Summary
Recent advances in the study of spontaneous brain activity have demonstrated activity patterns that emerge with no task performance or sensory stimulation; these discoveries hold promise for the study of higher-order associative network functionality. Additionally, such advances are argued to be relevant in pathological states, such as disorders of consciousness (DOC), i.e., coma, vegetative and minimally conscious states. Recent studies on resting state activity in DOC, measured with functional magnetic resonance imaging (fMRI) techniques, show that functional connectivity is disrupted in the task-negative or the default mode network. However, the two main approaches employed in the analysis of resting state functional connectivity data (i.e., hypothesis-driven seed-voxel and data-driven independent component analysis) present multiple methodological difficulties, especially in non-collaborative DOC patients. Improvements in motion artifact removal and spatial normalization are needed before fMRI resting state data can be used as proper biomarkers in severe brain injury. However, we anticipate that such developments will boost clinical resting state fMRI studies, allowing for easy and fast acquisitions and ultimately improve the diagnosis and prognosis in the absence of DOC patients’ active collaboration in data acquisition.
Keywords: coma , consciousness , default network , functional magnetic resonance imaging , resting state , spontaneous activity
Introduction
According to present-day understanding, the vacuum state (also called the quantum vacuum) is by no means a “simple empty space” ( 1 ) or some “absolutely empty void” ( 2 ) . In fact, the modern theory of quantum mechanics tells us that the vacuum state contains fleeting electromagnetic waves and particles that pop into and out of existence. Thanks to this new theory of the vacuum it is now possible to predict with remarkable precision the most “intimate” properties of atoms. If a proper theory of the vacuum in physics was an essential step in arriving at a comprehensive modern theory of the “particle” world, we hypothesize that in neuroscience the development of a proper theory of what is called the “baseline” activity of the brain could be a critical step towards a deeper understanding of the “neuronal” world.
Much of what is currently known about brain function derives from studies in which a task or stimulus is administered and the resulting changes in neuronal activity and behavior are measured. Recent studies on brain energy metabolism, however, have shown that task-related increases in neuronal metabolism are usually small when compared to resting energy consumption ( 3 ) . It is thus clear that in order to fully investigate the brain’s activity, one also needs to study its baseline energy-consuming features. Recent advances in the study of spontaneous brain activity ( 4 – 14 ) have demonstrated activity patterns that emerge with no task performance or sensory stimulation; these discoveries hold promise for the study of higher-order associative network functionality and its potential involvement in stimulus-induced activity ( 15 ) and in pathology ( 16 – 19 ) .
In this paper we review studies on resting state activity measured with functional magnetic resonance imaging (fMRI) techniques in patients with disorders of consciousness (DOC), namely coma, vegetative and minimally conscious states. While progress has been made in describing DOC from the clinical perspective ( 20 ) , we here focus on examining DOC patients from the point of view of recent developments deriving from studies of the healthy human brain. In our view, new insights obtained by studying blood oxygen level-dependent (BOLD) resting state activity ( 21 , 22 ) could help to improve understanding of DOC, opening the way for better diagnosis of these conditions, and possibly for prognosis and treatment ( 23 – 26 ) .
Disorders of consciousness
Disorders of consciousness encompass a spectrum of clinical conditions involving profound disruption of global consciousness due to massive brain lesions ( 27 – 29 ) . Clinical characterization of the different DOC is based on two main distinct components of human consciousness: arousal and awareness ( 30 ) . Whereas arousal refers to the behavioral alternation of sleep and wakefulness, awareness refers to the collective thoughts and feelings of an individual ( 31 ) . Coma is characterized by the absence of arousal and hence of awareness. Vegetative state patients are aroused but unaware of the environment and of themselves ( 32 , 33 ) . Minimally conscious state patients show reproducible behavioral evidence of awareness of the environment or the self but are unable, reliably, to communicate ( 28 ) . Locked-in syndrome patients ( 30 ) are fully conscious but are completely paralyzed, except for small movements of the eyes or eyelids. Behavioral assessment is one of the main methods used to detect awareness in severely brain injured patients recovering from coma ( 20 ) . However, detecting unambiguous signs of conscious perception in such patients can be very challenging, as is illustrated by the high frequency of misdiagnosis (up to 43%) in these patients ( 34 – 36 ) . New technical methods such as fMRI offer specific assessment procedures and the possibility of determining objectively whether an unresponsive patient is aware, in the absence of explicit verbal or motor responses.
