Summary
We all share the need to optimise the evaluation of patients with disorders of consciousness (DOC), given the high rate of misdiagnosis of vegetative state based on clinical examination. We believe that one way to do this is to optimise assessment from the early stages, in order to reduce discontinuity between the hospital and rehabilitation phases.
While clinical observation remains the “gold standard” for the diagnostic assessment of patients with DOC, neurophysiological investigations (electroencephalography, short latency evoked potentials and event-related potentials) could help to further understanding of the pathophysiology underlying the state of unresponsiveness, differentiate coma from other apparently similar conditions (i.e., locked-in and locked-in-like syndromes), and potentially integrate prognostic evaluation with monitoring of the evolution of the clinical state. Moreover, these techniques have the considerable advantage of being available at the bedside.
Discontinuity between the hospital and rehabilitation phases is rightly considered to be one of the critical points in the assessment of patients with DOC. In our view, a continuum of expert neurological assessment that begins with monitoring of the acute phase (focusing on evolution of primary brain damage and secondary complications) and follows through to the patient’s discharge from the intensive care unit (focusing on the pathophysiology of brain damage and prognostication based on clinical, neuroimaging and neurophysiological tests) could help to: i) optimise the rehabilitation programme according to the expectations of recovery; ii) provide a basis for comparison with subsequent periodic re-evaluations; iii) ensure uniformity of assessment regardless of the heterogeneity of care facilities; and iv) characterise a subset of patients who, showing discrepancies between neurophysiological tests and clinical status, are more likely to undergo unexpected recovery.
Keywords: coma , EEG , ERP , minimally conscious state , SEP , vegetative state
Introduction
Unlike other neurological conditions, the heterogeneous pathology linked to disorders of consciousness (DOC) currently excludes the possibility of differential diagnosis of clinical states on the basis of instrumental investigations. Clinical assessments are therefore based on the patient’s clinical history and behaviour. This creates a particular challenge for the clinician, who has to decide whether a certain behaviour, which might be inconsistent or incomplete, reflects a conscious or an unconscious process. It is important to note that the classification of the clinical stages of a patient in a coma or a minimally responsive state is, at present, based on the use of different clinical scales for the acute, sub-acute and chronic phases. Standardised scales allow for a more objective approach to the classification of clinical status, and also make it possible to quantify the evolution of behavioural changes. The problem of misdiagnosis of the vegetative state (VS), reported in some studies ( 1 ) , has made headlines and caused deep concern among both the public and the medical community.
Sensitive clinical scales, if used appropriately and applied at an expert level, can significantly reduce the error rate. There is also the possibility of combining behavioural assessments with instrumental neurophysiological tests (electroencephalography, EEG, and short- and long-latency evoked potentials, EPs), which can provide prognostic information and help to establish the clinical state beyond clinical evidence.
Clinical evaluation
Different scales are needed for the clinical evaluation of acute and transitional/protracted stages of DOC. In the acute phase, the widely applied Glasgow Coma Scale (GCS) is irreplaceable. In acute and transitional stages, the Full Outline of UnResponsiveness (FOUR) score ( 2 ) has been proposed to overcome some limitations of the GCS but its use is still not sufficiently widespread. Among scales able to differentiate VS from a minimally conscious state (MCS) and to indicate emergence from an MCS, the Coma Recovery Scale-Revised (CRS-R) ( 3 ) is the one most used to date. Individualised quantitative behavioural assessment (IQBA) techniques are a second means of objectively investigating behaviour in patients with DOC. IQBA is a useful adjunct to standardised assessment strategies and is an essential component of behavioural-based assessments ( 4 ) , but no studies involving IQBA have been published in recent years. Provided clinical observation remains the gold standard, there are instrumental techniques that can help to further understanding of the major pathophysiological mechanisms that cause the state of coma or of unresponsiveness in a patient and to differentiate these states from various other clinical conditions, e.g., locked-in syndrome (LIS) and diffuse neuromuscular weakness. Some instrumental tools are commonly used for clinical diagnosis while others are still regarded as tools of scientific investigation, since they have not been validated in extensive case studies. The former include conventional neuroimaging (computed tomography, CT, magnetic resonance imaging, MRI) and neurophysiological (EEG and short-latency EPs) tests, and the latter long-latency EPs, event-related potentials (ERPs) and functional and structural neuroimaging (functional MRI, fMRI, diffuse weighted imaging, DWI, and diffusion tensor imaging, DTI).
Neurophysiological investigations
Clinical neurophysiology is an extension of clinical examination and an integration of neuroimaging. Clinical neurophysiology plays a role in diagnosis, prognosis and monitoring. We believe that the potential of clinical neurophysiology in comatose patients is not yet sufficiently understood or exploited.
Neurophysiological tests have the following advantages: i) they can be performed at the bedside; ii); they can be performed many times along with the clinical examinations; iii) they are independent of the efferent channel of motor behaviour (intentional limb movements, verbalisation, eye movements and emotional facial expressions) on which clinical evidence of consciousness is based. Possible methodological drawbacks include heterogeneity of recordings and interpretation of neurophysiological tests. In this regard, there exists, as reported in the literature, sufficient clinical consensus on the most useful diagnostic-prognostic tools and procedures and on their simplified interpretation ( 5 ) . Technicians and neurophysiologists should be specifically trained in the use of these instruments and in the clinical questions posed by DOC patients.
