Abstract
Lymphatic vessels surround follicles within the ovary, but their roles in folliculogenesis and pregnancy, as well as the necessity of lymphangiogenesis in follicle maturation and health, are undefined. We used systemic delivery of mF4-31C1, a specific antagonist vascular endothelial growth factor receptor 3 (VEGFR-3) antibody to block lymphangiogenesis in mice. VEGFR-3 neutralization for 2 weeks before mating blocked ovarian lymphangiogenesis at all stages of follicle maturation, most notably around corpora lutea, without significantly affecting follicular blood angiogenesis. The numbers of oocytes ovulated, fertilized, and implanted in the uterus were normal in these mice; however, pregnancies were unsuccessful because of retarded fetal growth and miscarriage. Fewer patent secondary follicles were isolated from treated ovaries, and isolated blastocysts exhibited reduced cell densities. Embryos from VEGFR-3–neutralized dams developed normally when transferred to untreated surrogates. Conversely, normal embryos transferred into mF4-31C1–treated dams led to the same fetal deficiencies observed with in situ gestation. Although no significant changes were measured in uterine blood or lymphatic vascular densities, VEGFR-3 neutralization reduced serum and ovarian estradiol concentrations during gestation. VEGFR-3–mediated lymphangiogenesis thus appears to modulate the folliculogenic microenvironment and may be necessary for maintenance of hormone levels during pregnancy; both of these are novel roles for the lymphatic vasculature.
Ovarian neovascularization provides a unique environment in which to study physiological adult vasculogenesis apart from the traditional settings of wound healing and cancer pathologies. Lymphatic circulation plays a central role in fluid, lipid, and cellular transport,1 and lymphatic vessels are present within the ovary and surround follicles during development and maturation,2–5 but the importance of the lymphatic vasculature and lymphangiogenesis in the ovary is unclear. Consequently, the potential roles of lymphatic vessels in follicle maturation and pregnancy, and the extent of involvement or even necessity of maternal lymphangiogenesis in reproduction, are undefined. This contrasts with ovarian blood angiogenesis, whose critical roles in follicular nourishment and maturation and in the formation and maintenance of the corpus luteum are well appreciated; indeed, oocyte fertilization, embryonic implantation, uterine expansion, and successful gestation all require blood angiogenesis.6–8 Lymphangiogenesis, which is often concurrent with blood angiogenesis,9 may also play an important role in these processes.
Adult blood angiogenesis requires signaling via vascular endothelial growth factor receptor 2 (VEGFR-2), most potently by VEGF ligation.10,11 In murine ovaries, VEGF expression increases during angiogenic growth phases,12 and blockade of VEGFR-2 signaling in mice effectively prevents angiogenesis, resulting in a marked decrease in ovarian weight, blood vessel density, and number of corpora lutea, and in infertility.13–15 Because gonadotropin treatment apparently does not correct these deficiencies,16 it is likely that follicle maturation and successful pregnancy are highly dependent on VEGFR-2–mediated neovascularization in the ovary.6,17 Vascularization also occurs in the uterine wall and decidua during pregnancy, and significant disruption of angiogenesis by VEGFR-2 blockade in these tissues after fertilization has been shown to greatly reduce pregnancy success.18
VEGFR-3 is expressed primarily on lymphatic endothelial cells in adult tissue,19,20 and its signaling, via ligation by VEGF-C or VEGF-D, is necessary for lymphangiogenesis by inducing lymphatic endothelial cell proliferation and migration.19–23 Blockade of VEGFR-3 signaling using a function-blocking antibody such as mF4-31C1 (ImClone Systems; Eli Lilly, Indianapolis, IN) completely blocks the initiation of new lymphatic vessels in adult mice without affecting pre-existing lymphatic morphology or function and without apparently affecting blood angiogenesis.18,21,22 The ovary contains a dense lymphatic network that has been morphologically assessed in large rodents.24–26 Recent studies in which murine ovarian lymphatic vessel expansion was impaired during development found the dams to be infertile as adults.3
We investigated VEGFR-3–mediated lymphangiogenesis and the roles of new lymphatic vessels and lymphangiogenesis in female reproduction and found that lymphangiogenesis occurs within the murine ovary during reproductive cycles and folliculogenesis and that VEGFR-3 neutralization prevents viable, full-term pregnancies. Using combined in vivo, ex vivo, and in vitro methods, we examined which aspects of female fertility are influenced by inhibited maternal lymphangiogenesis including oocyte and follicular development and maturation, uterine implantation, and embryonic development. After we had eliminated direct effects on fetal and uterine VEGFR-3–mediated neovascularization, our results suggested that the new ovarian lymphatic vessels specifically modulate follicle development and hormone production, demonstrating a critical and novel role for ovarian lymphangiogenesis in reproduction.