Spontaneous fMRI activity patterns as a diagnostic tool in disorders of consciousness
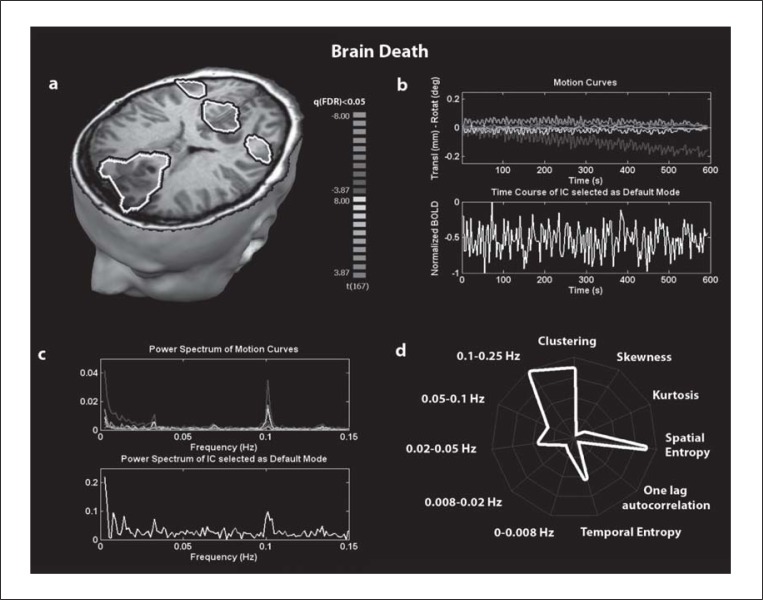
The potential power of the functional brain imaging and, particularly, the fMRI approach has been shown in both the diagnosis ( 37 – 39 ) and the prognosis ( 40 , 41 ) of DOC. However, in the absence of a full understanding of the neural correlates of consciousness, even a near-to-normal activation in response to passive stimulations (e.g. auditory, tactile or visual stimuli) cannot be taken as proof of preserved awareness. Instead, all that can be inferred is that a specific brain region is, to some degree, still able to activate and process sensory stimuli. Similarly, in the absence of cerebral activation during passive fMRI paradigms, it could be argued that the patient may have intact cognition but failing peripheral sensory systems (e.g., may be deaf or have a hearing impairment) or, in the case of visual stimuli, did not look at or fixate the presented visual targets (the latter investigation involves the use of eye-tracking devices which are difficult to calibrate in non-collaborative patients). Resting state fMRI acquisitions are easy to perform (i.e., do not need auditory, visual or somatosensory stimulation equipment in the fMRI environment) and could have a potentially broader and faster translation into clinical practice. Indeed, recent advances in the investigation of spontaneous brain activity have highlighted the clinical relevance of these studies in the absence of patient collaboration, an aspect particularly important in vegetative and minimally conscious patients. Cauda and co-workers ( 42 ) , for example, showed a dysfunctional default mode network (defined as a set of areas encompassing the posterior-cingulate/precuneus, anterior cingulated/ mesiofrontal cortex and temporo-parietal junctions, showing more activity at rest than during attention-demanding tasks) in three patients in a vegetative state. They found decreased connectivity in several brain regions, including the dorsolateral prefrontal cortex and anterior cingulated cortex, especially in the right hemisphere. Boly and co-workers ( 17 ) demonstrated absent cortico-thalamic functional connectivity but partially preserved cortico-cortical connectivity within the default network in a vegetative state patient studied 2.5 years following cardio-respiratory arrest. In this patient, anti-correlations could also be observed between the posterior cingulate/precuneus and a previously identified task-positive cortical network, but both correlations and anti-correlations were significantly reduced as compared to healthy controls. In the same study, a brain death patient studied two days after a massive cranial hemorrhage and evolution to a comatose state showed no residual functional connectivity. Brain death is an irreversible coma with absent brainstem reflexes – complementary examinations such as the electroencephalogram show an isoelectric pattern, while angiography shows absent intracranial arterial flow ( 43 ) . A non-zero BOLD signal measured in a brain totally lacking neuronal activity and arterial blood flow (i.e. in the presence of brain death) can be taken to be an artifactual signal. In such a situation, we were indeed able to show that head motion (see figure 1 and its caption for details of the analysis), due to heart pumping, was the main source of the measured BOLD signal, as confirmed by comparing its power spectrum with the power spectrum of the motion curves. We calculated the linear correlation of the power spectrum of the time course of the component selected as default mode with the power spectrum of each motion curve, obtaining respectively 0.58, 0.96, 0.95, 0.90, 0.93 and 0.59. These high correlation values, especially for the correlation with Y and Z translations and X and Y rotations, strongly point to the head motion origin of the BOLD signal. As reported by Boly et al. ( 17 ) , the absence, in brain death, of any connectivity pattern is an important indication that the generally used pre-processing technique ( 7 ) employed in the resting state data and seed-voxel connectivity analysis approaches, and indeed also applied by Boly et al. ( 17 ) , is quite effective and reliable.