Some of the neurophysiological tests that can be performed in the intensive care unit (ICU), e.g. EEG and somatosensory evoked potentials (SEPs), provide more information on the acute phase, while others (long-latency EPs/ERPs) inform on the post-acute phase in patients who are not yet responsive. The use of clinical neurophysiology should mean the application not of a standard battery of tests but rather of a choice of tests, i.e. the one or ones likely, on the basis of the evidence, to be most informative from the perspective of the given clinical question ( 5 ) . It is important to point out that the EEG, indispensable for diagnostic purposes in the ICU, should be supplemented by SEPs, which are reliable indicators of the severity of acute brain injury (hypoxic-ischaemic, traumatic and haemorrhagic). It is important to balance the high variability of the EEG and its sensitivity to neurosedation with the stability of SEPs, which are more resistant to sedation and have waveforms that are easily interpretable and comparable. There are conditions, such as LIS, locked-in-like states and diffuse neuromuscular weakness, in which the absence of a neuro-physiological assessment can contribute to a delay, of weeks or even months, in the observation of the real state of consciousness.
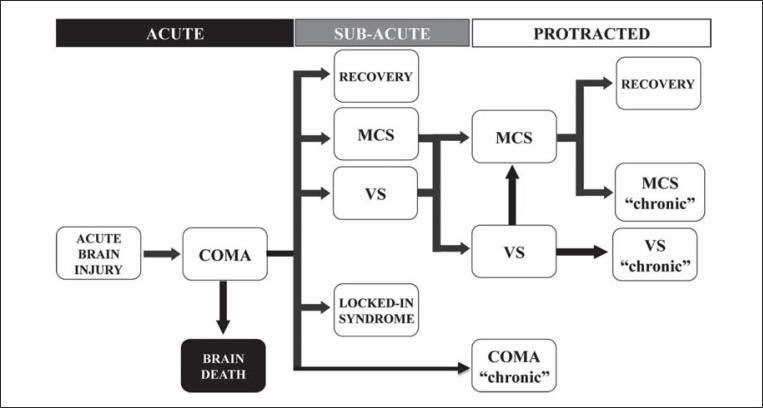
Here, we describe the use of clinical neurophysiology techniques in the phases following the onset of a coma ( Fig. 1 ), indicating the most informative tests depending on the clinical question and the aetiology of the brain damage.
Figure 1 .
Temporal phases in the clinical evolution of acute brain injury.
Abbreviations: MCS=minimally conscious state; VS=vegetative state.
Operationally, we divided the post-coma-onset state into three phases: acute, sub-acute and protracted. The acute phase usually corresponds to a coma duration of up to two weeks; the sub-acute phase includes different clinical states and extends to 6–8 weeks post-coma onset, corresponding to the period spent under treatment in hospital intensive and sub-intensive care units; instead, the last phase, of variable duration depending on the aetiology, usually corresponds to the time spent in the rehabilitation setting and results in a chronic state.
In all the phases, neurophysiological evaluation has a primarily prognostic role although it may also contribute to diagnosis of the state beyond clinical evidence. In this paper we refer mainly to the electroclinical assessment of patients in the acute and sub-acute phases.
Electroclinical assessment in the acute phase
In the acute phase, the EEG and SEPs have a major prognostic role with known patterns for poor outcomes in the following aetiologies: hypoxic-ischaemic encephalopathy, traumatic brain injury (TBI) and intracranial haemorrhage.
Hypoxic-ischaemic coma
As regards the early prognosis of hypoxic-ischaemic coma, it is necessary to consider the evidence provided by five meta-analyses investigating the usefulness of SEPs ( 6 – 10 ) . Four of these studies ( 6 – 9 ) agree that the absence of bilateral cortical SEPs at 24 hours has an unfavourable prognostic significance of 100% (all patients die or remain in a VS). Recently, Lee et al. ( 10 ) showed by means of summary receiver operating curve (SROC) analysis that the SEP predictive value for poor outcome was only marginally better than that of the GCS motor item (M≤3) and urged caution “against the tendency to generalize that SEP is a better marker than clinical signs”. However, as emerges from the electronic supplementary material, bilateral absence of cortical SEPs recorded on day 1 after cardiac arrest accurately predicted a poor outcome and no false positives were found except in one case ( 11 ) , whereas false positives were more common (30 cases) in patients with a GSC motor score of or less than 3. The similar SROC results for SEPs and the GCS reported in this paper ( 10 ) is a correct statistical representation but one that fails to consider the real usefulness of a test in a single case to avoid a falsely pessimistic prognosis. Absent SEPs with their very high specificity correspond to this requirement. Indeed, SEPs have been included in the practice parameter of the American Academy for Prediction of Outcome in Comatose Survivors after Cardiopulmonary Resuscitation ( 12 ) .
Despite their high specificity, SEPs have a low sensitivity, which explains the findings of the SROC analysis. A further limitation of SEPs is their poor ability to predict a good recovery. However, all patients who recover do have cortical SEPs. To further improve prognostication in these patients when outcome is uncertain after initial diagnosis, it might be valuable to apply additional investigations, such as long-latency EPs/ERPs and neuroimaging, which can be used from the first week ( 13 , 14 ) .
Another limitation of the previously cited studies is the follow ups: these were not usually longer than 3 to 6 months, whereas a recent study ( 15 ) reported some cases of hypoxic-ischaemic patients who showed a late recovery of consciousness (19 to 24 months). However, neurophysiological characterisation, in our view useful when dealing with studies on late clinical evolution, was not available for these patients. An enormously interesting question is whether the absence of SEPs retains its ominous prognostic value even in the chronic stages, as demonstrated in the study by Fischer and colleagues. Short-latency SEPs were always found to be absent along with the absence of long-latency EPs and ERPs in patients with chronic VS ( 16 ) .
The application of therapeutic mild hypothermia (32 to 34 °C) after cardiac arrest raises the issue of whether the American Academy of Neurology prognostic criteria ( 12 ) are still reliable after this procedure ( 17 ) . Indeed, according to some authors, motor responses are less reliable within 48 to 72 hours following the procedure ( 18 ) . However, it has been demonstrated that SEP amplitudes do not decrease during mild hypothermia ( 19 , 20 ) and that the bilateral absence of cortical SEP responses still suggested an ominous prognosis in hypothermic (32 to 34 °C) patients ( 21 , 22 ) . In a recent prospective study, Rossetti and colleagues ( 23 ) confirmed the 0% false-positive rate of absent SEPs as an indicator of poor outcome in hypoxic-ischaemic coma patients treated with hypothermia.