Materials and Methods
Animal Procedures
All protocols were approved by the Veterinary Authorities of the Canton Vaud according to Swiss law (protocols 1687, 1988, and 1988.1). mF4-31C1, a function-blocking antibody against murine VEGFR-3, was kindly provided by ImClone Systems.22 For 2 weeks before mating, 0.25 mL of 2.5 mg/mL mF4-31C1 (approximately 32 g/kg) was injected intraperitoneally every 2 days. For some control groups 0.25 mL saline solution was similarly injected with no adverse effects on reproductive potential. Three mice were used in each initial group to test blockade effects.
For studies without fertilization, 3-week-old female F1 hybrid mice (C57Bl/6JxCBA/caj; Charles River Laboratories International, L'Arbresle, France; Wilmington, MA) were treated for 2 weeks and then sacrificed (n = 5 mice per group). Follicles and ovaries were collected for subsequent in vitro culture and histological examination.
In studies requiring fertilization, 5- to 6-week-old female F1 hybrid (C57Bl/6JxCBA/caj) mice were treated for 2 weeks before mating (to ensure spanning two full estrus cycles). At approximately 7 to 8 weeks of age, mice in estrus were mated and coitus was evaluated by the presence of a vaginal plug 16 hours after mating. For embryo retrieval, ex vivo culture, and transplantation, mice were sacrificed 42 hours after mating and two-cell embryos were collected by oviduct flushing with M2 medium (Sigma-Aldrich, St. Louis, MO).
Embryos were implanted into pseudo-pregnant recipient NMRI mice (Charles River Laboratories International) according to standard implantation protocols. In brief, embryos from mF4-31C1–treated and untreated F1 hybrid donors were implanted into treated and untreated recipients. Recipient dams (n = 3 per group) were anesthetized using 100 mg/kg i.p. injection of ketamine and 10 mg/kg i.p. xylazine). A small midsagittal incision was made over each oviduct, and the donor embryos were deposited into each oviduct by mouth pipetting under a stereomicroscope. The incision was then sutured, and pregnancies were permitted to continue through day 17. Implantation success was consistently >90% for all groups.
For examination of fetal development and uterine implantation, mice were sacrificed at pregnancy day 17 or after birth. Implantation spots were counted in the uterus, and remaining fetuses were scored as normal, grade 1 (normal size but abnormal coloration), grade 2 (underdeveloped fetus, in size or limb development), or grade 3 (implanted cell mass or necrotic fetus). Fetuses were fixed overnight in 4% paraformaldehyde and then were stored in 70% ethanol until photographed.
In Vitro Follicle Culture and Maturation
To determine the direct effects of in vivo mF4-31C1 treatment on normal follicle maturation potential, ovaries were isolated from 5-week-old mice after 2 weeks of antibody treatment. In vitro maturation of preantral follicles was performed as described previously.27,28 In brief, whole ovaries were placed in 3 mL of L-15 Leibovitz–GlutaMAX medium (Life Technologies, Carlsbad, CA) with 10% fetal bovine serum (HyClone Laboratories, Logan, UT) and 1% penicillin–streptomycin solution (Life Technologies). Preantral follicles with diameters of 100 to 130 μm were mechanically separated from the ovaries, washed, and transferred to individual 10-μL droplets of MEM-α GlutaMAX medium containing 5% fetal bovine serum, 1% ITS (5 μg/mL, insulin, 5 μg/mL, transferrin, and 5 μg/mL selenium mixture solution; all Life Technologies), 1% penicillin-streptomycin, and 100 mIU/mL recombinant human follicle-stimulating hormone (Organon; Merck Sharpe & Dohme, Pfäffikon, Switzerland; EMD Merck, Billerica, MA). For direct in vitro effects of VEGFR-3 blocking, 10 μg/mL mF4-31C1 was added to the culture medium of wild-type follicles (n = 14 control; n = 13 test); diameters of 10 surviving follicles are reported.
