Abstract
The vascular endothelium regulates blood flow in response to physiological needs. Endothelial dysfunction is closely related to atherosclerosis and its risk factors, and it constitutes an intermediate step on the progression to adverse events throughout the natural history of coronary artery disease (CAD), often affecting clinical outcomes. Understanding the relation of endothelial function with CAD provides an important pathophysiological insight, which can be useful both in clinical and research management. In this review, we summarize the current knowledge on endothelial dysfunction and its prognostic influence throughout the natural history of CAD, from early atherosclerosis to post-transplant management.
Keywords: Endothelial dysfunction, Coronary heart disease, Heart failure, Heart transplant, Acetylcholine
Introduction
The aim of this review is to provide a summary of the current knowledge on endothelial dysfunction, particularly focused on its clinical implications in coronary artery disease (CAD). Both coronary and peripheral endothelial dysfunction will be discussed, as peripheral endothelial function often serves as a surrogate for coronary vascular function.
The vascular endothelium is a monolayer of cells covering the internal lumen of all blood vessels, thereby separating the blood from the vascular wall and organ tissues. The vascular endothelium serves different functions: (i) it is an anti-coagulant surface1; (ii) it regulates fluid and molecule traffic between blood and tissues2; (iii) it contributes to the vascular homeostasis and repair3; (iv) it plays a crucial role in vascular tone and blood flow regulation.4,5
Cardiovascular risk factors produce endothelial dysfunction by various complex mechanisms, among which oxidative stress is considered an important agent. Increased intracellular superoxide produces endothelial dysfunction by several mechanisms such as NO inactivation and formation of peroxinitrite, NO synthase uncoupling, prostacyclin formation inhibition, endothelin expression stimulation, and a reduced NO signalling due to inhibition of soluble guanylate cyclase activity.6 All these mechanisms are combined to promote a vasoconstrictive and pro-coagulant milieu in endothelial dysfunction.
When endothelial function is altered, any of its functions could be impaired. Yet, for practical reasons, it is the current standard to measure endothelial function by the study of its vasomotor regulation function. It is also customary to use the generic term ‘endothelial function’ as a synecdoche for endothelium-dependent vascular reactivity.
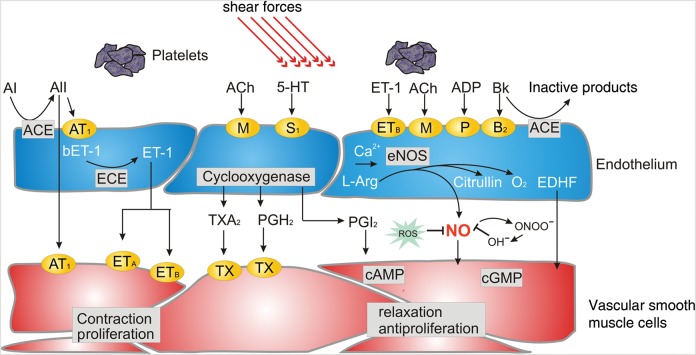
Since myocardial oxygen extraction is very high at basal conditions, any additional metabolic demands must be met by an increase in myocardial blood supply. In normal conditions, the coronary circulation is capable of increasing its basal flow by at least three times. This maximal increase in coronary blood flow is known as coronary flow reserve (CFR). The main agent in this blood flow regulation is the coronary endothelium, which produces vasodilator substances such as nitric oxide (NO) and prostacyclin and vasoconstrictor substances, mainly endothelin-1, in response to different physiological stimuli.7 Endothelial shear stress caused by arterial blood flow, and autacoids such as acetylcholine, histamine, and bradykinin are the main physiological triggers for endothelium release of NO, which in turn produces a guanylyl-cyclase-mediated relaxation of vascular smooth muscle (Figure 1).
Figure 1.
Endothelium-derived vasoactive substances. Shear stress and activation of a variety of receptors leads to a release of nitric oxide by inducing endothelial nitric oxide synthase. It exerts relaxation of vascular smooth muscle cells and exerts antiproliferative effects as well as inhibits thrombocyte aggregation and leucocyte adhesion. Other endothelium-derived relaxing factors, including endothelium-derived hyperpolarizing factor and prostacyclin, are also shown. ACE, angiotensin-converting enzyme; Ach, acetylcholine; AI, angiotensin I; AII, angiotensin II; AT1, angiotensin 1 receptor; Bk, bradykinin; COX, cyclooxygenase; ECE, ET-converting enzyme; EDHF, endothelium-derived hyperpolarizing factor; ETA and ETB, endothelin A and B receptors; ET-1, endothelin-1; l-Arg, l-arginine; M, muscarinic acetylcholine receptor; PGH2, prostaglandin H2; ROS, reactive oxygen species; S1, serotoninergic receptor; TX, thromboxane receptor; TXA2, thromboxane; 5-HT, serotonin. Reproduced with kind permission from Springer Science and Business Media. Source: Springer. Pflügers Arch- Eur J Physiol (2010) 459:1005–1013. Human endothelial dysfunction: EDRFs. Flammer and Lüscher.101
However, important, the vascular endothelium is not the only determinant of coronary blood flow. Coronary microvascular function can also be impaired due to other mechanisms as thrombi, debris embolization, ventricular hypertrophy, myocardial, and vascular oedema, smooth muscle dysfunction, etc.7,8 When microvascular function is impaired by an endothelium-independent factor, CFR is reduced, limiting the overall vasodilator capacity. Endothelium-independent microvascular dysfunction has been shown as a predictor of adverse cardiovascular events in diverse settings from early atherosclerosis9 to post-stenting10,11 and acute myocardial infarction.12 Thus, although the focus of this article is set on the prognostic importance of endothelial dysfunction, it has to be acknowledged that microvascular endothelium-independent dysfunction can also be an important source of flow dysregulation and adverse clinical outcomes.
Assessment of endothelial function
There are several ways to measure endothelial function in the clinical setting. Although it is far beyond the scope of this review to discuss the methodology in detail, we will very briefly summarize the most common possibilities. For further reading, we refer to recent comprehensive reviews.13,14 All the techniques have in common that they measure the response of the vessels to endothelial-dependent stimuli, mainly reactive hyperaemia (shear stress) or vasoactive substances.
The coronary vasomotion can be assessed directly and invasively by coronary angiography.15–17 In principle, intracoronary vasoactive stimuli, mainly acetylcholine, are infused to trigger an endothelium-dependent vascular reaction. A functioning endothelium releases NO in response to acetylcholine, causing vasodilation in the epicardial arteries and the coronary microcirculation. Epicardial vasodilation is measured by quantitative angiography or intravascular ultrasound (IVUS); vasodilation in the microcirculation is assessed measuring the coronary blood flow with a Doppler wire, since the microcirculation is the main determinant of coronary resistance, and therefore of coronary blood flow. If the coronary endothelium is dysfunctional, NO release is deficient, and paradoxical vasoconstriction, due to direct muscarinic smooth muscle stimulation, is observed in the epicardial arteries or the microcirculation. Non-endothelial vasodilation can be measured using other drugs, such as adenosine or nitroprusside for the microcirculation, and nitroglycerine or nitroprusside for the epicardial arteries. Other non-pharmacological approaches which have been described for coronary endothelium-dependent vasodilation assessment are based on flow-mediated dilation (FMD) in response to hyperaemia, like exercise, mental stress or pacing, or on sympathetic nervous system activation, like the cold pressor test.
