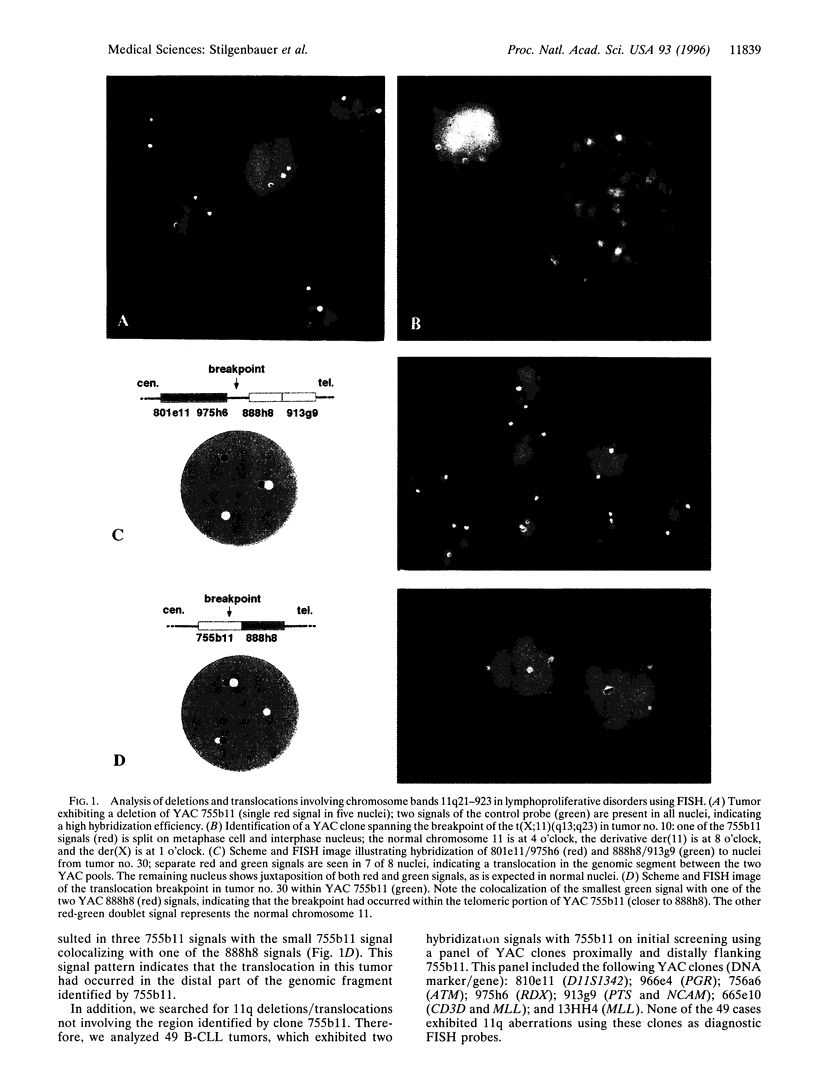
Abstract
Aberrations of the long arm of chromosome 11 are among the most common chromosome abnormalities in lymphoproliferative disorders (LPD). Translocations involving BCL1 at 11q13 are strongly associated with mantle cell lymphoma. other nonrandom aberrations, especially deletions and, less frequently, translocations, involving bands 11q21-923 have been identified by chromosome banding analysis. To date, the critical genomic segment and candidate genes involved in these deletions have not been identified. In the present study, we have analyzed tumors from 43 patients with LPD (B-cell chronic lymphocytic leukemia, n = 40; mantle cell lymphoma, n = 3) showing aberrations of bands 11q21-923 by fluorescence in situ hybridization. As probes we used Alu-PCR products from 17 yeast artificial chromosome clones spanning chromosome bands 11q14.3-923.3, including a panel of yeast artificial chromosome clones recognizing a contiguous genomic DNA fragment of approximately 9-10 Mb in bands 11q22.3-923.3. In the 41 tumors exhibiting deletions, we identified a commonly deleted segment in band 11q22.3-923.1; this region is approximately 2-3 Mb in size and contains the genes coding for ATM (ataxia telangiectasia mutated), RDX (radixin), and FDX1 (ferredoxin 1). Furthermore, two translocation break-points were localized to a 1.8-Mb genomic fragment contained within the commonly deleted segment. Thus, we have identified a single critical region of 2-3 Mb in size in which 11q14-923 aberrations in LPD cluster. This provides the basis for the identification of the gene(s) at 11q22.3-923.1 that are involved in the pathogenesis of LPD.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akao Y., Seto M., Yamamoto K., Iida S., Nakazawa S., Inazawa J., Abe T., Takahashi T., Ueda R. The RCK gene associated with t(11;14) translocation is distinct from the MLL/ALL-1 gene with t(4;11) and t(11;19) translocations. Cancer Res. 1992 Nov 1;52(21):6083–6087. [PubMed] [Google Scholar]
- Bentz M., Cabot G., Moos M., Speicher M. R., Ganser A., Lichter P., Döhner H. Detection of chimeric BCR-ABL genes on bone marrow samples and blood smears in chronic myeloid and acute lymphoblastic leukemia by in situ hybridization. Blood. 1994 Apr 1;83(7):1922–1928. [PubMed] [Google Scholar]
- Bianchi A. B., Hara T., Ramesh V., Gao J., Klein-Szanto A. J., Morin F., Menon A. G., Trofatter J. A., Gusella J. F., Seizinger B. R. Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nat Genet. 1994 Feb;6(2):185–192. doi: 10.1038/ng0294-185. [DOI] [PubMed] [Google Scholar]
- Bosch F., Jares P., Campo E., Lopez-Guillermo A., Piris M. A., Villamor N., Tassies D., Jaffe E. S., Montserrat E., Rozman C. PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: a highly specific marker of mantle cell lymphoma. Blood. 1994 Oct 15;84(8):2726–2732. [PubMed] [Google Scholar]