Preantral follicles from control and treated females (n = 3 mice per group) were induced to mature on day 12 of in vitro culture by exposure to medium lacking hFSH, but supplemented with 2.5 IU/mL hCG-Pregnyl (Organon; Merck) and 5 ng/mL murine epidermal growth factor (Sigma-Aldrich). After 16 hours, oocytes were retrieved by removing the follicular cumulus cells using 200 IU/mL of hyaluronidase (Sigma-Aldrich). Oocytes were classified by the following maturation states: germinal vesicle, germinal vesicle breakdown, and metaphase II.
Ex Vivo Development of Two-Cell Embryos
To determine the direct effects of VEGFR-3 inhibition on preimplantation embryonic development, two-cell embryos were retrieved from oviducts (n = 3 mice per group) and were cultured in four-well dishes containing 400 μL of M16 medium (Sigma-Aldrich). The number of embryos that developed into the eight-cell, morula, and blastocyst stages were quantified and imaged under a Nikon SMZ1000 stereomicroscope equipped with a Nikon DS-5M monochrome camera at 66, 90, and 114 hours after mating, respectively.
Differential Staining of Inner Cell Mass and Trophectoderm Cells
To quantify embryonic development, the numbers of total blastomere, inner cell mass cells, and trophectoderm cells in blastocysts were evaluated 72 hours after culture by a modification of the method of Thouas et al.29 In brief, blastocysts were treated with 1% Triton X-100 (Sigma-Aldrich) and 0.1 mg/mL propidium iodide (Sigma-Aldrich) for 5 seconds at room temperature. After two washes with M2 medium, they were incubated in 0.05 mg/mL bisbenzimide (Sigma-Aldrich) in 100% ethanol for 2 hours at 4°C. Inner cell mass cells were stained blue by bisbenzimide, and trophectoderm cells were stained pink by both blue-staining bisbenzimide and red-staining propidium iodide. The numbers of cells in each body were counted under a Zeiss Axiovert 200M microscope by two experienced researchers (J.E.I. and S.T.L.).
Immunofluorescence, Immunohistochemistry, and Histology
To visualize lymphatic vessels, ovary and uterus cryosections (6 μm thick; cold acetone–fixed) were labeled with a primary antibody to the lymphatic-specific marker LYVE-1 (1:500, Upstate 07-538; EMD Millipore). For blood vessels, ovaries were labeled with a FITC-conjugated primary antibody to CD31/PECAM-1 (1:200, 550274; BD Pharmingen, San Jose, CA). Vessels were also colabeled for VEGFR-3 (1:100, AF743; R&D Systems, Minneapolis, MN). Sections were also labeled for the macrophage-specific surface marker F4/80 (1:50, MCA497; AbD Serotec, Kidlington, UK; Raleigh, NC), collagen IV (1:1000, 10760; MP Biomedicals, Santa Ana, CA), and Ki-67 (1:100, M7249; Dako, Carpinteria, CA). These antibodies were detected with Alexa Fluor 488– or 594–conjugated donkey, rabbit, or goat IgG secondary antibodies (1:200, Life Technologies), and nuclei were labeled with DAPI (Vector Laboratories, Burlingame, CA). Fluorescent labeling was observed and imaged using a Zeiss Axiovert 200M microscope with a Zeiss AxioCam MRm camera. Slides were then rinsed and counterstained with H&E, dehydrated, mounted with Fluka Eukitt mounting medium (Sigma-Aldrich), and imaged again using a Zeiss AxioCam MRc color camera. The corresponding fluorescence and chromogenic images were then compared for identification, and subsequent quantification, of follicular development.
To label apoptotic cells, a fluorescence TUNEL kit was used according to the manufacturer's instructions (Roche Diagnostics, Rotkreuz, Switzerland; Indianapolis, IN). Oil Red O (Sigma-Aldrich) was used to stain lipids in ovarian frozen sections. Sections were counterstained with hematoxylin and immediately mounted with Möwiol-based mounting medium for imaging.
Hormone Analysis
Serum was collected from all mice when sacrificed and analyzed using enzyme-linked immunosorbent assay (ELISA) kits for levels of estradiol (Calbiotech, Spring Valley, CA), progesterone (BioSource; Life Technologies), follicle-stimulating hormone (Endocrine Technologies, Newark, CA), and luteinizing hormone (Endocrine Technologies), according to the manufacturer's instructions. Absorbance was measured using a Tecan Safire2 plate reader (Tecan, Männedorf, Switzerland). Total ovarian estradiol content was obtained by homogenizing one ovary in 400 μL of PBS, centrifuging, and then analyzing the supernatant with ELISA. Ovarian estradiol was normalized by ovary weight.