Figure 1 .
Independent component (IC) selected as default mode in brain death (female, age 50 years). The selection criteria were based on similarity testing with an averaged default mode map obtained from an independent control group of 20 healthy volunteers (black and white contour). The similarity value was 0.11. fMRI data were pre-processed using the “BrainVoyager” software package (Brain Innovation, Maastricht, The Netherlands). Pre-processing of functional scans included 3D motion correction, linear trend removal, slice scan time correction and filtering out of low frequencies up to 0.005 Hz. The data were spatially smoothed with a Gaussian filter of full width at half-maximum value of 8 mm. The functional images from each subject were aligned to the participant’s own anatomical scan and warped into the standard anatomical space of Talairach and Tournoux (1988). ICA was performed using the “BrainVoyager” software package with thirty components.
(a) Spatial map of the IC selected as default mode; (b) Motion curves illustrating translation parameters (in mm) for x (red), y (green) and z (blue) and rotation (in °) for pitch (yellow), roll (purple) and yaw (cyan) parameters together with the time course of the IC selected as default mode; (c) Power spectrum of the motion curves and of the time course of the IC selected as default mode; (d) Graphic representation (i.e., fingerprint) of default mode temporal properties showing five frequency bands, temporal entropy and one-lag autocorrelation and spatial properties (spatial entropy, skewness, kurtosis and clustering) (normalized values).
In a more comprehensive study ( 44 ) , fourteen non-communicative brain-damaged patients and fourteen healthy controls participated in a resting state fMRI protocol. Connectivity was investigated by using probabilistic independent component analysis (ICA) and an automated template-matching component selection approach. Functional connectivity in all default network areas was found to be non-linearly correlated with the degree of consciousness, ranging from healthy volunteers and locked-in syndrome, to minimally conscious, vegetative and comatose patients. Furthermore, connectivity in the precuneus was found to be significantly stronger in minimally conscious patients compared with vegetative state patients, while locked-in syndrome patients’ default network connectivity was shown to be not significantly different from that of healthy control subjects.
The level of connectivity in the brain that seems to be confirmed as an important ingredient for sustaining consciousness also plays a key role in theoretical models of consciousness, such as Tononi’s integrated information theory ( 45 ) . According to this approach it is integration (through connectivity) that can sustain consciousness in the brain, and the minimally conscious state case presented, with a unilaterally functioning brain ( Fig. 2 , over), illustrates that integration can be limited to one hemisphere, allowing the maintenance of a minimal level of consciousness.
Figure 2 .
Independent component selected as default mode in a healthy subject (male, age 39), a locked-in syndrome patient (female, age 24 years with a brainstem stroke), a minimally conscious state (MCS) patient (male, age 24 years, post-traumatic) and a vegetative state (VS) patient (male, age 87 years, post-traumatic). The selection criteria were based on similarity testing with an averaged default mode map obtained from an independent control group of 20 healthy volunteers (black and white contour). Similarity values were respectively 0.65, 0.52 and 0.10. Note that in the case of the minimally conscious patient this method failed to select the proper default mode because of the very asymmetrical brain damage and network selection; hence, it was based on visual inspection of both the spatial maps and time courses. For the pre-processing and analysis of the data see figure 1 .