- Brown A. G., Ross F. M., Dunne E. M., Steel C. M., Weir-Thompson E. M. Evidence for a new tumour suppressor locus (DBM) in human B-cell neoplasia telomeric to the retinoblastoma gene. Nat Genet. 1993 Jan;3(1):67–72. doi: 10.1038/ng0193-67. [DOI] [PubMed] [Google Scholar]
- Cerretti D. P., Hollingsworth L. T., Kozlosky C. J., Valentine M. B., Shapiro D. N., Morris S. W., Nelson N. Molecular characterization of the gene for human interleukin-1 beta converting enzyme (IL1BC). Genomics. 1994 Apr;20(3):468–473. doi: 10.1006/geno.1994.1202. [DOI] [PubMed] [Google Scholar]
- Chang C. Y., Wu D. A., Lai C. C., Miller W. L., Chung B. C. Cloning and structure of the human adrenodoxin gene. DNA. 1988 Nov;7(9):609–615. doi: 10.1089/dna.1988.7.609. [DOI] [PubMed] [Google Scholar]
- Chen Z., Brand N. J., Chen A., Chen S. J., Tong J. H., Wang Z. Y., Waxman S., Zelent A. Fusion between a novel Krüppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 1993 Mar;12(3):1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilder M. C., François S., Bosic C., Moreau A., Mellerin M. P., Le Paslier D., Bataille R., Moisan J. P. Deletion cartography around the D13S25 locus in B cell chronic lymphocytic leukemia and accurate mapping of the involved tumor suppressor gene. Cancer Res. 1995 Mar 15;55(6):1355–1357. [PubMed] [Google Scholar]
- Döhner H., Fischer K., Bentz M., Hansen K., Benner A., Cabot G., Diehl D., Schlenk R., Coy J., Stilgenbauer S. p53 gene deletion predicts for poor survival and non-response to therapy with purine analogs in chronic B-cell leukemias. Blood. 1995 Mar 15;85(6):1580–1589. [PubMed] [Google Scholar]
- Döhner H., Pohl S., Bulgay-Mörschel M., Stilgenbauer S., Bentz M., Lichter P. Trisomy 12 in chronic lymphoid leukemias--a metaphase and interphase cytogenetic analysis. Leukemia. 1993 Apr;7(4):516–520. [PubMed] [Google Scholar]
- Gaidano G., Ballerini P., Gong J. Z., Inghirami G., Neri A., Newcomb E. W., Magrath I. T., Knowles D. M., Dalla-Favera R. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidano G., Hauptschein R. S., Parsa N. Z., Offit K., Rao P. H., Lenoir G., Knowles D. M., Chaganti R. S., Dalla-Favera R. Deletions involving two distinct regions of 6q in B-cell non-Hodgkin lymphoma. Blood. 1992 Oct 1;80(7):1781–1787. [PubMed] [Google Scholar]
- James M. R., Richard C. W., 3rd, Schott J. J., Yousry C., Clark K., Bell J., Terwilliger J. D., Hazan J., Dubay C., Vignal A. A radiation hybrid map of 506 STS markers spanning human chromosome 11. Nat Genet. 1994 Sep;8(1):70–76. doi: 10.1038/ng0994-70. [DOI] [PubMed] [Google Scholar]
- Johansson B., Mertens F., Mitelman F. Cytogenetic deletion maps of hematologic neoplasms: circumstantial evidence for tumor suppressor loci. Genes Chromosomes Cancer. 1993 Dec;8(4):205–218. doi: 10.1002/gcc.2870080402. [DOI] [PubMed] [Google Scholar]
- Juliusson G., Oscier D. G., Fitchett M., Ross F. M., Stockdill G., Mackie M. J., Parker A. C., Castoldi G. L., Guneo A., Knuutila S. Prognostic subgroups in B-cell chronic lymphocytic leukemia defined by specific chromosomal abnormalities. N Engl J Med. 1990 Sep 13;323(11):720–724. doi: 10.1056/NEJM199009133231105. [DOI] [PubMed] [Google Scholar]