F(Ab′)2 Preparation and Verification
The approximately 30-kDa Fc region of mF4-31C1 was removed by enzymatic digestion using a Pierce F(ab′)2 preparation kit (Thermo Fisher Scientific, Rockford, IL) according to the manufacturer's instructions, except that 2 hours of additional digestion time was used. The cleaved antibody was purified overnight using a Pierce antibody clean-up kit. Compared with 10 μg/mL for the full antibody, for the F(ab′)2 fragment 50 μg/mL was necessary to achieve complete blockade of VEGFR-3 phosphorylation in human lymphatic endothelial cells treated with 100 ng/mL VEGF-C (R&D Systems). Approximately 150 g/kg was used in vivo, as above.
Image Analysis and Quantification
To quantify macrophages, Oil Red O–labeled and TUNEL-labeled slides and images of entire ovaries were assembled, the ovary body was outlined, and the percentage of positive area measured using MetaMorph software version 6.3 (Molecular Devices, Sunnyvale, CA). For lymphatic and blood vessel quantification, we considered each follicular maturation state. Each follicle was outlined using a CintiQ freehand graphics tablet–display monitor (Wacom, Otono, Saitama, Japan). Vessel labeling was defined by fluorescence threshold, and the number of positive pixels for each follicle was determined. The average vessel area for each maturation state was calculated. Follicle maturation states were scored as follows: 1, preantral follicle (secondary follicle); 2, antral follicle (small follicle with formed atrium); 3, Graafian follicle (large follicle with significant atrium); and 4, corpus luteum (Supplemental Figure S1, A–C). These divisions were consistently identified across multiple examiners.
Statistical Analysis
For determining statistical significance between treatments in follicle vascularization over the stages of maturation, follicle survival and maturation, and embryo development, Student's t-tests were used to compare factors in treated versus untreated groups. Two-way analysis of variance followed by Bonferroni post hoc analysis was used for hormone levels. Data are expressed as means ± SEM.
Results
VEGFR-3 Neutralization Prevents Successful Murine Pregnancy
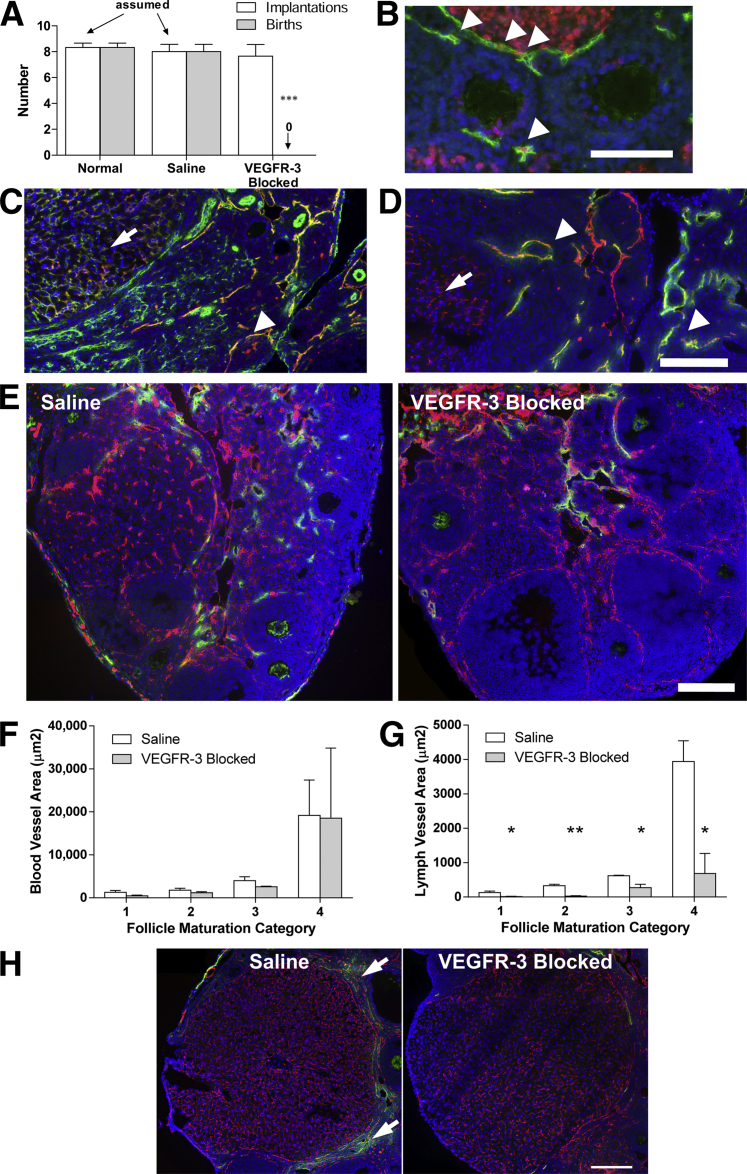