Methodological issues in fMRI resting state assessment
There are two main ways of analyzing resting state-functional connectivity MRI (rs-fcMRI): i) the hypothesis-driven seed-voxel approach ( 7 ) and ii) data-driven ICA approach ( 46 ) , each offering their own advantages and limitations ( 47 ) . The seed-voxel approach consists of extracting the BOLD time course from a region of interest (ROI) and it determines the temporal correlation between this signal (seed) and the time course from all other brain voxels ( 7 ) . To better visualize the correlation pattern and better analyze its properties, the seed approach can be integrated with graph theory methods (e.g., 48 , 49 ). In graphic rs-fcMRI representations, the resting state BOLD time series for each of the ROIs extracted from the network under investigation are correlated among each other giving a correlation matrix which can be represented as a weighted graph. To reduce spurious variance unlikely to reflect neuronal activity, the BOLD signal is pre-processed by temporal bandpass filter, spatial smoothing, and by regressing out head motion curves, whole brain signal and ventricular and white matter signal (and each of their first-order derivative terms) ( 7 ) . This method, which is quite straightforward and gives very intuitive results has been widely adopted and seems to give very consistent results ( 3 ) . On the other hand, it has raised some controversial issues mostly related to the pre-processing procedure, especially concerning the regressing out of the global activity from the BOLD signal (an important step to obtain zero connectivity in brain death, 17), which might induce spurious anti-correlations ( 50 , 51 ) .
Contrary to the seed-voxel connectivity assessment, ICA-based assessments ( 46 ) do not require an a priori definition of ROIs. ICA analyzes the entire BOLD dataset and decomposes it into components that are maximally statistically independent ( 52 ) . A number of studies have shown that ICA is a powerful tool which can simultaneously extract a variety of different coherent neuronal networks ( 6 , 18 , 46 , 53 – 55 ) and separate them from other signal modulations, such as those induced by head motion or physiological confounds (e.g., cardiac pulsation, respiratory cycle and slow changes in the depth and rate of breathing, 56 – 58 ). Still, performing group studies in ICA remains a debated issue, i.e. estimated independent components (ICs) must be matched across subjects and one may need to deal with different numbers of ICs extracted for different subjects if a fixed number of ICs is not empirically chosen before performing the ICA. Different strategies have been proposed in the recent literature, such as principal component back-projection techniques ( 59 ) and, more recently, general linear model dual regression approaches ( 60 ) .
Patients with a chronic DOC develop brain atrophy and secondary hydrocephalus (i.e., ex-vacuo dilation of the ventricles) and traumatic brain injuries and focal hemorrhages will considerably deform the brain. This implies that even if a normalization procedure of the brain has been performed, the selection of a proper seed region becomes difficult and requires visual inspection by an expert eye. Instead, the ICA approach (as illustrated in the minimally conscious patient shown in figure 2 ) is able to automatically identify a cerebral resting state network (RSN) even if the brain is distorted and if the regions are not localized as expected. Recognizing the network (even if it involves only half of a brain as in the reported case) thus becomes an easier task. Of course the problem of automatically selecting the right IC in cases like the one reported remains a delicate issue. Simply performing a similarity test ( 61 ) has been shown not to be successful for choosing the right component (e.g., the component presented in figure 2 was selected by visual inspection). Different approaches could be implemented, such as masking, which can restrict the similarity test only to the part of the brain which, on the basis of expert inspection of structural imaging or other neuroimaging data, is considered less damaged and, hence, still able to present a pattern of activity similar to that of healthy subjects.
Another important issue in the study of spontaneous BOLD signal fluctuations, especially in the case of patients showing a significantly reduced neuronal activity, is the possible contamination by artifact and noise sources ( 9 , 62 , 63 ) . When dealing with non-collaborative patients, major confounds in resting state fMRI acquisitions and analysis are movement, pulse and respiration artifacts. ICA seems to offer the advantage of better isolating physiological artifacts from the neuronal components and this is why data-driven approaches are now being commonly adopted in this field ( 55 , 56 , 64 ) . Clinical studies should preferably perform heart rate and respiratory rate recording ( 65 ) and simultaneous real movement monitoring which would improve resting state analyses.
Finally a more drastic approach, but which may really solve the movement problem, is to mildly sedate the patients. It has been shown that sedatives (e.g., midazolam; 66 ) and anesthetics (e.g., sevoflurane; 67 ) only partially reduce the connectivity in the default mode network. Accordingly, it could become feasible to compare brain connectivity between mildly sedated DOC patients and mildly sedated healthy controls. Given the known effect of sleep on RSN connectivity ( 68 ) it is also important to ascertain that DOC patients and conscious control groups are scanned at comparable vigilance levels.
Concluding remarks
Recent advances in the study of spontaneous brain activity not only increase understanding of human brain functionality, but also offer new opportunities for clinical investigations of the most disparate neurological pathologies. The world of DOC research has turned its attention to the study of spontaneous activity, and studies of resting metabolism and spontaneous electrical activity at scalp level in DOC patients can now be supported by BOLD activity studies during rest. What makes fMRI approaches very appealing is the widespread availability, in medical facilities, of new-generation MRI scanners, now able to perform not only structural but also functional sequences. Easy as a resting state protocol using a common echo planar imaging sequence may appear (and thus suitable for clinical purposes), rapid transient “clonic” motions during the acquisition (which induce large artifacts on the BOLD measurements) and DOC patients’ highly deformed brains (making comparisons with healthy normal brains difficult) render the analysis and interpretation of these data challenging.