The same principle of FMD can also be applied to the peripheral vasculature18: Briefly, ultrasound images of the brachial artery are used to determine arterial diameter before and after reactive hyperaemia-induced vasodilation. Reactive hyperaemia is achieved by causing limb ischaemia with a blood pressure cuff for a few minutes. Flow-mediated dilation is commonly expressed as per cent change in artery diameter. This technique correlates with invasively measured epicardial coronary function19 and has been widely used in clinical research.
Peripheral arterial tonometry (PAT) is a newer technique based on the change in finger pulse wave amplitude in response to reactive hyperaemia.20 A mini-cuff is used on the finger to record pulse amplitude, and another on the contralateral arm to serve as a control. This relatively simple technique with a low observer-dependency indeed correlates with microvascular coronary endothelial function.21 However, the observed reaction is only partly endothelial dependent,22 and other factors affecting the microcirculation, such as the sympathetic nervous system may affect this measurement.20
Endothelial dysfunction in early asymptomatic atherosclerosis
The development of atherosclerosis is a continuous process starting already early in life and has a long asymptomatic initial phase and a slow progression caused and accelerated by the presence of different risk factors.23 Importantly, endothelial dysfunction is one of the first recognizable signs of its development and is present long before the sometimes-devastating consequences of atherosclerosis appear. Endothelial dysfunction has been reported in relation with most, if not all, risk factors for atherosclerosis,24–26 such as hypertension,27 diabetes,28 hyperlipidaemia,29 and ageing.30,31 Endothelial dysfunction not only is a marker of atherosclerosis but it itself contributes to the progression of atherosclerosis32 by various mechanisms, by promoting coagulation, vasoconstriction, and deficient or pathological vascular repair.33 Interestingly, endothelial dysfunction itself can cause myocardial ischaemia even in the absence of relevant coronary stenosis.34 Thus, it has been proposed that endothelial dysfunction constitutes a first stage of atherosclerosis, summarizing the influence of all cardiovascular risk factors, and can itself be the cause of cardiovascular events.35 As endothelial function is associated with risk factors, the absence of endothelial dysfunction might depict a particularly favourable state.36,37
In primary prevention, physicians mainly rely on classical risk scores (e.g. Framingham or SCORE) to assess the risk for patients. The measurement of endothelial function, however, as it depicts the overall burden of risk and includes so far unknown factors, might be a better measure for re-classification and personalized decision-making.
For example, Li et al.38 recently showed a worse endothelial function, as measured by PAT, in patients with the metabolic syndrome compared with those without it, even though both had the same number of classical risk factors present. Interestingly, the Northern Manhattan Study39 prospectively followed 819 subjects during a mean of 81 months for cardiovascular events, focusing on the incremental predictive value of FMD and its relation to metabolic syndrome. Patients with metabolic syndrome had a higher rate of events (HR: 1.5), but patients with metabolic syndrome and endothelial dysfunction had the highest rate (HR: 2.6), which shows an incremental prognostic value of endothelial dysfunction. In type 2 diabetes a normal peripheral endothelium-dependent vasodilation (as established by an FMD >8%) has an excellent negative predictive value for CAD.37
The cardiovascular Health Study40 evaluated the relation between brachial FMD and cardiovascular events in a cohort of 2700 elderly patients without known cardiovascular disease. Over a 5-year follow-up period, FMD was a predictor of cardiovascular events, even after adjustment for other risk factors. More recently, the multi-ethnic study of atherosclerosis (MESA) study, by the same group, proved the prognostic value of FMD independently of the Framingham Risk Score in over 3000 patients followed over 5 years.41 Similar results were obtained in a cohort of 2264 asymptomatic postmenopausal women42 followed up for a mean period of 45 months, in whom endothelial function was also evaluated by FMD. However, in other series, as the FATE study43 and the PIVUS study,44 FMD failed to add incremental prediction value or even correlate with events (Table 1). However, in the FATE study hyperaemic velocity, a correlate of microvascular dilation, did correlate with events and it provided additional independent prognostic value. Methodological differences and a younger and presumably healthier population in the FATE study may be accountable for this disparity.
Table 1.
Studies of cardiovascular events in patients with endothelial dysfunction and early or stable coronary artery disease
| Study | Population | Number | Method | Endpoints | Results |
|---|---|---|---|---|---|
| NOMAS39 | Asymptomatic, >40 years old | 819 | FMD | MACE (Stroke + MI + CV death) | OR 2.89 for patients with endothelial dysfunction and metabolic syndrome; OR 1.64 (NS) for endothelial dysfunction alone. |
| Cardiovascular Health Study40 | Asymptomatic, elderly (72–98 years old) | 2792 | FMD | MACE, heart failure, revascularization, claudication | Event free survival at 5 years 78.3 vs. 73.6 (FMD >median vs. FMD <median) P = 0.006 |
| MESA41 | Asymptomatic | 3026 | FMD | CV death, MI, angina, revascularization, cardiac arrest, stroke | OR 0.79 FMD/unit SD; 29% correct reclassification of risk. |
| Rossi42 | Asymptomatic postmenopausal women | 2264 | FMD | MACE | OR 4.42 for lower FMD tertile vs. higher FMD tertile |
| FATE43 | Healthy, male, low-intermediate FRS | 1574 | FMD | MACE | OR 0.92, NS |
| Papaioannou37 | Asymptomatic, type 2 diabetic | 75 | FMD | Nuclear myocardial perfusion imaging | FMD >8% had a negative predictive value of 93% for myocardial ischaemia |
| PIVUS44 | Asymptomatic, over 70 years old | 1016 | Invasive brachial—FMD—radial tonometry | MACE | Invasive brachial OR 0.72/SD (P = 0.01). FMD and tonometry no prognostic value |
| Framingham third generation PAT100 | General population, Framingham 3rd generation | 1957 | PAT | Relation to CV risk factors | Correlation with male, BMI, total chol/HDL, smoking, diabetes, lipid-lowering treatment |
| Rubinhstein50 | Symptomatic; low risk stress tests or normal coronary angiogram | 270 | PAT | MACE + Revascularization + CV hospitalization | Events 48 vs. 28% at 7 years, P = 0.03 |
| Suwaidi17 | Symptomatic; no significant coronary atherosclerosis | 157 | Direct coronary | MI + CV death + heart failure + revascularization | Only patients with severe endothelial dysfunction had events (14%) |
| Schachinger47 | Symptomatic; no stenosis or single vessel | 147 | Direct coronary | MI + CV death + heart failure + revascularization + angina + stroke + peripheral revascularization | Patients with events had significantly poorer vascular response to acetylcholine |
| Halcox51 | Symptomatic; with and without CAD | 308 (132 with CAD; 176 without) | Direct coronary | CV death + AMI + unstable angina + stroke | Patients with MACE had poorer response, both micro and macrovascular |
| Heitzer52 | Documented CAD | 281 | Brachial plethysmography | CV death, MI, stroke, revascularization | Patients with events had lower responses |
| Nitenberg49 | Diabetic patients, no obstructive CAD; vs. non-diabetic controls | 72 diabetics 56 controls | Direct coronary, cold pressor test | Sudden cardiac death, MI, angina, stroke, TIA, revascularization | Diabetics with abnormal cold pressor test showed higher MACE rates |
Thus, the presence of endothelial function may serve as an important tool to re-classify the risk of the patients beyond the conventional risk factors. Future studies thus have to better delineate which patients benefit most from a measurement and which method is best to assess endothelial dysfunction.
