 (m, H2C(172)), 2.15 (s, H3C(121)), 2.21 (H3C(71)) and 2.23 (HC(17)), superimposed by water signal, 2.51 (br d, J∼7.2 Hz, HC(18)), 2.60 (m, H2C(81)), 2.63 (m, HAC(20)), 2.69 (m, H2C(2′′)), 3.02 (dd, J=4.1/18.5 Hz, HBC(20)), 3.06 (app. t, J∼8.1/9.1 Hz, HC(2′)), 3.21 (app. t, J∼9.2/9.4 Hz, HC(4′)), 3.28 (app. t, J∼9.0/9.1 Hz, HC(3′)), 3.40 (m, HC(5′)), 3.42 (m, HC(82)), 3.58 (dd, J=7.6/9.2, HAC(1′′)), 3.62 (s, H3C(135)), 3.85 (m, HBC(1′′)), 3.91/4.01 (AB system, J=15.8 Hz, H2C(10)), 4.14 (dd, J=6.4/12.0 Hz, HAC(6′)), 4.21 (dd, J∼2.1/12.1 Hz, HBC(6′)), 4.24 (d, J=7.9 Hz, HC(1′)), 4.30 (s, HC(132)), 4.47 (m, HC(1)), 5.32 (dd, J=2.2/11.7 Hz, HAC(32)), 6.26 (dd, J=2.3/17.7 Hz, HBC(32)), 6.49 (dd, J=11.6/17.6 Hz, HC(31)), 6.53 (dd, J=1.8/8.2 Hz, HC(8′′)), 6.67 (d, J=8.1 Hz, HC(7′′)), 6.69 (d, J=1.8 Hz, HC(4′′)), 9.45 ppm (s, HC(5)); 13C NMR (CD3CN): δ=8.8 (71), 9.4 (121), 12.4 (21), 18.0 (181), 22.7 (10), 27.8 (81), 28.1 (171), 31.0 (172), 32.3 (20), 35.9 (2′′), 46.4 (17), 52.8 (18), 53.0 (135), 58.0 (1), 61.1 (132), 62.5 (82), 64.2 (6′), 70.9 (4′), 71.6 (1′′), 74.3 (2′), 74.4 (5′), 77.1 (3′), 103.6 (1′), 111.4 (12), 115.0 (15), 116.1 (7′′), 117.0 (4′′), 119.0 (32), 120.3 (8), 121.3 (8′′), 126.5 (13), 126.9 (31), 129.4 (3), 129.8 (6), 131.5 (3′′), 133.3 (7), 136.7 (11), 137.7 (9), 143.9 (6′′), 145.4 (5′′), 154.5 (16), 155.6 (2), 170.0 (133), 173.7 (173), 175.7 (4), 177.8 (5), 185.4 (19), 187.4 ppm (131); ESI-MS m/z (%): 1019.1 (6, [M−H+2 K]+); 1003.2 (8, [M−H+K+Na]+); 997.3 (13); 983.3 (23), 982.3 (52), 981.4 (100, [M+K]+); 965.5 (72, [M+Na]+); 949.5 (7, [M−CH3OH+K]+); 945.1 (2), 944.2 (5), 943.1 (11, C49H59N4O15+, [M+H]+); 829.4 (3, [M−C8H8O2+Na]+); 789.2 (3, [M−C8H10O3+H]+).
Isolation by HPLC and spectroanalytical data of minor FCC fractions
Ma-FCC-57: UV/Vis (c=3.4×10−5 m)=385 (sh, 0.36), 364 (0.56), 319 (1.00), 272 (sh, 0.99), 232 nm (sh, 1.59); ESI-MS m/z (%): 1081.4 (43, [M+K]+); 1067.5 (27), 1066.5 (55), 1065.5 (100, [M+Na]+); 1045.3 (16), 1044.2 (27), 1043.3 (43, [M+H]+).
Ma-FCC-60: UV/Vis (c=3.1×10−5 m)=386 (sh, 0.32), 363 (0.51), 318 (1.00), 271 (sh, 0.98), 234 nm (sh, 1.56); ESI-MS m/z (%): 847.3 (29), 846.4 (62), 845.4 (100, [M+K]+); 829.3 (85, [M+Na]+); 809.4 (11), 808.2 (31), 807.2 (49, [M+H]+).