Zandbergen et al. ( 24 ) proposed clinical guidelines for improving the prediction of an unfavourable outcome in hypoxic-ischaemic coma patients, postponing the prognostic evaluation to 72 hours and limiting SEP recording in patients with absent photomotor reflex or GCS≤5 (M≤3). Recent European guidelines on the use of neurophysiological testing in the ICU suggest a prognostic evaluation at 24 hours ( 5 ) . We have adopted a protocol for recording EEG and SEPs, defined by our institute’s interventional cardiologists and ICU for early prognosis of hypoxic-ischaemic coma. This protocol involves recording EEG and SEPs at 24 hours in a patient with GCS M≤3. If the EEG and SEPs show unfavourable patterns for the recovery of consciousness (bilateral absence of SEPs and severe EEG abnormalities), we perform a confirmatory test at 72 hours.
What are the clinical implications of the finding of absent SEPs during hypothermia? Prognostic determination can be used, in a practical sense, to counsel families of cardiac arrest survivors at a number of stages during the post-resuscitation period. Soon after hospital admission, families can be consulted over the decision of whether or not to use highly invasive procedures. After therapeutic hypothermia and re-warming an appropriate plan of care, tailored to the expectations of neurological recovery, can be established. This may diminish uncertainty among family members and allow them to get accustomed to the idea that their loved one will die or not wake up again and to understand that their loved one will not benefit from intensive rehabilitation.
Traumatic brain injury
The EEG and the GCS are less reliable early prognostic indicators in post-traumatic compared with hypoxicischaemic coma ( 25 ) . At the early stage, the presence of neurosedation hampers clinical evaluation and makes the interpreting of background EEG activity and the presence of reactivity less reliable. The early SEP component does not have these limitations, being sensitive only to structural damage. In a systematic review of the literature, Robinson et al. ( 9 ) not only confirmed the high prognostic value of bilateral absence of cortical SEPs in post-traumatic coma (90 to 95% of non-awakening) but also highlighted the favourable prognostic significance of bilaterally normal cortical SEPs (over 90% of awakening).
By classifying the patterns of SEP alterations on both hemispheres (normal, absent and pathological, respectively N, A and P), it is possible to aggregate the changes into three different prognostic categories: grade 1 [(NN, NP): normal] with good prognosis, grade 3 [(AA): absent] with poor prognosis and grade 2 [(NA, PP, AP):pathological] where prognosis is uncertain. It should be noted that grades 1 and 3 allow the correct classification of 65–70% of patients with severe post-traumatic coma. In addition, the normal pattern (grade 1) in our series had a positive predictive value of more than 90% for awakening and of more than 80% for good functional recovery, both assessed using the Glasgow Outcome Scale (GOS) ( 26 ) . In our series, severe disability (GOS=3) was associated with virtually 100% of cases with monolaterally or bilaterally absent cortical SEPs. Thus, in severe TBI from the first to second day of coma, we can expect two very different clinical courses, depending on the recording of normal or absent SEPs. In the latter case, for example, it is possible to envisage a long stay in the ICU, the need for a tracheostomy and a very long rehabilitation process with an almost certain outcome of severe disability in the minority of patients that do recover a state of consciousness.
A recent comparative meta-analysis examined the single best early indicator of prognosis among SEPs, CT, EEG and GCS photomotor reflex in coma and post-traumatic and hypoxic-ischaemic patients ( 27 ) . The study selected 26 comparable papers including over 800 patients and it was concluded that SEPs were the best single prognostic indicator. On the basis of this evidence, SEPs should always be associated with clinical assessment for early prognosis of coma after acute brain injury. We indeed wondered why a recent review of the literature ( 28 ) , assessing the prognostic indicators of trauma, did not take SEPs into account. Therefore, we agree with Coleman and colleagues ( 29 ) , who remark on the underuse of these prognostic indicators: “despite the clear utility of short-latency sensory EPs and widespread availability in most regional hospitals, these simple measures are rarely used” ( 29 ) .
Intracranial haemorrhage
Available data on the prognostic value of SEPs in coma caused by intracranial haemorrhage (subarachnoid and intraparenchymal) refer to smaller series. Similarly to what was seen in hypoxic-ischaemic coma, these data indicate that SEPs can be used to make an early prognosis of unfavourable outcome with 100% specificity. In our extended series of 165 intracranial haemorrhages (unpublished data) with a GCS score <9, patients with absent SEPs at the first assessment (25% of the total) all died before discharge from the ICU. Patients deemed brain-dead at the first examination were excluded from the series.
The considerations expressed above apply to adult patients. There are few data available on children. In a recent meta-analysis ( 30 ) , however, it was thought appropriate to indicate to clinicians the usefulness of incorporating SEPs into the integrated process of outcome prediction after acute brain injury in children. Caution is, nonetheless, recommended in predicting unfavourable outcomes in comatose paediatric patients with absent SEPs. The possibility of withdrawing treatment cannot be considered even in patients with coma due to hypoxic-ischaemic encephalopathy because awakening can occur despite bilaterally absent SEPs.
If the neurophysiological methods have high diagnostic and prognostic values in acute brain injury, it is reasonable to expect that their use in serial/continuous monitoring of coma patients at risk of deterioration and secondary complications will have a valuable impact on clinical and therapeutic strategies. The available data are scarce in this respect but the necessity of monitoring brain function in coma patients is gaining increasing interest. Indeed, neurophysiological data (EEG/SEPs) are beginning to be included in ICU multiparameter monitoring systems ( 31 , 32 ) .