Thanks to recent progress in data analysis, we now have a fuller understanding of the properties of the BOLD signal and its spatial patterns in cohorts of healthy subjects, where head motions are controllable and the brain’s structure does not show excessive between-subject variability. For clinical purposes, we need to go from group-level analysis to reliable individual assessments. Then, of course, it will become mandatory to tackle the problem of defining thresholds of the various biomarkers capable of distinguishing between the different DOC states. Of course, in traumatic cases like the MCS reported in figure 2 , where only half of the brain is working but still seems sufficient to ensure a certain level of consciousness, the problem of threshold definition becomes even more difficult. The introduction of effective biomarkers able to weight the important asymmetries of the brain is thus strongly recommended, and this could be addressed, in part, by attaching more importance to the time domain properties than has, in the main, been the case so far using the spatially driven analyses. Before the technique can be employed in clinical routine as a diagnostic or prognostic marker at single subject level, we will need to tackle the problems of spatial normalization (e.g., novel warping methods; 69 , 70 ) and movement correction together with the issue of strongly asymmetric brains in DOC.
Acknowledgments
AV was funded by French Speaking Community Concerted Research Action (ARC 06/11-340). AD is funded by the DISCOS Marie-Curie Research Training Network. This work was also supported by the European Commission (Mindbridge, DECODER, COST-CATIA), McDonnell Foundation, Mind Science Foundation, FRS, Reine Elisabeth Medical Foundation and University and University Hospital of Liège.
References
- 1. Lambrecht A . Observing mechanical dissipation in the quantum vacuum: an experimental challenge . In: Figger H , Meschede D , Zimmermann C , editors. Laser Physics at the Limits . Berlin/New York: Springer ; 2002 . [Google Scholar]
- 2. Ray C . Time, Space and Philosophy . London/New York: Routledge ; 1991 . [Google Scholar]
- 3. Fox MD , Raichle ME . Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging . Nat Rev Neurosci . 2007 ; 8 : 700 – 711 . doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 4. Xiong J , Parsons LM , Gao JH , Fox PT . Interregional connectivity to primary motor cortex revealed using MRI resting state images . Hum Brain Mapp . 1999 ; 8 : 151 – 156 . doi: 10.1002/(SICI)1097-0193(1999)8:2/3<151::AID-HBM13>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mitra PP , Ogawa S , Hu X , Ugurbil K . The nature of spatiotemporal changes in cerebral hemodynamics as manifested in functional magnetic resonance imaging . Magn Reson Med . 1997 ; 37 : 511 – 518 . doi: 10.1002/mrm.1910370407. [DOI] [PubMed] [Google Scholar]
- 6. Greicius MD , Krasnow B , Reiss AL , Menon V . Functional connectivity in the resting brain: a network analysis of the default mode hypothesis . Proc Natl Acad Sci U S A . 2003 ; 100 : 253 – 258 . doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fox MD , Snyder AZ , Vincent JL , Corbetta M , Van Essen DC , Raichle ME . The human brain is intrinsically organized into dynamic, anticorrelated functional networks . Proc Natl Acad Sci U S A . 2005 ; 102 : 9673 – 9678 . doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biswal B , Yetkin FZ , Haughton VM , Hyde JS . Functional connectivity in the motor cortex of resting human brain using echo-planar MRI . Magn Reson Med . 1995 ; 34 : 537 – 541 . doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 9. Cordes D , Haughton VM , Arfanakis K , et al. Mapping functionally related regions of brain with functional connectivity MR imaging . AJNR Am J Neuroradiol . 2000 ; 21 : 1636 – 1644 . [PMC free article] [PubMed] [Google Scholar]