- Kearney L., Bower M., Gibbons B., Das S., Chaplin T., Nacheva E., Chessells J. M., Reeves B., Riley J. H., Lister T. A. Chromosome 11q23 translocations in both infant and adult acute leukemias are detected by in situ hybridization with a yeast artificial chromosome. Blood. 1992 Oct 1;80(7):1659–1665. [PubMed] [Google Scholar]
- Kobayashi H., Espinosa R., 3rd, Fernald A. A., Begy C., Diaz M. O., Le Beau M. M., Rowley J. D. Analysis of deletions of the long arm of chromosome 11 in hematologic malignancies with fluorescence in situ hybridization. Genes Chromosomes Cancer. 1993 Dec;8(4):246–252. doi: 10.1002/gcc.2870080407. [DOI] [PubMed] [Google Scholar]
- Lengauer C., Green E. D., Cremer T. Fluorescence in situ hybridization of YAC clones after Alu-PCR amplification. Genomics. 1992 Jul;13(3):826–828. doi: 10.1016/0888-7543(92)90160-t. [DOI] [PubMed] [Google Scholar]
- Lichter P., Tang C. J., Call K., Hermanson G., Evans G. A., Housman D., Ward D. C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science. 1990 Jan 5;247(4938):64–69. doi: 10.1126/science.2294592. [DOI] [PubMed] [Google Scholar]
- Liu Y., Szekely L., Grandér D., Söderhäll S., Juliusson G., Gahrton G., Linder S., Einhorn S. Chronic lymphocytic leukemia cells with allelic deletions at 13q14 commonly have one intact RB1 gene: evidence for a role of an adjacent locus. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8697–8701. doi: 10.1073/pnas.90.18.8697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Meerabux J., Yaspo M. L., Roebroek A. J., Van de Ven W. J., Lister T. A., Young B. D. A new member of the proprotein convertase gene family (LPC) is located at a chromosome translocation breakpoint in lymphomas. Cancer Res. 1996 Feb 1;56(3):448–451. [PubMed] [Google Scholar]
- Miura M., Zhu H., Rotello R., Hartwieg E. A., Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993 Nov 19;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Mérel P., Hoang-Xuan K., Sanson M., Moreau-Aubry A., Bijlsma E. K., Lazaro C., Moisan J. P., Resche F., Nishisho I., Estivill X. Predominant occurrence of somatic mutations of the NF2 gene in meningiomas and schwannomas. Genes Chromosomes Cancer. 1995 Jul;13(3):211–216. doi: 10.1002/gcc.2870130311. [DOI] [PubMed] [Google Scholar]
- Okamura T., John M. E., Zuber M. X., Simpson E. R., Waterman M. R. Molecular cloning and amino acid sequence of the precursor form of bovine adrenodoxin: evidence for a previously unidentified COOH-terminal peptide. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5705–5709. doi: 10.1073/pnas.82.17.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini J. H., Walsh M. E., DiMare C., Chen X. N., Korenberg J. R., Weaver D. T. Isolation and characterization of the human MRE11 homologue. Genomics. 1995 Sep 1;29(1):80–86. doi: 10.1006/geno.1995.1217. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H. Chromosomal translocations in human cancer. Nature. 