Electroclinical assessment in the subacute-transitional phase
In poorly patients or those who are not responsive after the end of coma despite clinical optimisation (remission of sedation effects and septic-metabolic comorbidities), a mutimodal neurophysiological approach is recommended not only to shed light on the pathophysiology of their brain damage but also to contribute to defining states of consciousness beyond clinical evidence and to obtain more clues for prognosis.
The EEG is useful during the transitional stage as an indicator of the reorganisation of posterior rhythms or the presence of sleep elements, both associated with a favourable outcome ( 33 , 34 ) . Repeating recording of SEPs, for example, can confirm the evolution of the diffuse axonal injury, characterised by a minor variation in amplitude of early cortical components compared to a recovery in amplitude of the middle latency components favoured by the reduction or discontinuation of sedation. At this stage, if sedation were discontinued and septic or metabolic disorders were absent, we could start to use long-latency EPs/ERPs. These reflect the parallel activation of distributed, often ill-defined, cortical generators. Although their presence also depends on the integrity of sub-cortical pathways, a moderate decrease in brainstem and thalamo-cortical transmission is usually insufficient to cause significant latency and amplitude abnormalities. Specific peaks (e.g., auditory N100, MMN and P300) can be rated as present or absent. ERPs are also rated according to their complexity (number of preserved components). In comatose patients, they were recently demonstrated to be predictors of a good outcome ( 13 , 35 – 38 ) . Albeit used less often in the ICU than short-latency EP recording, the recording of long-latency EPs/ERPs should be recommended because of the capacity of these EPs to predict with a very high probability a favourable outcome in comatose patients ( 39 ) . They are also indicated as a tool for helping to define states of consciousness beyond clinical evidence.
We here report, in detail, a case in which the neurophysiological integrated approach was used to help define the actual status of a patient.
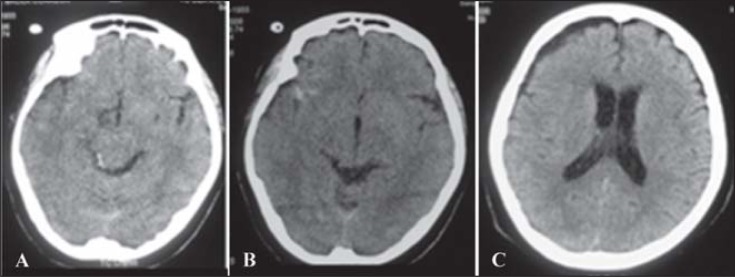
A woman after severe TBI at 11 days showed inexplicable absence of motor responses without relevant traumatic lesions during repeated brain CT ( Fig. 2 ). She showed a complete 3rd right cranial nerve palsy, open left eye with fixation and inconsistent eye tracking, and gaze sometimes appearing attentive despite no motor or verbal responses. Furthermore, no pyramidal signs were present while an electromyographic examination excluded neuromyopathy. Thus, a low grade of MCS or a sort of akinetic mutism was suspected.
Figure 2 .
Brain CT showing no relevant parenchymal lesions (A); a small hyperintense lesion in the right temporal region (B) and a right frontal mild hygroma (C) were present.
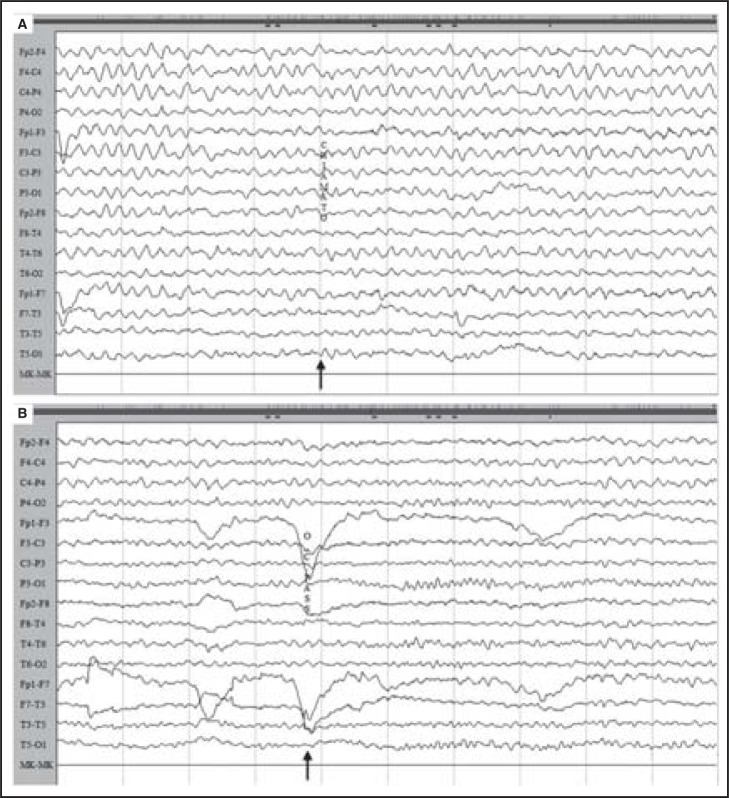
The EEG ( Fig. 3A ) on day 12 was dominated by slow monotonous activity at 5Hz for the first 30 minutes of recording and was not reactive to different stimuli. After this phase, the EEG showed a spontaneous attenuation of slow activity and passive eye closure elicited clear-cut reactivity with the appearance of a posterior 9 Hz alpha rhythm in both hemispheres ( Fig. 3B ).
Figure 3 .
EEG on day 12 dominated by slow monotonous activity at 4–5 Hz for the first 30 minutes of recording (A), not reactive to different stimuli (arrow). After this phase (B), the EEG showed spontaneous attenuation of slow activity and passive eye closure (arrow) elicited clear-cut reactivity with the appearance of a posterior 9 Hz alpha rhythm in both hemispheres.