Ma-FCC-65: UV/Vis (c=2.5×10−5 m)=385 (sh, 0.39), 362 (0.61), 319 (1.00), 269 (sh, 0.96), 243 nm (sh, 1.36); ESI-MS m/z (%): 847.3 (29), 846.4 (53), 845.4 (100, [M+K]+); 829.5 (82, [M+Na]+); 809.2 (5), 808.3 (12), 807.2 (24, [M+H]+).
Ma-FCC-68: UV/Vis (c=1.9×10−5 m)=384 (sh, 0.38), 362 (0.57), 318 (1.00), 274 (sh, 0.99), 233 nm (sh, 1.57); ESI-MS m/z (%): 847.4 (22), 846.4 (51), 845.4 (100, [M+K]+); 829.4 (95, [M+Na]+); 809.2 (8), 808.3 (11), 807.2 (19, [M+H]+).
Equilibration between anomeric Ma-FCC-63 and Ma-FCC-64: In an analytical experiment samples of both isomers were stored in a 1:1 (v/v) mixture of methanol and water under argon atmosphere at room temperature in the dark. Over the time course of 48 h several aliquots of the solution were taken and applied to analysis by HPLC. Two hours after starting the experiment, about one third of Ma-FCC-63 was converted into Ma-FCC-64 and vice versa. After approximately 24 h the equilibrium was reached in both cases (see Figure 7).
Co-injection of Ma-FCC-69 and Sw-FCC-62: Ma-FCC-69 was isolated from yellow banana leaves and characterized as described above. The spectroscopic data were similar to the ones of Sw-FCC-62 from Spathiphyllum wallisii.[37] Purified samples of Ma-FCC-69 and of Sw-FCC-62 were separately applied to analytical HPLC, as well as a 1:1 mixture of both of them (Figure 9). These standardized analytical HPLC-experiments showed Ma-FCC-69 to differ from Sw-FCC-62.
Acknowledgments
We would like to thank Thomas Müller and Christoph Kreutz for invaluable mass spectrometric and NMR spectroscopic help, Andreas Holzinger (Botanical Institute, University of Innsbruck) for a fluorescence image, and we are grateful to the Austrian National Science Foundation (FWF, projects No. L-472 and I-563) for financial support.
References
- 1.Hörtensteiner S, Lee DW. In: Senescence Processes in Plants. Gan S, editor. Oxford, UK: Blackwell; 2007. p. 12. [Google Scholar]
- 2.Barry CS. Plant Sci. 2009;176:325. [Google Scholar]
- 3.Matile P. Chimia. 1987;41:376. [Google Scholar]
- 4.Kräutler B, Jaun B, Bortlik K, Schellenberg M, Matile P. Angew. Chem. 1991;103:1354. [Google Scholar]
- Angew. Chem. Int. Ed. Engl. 1991;30:1315. [Google Scholar]
- 5.Kräutler B. Photochem. Photobiol. Sci. 2008;7:1114. doi: 10.1039/b802356p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moser S, Müller T, Oberhuber M, Kräutler B. Eur. J. Org. Chem. 2009:21. doi: 10.1002/ejoc.200800804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kräutler B, Matile P. Acc. Chem. Res. 1999;32:35. [Google Scholar]
- 8.Hörtensteiner S, Kräutler B. Biochim. Biophys. Acta. 2011;1807:977. doi: 10.1016/j.bbabio.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Mühlecker W, Ongania KH, Kräutler B, Matile P, Hörtensteiner S. Angew. Chem. 1997;109:401. [Google Scholar]
- Angew. Chem. Int. Ed. Engl. 1997;36:401. [Google Scholar]
- 10.Kräutler B, Hörtensteiner S. In: The Handbook of Porphyrin Science Vol. 28. Ferreira GC, Kadish KM, Smith KM, Guilard R, editors. USA: World Scientific Publishing; 2013. pp. 117–185. [Google Scholar]
- 11.Kräutler B. In: The Handbook of Porphyrin Science Vol. 13. Kadish KM, Smith KM, Guilard R, editors. Oxford: Elsevier; 2003. p. 183. [Google Scholar]