Endothelial dysfunction in symptomatic patients without obstructive coronary artery disease
Over 20% of the patients referred to a coronary angiogram due to chest pain do not present significant obstructive coronary stenosis.45 Yet, a considerable proportion of these patients suffer from typical angina, and often myocardial perfusion defects can be demonstrated. Many of these patients present an abnormal coronary endothelial function, which can result in chronic ischaemia and facilitate acute ischaemic events, as explained below.
A study in 27 patients showed how abnormal flow response to selective infusion of acetylcholine in the left anterior descending artery (LAD) in patients with angina and non-obstructive CAD was related to concordant exercise-induced myocardial perfusion defects on SPECT.46 Lerman demonstrated that the perfusion defect could be provoked at rest using the same method of selective LAD acetylcholine infusion.34 The results of these two studies show how coronary vascular dysfunction without severe obstructive disease can be the source of myocardial ischaemia due to endothelial dysfunction and the resulting flow dysregulation.
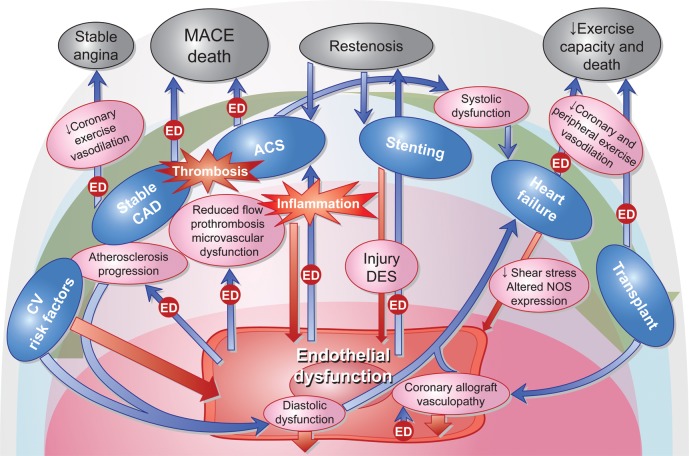
Because the vascular endothelium not only regulates vascular tone and flow, but has also important roles in vascular permeability and thrombosis homeostasis, endothelial dysfunction not only may lead to chronic stable myocardial ischaemia, but can also be the source atherosclerosis progression32 and acute ischaemic events (Figures 2). One prospective study from the Mayo Clinic17 in 157 patients with mild CAD, and an invasive coronary endothelial function test by acetylcholine infusion in the LAD, showed coronary endothelial dysfunction to be an independent predictor of cardiovascular events. Schachinger et al.47 had similar results in another study with 147 patients, using the same method. In two other studies performed in diabetic patients without obstructive CAD, in whom coronary endothelial function was tested by the cold pressor test, coronary endothelial dysfunction showed a correlation with myocardial perfusion defects48 and long-term adverse cardiovascular events.49
Figure 2.
Illustration of the reciprocal interaction between endothelial dysfunction, inflammation and the natural history of coronary artery disease. Blue arrows marked with the box ‘ED’ represent processes in which endothelial dysfunction modifies the evolution or prognosis of coronary artery disease. Red arrows represent ways in which coronary artery disease contributes to a worse endothelial function.
In symptomatic patients without obstructive CAD, non-invasive peripheral microvascular endothelial function measured by PAT is also able to discriminate subjects at high risk for adverse cardiovascular events.50
Endothelial dysfunction in stable coronary artery disease
In patients with stable obstructive CAD, endothelial function is an independent predictor of symptoms and cardiovascular risk. In one study, 308 patients with and without CAD were studied by direct coronary endothelial function testing. Abnormal response to selective intracoronary acetylcholine infusion, both in the epicardial and microvascular circulation, was an independent predictor of MACE, even after adjusting for the presence of CAD.51 Another study in 281 patients with documented CAD showed that peripheral endothelial dysfunction also identifies subjects at a higher risk for cardiovascular events.52
Diabetic patients constitute a high-risk population for symptomatic and silent myocardial ischaemia. Two substudies of the Detection of Ischemia in Asymptomatic Diabetics study evaluated the utility of FMD to predict silent myocardial ischaemia37 and microvascular damage as expressed by microalbuminuria.53 In these studies, a cutoff value of 8% FMD showed a high negative predictive value for silent myocardial ischaemia, and microalbuminuria correlated with abnormal endothelium-dependent vasoreactivity.
Although endothelial dysfunction is a systemic condition, and thus non-invasive functional tests correlate with invasive tests, endothelial dysfunction in the epicardial arteries can also be focal. One study showed a correlation between focal endothelial dysfunction and a larger necrotic core, as measured by IVUS.54 This study provides a rationale for endothelial dysfunction being related to acute coronary syndrome (ACS). Whether in this case endothelial dysfunction is the cause or the expression of a vulnerable plaque is uncertain.