Female mice were mated at 6 to 8 weeks of age after 2 weeks of treatment with i) anti–VEGFR-3 neutralizing antibody (mF4-31C1), ii) saline (control), or iii) no injection (normal). All treatments were stopped 2 to 3 days before mating. Normal and control mice were equally successful in giving birth to normal and healthy pups. Mice treated with mF4-31C1, however, failed to produce a single live birth in the three animals initially tested (Figure 1A), despite a normal number of uterine implantation spots. This unexpected response suggested that VEGFR-3 signaling, likely mediating lymphangiogenesis, is a necessary process in murine pregnancy. Indeed, Ki-67 lymphatic endothelial cells were identified surrounding maturing follicles (Figure 1B), indicative of a proliferating and expanding lymphatic vasculature.
Figure 1.
Ovarian and follicular lymphangiogenesis (but not blood angiogenesis) is inhibited by VEGFR-3 blockade in the ovary, preventing successful pregnancy. A: Number of embryo implantations (white bars) in the uterus of normal, saline-injected, and VEGFR-3–blocked dams and live births (gray bars) assessed after delivery. Dams of viable pups were nursing, and uterine implantations were assumed to equal pups born. B: Ki-67+ (red) lymphatic endothelial cell (LYVE-1; green) nuclei in lymphatic vessels surrounding follicles (arrowheads). C: Blood vessels (CD31; green) are mostly negative for VEGFR-3 (red), with the exception of those within the corpora lutea (arrow). Because lymphatic vessels also express CD31, the strong VEGFR-3 colocalization likely marks lymphatic vessels (arrowhead). D: Ovarian lymphatic vessels (LYVE-1; green) are positive for VEGFR-3 (red) (arrowheads). LYVE-1+/VEGFR-3+ vessels within the corpora lutea are blood vessels (arrows). E: The extent of lymphatic vasculature (LYVE-1; green) surrounding follicles within the ovary was reduced in VEGFR-3–blocked ovaries; the blood vasculature and blood angiogenesis (CD31; red) were unaffected by the treatment. F: Quantification of blood and lymphatic vessel coverage in preantral follicles (maturation category 1), small follicles with formed atrium (maturation category 2), large follicles with significant atrium (maturation category 3), and corpora lutea (maturation category 4) demonstrated no changes in blood vessel density with VEGFR-3 blockade, despite some vessels expressing VEGFR-3. G: There was a significant reduction in lymphatic vessels at each maturation state with VEGFR-3 blockade. Note the normal increase in vascularization with folliculogenesis for both lymphatic and blood vessels; lymphangiogenesis was specifically blocked by VEGFR-3 neutralization. H: Lymphatic vessels (LYVE-1; green) are clearly visualized around the hormone-producing corpora lutea (category 4) (arrows) in normal ovaries (right), but lacking in VEGFR-3–blocked ovaries (left). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bar = 200 μm.