- 12.Oberhuber M, Berghold J, Breuker K, Hörtensteiner S, Kräutler B. Proc. Natl. Acad. Sci. USA. 2003;100:6910. doi: 10.1073/pnas.1232207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberhuber M, Berghold J, Kräutler B. Angew. Chem. 2008;120:3100. doi: 10.1002/anie.200705330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2008;47:3057. [Google Scholar]
- 14.Frankenberg N, Lagarias JC. In: The Porphyrin Handbook, Vol. 13. Kadish KM, Smith KM, Guilard R, editors. Oxford, UK: Elsevier; 2003. p. 211. [Google Scholar]
- 15.Falk H. Chemistry of Linear Oligopyrroles and Bile Pigments. Wien: Springer; 1989. [Google Scholar]
- 16.Curty C, Engel N. Phytochemistry. 1996;42:1531. [Google Scholar]
- 17.Losey FG, Engel N. J. Biol. Chem. 2001;276:8643. doi: 10.1074/jbc.M009288200. [DOI] [PubMed] [Google Scholar]
- 18.Müller T, Rafelsberger M, Vergeiner C, Kräutler B. Angew. Chem. 2011;123:10912. doi: 10.1002/anie.201103934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2011;50:10724. [Google Scholar]
- 19.Djapic N, Djuric A, Pavlovic A. Res. J. Agr. Sci. 2009;41:256. [Google Scholar]
- 20.Moser S, Müller T, Ebert M-O, Jockusch S, Turro NJ, Kräutler B. Angew. Chem. 2008;120:9087. doi: 10.1002/anie.200803189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2008;47:8954. [Google Scholar]
- 21.Moser S, Müller T, Holzinger A, Lütz C, Jockusch S, Turro NJ, Kräutler B. Proc. Natl. Acad. Sci. USA. 2009;106:15538. doi: 10.1073/pnas.0908060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller T, Kräutler B. Gerontology. 2011;57:521. doi: 10.1159/000321877. [DOI] [PubMed] [Google Scholar]
- 23.Banala S, Moser S, Müller T, Kreutz C, Holzinger A, Lütz C, Kräutler B. Angew. Chem. 2010;122:5300. doi: 10.1002/anie.201000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2010;49:5174. [Google Scholar]
- 24.Kräutler B, Hörtensteiner S. In: Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications, Adv. Photosynth. Res. Vol. 25. Grimm B, Porra R, Rüdiger W, Scheer H, editors. Dordrecht: Springer; 2006. p. 237. [Google Scholar]
- 25.Kräutler B, Jaun B, Amrein W, Bortlik K, Schellenberg M, Matile P. Plant Physiol. Biochem. 1992;30:333. [Google Scholar]
- 26.Kräutler B, Puffer B, Müller T. Nachr. Chem. 2012;60:1084. [Google Scholar]
- 27.Porra RJ, Thompson WA, Kriedemann PE. Biochim. Biophys. Acta. 1989;975:384. [Google Scholar]
- 28.Moser S, Müller T, Holzinger A, Lütz C, Kräutler B. Chem. Eur. J. 2012;18:10873. doi: 10.1002/chem.201201023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moser S, Ulrich M, Müller T, Kräutler B. Photochem. Photobiol. Sci. 2008;7:1577. doi: 10.1039/b813558d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulrich M, Moser S, Müller T, Kräutler B. Chem. Eur. J. 2011;17:2285. doi: 10.1002/chem.201003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kessler H, Gehrke M, Griesinger C. Angew. Chem. 1988;100:507. [Google Scholar]
- Angew. Chem. Int. Ed. Engl. 1988;27:490. [Google Scholar]
- 32.Ernst RR, Bodenhausen G, Wokaun A. Principles of Nuclear Magnetic Resonance in One and Two Dimensions. Oxford: Clarendon; 1987. [Google Scholar]
- 33.Pretsch E, Bühlmann P, Affolter C. Structure Determination of Organic Compounds. Berlin: Springer; 2000. [Google Scholar]
- 34.Kırmızıbekmez H, Celep E, Masullo M, Bassarello C, Yeşilada E, Piacente S. Helv. Chim. Acta. 2009;92:1845. [Google Scholar]
- 35.King-Morris MJ, Serianni AS. J. Am. Chem. Soc. 1987;109:3501. [Google Scholar]