Endothelial dysfunction after coronary artery stenting
Coronary stenting results in acute endothelial injury, sometimes even associated with arterial dissection or haematoma, and in the presence of prosthetic metallic material in the vessel lumen; in the case of drug-eluting stents (DES), this material is associated with an artificial polymer and a drug. A functional vascular endothelium prevents platelet adhesion, aggregation, and activation by the secretion of prostacyclin and NO, and maintains an adequate balance between pro-coagulant (tissue factor) and anti-coagulant (heparin, protein C/S) and thrombolytic factors (tissue plasminogen activator)33; an injured or dysfunctional endothelium loses its antiplatelet function and may favour pro-coagulant activity, which in combination with a reduced blood flow due to vasoconstriction can increase the risk of stent thrombosis.55
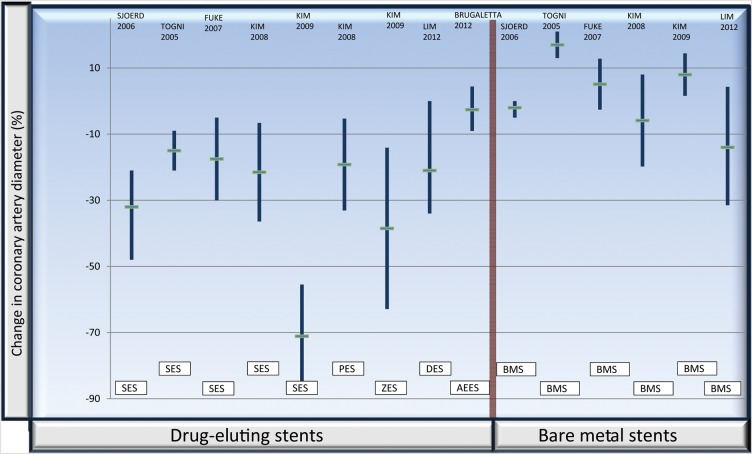
Several studies with DES have shown a persistently abnormal endothelium-dependent vasoreactivity in coronary arteries after DES implantation (Figure 3). Both the sirolimus56,57 and the paclitaxel58 stents exhibit a detrimental effect extending over at least 6 months, while the second-generation zotarolimus stent seems to have a more benign albeit not neutral behaviour.59 In the study by Togni, endothelial response was evaluated by bicycle exercise, while in the rest of the studies the method used was acetylcholine infusion in the left coronary artery.
Figure 3.
Illustration of the post-stent epicardial reactivity to acetylcholine in the various studies referenced in the text56–62. The values express the change in coronary artery lumen diameter (%) to acetylcholine or exercise, measured by angiography; horizontal short lines represent average, and vertical bars standard deviation. The red vertical line separates drug-eluting stents from bare metal stents. As can be appreciated, patients with drug-eluting stents exhibit a vasoconstrictive response, while patients with bare metal stents have a roughly neutral vascular response. AEES, absorbable everolimus eluting scaffold; BMS, bare metal stent; PES, paclitaxel eluting stent; DES, drug eluting stent; SES, sirolimus eluting stent; ZES, zotarolimus eluting stent.
The new bioresorbable coronary scaffolds may be promising in their ability to allow long-term restoration of coronary vasomotion, especially at the stented segments. Data from the ABSORB stent60,61 (a polylactide bioresorbable everolimus-eluting scaffold) showed an abnormal response of the segment distal to the scaffold at 12 months, which improved significantly at 24 months; interestingly, the scaffolded segments showed improved endothelial-dependent and independent vasomotion at 24 months of implantation, which was related to scaffold degradation assessed by IVUS.
Lim et al. recently showed that in patients who were evaluated in the catheterization laboratory due to chest pain (in average >1 year after stent placement) coronary vascular function was not worse than in controls.62 Thus, although it is generally accepted that coronary endothelial dysfunction may be impaired in the short term, there might not be long-term relevance. Interestingly, however, endothelial dysfunction as assessed by brachial FMD has been reported as a predictor of in-stent restenosis.63
Endothelial dysfunction in acute coronary syndrome
Most frequently, in ACS there is a local inflammatory status which affects endothelial function and can make atherosclerotic plaques more prone to rupture and platelet adhesion, vasospasm, and stasis, which can precipitate coronary thrombosis.64 Microvascular function is frequently impaired, but it is uncertain whether this is a contributor or a consequence of the ACS, and it may be to a large extent endothelial independent, due to thrombi and debris embolization, myocardial oedema, and other factors. Endothelial function testing is particularly challenging in ACS. The unpredictable nature of ACS onset, the unstable condition of the patient and the early dramatic impairment in microvascular function, make it very difficult to obtain and interpret data concerning vascular reactivity in this context.
Coronary endothelial dysfunction seems to be present in most patients with acute coronary syndrome, and is reversible in a matter of months.65 In patients with unstable angina, endothelium-dependent vasoreactivity parallels inflammatory activity as expressed by C-reactive protein levels.66
Endothelial function after ACS, measured in the peripheral circulation, has been shown as an independent predictor of MACE, and subsequent normalization of endothelial function in these patients predicts a lower risk of events.67,68 In patients with ST elevation ACS treated with primary angioplasty, FMD improvement 6 months after the event also correlates with a lower end-diastolic left ventricular volume.69
Endothelial dysfunction and heart failure
Diastolic function is most frequently impaired in CAD, as a result of hypertension, obesity or hypertrophy, but also due to a reduced NO bioavailability in the myocardium. Nitric oxide plays a role in myocardial as well as vascular relaxation,70 and there is animal evidence that reduced NO availability due to reactive oxygen species (ROS) and NOS uncoupling is a causal factor in the development of diastolic dysfunction.71 In humans with CAD a high prevalence of diastolic dysfunction has been found, which correlates with endothelial dysfunction as assessed by FMD.72 Myocardial damage in CAD is frequently not limited to diastolic function, but can lead to systolic dysfunction due to myocardial necrosis and adverse remodelling, and it has already been mentioned how endothelial dysfunction impairs cardiac remodelling after an acute myocardial infarction.69
Independently of the initial mechanism for heart failure, endothelial dysfunction plays a major role in the progression of the disease and has an important impact on clinical outcomes.73–75 The reduced stroke volume produces a lower endothelial shear stress, which causes a dysregulation in NO synthase isoforms gene expression,76,77 eventually leading to a reduced NO bioavailability.78 Furthermore, there is an additional reduction in NO bioavailability caused by direct NO destruction by ROS, mainly driven by an increase in angiotensin II and aldosterone activity, and purine metabolism.79
Both coronary and peripheral endothelial dysfunction have been found in ischaemic as well as non-ischaemic heart failure patients,80–82 although peripheral endothelial dysfunction, measured by brachial FMD, seems to be less important in non-ischaemic patients,83 suggesting an incremental role of atherosclerosis in ischaemic patients.
The impaired peripheral endothelium-dependent vasodilation in response to exercise may limit the oxygen supply to skeletal muscles and thus have a negative impact on functional class. Peripheral endothelial dysfunction in heart failure is related to a worse clinical outcome.73–75 Conversely, regular exercise training has proved capable of restoring peripheral endothelial vasomotor function in patients with heart failure,84,85 which is accompanied by a higher exercise capacity. Thus, the systemic endothelial dysfunction in heart failure underscores the systemic nature of the disease and may be used to assess the effectiveness of therapy and predict events.
Endothelial dysfunction in heart failure, measured by brachial FMD, has been shown to be a pre-procedural predictor of good response to cardiac resynchronization therapy (CRT) in one study. Also, endothelial dysfunction improvement after CRT correlated with functional improvement.86 Two other studies have shown that CRT improves endothelial dysfunction in heart failure, and that this improvement is related to an increase in cardiac output, thus probably shear-stress mediated.87,88 This may be one path by which CRT improves symptoms in heart failure.