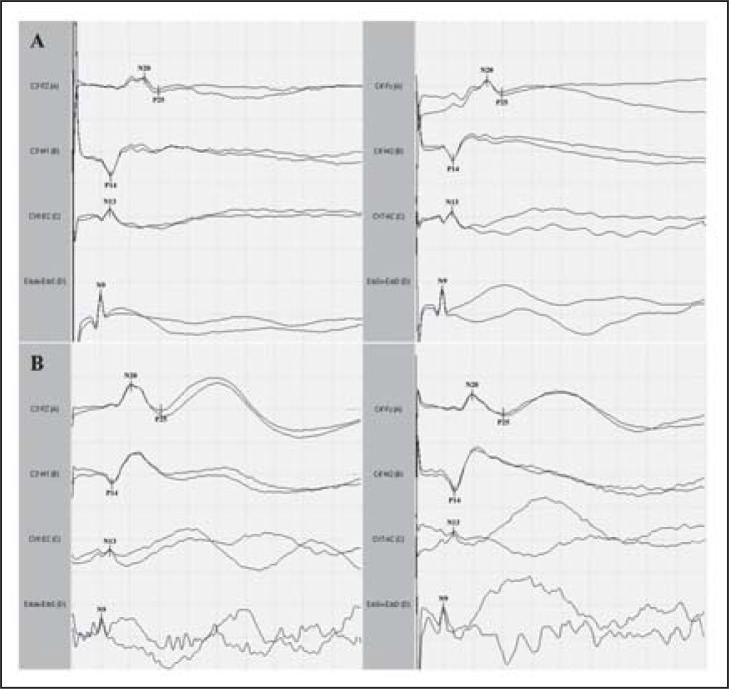
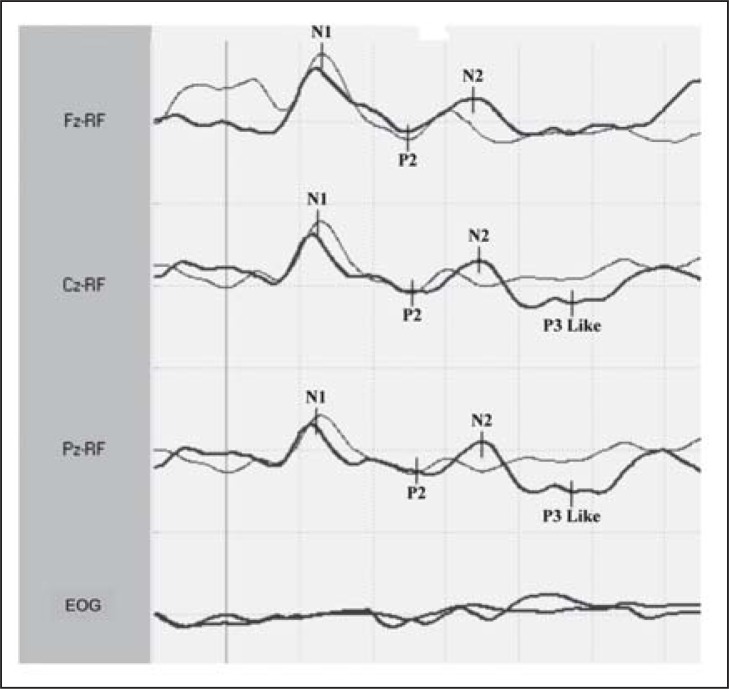
Median-nerve SEPs ( Fig. 4 , over) were obtained 2 and 12 days after admission to the ICU. In the first recording, the early cortical component (N20-P25) was present in both hemispheres with reduced amplitude and increased central conduction time; middle SEP components were not present. In the second recording, cortical SEP components showed an increase in amplitude associated with the presence of SEP middle components. Considering that a state of akinetic mutism might have prevented recognition of an initial recovery of consciousness, we recorded ERPs on day 12. They were recorded by a passive odd-ball paradigm using auditory stimulation. ERPs consisted of the N1 and P2 components at both frequent and rare tones. N2 and a wide positivity at Cz and Pz (in the 300 to 500 ms range), which is more likely to correspond to a “P3b-like” component, were also elicited by target stimuli (rare tones) ( Fig. 5 , over). A clear N2-P3 complex was present on the ERP recording, albeit with prolonged latency indicating the presence of uncovered cognitive activity. We interpreted the reorganisation of the posterior alpha rhythm, the increase in amplitude of cortical SEPs and the presence of ERP components as indicators of recovery of functional thalamo-cortical and intracortical connectivity during the post-traumatic transitional stage. The patient regained motor and verbal responses a month later and she had a good functional outcome at six months.
Figure 4 .
Median-nerve somatosensory evoked potentials (SEPs) were obtained respectively two (A) and 12 (B) days after admission to the ICU. In the first recording, the early cortical component (N20-P25) was present in both hemispheres with reduced amplitude and increased central conduction time; middle SEP components were not present. In the second recording, cortical SEP components showed an increase in amplitude associated with the presence of middle SEP components. Sensitivity: 5μV/div; sweep time: 10 ms/div.
Figure 5 .
Event-related potentials in response to frequent and rare tones obtained on day 12. ERP components N1 and P2 are present in response to both ‘frequent’ and ‘rare’ tones. A wide positivity (in the 300 to 500 ms range) was also recorded at Cz and Pz in response to rare tones. This is very likely to correspond to a “P3b-like” component. Sensitivity: 10μV/div; sweep time: 100 ms/div.
We therefore propose that recordings of ERPs be included in regular clinical and instrumental assessments of VS and MCS patients in transitional/protracted stages in order to seek cortical electrophysiological activity as an additional prognostic factor anticipating a noticeable clinical recovery, such as the case described above and a case of post-traumatic locked-in syndrome ( 40 ) .