1994 Nov 10;372(6502):143–149. doi: 10.1038/372143a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg C. L., Wong E., Petty E. M., Bale A. E., Tsujimoto Y., Harris N. L., Arnold A. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9638–9642. doi: 10.1073/pnas.88.21.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D., Diaz M. O., Espinosa R., 3rd, Patel Y. D., van Melle E., Ziemin S., Taillon-Miller P., Lichter P., Evans G. A., Kersey J. H. Mapping chromosome band 11q23 in human acute leukemia with biotinylated probes: identification of 11q23 translocation breakpoints with a yeast artificial chromosome. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9358–9362. doi: 10.1073/pnas.87.23.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitsky K., Bar-Shira A., Gilad S., Rotman G., Ziv Y., Vanagaite L., Tagle D. A., Smith S., Uziel T., Sfez S. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995 Jun 23;268(5218):1749–1753. doi: 10.1126/science.7792600. [DOI] [PubMed] [Google Scholar]
- Stilgenbauer S., Leupolt E., Ohl S., Weiss G., Schröder M., Fischer K., Bentz M., Lichter P., Döhner H. Heterogeneity of deletions involving RB-1 and the D13S25 locus in B-cell chronic lymphocytic leukemia revealed by fluorescence in situ hybridization. Cancer Res. 1995 Aug 15;55(16):3475–3477. [PubMed] [Google Scholar]
- Taylor A. M., Metcalfe J. A., Thick J., Mak Y. F. Leukemia and lymphoma in ataxia telangiectasia. Blood. 1996 Jan 15;87(2):423–438. [PubMed] [Google Scholar]
- Thirman M. J., Gill H. J., Burnett R. C., Mbangkollo D., McCabe N. R., Kobayashi H., Ziemin-van der Poel S., Kaneko Y., Morgan R., Sandberg A. A. Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations. N Engl J Med. 1993 Sep 23;329(13):909–914. doi: 10.1056/NEJM199309233291302. [DOI] [PubMed] [Google Scholar]
- Trofatter J. A., MacCollin M. M., Rutter J. L., Murrell J. R., Duyao M. P., Parry D. M., Eldridge R., Kley N., Menon A. G., Pulaski K. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993 Mar 12;72(5):791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Jaffe E., Cossman J., Gorham J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11;14) chromosome translocation. Nature. 1985 May 23;315(6017):340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- Wilgenbus K. K., Milatovich A., Francke U., Furthmayr H. Molecular cloning, cDNA sequence, and chromosomal assignment of the human radixin gene and two dispersed pseudogenes. Genomics. 1993 Apr;16(1):199–206. doi: 10.1006/geno.1993.1159. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. ATM-related genes: what do they tell us about functions of the human gene? Cell. 1995 Sep 8;82(5):685–687. doi: 10.1016/0092-8674(95)90463-8. [DOI] [PubMed] [Google Scholar]
- Ziemin-van der Poel S., McCabe N. R., Gill H. J., Espinosa R., 3rd, Patel Y., Harden A., Rubinelli P., Smith S. D., LeBeau M. M., Rowley J. D. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10735–10739. doi: 10.1073/pnas.88.23.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]