- 10. Damoiseaux JS , Rombouts SA , Barkhof F , et al. Consistent resting-state networks across healthy subjects . Proceedings of the Proc Natl Acad Sci U S A . 2006 ; 103 : 13848 – 13853 . doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lowe MJ , Mock BJ , Sorenson JA . Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations . Neuroimage . 1998 ; 7 : 119 – 132 . doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- 12. Nir Y , Hasson U , Levy I , Yeshurun Y , Malach R . Widespread functional connectivity and fMRI fluctuations in human visual cortex in the absence of visual stimulation . Neuroimage . 2006 ; 30 : 1313 – 1324 . doi: 10.1016/j.neuroimage.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 13. Vincent JL , Patel GH , Fox MD , et al. Intrinsic functional architecture in the anaesthetized monkey brain . Nature . 2007 ; 447 : 83 – 86 . doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 14. Beckmann CF , DeLuca M , Devlin JT , Smith SM . Investigations into resting-state connectivity using independent component analysis . Philos Trans R Soc Lond B Biol Sci . 2005 ; 360 : 1001 – 1013 . doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boly M , Balteau E , Schnakers C , et al. Baseline brain activity fluctuations predict somatosensory perception in humans . Proc Natl Acad Sci U S A . 2007 ; 104 : 12187 – 12192 . doi: 10.1073/pnas.0611404104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boly M , Phillips C , Tshibanda L , et al. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? . Ann N Y Acad Sci . 2008 ; 1129 : 119 – 129 . doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boly M , Tshibanda L , Vanhaudenhuyse A , et al. Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient . Hum Brain Mapp . 2009 ; 30 : 2393 – 2400 . doi: 10.1002/hbm.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greicius MD , Menon V . Default-mode activity during a passive sensory task: uncoupled from deactivation but impacting activation . J Cogn Neurosci . 2004 ; 16 : 1484 – 1492 . doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 19. Rombouts SA , Damoiseaux JS , Goekoop R , et al. Model-free group analysis shows altered BOLD FMRI networks in dementia . Hum Brain Mapp . 2009 ; 30 : 256 – 266 . doi: 10.1002/hbm.20505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Majerus S , Gill-Thwaites H , Andrews K , Laureys S . Behavioral evaluation of consciousness in severe brain damage . Prog Brain Res . 2005 ; 150 : 397 – 413 . doi: 10.1016/S0079-6123(05)50028-1. [DOI] [PubMed] [Google Scholar]
- 21. Raichle ME , MacLeod AM , Snyder AZ , Powers WJ , Gusnard DA , Shulman GL . A default mode of brain function . Proc Natl Acad Sci USA . 2001 ; 98 : 676 – 682 . doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Raichle ME , Snyder AZ . A default mode of brain function: a brief history of an evolving idea . Neuroimage . 2007 ; 37 : 1083 – 1099 . doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 23. Giacino JT , Hirsch J , Schiff N , Laureys S . Functional neuroimaging applications for assessment and rehabilitation planning in patients with disorders of consciousness . Arch Phys Med Rehabil . 2006 ; 87 : S67 – 76 . doi: 10.1016/j.apmr.2006.07.272. [DOI] [PubMed] [Google Scholar]
- 24. Laureys S , Giacino JT , Schiff ND , Schabus M , Owen AM . How should functional imaging of patients with disorders of consciousness contribute to their clinical rehabilitation needs? . Curr Opin Neurol . 2006 ; 19 : 520 – 527 . doi: 10.1097/WCO.0b013e3280106ba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schiff ND . Measurements and models of cerebral function in the severely injured brain . J Neurotrauma . 2006 ; 23 : 1436 – 1449 . doi: 10.1089/neu.2006.23.1436. [DOI] [PubMed] [Google Scholar]
- 26. Schiff ND . Multimodal neuroimaging approaches to disorders of consciousness . J Head Trauma Rehabil . 2006 ; 21 : 388 – 397 . doi: 10.1097/00001199-200609000-00003. [DOI] [PubMed] [Google Scholar]
- 27. Bernat JL . Chronic disorders of consciousness . Lancet . 2006 ; 367 : 1181 – 1192 . doi: 10.1016/S0140-6736(06)68508-5. [DOI] [PubMed] [Google Scholar]
- 28. Giacino JT , Ashwal S , Childs N , et al. The minimally conscious state: definition and diagnostic criteria . Neurology . 2002 ; 58 : 349 – 353 . doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 29. Laureys S , Owen AM , Schiff ND . Brain function in coma, vegetative state, and related disorders . Lancet Neurol . 2004 ; 3 : 537 – 546 . doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- 30. Posner J , Saper C , Schiff N , Plum F , editors. Plum and Posner’s Diagnosis of Stupor and Coma . 4th ed. New York : Oxford University Press ; 2007 . [Google Scholar]