Endothelial function after heart transplant
After heart transplant the coronary endothelium is replaced by a presumably healthier one, stroke volume increases and so does endothelial shear stress, one of the main inducers of NO formation. However, the restoration of endothelial function is not immediate or complete, probably due to the persistence of atherosclerosis risk factors, inflammation and the use of immunosuppressive drugs such as cyclosporine.
Indeed, a high prevalence of coronary endothelial dysfunction has been described early after heart transplant.89 Coronary endothelial dysfunction in early post-transplant patients has been correlated with a higher risk of developing cardiac allograft vasculopathy (CAV) as expressed by intimal thickening90 and clinical events.91 When allograft vasculopathy is evident, coronary endothelial dysfunction is the norm.89 Immunosuppression with cyclosporine contributes to the development of endothelial dysfunction in post-transplant patients through various mechanisms, such as a decreased synthesis of NO,92 increased levels of endothelin93 and increased production of ROS.94 Sirolimus seems to have a more benign effect on endothelial function than cyclosporine.95
The evolution of peripheral endothelial function after heart transplant remains controversial. Although it has been reported to improve early after transplantation,96 other studies have not confirmed this observation,97,98 and indeed have found a high and persistent prevalence of peripheral endothelial dysfunction in the first year post-transplantation. Peripheral endothelial dysfunction limits the vasodilator response to exercise, correlates with functional capacity and may be the cause why heart transplant recipients often do not achieve a normal functional status.99 Also, it has been found to correlate with the risk of developing CAV in the first year.97
Conclusion
The assessment of endothelial function provides us with the ability to obtain information on the functional significance of cardiovascular disease at the different stages of the disease. There is a growing body of evidence to suggest that mainly the non-invasive assessment may intergrade into our practice to enable us to classify the risk of the patients with CAD and assess the success of therapy.
Conflict of interest: A.L. is on the advisory board of Itamar Medical. A.L.'s research is funded by the National Institutes of Health (HL92954, HL77131, DK73608, AG31750) and the Mayo Foundation.
Acknowledgements
The authors wish to acknowledge Dr Bernard Gersh for his valued advice and encouragement.
References
- 1.de Agostini AI, Watkins SC, Slayter HS, Youssoufian H, Rosenberg RD. Localization of anticoagulantly active heparan sulfate proteoglycans in vascular endothelium: antithrombin binding on cultured endothelial cells and perfused rat aorta. J Cell Biol. 1990;111:1293–1304. doi: 10.1083/jcb.111.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Hinsbergh WM. Endothelial permeability for macromolecules. Mechanistic aspects of pathophysiological modulation. Arterioscler Thromb Vasc Biol. 1997;17:1018–1023. doi: 10.1161/01.atv.17.6.1018. [DOI] [PubMed] [Google Scholar]
- 3.Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 4.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 5.Griendling KK, Harrison DG, Alexander RW. Biology of the vessel wall. In: Fuster V, Walsh R, Harrington R, editors. Hurst's The Heart. 13th ed. Mcgraw-hill; 2010. [Google Scholar]
- 6.Munzel T, Gori T, Bruno RM, Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 7.Lüscher TF, Flammer AJ, Lerman A. Assessment of coronary vasoreactivity and the microcirculation. In: Eeckhout E SP, Wijns W, Vahanian A, van Sambeek M, De Palma R, editors. Percutaneous Interventional Cardiovascular Medicine: The PCR-EAPCI Textbook. Toulouse, France: Europa Edition; 2012. [Google Scholar]
- 8.Lerman A, Holmes DR, Herrmann J, Gersh BJ. Microcirculatory dysfunction in ST-elevation myocardial infarction: cause, consequence, or both? Eur Heart J. 2007;28:788–797. doi: 10.1093/eurheartj/ehl501. [DOI] [PubMed] [Google Scholar]
- 9.Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis. 2004;15:259–264. doi: 10.1097/01.mca.0000134590.99841.81. [DOI] [PubMed] [Google Scholar]
- 10.Albertal M, Voskuil M, Piek JJ, de Bruyne B, Van Langenhove G, Kay PI, Costa MA, Boersma E, Beijsterveldt T, Sousa JE, Belardi JA, Serruys PW. Coronary flow velocity reserve after percutaneous interventions is predictive of periprocedural outcome. Circulation. 2002;105:1573–1578. doi: 10.1161/01.cir.0000012514.15806.dd. [DOI] [PubMed] [Google Scholar]
- 11.Herrmann J, Haude M, Lerman A, Schulz R, Volbracht L, Ge J, Schmermund A, Wieneke H, von Birgelen C, Eggebrecht H, Baumgart D, Heusch G, Erbel R. Abnormal coronary flow velocity reserve after coronary intervention is associated with cardiac marker elevation. Circulation. 2001;103:2339–2345. doi: 10.1161/01.cir.103.19.2339. [DOI] [PubMed] [Google Scholar]
- 12.Sorajja P, Gersh BJ, Costantini C, McLaughlin MG, Zimetbaum P, Cox DA, Garcia E, Tcheng JE, Mehran R, Lansky AJ, Kandzari DE, Grines CL, Stone GW. Combined prognostic utility of ST-segment recovery and myocardial blush after primary percutaneous coronary intervention in acute myocardial infarction. Eur Heart J. 2005;26:667–674. doi: 10.1093/eurheartj/ehi167. [DOI] [PubMed] [Google Scholar]
- 13.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, Cosentino F, Deanfield J, Gallino A, Ikonomidis I, Kremastinos D, Landmesser U, Protogerou A, Stefanadis C, Tousoulis D, Vassalli G, Vink H, Werner N, Wilkinson I, Vlachopoulos C. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Circulation. Eur J Cardiovasc Prev Rehabil. 2011;18:775–789. doi: 10.1177/1741826711398179. [DOI] [PubMed] [Google Scholar]