The prognostic value of ERPs has been tested in four cohort studies ( 41 – 44 ) and in one case report ( 45 ) , involving a total of 128 patients (87 VS and 41 MCS), with evaluation of the MMN, P3, P600 and N400 components. The presence of MMN was significantly correlated with subsequent recovery of responsiveness in VS patients ( 41 – 43 ) . In particular, Wijnen et al. ( 42 ) showed that the occurrence of an MMN amplitude greater than −1 μV anticipated recovery of consciousness in the following two years (p=0.02). The presence of P3 predicted recovery of contact in VS patients ( 44 ) in the following 12 months, while its absence did not exclude the possibility of recovery. In a case of post-traumatic VS described by Faran et al. ( 45 ) , it was possible to see cognitive components (P3, N400 and P600) one year before clinical improvement to MCS.
Concluding remarks
Most of the new studies in DOC patients have focused on the protracted stages, using methods such as fMRI that may offer a complementary approach to the assessment of consciousness in low responsive brain-injured patients ( 46 – 48 ) . However, in the absence of large-scale multicentre studies ( 49 , 50 ) , the real contribution of functional neuroimaging remains investigational and not yet clinical ( 51 ) . Moreover, these techniques are complex, time-consuming and are not available at the bedside.
In our view it is critically important to improve assessment of DOC from the early stages. It is also important to underline that neurophysiological tests are the only instrumental methods validated for the prognosis of brain damage in the acute and post-acute phases.
Taken as a whole, neurophysiological tests would appear to represent a generally available and objective screening method, capable of identifying patients with a poor outcome and those with uncertain or probably good prognoses and, among these, those who might harbour hidden cognitive activities and would thus benefit from further investigation using non-conventional neuroimaging techniques (fMRI and DWI/DTI).
We agree with authors who point to the discontinuity of assessment (between the intensive or sub-intensive and the chronic phases of care) among the causes of misdiagnosis of DOC ( 51 ) . We believe that a comprehensive multimodal neurophysiological approach, using the more informative tests (EEG and short- and long-latency EPs/ERPs), should be included in protocols for evaluation of poorly-responsive patients with severe head trauma in order to establish the patient’s actual clinical state and prevent a LIS/LIS-like state from being mistaken for prolonged coma, VS or low-grade MCS. This, in turn, will drastically reduce late “discoveries” and differentiate them from very exceptional late recoveries.
Ideally, every single non-responsive patient at discharge should be accompanied by a card including the following information: aetiology of acute brain injury, time from onset, clinical evaluation using GCS for the acute stage and CRS-R for the transitional stage, neuroimaging of brain damage (CT and MRI), EEG (dominant frequency and reactivity), short-latency SEPs (classified as A, N and P), long-latency EPs/ERPs (A and P), and EMG (presence/absence of neuromyopathy).
Finally, a prognostic hypothesis should be indicated signalling eventual discrepancies between clinical status and electrophysiological findings.
We believe that our proposal on early electroclinical assessment to reduce discontinuity between the hospital and rehabilitation stages could constitute the first step in the evaluation of the natural history of a patient’s DOC ( 52 ) .
References
- 1. Schnakers C , Vanhaudenhuyse A , Giacino J , et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment . BMC Neurol . 2009 ; 9 : 35 . doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wijdicks EF , Bamlet WR , Maramattom BV , Manno EM , McClelland RL . Validation of a new coma scale: The FOUR score . Ann Neurol . 2005 ; 58 : 585 – 593 . doi: 10.1002/ana.20611. [DOI] [PubMed] [Google Scholar]
- 3. Giacino JT , Kalmar K , Whyte J . The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility . Arch Phys Med Rehabil . 2004 ; 85 : 2020 – 2029 . doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 4. Giacino JT , Smart CM . Recent advances in behavioral assessment of individuals with disorders of consciousness . Curr Opin Neurol . 2007 ; 20 : 614 – 619 . doi: 10.1097/WCO.0b013e3282f189ef. [DOI] [PubMed] [Google Scholar]
- 5. Guérit JM , Amantini A , Amodio P , et al. Consensus on the use of neurophysiological tests in the intensive care unit (ICU): electroencephalogram (EEG), evoked potentials (EP), and electroneuromyography (ENMG) . Neurophysiol Clin . 2009 ; 39 : 71 – 83 . doi: 10.1016/j.neucli.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 6. Zandbergen EG , de Haan RJ , Stoutenbeek CP , Koelman JH , Hijdra A . Systematic review of early prediction of poor outcome in anoxic-ischaemic coma . Lancet . 1998 ; 352 : 1808 – 1812 . doi: 10.1016/S0140-6736(98)04076-8. [DOI] [PubMed] [Google Scholar]
- 7. Attia J , Cook DJ . Prognosis in anoxic and traumatic coma . Crit Care Clin . 1998 ; 14 : 497 – 511 . doi: 10.1016/s0749-0704(05)70013-0. [DOI] [PubMed] [Google Scholar]
- 8. Rothstein TL . The role of evoked potentials in anoxic-ischemic coma and severe brain trauma . J Clin Neurophysiol . 2000 ; 17 : 486 – 497 . doi: 10.1097/00004691-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 9. Robinson LR , Micklesen PJ , Tirschwell DL , Lew HL . Predictive value of somatosensory evoked potentials for awakening from coma . Crit Care Med . 2003 ; 31 : 960 – 967 . doi: 10.1097/01.CCM.0000053643.21751.3B. [DOI] [PubMed] [Google Scholar]