- 31. Laureys S . The neural correlate of (un)awareness: lessons from the vegetative state . Trends Cogn Sci . 2005 ; 9 : 556 – 559 . doi: 10.1016/j.tics.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 32. Bastianello S , Luccichenti G , Giugni E , Pezzella F , Calliada F . Splitting the technology: functional imaging between open and high-field magnetic resonance . Funct Neurol . 2009 ; 24 : 69 – 70 . [PubMed] [Google Scholar]
- 33. Jennett B , Plum F . Persistent vegetative state after brain damage. A syndrome in search of a name . Lancet . 1972 ; 1 : 734 – 737 . doi: 10.1016/s0140-6736(72)90242-5. [DOI] [PubMed] [Google Scholar]
- 34. Andrews K , Murphy L , Munday R , Littlewood C . Misdiagnosis of the vegetative state: retrospective study in a rehabilitation unit . BMJ . 1996 ; 313 : 13 – 16 . doi: 10.1136/bmj.313.7048.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Childs NL , Mercer WN . Misdiagnosing the persistent vegetative state. Misdiagnosis certainly occurs . BMJ . 1996 ; 313 : 944 . doi: 10.1136/bmj.313.7062.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schnakers C , Vanhaudenhuyse A , Giacino JT , et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment . BMC Neurol . 2009 ; 9 : 35 . doi: 10.1186/1471-2377-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laureys S , Faymonville ME , Degueldre C , et al. Auditory processing in the vegetative state . Brain . 2000 ; 123 : 1589 – 1601 . doi: 10.1093/brain/123.8.1589. [DOI] [PubMed] [Google Scholar]
- 38. Hirsch J . Functional neuroimaging during altered states of consciousness: how and what do we measure? . Prog Brain Res . 2005 ; 150 : 25 – 43 . doi: 10.1016/S0079-6123(05)50003-7. [DOI] [PubMed] [Google Scholar]
- 39. Schiff ND , Rodriguez-Moreno D , Kamal A , et al. fMRI reveals large-scale network activation in minimally conscious patients . Neurology . 2005 ; 64 : 514 – 523 . doi: 10.1212/01.WNL.0000150883.10285.44. [DOI] [PubMed] [Google Scholar]
- 40. Di H , Boly M , Weng X , Ledoux D , Laureys S . Neuroimaging activation studies in the vegetative state: predictors of recovery? . Clin Med . 2008 ; 8 : 502 – 507 . doi: 10.7861/clinmedicine.8-5-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Coleman MR , Davis MH , Rodd JM , et al. Towards the routine use of brain imaging to aid the clinical diagnosis of disorders of consciousness . Brain . 2009 ; 132 : 2541 – 2552 . doi: 10.1093/brain/awp183. [DOI] [PubMed] [Google Scholar]
- 42. Cauda F , Micon BM , Sacco K , et al. Disrupted intrinsic functional connectivity in the vegetative state . J Neurol Neurosurg Psychiatry . 2009 ; 80 : 429 – 431 . doi: 10.1136/jnnp.2007.142349. [DOI] [PubMed] [Google Scholar]
- 43. Wijdicks EF . Determining brain death in adults . Neurology . 1995 ; 45 : 1003 – 1011 . doi: 10.1212/wnl.45.5.1003. [DOI] [PubMed] [Google Scholar]
- 44. Vanhaudenhuyse A , Noirhomme Q , Tshibanda LJ , et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients . Brain . 2010 ; 133 : 161 – 171 . doi: 10.1093/brain/awp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tononi G . Consciousness as integrated information: a provisional manifesto . Biol Bull . 2008 ; 215 : 216 – 242 . doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- 46. McKeown MJ , Makeig S , Brown GG , et al. Analysis of fMRI data by blind separation into independent spatial components . Hum Brain Mapp . 1998 ; 6 : 160 – 188 . doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cole DM , Smith SM , Beckmann CF . Advances and pitfalls in the analysis and interpretation of resting-state fMRI data . Front Syst Neurosci . 2010 ; 4 : 8 . doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fair DA , Cohen AL , Dosenbach NU , et al. The maturing architecture of the brain’s default network . Proc Natl Acad Sci U S A . 2008 ; 105 : 4028 – 4032 . doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hagmann P , Cammoun L , Gigandet X , et al. Mapping the structural core of human cerebral cortex . PLoS Biol . 2008 ; 6 : e159 . doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Murphy K , Birn RM , Handwerker DA , Jones TB , Bandettini PA . The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? . Neuroimage . 2009 ; 44 : 893 – 905 . doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fox MD , Zhang D , Snyder AZ , Raichle ME . The global signal and observed anticorrelated resting state brain networks . J Neurophysiol . 2009 ; 101 : 3270 – 3283 . doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hyvarinen A , Karhunen J , Oja E . Independent Component Analysis . John Wiley & Sons ; 2001 . [Google Scholar]