- 15.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 16.Cox DA, Vita JA, Treasure CB, Fish RD, Alexander RW, Ganz P, Selwyn AP. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989;80:458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- 17.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 18.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 19.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrage D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulation. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 20.Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, Karas RH, Udelson JE. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003;146:168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 21.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 22.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101:545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 23.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG, Lauer MS, Shaw LJ, Smith SC, Jr, Taylor AJ, Weintraub WS, Wenger NK, Jacobs AK. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 24.Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81:491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- 25.Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833–843. doi: 10.1016/0735-1097(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 26.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–175. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 27.Panza JA, Quyyumi AA, Brush JJ, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 28.Makimattila S, Virkamaki A, Groop PH, Cockcroft J, Utriainen T, Fagerudd J, Yki-Jarvinen H. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94:1276–1282. doi: 10.1161/01.cir.94.6.1276. [DOI] [PubMed] [Google Scholar]
- 29.Casino PR, Kilcoyne CM, Quyyumi AA, Hoeg JM, Panza JA. The role of nitric oxide in endothelium-dependent vasodilation of hypercholesterolemic patients. Circulation. 1993;88:2541–2547. doi: 10.1161/01.cir.88.6.2541. [DOI] [PubMed] [Google Scholar]
- 30.Yasue H, Matsuyama K, Okumura K, Morikami Y, Ogawa H. Responses of angiographically normal human coronary arteries to intracoronary injection of acetylcholine by age and segment. Possible role of early coronary atherosclerosis. Circulation. 1990;81:482–490. doi: 10.1161/01.cir.81.2.482. [DOI] [PubMed] [Google Scholar]
- 31.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. The ageing endothelium, cardiovascular risk and disease in man. Exp Physiol. 2009;94:317–321. doi: 10.1113/expphysiol.2008.043356. [DOI] [PubMed] [Google Scholar]
- 32.Lerman A, Cannan CR, Higano SH, Nishimura RA, Holmes DR., Jr Coronary vascular remodeling in association with endothelial dysfunction. Am J Cardiol. 1998;81:1105–1109. doi: 10.1016/s0002-9149(98)00135-0. [DOI] [PubMed] [Google Scholar]
- 33.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 34.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 35.Reriani MK, Lerman LO, Lerman A. Endothelial function as a functional expression of cardiovascular risk factors. Biomark Med. 2010;4:351–360. doi: 10.2217/bmm.10.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Flammer AJ, Nelson RE, Gulati R, Friedman PA, Thomas RJ, Sandhu NP, Reriani MK, Lerman LO, Lerman A. Normal vascular function as a prerequisite for the absence of coronary calcification in patients free of cardiovascular disease and diabetes. Circ J. 2012;76:2705–2710. doi: 10.1253/circj.cj-12-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papaioannou GI, Kasapis C, Seip RL, Grey NJ, Katten D, Wackers FJ, Inzucchi SE, Engel S, Taylor A, Young LH, Chyun DA, Davey JA, Iskandrian AE, Ratner RE, Robinson EC, Carolan S, Heller GV. Value of peripheral vascular endothelial function in the detection of relative myocardial ischemia in asymptomatic type 2 diabetic patients who underwent myocardial perfusion imaging. J Nucl Cardiol. 2006;13:362–368. doi: 10.1016/j.nuclcard.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Flammer AJ, Lennon RJ, Nelson RE, Gulati R, Friedman PA, Thomas RJ, Sandhu NP, Hua Q, Lerman LO, Lerman A. Comparison of the effect of the metabolic syndrome and multiple traditional cardiovascular risk factors on vascular function. Mayo Clin Proc. 2012;87:968–975. doi: 10.1016/j.mayocp.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki T, Hirata K, Elkind MS, Jin Z, Rundek T, Miyake Y, Boden-Albala B, Di Tullio MR, Sacco R, Homma S. Metabolic syndrome, endothelial dysfunction, and risk of cardiovascular events: the Northern Manhattan Study (NOMAS) Am Heart J. 2008;156:405–410. doi: 10.1016/j.ahj.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 41.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi R, Nuzzo A, Origliani G, Modena MG. Prognostic role of flow-mediated dilation and cardiac risk factors in post-menopausal women. J Am Coll Cardiol. 2008;51:997–1002. doi: 10.1016/j.jacc.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 43.Anderson TJ, Charbonneau F, Title LM, Buithieu J, Rose MS, Conradson H, Hildebrand K, Fung M, Verma S, Lonn EM. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123:163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 44.Lind L, Berglund L, Larsson A, Sundstrom J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation. 2011;123:1545–1551. doi: 10.1161/CIRCULATIONAHA.110.984047. [DOI] [PubMed] [Google Scholar]
- 45.Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, Pijls NH, Siebes M, Spaan JA. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321–1341. doi: 10.1161/CIRCULATIONAHA.106.177276. [DOI] [PubMed] [Google Scholar]
- 46.Zeiher AM, Krause T, Schachinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345–2352. doi: 10.1161/01.cir.91.9.2345. [DOI] [PubMed] [Google Scholar]
- 47.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 48.Nitenberg A, Ledoux S, Valensi P, Sachs R, Attali JR, Antony I. Impairment of coronary microvascular dilation in response to cold pressor—induced sympathetic stimulation in type 2 diabetic patients with abnormal stress thallium imaging. Diabetes. 2001;50:1180–1185. doi: 10.2337/diabetes.50.5.1180. [DOI] [PubMed] [Google Scholar]
- 49.Nitenberg A, Valensi P, Sachs R, Cosson E, Attali JR, Antony I. Prognostic value of epicardial coronary artery constriction to the cold pressor test in type 2 diabetic patients with angiographically normal coronary arteries and no other major coronary risk factors. Diabetes Care. 2004;27:208–215. doi: 10.2337/diacare.27.1.208. [DOI] [PubMed] [Google Scholar]
- 50.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 51.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 52.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 53.Papaioannou GI, Seip RL, Grey NJ, Katten D, Taylor A, Inzucchi SE, Young LH, Chyun DA, Davey JA, Wackers FJ, Iskandrian AE, Ratner RE, Robinson EC, Carolan S, Engel S, Heller GV. Brachial artery reactivity in asymptomatic patients with type 2 diabetes mellitus and microalbuminuria (from the Detection of Ischemia in Asymptomatic Diabetics-brachial artery reactivity study) Am J Cardiol. 2004;94:294–299. doi: 10.1016/j.amjcard.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 54.Lavi S, Bae JH, Rihal CS, Prasad A, Barsness GW, Lennon RJ, Holmes DR, Jr, Lerman A. Segmental coronary endothelial dysfunction in patients with minimal atherosclerosis is associated with necrotic core plaques. Heart. 2009;95:1525–1530. doi: 10.1136/hrt.2009.166017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamasaki S, Tei C. Effect of coronary endothelial function on outcomes in patients undergoing percutaneous coronary intervention. J Cardiol. 2011;57:231–238. doi: 10.1016/j.jjcc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 56.Togni M, Windecker S, Cocchia R, Wenaweser P, Cook S, Billinger M, Meier B, Hess OM. Sirolimus-eluting stents associated with paradoxic coronary vasoconstriction. J Am Coll Cardiol. 2005;46:231–236. doi: 10.1016/j.jacc.2005.01.062. [DOI] [PubMed] [Google Scholar]