- 10. Lee YC , Phan TG , Jolley DJ , Castley HC , Ingram DA , Reutens DC . Accuracy of clinical signs, SEP, and EEG in predicting outcome of hypoxic coma. A meta-analysis . Neurology . 2010 ; 74 : 572 – 580 . doi: 10.1212/WNL.0b013e3181cff761. [DOI] [PubMed] [Google Scholar]
- 11. Young GB , Doig G , Ragazzoni A . Anoxic-ischemic encephalopathy: clinical and electrophysiological associations with outcome . Neurocrit Care . 2005 ; 2 : 159 – 164 . doi: 10.1385/NCC:2:2:159. [DOI] [PubMed] [Google Scholar]
- 12. Wijdicks EF , Hijdra A , Young GB , Bassetti CL , Wiebe S . Quality Standards Subcommittee of the American Academy of Neurology. Practice Parameter: Prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology . Neurology . 2006 ; 67 : 203 – 210 . doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 13. Fischer C , Luauté J , Némoz C , Morlet D , Kirkorian G , Mauguière F . Improved prediction of awakening or nonawakening from severe anoxic coma using tree-based classification analysis . Crit Care Med . 2006 ; 34 : 1520 – 1524 . doi: 10.1097/01.CCM.0000215823.36344.99. [DOI] [PubMed] [Google Scholar]
- 14. Wijman CA , Mlynash M , Caulfield AF , et al. Prognostic value of brain diffusion-weighted imaging after cardiac arrest . Ann Neurol . 2009 ; 65 : 394 – 402 . doi: 10.1002/ana.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Estraneo A , Moretta P , Loreto V , Lanzillo B , Santoro L , Trojano L . Late recovery after traumatic, anoxic, or hemorrhagic long-lasting vegetative state . Neurology . 2010 ; 75 : 239 – 245 . doi: 10.1212/WNL.0b013e3181e8e8cc. [DOI] [PubMed] [Google Scholar]
- 16. Fischer C , Luaute J , Morlet D . Event-related potentials (MMN and novelty P3) in permanent vegetative or minimally conscious states . Clin Neurophysiol . 2010 ; 121 : 1032 – 1042 . doi: 10.1016/j.clinph.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 17. Geocadin RG , Eleff SM . Cardiac arrest resuscitation: neurologic prognostication and brain death . Curr Opin Crit Care . 2008 ; 14 : 261 – 268 . doi: 10.1097/MCC.0b013e3282fd68ea. [DOI] [PubMed] [Google Scholar]
- 18. Al Thenayan E , Savard M , Sharpe M , Norton L , Young B . Predictors of poor neurologic outcome after induced mild hypothermia following cardiac arrest . Neurology . 2008 ; 71 : 1535 – 1537 . doi: 10.1212/01.wnl.0000334205.81148.31. [DOI] [PubMed] [Google Scholar]
- 19. Guérit JM . Intraoperative monitoring during cardiac surgery . In: Nuwer MR , editor. Intraoperative Monitoring of Neural Function: Handbook of Clinical Neurophysiology, 8 . Amsterdam : Elsevier ; 2008 . pp. 829 – 838 . [Google Scholar]
- 20. Kottenberg-Assenmacher E , Armbruster W , Bornfeld N , Peters J . Hypothermia does not alter somatosensory evoked potential amplitude and global cerebral oxygen extraction during marked sodium nitroprusside-induced arterial hypotension . Anesthesiology . 2003 ; 98 : 1112 – 1118 . doi: 10.1097/00000542-200305000-00013. [DOI] [PubMed] [Google Scholar]
- 21. Tiainen M , Kovala TT , Takkunen OS , Roine RO . Somatosensory and brainstem auditory evoked potentials in cardiac arrest patients treated with hypothermia . Crit Care Med . 2005 ; 33 : 1736 – 1740 . doi: 10.1097/01.ccm.0000171536.63641.d9. [DOI] [PubMed] [Google Scholar]
- 22. Bouwes A , Binnekade JM , Zandstra DF , et al. Somatosensory evoked potentials during mild hypothermia after cardiopulmonary resuscitation . Neurology . 2009 ; 73 : 1457 – 1461 . doi: 10.1212/WNL.0b013e3181bf98f4. [DOI] [PubMed] [Google Scholar]
- 23. Rossetti AO , Urbano LA , Delodder F , Kaplan PW , Oddo M . Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest . Crit Care . 2010 ; 14 : R173 . doi: 10.1186/cc9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zandbergen EG , Hijdra A , Koelman JH , et al. PROPAC Study Group Prediction of poor outcome within the first 3 days of postanoxic coma . Neurology . 2006 ; 66 : 62 – 68 . doi: 10.1212/01.wnl.0000191308.22233.88. [DOI] [PubMed] [Google Scholar]
- 25. Carter BG , Butt W . Review of the use of somatosensory evoked potentials in the prediction of outcome after severe brain injury . Crit Care Med . 2001 ; 29 : 178 – 186 . doi: 10.1097/00003246-200101000-00036. [DOI] [PubMed] [Google Scholar]
- 26. Amantini A , Grippo A , Fossi S , et al. Prediction of ‘awakening’ and outcome in prolonged acute coma from severe traumatic head injury: evidence for validity of short latency SEPs . Clin Neurophysiol . 2005 ; 116 : 229 – 235 . doi: 10.1016/j.clinph.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 27. Carter BG , Butt W . Are somatosensory evoked potentials the best predictor of outcome after severe brain injury? A systematic review . Intensive Care Med . 2005 ; 31 : 765 – 775 . doi: 10.1007/s00134-005-2633-1. [DOI] [PubMed] [Google Scholar]
- 28. Maas AI , Stocchetti N , Bullock R . Moderate and severe traumatic brain injury in adults . Lancet Neurol . 2008 ; 7 : 728 – 741 . doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 29. Coleman MR , Davis MH , Rodd JM , et al. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness . Brain . 2009 ; 132 : 2541 – 2552 . doi: 10.1093/brain/awp183. [DOI] [PubMed] [Google Scholar]
- 30. Carrai R , Grippo A , Lori S , Pinto F , Amantini A . Prognostic value of somatosensory evoked potentials in comatose children: a systematic literature review . Intensive Care Med . 2010 ; 36 : 1112 – 1126 . doi: 10.1007/s00134-010-1884-7. [DOI] [PubMed] [Google Scholar]