- 53. De Luca M , Beckmann CF , De Stefano N , Matthews PM , Smith SM . fMRI resting state networks define distinct modes of long-distance interactions in the human brain . Neuroimage . 2006 ; 29 : 1359 – 1367 . doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 54. Greicius MD , Srivastava G , Reiss AL , Menon V . Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI . Proc Natl Acad Sci U S A . 2004 ; 101 : 4637 – 4642 . doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Esposito F , Aragri A , Pesaresi I , et al. Independent component model of the default-mode brain function: combining individual-level and population-level analyses in resting-state fMRI . Magn Reson Imaging . 2008 ; 26 : 905 – 913 . doi: 10.1016/j.mri.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 56. Birn RM , Murphy K , Bandettini PA . The effect of respiration variations on independent component analysis results of resting state functional connectivity . Hum Brain Mapp . 2008 ; 29 : 740 – 750 . doi: 10.1002/hbm.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Perlbarg V , Bellec P , Anton JL , Pélégrini-Issac M , Doyon J , Benali H . CORSICA: correction of structured noise in fMRI by automatic identification of ICA components . Magn Reson Imaging . 2007 ; 25 : 35 – 46 . doi: 10.1016/j.mri.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 58. Perlbarg V , Marrelec G . Contribution of exploratory methods to the investigation of extended large-scale brain networks in functional MRI: methodologies, results, and challenges . Int J Biomed Imaging . 2008 ; 2008 : 218519 . doi: 10.1155/2008/218519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Calhoun VD , Adali T , Pearlson GD , Pekar JJ . A method for making group inferences from functional MRI data using independent component analysis . Hum Brain Mapp . 2001 ; 14 : 140 – 151 . doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Filippini N , Macintosh BJ , Hough MG , et al. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele . P Proc Natl Acad Sci U S A . 2009 ; 106 : 7209 – 7214 . doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Esposito F , Scarabino T , Hyvarinen A , et al. Independent component analysis of fMRI group studies by self-organizing clustering . Neuroimage . 2005 ; 25 : 193 – 205 . doi: 10.1016/j.neuroimage.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 62. Birn RM , Diamond JB , Smith MA , Bandettini PA . Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fMRI . Neuroimage . 2006 ; 31 : 1536 – 1548 . doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 63. Chuang KH , Chen JH . IMPACT: image-based physiological artifacts estimation and correction technique for functional MRI . Magn Reson Med . 2001 ; 46 : 344 – 353 . doi: 10.1002/mrm.1197. [DOI] [PubMed] [Google Scholar]
- 64. Beall EB , Lowe MJ . Isolating physiologic noise sources with independently determined spatial measures . Neuroimage . 2007 ; 37 : 1286 – 1300 . doi: 10.1016/j.neuroimage.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 65. Gray MA , Minati L , Harrison NA , Gianaros PJ , Napadow V , Critchley HD . Physiological recordings: basic concepts and implementation during functional magnetic resonance imaging . Neuroimage . 2009 ; 47 : 1105 – 1115 . doi: 10.1016/j.neuroimage.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Greicius MD , Supekar K , Menon V , Dougherty RF . Resting-state functional connectivity reflects structural connectivity in the default mode network . Cereb Cortex . 2009 ; 19 : 72 – 78 . doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peltier SJ , Kerssens C , Hamann SB , Sebel PS , Byas-Smith M , Hu X . Functional connectivity changes with concentration of sevoflurane anesthesia . Neuroreport . 2005 ; 16 : 285 – 288 . doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- 68. Horovitz SG , Braun AR , Carr WS , et al. Decoupling of the brain’s default mode network during deep sleep . Proc Natl Acad Sci USA . 2009 ; 106 : 11376 – 11381 . doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ashburner J . A fast diffeomorphic image registration algorithm . Neuroimage . 2007 ; 38 : 95 – 113 . doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 70. Giugni E , Vadalà R , De Vincentiis C , Colica C , Bastianello S . The brain’s default mode network: a mind “sentinel” role? . Funct Neurol . 2010 ; 25 : 189 – 190 . [PubMed] [Google Scholar]