- 57.Fuke S, Maekawa K, Kawamoto K, Saito H, Sato T, Hioka T, Ohe T. Impaired endothelial vasomotor function after sirolimus-eluting stent implantation. Circ J. 2007;71:220–225. doi: 10.1253/circj.71.220. [DOI] [PubMed] [Google Scholar]
- 58.Kim JW, Suh SY, Choi CU, Na JO, Kim EJ, Rha SW, Park CG, Seo HS, Oh DJ. Six-month comparison of coronary endothelial dysfunction associated with sirolimus-eluting stent vs. Paclitaxel-eluting stent. JACC Cardiovasc Interv. 2008;1:65–71. doi: 10.1016/j.jcin.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Kim JW, Seo HS, Park JH, Na JO, Choi CU, Lim HE, Kim EJ, Rha SW, Park CG, Oh DJ. A prospective, randomized, 6-month comparison of the coronary vasomotor response associated with a zotarolimus- vs. a sirolimus-eluting stent: differential recovery of coronary endothelial dysfunction. J Am Coll Cardiol. 2009;53:1653–1659. doi: 10.1016/j.jacc.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 60.Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hebert K, Veldhof S, Webster M, Thuesen L, Dudek D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897–910. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 61.Brugaletta S, Heo JH, Garcia-Garcia HM, Farooq V, van Geuns RJ, de Bruyne B, Dudek D, Smits PC, Koolen J, McClean D, Dorange C, Veldhof S, Rapoza R, Onuma Y, Bruining N, Ormiston JA, Serruys PW. Endothelial-dependent vasomotion in a coronary segment treated by ABSORB everolimus-eluting bioresorbable vascular scaffold system is related to plaque composition at the time of bioresorption of the polymer: indirect finding of vascular reparative therapy? Eur Heart J. 2012;33:1325–1333. doi: 10.1093/eurheartj/ehr466. [DOI] [PubMed] [Google Scholar]
- 62.Lim SH, Flammer AJ, Yoon MH, Lennon RJ, Gulati R, Mathew V, Rihal CS, Lerman LO, Lerman A. The long-term effect of coronary stenting on epicardial and microvascular endothelial function. Circ Cardiovasc Interv. 2012;5:523–529. doi: 10.1161/CIRCINTERVENTIONS.112.970111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patti G, Pasceri V, Melfi R, Goffredo C, Chello M, D'Ambrosio A, Montesanti R, Di Sciascio G. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111:70–75. doi: 10.1161/01.CIR.0000151308.06673.D2. [DOI] [PubMed] [Google Scholar]
- 64.Tousoulis D, Charakida M, Stefanadis C. Endothelial function and inflammation in coronary artery disease. Heart. 2006;92:441–444. doi: 10.1136/hrt.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elbaz M, Carrie D, Baudeux JL, Arnal JF, Maupas E, Lotterie JA, Perret B, Puel J. High frequency of endothelial vasomotor dysfunction after acute coronary syndromes in non-culprit and angiographically normal coronary arteries: a reversible phenomenon. Atherosclerosis. 2005;181:311–319. doi: 10.1016/j.atherosclerosis.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 66.Fichtlscherer S, Rosenberger G, Walter G, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein levels and impaired endothelial vasoreactivity in patients with coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 67.Fichtlscherer S, Breuer S, Zeiher AM. Prognostic value of systemic endothelial dysfunction in patients with acute coronary syndromes: further evidence for the existence of the ‘vulnerable’ patient. Circulation. 2004;110:1926–1932. doi: 10.1161/01.CIR.0000143378.58099.8C. [DOI] [PubMed] [Google Scholar]
- 68.Careri G, Nerla R, Di Monaco A, Russo G, Stazi A, Villano A, Sestito A, Lanza GA, Crea F. Clinical correlates and prognostic value of flow mediated dilation in patients with non-ST segment elevation acute coronary syndromes. Am J Cardiol. 2013;111:51–57. doi: 10.1016/j.amjcard.2012.08.049. [DOI] [PubMed] [Google Scholar]
- 69.Bissinger A, Grycewicz T, Grabowicz W, Lubinski A. Endothelial function and left ventricular remodeling in diabetic and non-diabetic patients after acute coronary syndrome. Med Sci Monit. 2011;17:CR73–CR77. doi: 10.12659/MSM.881390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michel T. NO way to relax: the complexities of coupling nitric oxide synthase pathways in the heart. Circulation. 2010;121:484–486. doi: 10.1161/CIR.0b013e3181d1e24e. [DOI] [PubMed] [Google Scholar]
- 71.Silberman GA, Fan TH, Liu H, Jiao Z, Xiao HD, Lovelock JD, Boulden BM, Widder J, Fredd S, Bernstein KE, Wolska BM, Dikalov S, Harrison DG, Dudley SC., Jr Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation. 2010;121:519–528. doi: 10.1161/CIRCULATIONAHA.109.883777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ma LN, Zhao SP, Gao M, Zhou QC, Fan P. Endothelial dysfunction associated with left ventricular diastolic dysfunction in patients with coronary heart disease. Int J Cardiol. 2000;72:275–279. doi: 10.1016/s0167-5273(99)00203-x. [DOI] [PubMed] [Google Scholar]
- 73.Shechter M, Matetzky S, Arad M, Feinberg MS, Freimark D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure†. Eur J Heart Fail. 2009;11:588–593. doi: 10.1093/eurjhf/hfp053. [DOI] [PubMed] [Google Scholar]
- 74.Fischer D, Rossa S, Landmesser U, Spiekermann S, Engberding N, Hornig B, Drexler H. Endothelial dysfunction in patients with chronic heart failure is independently associated with increased incidence of hospitalization, cardiac transplantation, or death. Eur Heart J. 2005;26:65–69. doi: 10.1093/eurheartj/ehi001. [DOI] [PubMed] [Google Scholar]
- 75.de Berrazueta JR, Guerra-Ruiz A, García-Unzueta MT, Martín Toca G, Sainz Laso R, Sáez de Adana M, Casanova Martín MA, Cobo M, Llorca J. Endothelial dysfunction, measured by reactive hyperaemia using strain-gauge plethysmography, is an independent predictor of adverse outcome in heart failure. Eur J Heart Fail. 2010;12:477–483. doi: 10.1093/eurjhf/hfq036. [DOI] [PubMed] [Google Scholar]
- 76.Damy T, Ratajczak P, Shah AM, Camors E, Marty I, Hasenfuss G, Marotte F, Samuel JL, Heymes C. Increased neuronal nitric oxide synthase-derived NO production in the failing human heart. Lancet. 2004;363:1365–1367. doi: 10.1016/S0140-6736(04)16048-0. [DOI] [PubMed] [Google Scholar]