- 36.Roslund MU, Tähtinen P, Niemitz M, Sjöholm R. Carbohydr. Res. 2008;343:101. doi: 10.1016/j.carres.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Kräutler B, Banala S, Moser S, Vergeiner C, Müller T, Lütz C, Holzinger A. FEBS Lett. 2010;584:4215. doi: 10.1016/j.febslet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 38.Matile P, Hörtensteiner S, Thomas H, Kräutler B. Plant Physiol. 1996;112:1403. doi: 10.1104/pp.112.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Müller T, Ulrich M, Ongania K-H, Kräutler B. Angew. Chem. 2007;119:8854. doi: 10.1002/anie.200703587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2007;46:8699. [Google Scholar]
- 40.Kress WJ, Prince LM, Hahn WJ, Zimmer EA. Syst. Biol. 2001;50:926. doi: 10.1080/106351501753462885. [DOI] [PubMed] [Google Scholar]
- 41.Hörtensteiner S, Rodoni S, Schellenberg M, Vicentini F, Nandi OI, Qui YL, Matile P. Plant Biol. 2000;2:63. [Google Scholar]
- 42.Dörmann P, Benning C. Trends Plant Sci. 2002;7:112. doi: 10.1016/s1360-1385(01)02216-6. [DOI] [PubMed] [Google Scholar]
- 43.Garrido JL, Otero J, Maestro MA, Zapata M. J. Phycol. 2000;36:497. doi: 10.1046/j.1529-8817.2000.99135.x. [DOI] [PubMed] [Google Scholar]
- 44.Fahr A, van Hoogevest P, May S, Bergstrand N, Leigh MLS. Eur. J. Pharm. Sci. 2005;26:251. doi: 10.1016/j.ejps.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Frey PA, Hegeman AD. Enzymatic Reaction Mechanisms. New York: Oxford University Press; 2007. [Google Scholar]
- 46.Hörtensteiner S, Feller U. J. Exp. Bot. 2002;53:927. doi: 10.1093/jexbot/53.370.927. [DOI] [PubMed] [Google Scholar]
- 47.Baranano DE, Rao M, Ferris CD, Snyder SH. Proc. Natl. Acad. Sci. USA. 2002;99:16093. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sedlak TW, Snyder SH. Pediatrics. 2004;113:1776. doi: 10.1542/peds.113.6.1776. [DOI] [PubMed] [Google Scholar]
- 49.Hörtensteiner S. Annu. Rev. Plant. Biol. 2006;57:55. doi: 10.1146/annurev.arplant.57.032905.105212. [DOI] [PubMed] [Google Scholar]
- 50.Archetti M, Brown SP. Proc. R. Soc. London Ser. B. 2004;271:1219. doi: 10.1098/rspb.2004.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Archetti M. Proc. R. Soc. London Ser. B. 2009;276:2575. doi: 10.1098/rspb.2009.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matile P. Bot. Helv. 1994;104:87. [Google Scholar]
- 53.Sumner P, Mollon JD. J. Exp. Biol. 2000;203:1987. doi: 10.1242/jeb.203.13.1987. [DOI] [PubMed] [Google Scholar]
- 54.Osorio D, Vorobyev M. Proc. R. Soc. London Ser. B. 1996;263:593. doi: 10.1098/rspb.1996.0089. [DOI] [PubMed] [Google Scholar]
- 55.Morton JF. Fruits of Warm Climates. Florida: Florida Flair Books; 1987. p. 29. [Google Scholar]
- 56.Stiles EW. Am. Nat. 1982;120:500. [Google Scholar]
- 57.Winter Y, Lopez J, von Helversen O. Nature. 2003;425:612. doi: 10.1038/nature01971. [DOI] [PubMed] [Google Scholar]
- 58.Sumner P, Mollon JD. J. Exp. Biol. 2000;203:1963. doi: 10.1242/jeb.203.13.1963. [DOI] [PubMed] [Google Scholar]
- 59.Zhao H, Rossiter SJ, Teeling EC, Li C, Cotton JA, Zhang S. Proc. Natl. Acad. Sci. USA. 2009;106:8980. doi: 10.1073/pnas.0813201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kurup R, Johnson AJ, Sankar S, Hussain AA, Kumar CS, Baby S. Plant Biol. 2013;15:611. doi: 10.1111/j.1438-8677.2012.00709.x. [DOI] [PubMed] [Google Scholar]
- 61.Gottlieb HE, Kotlyar V, Nudelman A. J. Org. Chem. 1997;62:7512. doi: 10.1021/jo971176v. [DOI] [PubMed] [Google Scholar]
- 62.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]