- 31. Fossi S , Amantini A , Grippo A , et al. Continuous EEG-SEP monitoring of severely brain injured patients in NICU: methods and feasibility . Neurophysiol Clin . 2006 ; 36 : 195 – 205 . doi: 10.1016/j.neucli.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 32. Amantini A , Fossi S , Grippo A , et al. Continuous EEG-SEP monitoring in severe brain injury . Neurophysiol Clin . 2009 ; 39 : 85 – 93 . doi: 10.1016/j.neucli.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 33. Bagnato S , Boccagni C , Prestandrea C , Sant’Angelo A , Castiglione A , Galardi G . Prognostic value of standard EEG in traumatic and non-traumatic disorders of consciousness following coma . Clin Neurophysiol . 2010 ; 121 : 274 – 280 . doi: 10.1016/j.clinph.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 34. Valente M , Placidi F , Oliveira AJ , et al. Sleep organization pattern as a prognostic marker at the subacute stage of post-traumatic coma . Clin Neurophysiol . 2002 ; 113 : 1798 – 1805 . doi: 10.1016/s1388-2457(02)00218-3. [DOI] [PubMed] [Google Scholar]
- 35. Kane NM , Curry SH , Butler SR , Cummins BH . Electro-physiological indicator of awakening from coma . Lancet . 1993 ; 341 : 688 . doi: 10.1016/0140-6736(93)90453-n. [DOI] [PubMed] [Google Scholar]
- 36. Guérit JM , Verougstraete D , de Tourtchaninoff M , Debatisse D , Witdoeckt C . ERPs obtained with the auditory oddball paradigm in coma and altered states of consciousness: clinical relationships, prognostic value, and origin of components . Clin Neurophysiol . 1999 ; 110 : 1260 – 1269 . doi: 10.1016/s1388-2457(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 37. Fischer C , Luauté J , Adeleine P , Morlet D . Predictive value of sensory and cognitive evoked potentials for awakening from coma . Neurology . 2004 ; 63 : 669 – 673 . doi: 10.1212/01.wnl.0000134670.10384.e2. [DOI] [PubMed] [Google Scholar]
- 38. Fischer C , Dailler F , Morlet D . Novelty P3 elicited by the subject’s own name in comatose patients . Clin Neurophysiol . 2008 ; 119 : 2224 – 2230 . doi: 10.1016/j.clinph.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 39. Daltrozzo J , Wioland N , Mutschler V , Kotchoubey B . Predicting coma and other low responsive patients outcome using event-related brain potentials: a meta-analysis . Clin Neurophysiol . 2007 ; 118 : 606 – 614 . doi: 10.1016/j.clinph.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 40. Carrai R , Grippo A , Fossi S , et al. Transient post-traumatic locked-in syndrome: a case report and a literature review . Neurophysiol Clin . 2009 ; 39 : 95 – 100 . doi: 10.1016/j.neucli.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 41. Kotchoubey B , Lang S , Mezger G , et al. Information processing in severe disorders of consciousness: vegetative state and minimally conscious state . Clin Neurophysiol . 2005 ; 116 : 2441 – 2453 . doi: 10.1016/j.clinph.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 42. Wijnen VJ , van Boxtel GJ , Eilander HJ , de Gelder B . Mismatch negativity predicts recovery from the vegetative state . Clin Neurophysiol . 2007 ; 118 : 597 – 605 . doi: 10.1016/j.clinph.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 43. Qin P , Di H , Yan X , et al. Mismatch negativity to the patient’s own name in chronic disorders of consciousness . Neurosci Lett . 2008 ; 448 : 24 – 28 . doi: 10.1016/j.neulet.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 44. Cavinato M , Freo U , Ori C , et al. Post-acute P300 predicts recovery of consciousness from traumatic vegetative state . Brain Inj . 2009 ; 23 : 973 – 980 . doi: 10.3109/02699050903373493. [DOI] [PubMed] [Google Scholar]
- 45. Faran S , Vatine JJ , Lazary A , Ohry A , Birbaumer N , Kotchoubey B . Late recovery from permanent traumatic vegetative state heralded by event-related potentials . J Neurol Neurosurg Psychiatry . 2006 ; 77 : 998 – 1000 . doi: 10.1136/jnnp.2005.076554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Owen AM , Coleman MR , Boly M , Davis MH , Laureys S , Pickard JD . Detecting awareness in the vegetative state . Science . 2006 ; 313 : 1402 . doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 47. Owen AM , Coleman MR . Functional MRI in disorders of consciousness: advantages and limitations . Curr Opin Neurol . 2007 ; 20 : 632 – 637 . doi: 10.1097/WCO.0b013e3282f15669. [DOI] [PubMed] [Google Scholar]
- 48. Luccichenti G , Giugni E , Péran P , et al. 3 Tesla is twice as sensitive as 1.5 Tesla magnetic resonance imaging in the assessment of diffuse axonal injury in traumatic brain injury patients . Funct Neurol . 2010 ; 25 : 109 – 114 . [PubMed] [Google Scholar]
- 49. Giugni E , Vadalà R , De Vincentiis C , Colica C , Bastianello S . The brain’s default mode network: a mind “sentinel” role? . Funct Neurol . 2010 ; 25 : 189 – 190 . [PubMed] [Google Scholar]
- 50. Laureys S , Boly M . What is it like to be vegetative or minimally conscious? . Curr Opin Neurol . 2007 ; 20 : 609 – 613 . doi: 10.1097/WCO.0b013e3282f1d6dd. [DOI] [PubMed] [Google Scholar]
- 51. Fins JJ , Shapiro ZE . Neuroimaging and neuroethics: clinical and policy considerations . Curr Opin Neurol . 2007 ; 20 : 650 – 654 . doi: 10.1097/WCO.0b013e3282f11f6d. [DOI] [PubMed] [Google Scholar]
- 52. Bernat JL . The natural history of chronic disorders of consciousness . Neurology . 2010 ; 75 : 206 – 207 . doi: 10.1212/WNL.0b013e3181e8e960. [DOI] [PubMed] [Google Scholar]