- 77.Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–1469. doi: 10.1016/j.jacc.2011.11.082. [DOI] [PubMed] [Google Scholar]
- 78.Winlaw DS, Smythe GA, Keogh AM, Schyvens CG, Spratt PM, Macdonald PS. Increased nitric oxide production in heart failure. Lancet. 1994;344:373–374. doi: 10.1016/s0140-6736(94)91403-6. [DOI] [PubMed] [Google Scholar]
- 79.Bauersachs J, Widder JD. Endothelial dysfunction in heart failure. Pharmacol Rep. 2008;60:119–126. [PubMed] [Google Scholar]
- 80.Treasure CB, Vita JA, Cox DA, Fish RD, Gordon JB, Mudge GH, Colucci WS, Sutton MG, Selwyn AP, Alexander RW. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. 1990;81:772–779. doi: 10.1161/01.cir.81.3.772. [DOI] [PubMed] [Google Scholar]
- 81.Bitar F, Lerman A, Akhter MW, Hatamizadeh P, Janmohamed M, Khan S, Elkayam U. Variable response of conductance and resistance coronary arteries to endothelial stimulation in patients with heart failure due to nonischemic dilated cardiomyopathy. J Cardiovasc Pharmacol Ther. 2006;11:197–202. doi: 10.1177/1074248406292574. [DOI] [PubMed] [Google Scholar]
- 82.Kubo SH, Rector TS, Bank AJ, Williams RE, Heifetz SM. Endothelium-dependent vasodilation is attenuated in patients with heart failure. Circulation. 1991;84:1589–1596. doi: 10.1161/01.cir.84.4.1589. [DOI] [PubMed] [Google Scholar]
- 83.Klosinska M, Rudzinski T, Grzelak P, Stefanczyk L, Drozdz J, Krzeminska-Pakula M. Endothelium-dependent and -independent vasodilation is more attenuated in ischaemic than in non-ischaemic heart failure. Eur J Heart Fail. 2009;11:765–770. doi: 10.1093/eurjhf/hfp091. [DOI] [PubMed] [Google Scholar]
- 84.Belardinelli R, Capestro F, Misiani A, Scipione P, Georgiou D. Moderate exercise training improves functional capacity, quality of life, and endothelium-dependent vasodilation in chronic heart failure patients with implantable cardioverter defibrillators and cardiac resynchronization therapy. Eur J Cardiovasc Prev Rehabil. 2006;13:818–825. doi: 10.1097/01.hjr.0000230104.93771.7d. [DOI] [PubMed] [Google Scholar]
- 85.Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 86.Akar JG, Al-Chekakie MO, Fugate T, Moran L, Froloshki B, Varma N, Santucci P, Wilber DJ, Matsumura ME. Endothelial dysfunction in heart failure identifies responders to cardiac resynchronization therapy. Heart Rhythm. 2008;5:1229–1235. doi: 10.1016/j.hrthm.2008.05.027. [DOI] [PubMed] [Google Scholar]
- 87.Enomoto K, Yamabe H, Toyama K, Matsuzawa Y, Yamamuro M, Uemura T, Morihisa K, Iwashita S, Kaikita K, Sugiyama S, Ogawa H. Improvement effect on endothelial function in patients with congestive heart failure treated with cardiac resynchronization therapy. J Cardiol. 2011;58:69–73. doi: 10.1016/j.jjcc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 88.Tesselaar E, Schiffer A, Widdershoven J, Broers H, Hendriks E, Luijten K, Creusen J. Effect of cardiac resynchronization therapy on endothelium-dependent vasodilatation in the cutaneous microvasculature. Pacing Clin Electrophysiol. 2012;35:377–384. doi: 10.1111/j.1540-8159.2011.03313.x. [DOI] [PubMed] [Google Scholar]
- 89.Fish RD, Nabel EG, Selwyn AP, Ludmer PL, Mudge GH, Kirshenbaum JM, Schoen FJ, Alexander RW, Ganz P. Responses of coronary arteries of cardiac transplant patients to acetylcholine. J Clin Invest. 1988;81:21–31. doi: 10.1172/JCI113297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davis SF, Yeung AC, Meredith IT, Charbonneau F, Ganz P, Selwyn AP, Anderson TJ. Early endothelial dysfunction predicts the development of transplant coronary artery disease at 1 year posttransplant. Circulation. 1996;93:457–462. doi: 10.1161/01.cir.93.3.457. [DOI] [PubMed] [Google Scholar]
- 91.Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Oberoi M, Johnson MR, Costanzo MR. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091–3096. doi: 10.1161/hc5001.100796. [DOI] [PubMed] [Google Scholar]
- 92.Sudhir K, MacGregor JS, DeMarco T, De Groot CJ, Taylor RN, Chou TM, Yock PG, Chatterjee K. Cyclosporine impairs release of endothelium-derived relaxing factors in epicardial and resistance coronary arteries. Circulation. 1994;90:3018–3023. doi: 10.1161/01.cir.90.6.3018. [DOI] [PubMed] [Google Scholar]
- 93.Edwards BS, Hunt SA, Fowler MB, Valantine HA, Anderson LM, Lerman A. Effect of cyclosporine on plasma endothelin levels in humans after cardiac transplantation. Am J Cardiol. 1991;67:782–784. doi: 10.1016/0002-9149(91)90545-v. [DOI] [PubMed] [Google Scholar]
- 94.Diederich D, Skopec J, Diederich A, Dai FX. Cyclosporine produces endothelial dysfunction by increased production of superoxide. Hypertension. 1994;23(6 Pt 2):957–961. doi: 10.1161/01.hyp.23.6.957. [DOI] [PubMed] [Google Scholar]
- 95.Raichlin E, Prasad A, Kremers WK, Edwards BS, Rihal CS, Lerman A, Kushwaha SS. Sirolimus as primary immunosuppression is associated with improved coronary vasomotor function compared with calcineurin inhibitors in stable cardiac transplant recipients. Eur Heart J. 2009;30:1356–1363. doi: 10.1093/eurheartj/ehp123. [DOI] [PubMed] [Google Scholar]
- 96.Kubo SH, Rector TS, Bank AJ, Tschumperlin LK, Raij L, Brunsvold N, Kraemer MD. Effects of cardiac transplantation on endothelium-dependent dilation of the peripheral vasculature in congestive heart failure. Am J Cardiol. 1993;71:88–93. doi: 10.1016/0002-9149(93)90716-p. [DOI] [PubMed] [Google Scholar]
- 97.Roig E, Cuppoletti A, Masotti M, Kianco R, Vallejos I, Sitges M, Ortiz J, Perez-Villa F. Assessment of peripheral endothelial-dependent vasodilatation within the first year after heart transplantation. J Heart Lung Transplant. 2009;28:299–304. doi: 10.1016/j.healun.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 98.Cuppoletti A, Sitges M, Perez Villa F, Orus J, Magrina J, Roig E. Impairment in forearm endothelium-dependent vasodilation after heart transplantation. Transplant Proc. 2003;35:2011–2013. doi: 10.1016/s0041-1345(03)00661-4. [DOI] [PubMed] [Google Scholar]
- 99.Andreassen AK, Kvernebo K, Jorgensen B, Simonsen S, Kjekshus J, Gullestad L. Exercise capacity in heart transplant recipients: relation to impaired endothelium-dependent vasodilation of the peripheral microcirculation. Am Heart J. 1998;136:320–328. doi: 10.1053/hj.1998.v136.89731. [DOI] [PubMed] [Google Scholar]
- 100.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flammer AJ, Luscher TF. Human endothelial dysfunction: EDRFs. Pflugers Arch. 2010;459:1005–1013. doi: 10.1007/s00424-010-0822-4. [DOI] [PubMed] [Google Scholar]