Ovarian Lymphangiogenesis, but Not Blood Angiogenesis, Is Inhibited by VEGFR-3 Blockade
Although mature blood vessels do not express VEGFR-3, angiogenic blood vessels have been observed to express VEGFR-3 both during normal development and in tumors and healing wounds,30–32 and there is evidence that VEGFR-3 inhibition may limit tumor angiogenesis.33,34 Because blood angiogenesis in the ovary is necessary for pregnancy,6–8,16,35 we first examined blood vessels to assess whether mF4-31C1 has any effects on ovarian angiogenesis. VEGFR-3 expression was not seen on most blood vessels; colocalization on CD31+/LYVE-1− vessels occurred only within corpora lutea (Figure 1, C and D).36 Importantly, however, blood vascularization area was not significantly affected by VEGFR-3 blockade (P = 0.168, P = 0.308, P = 0.194, and P = 0.972 for follicle maturation category 1 through 4, respectively) (Figure 1, E and F), consistent with that seen in dermal wound healing with mF4-31C1.21,22
LYVE-1+/VEGFR-3+ lymphatic vessels were observed surrounding nearly every follicle at all maturation states and did not penetrate into the follicular body or thecal layers (Figure 1, B–E).5 The extent of lymphatic vascularization was dependent on the maturation stage of the follicles, with secondary and preantral follicles exhibiting only a few sparse lymphatic vessels. Whereas antral follicles and corpora lutea exhibited a significantly greater peripheral lymphatic network (Figure 1, E–G). In ovaries from mice treated with mF4-31C1, the extent of lymphatic vascularization was greatly reduced at all four stages of follicular maturation, as measured by vessel density (P = 0.049, P = 0.002, P = 0.028, and P = 0.036, respectively) (Figure 1G). There was not a complete absence of lymphatic vessels within the ovary, consistent with our previous studies, in which VEGFR-3 neutralization prevented lymphangiogenesis but had no morphological effects on pre-existing lymphatic vessels.21,22 The reduced lymphatic vessel density, mostly around the follicles and corpora lutea (Figure 1H), indicates that lymphangiogenesis is an active process during folliculogenesis, because blockade of VEGFR-3 prevented only the expansion of new lymphatic vessels.
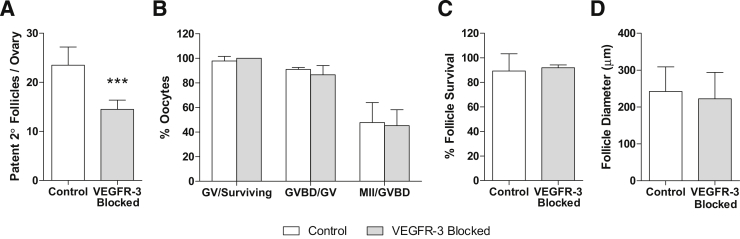
VEGFR-3 Neutralization Decreases the Number of Healthy Follicles but Has No Direct Effect on Their Quality
To explore the possible effects of VEGFR-3 signaling and lack of lymphatic vasculature on follicular maturation potential in virgin mice, preantral follicles (100 to 130 μm) were retrieved after 2 weeks of treatment for in vitro culture. Although histological examination of ovaries suggested greater numbers of preantral follicles in VEGFR-3–blocked mice (Supplemental Figure S1D), the numbers of healthy patent preantral follicles successfully retrieved was lower (P < 0.001) from mF4-31C1–treated mice than from saline-treated mice (Figure 2A). Fragile contact between the oocyte and granulosa cells in mF4-31C1–treated ovaries was observed. (Preantral follicles require interactions between surrounding granulosa and theca cell layers and the oocyte.27) These contacts were apparently not due to any loss of basal lamina integrity, however, as evidenced by collagen IV, laminin, and perlecan labeling (Supplemental Figure S2), because no differences were observed in the granulosa–thecal boundary. Although these contacts could affect the local in vivo environment, after separation and in vitro culture both groups of preantral follicles exhibited similar survival rates; surviving follicles were able to mature normally ex vivo28 to germinal vesicles and through the germinal vesicle breakdown and metaphase II phases with equal success (P = 0.373, P = 0.386, and P = 0.843, respectively) (Figure 2B). Preantral follicles isolated from untreated mice were cultured directly in the presence of 10 μg/mL mF4-31C1 in vitro; all follicles survived equally (P = 0.763) and grew to similar sizes (P = 0.539) (Figure 2, C and D). Thus, blocking VEGFR-3 in vitro had no direct effect on isolated follicle growth and maturation.
Figure 2.
Secondary follicles, once retrieved from the ovaries of treated mice, mature normally in vitro. A: Fewer patent follicles were successfully separated from collected ovaries in treated mice. B: Follicles and their oocytes separated from ovaries of VEGFR-3–treated mice survive normally; germinal vesicle oocytes from treated and control mice mature to germinal vesicle breakdown and metaphase II stages using the in vitro drop culture technique at equal rates. C: Survival of secondary follicles directly treated with the VEGFR-3–blocking antibody in vitro was not inhibited. D: Maturation potential, as determined by follicle size, was also unaffected by direct VEGFR-3 treatment on the follicles. ∗∗∗P < 0.001. GV, germinal vesicle; GVBD, germinal vesicle breakdown; MII, metaphase II; 2°, secondary.
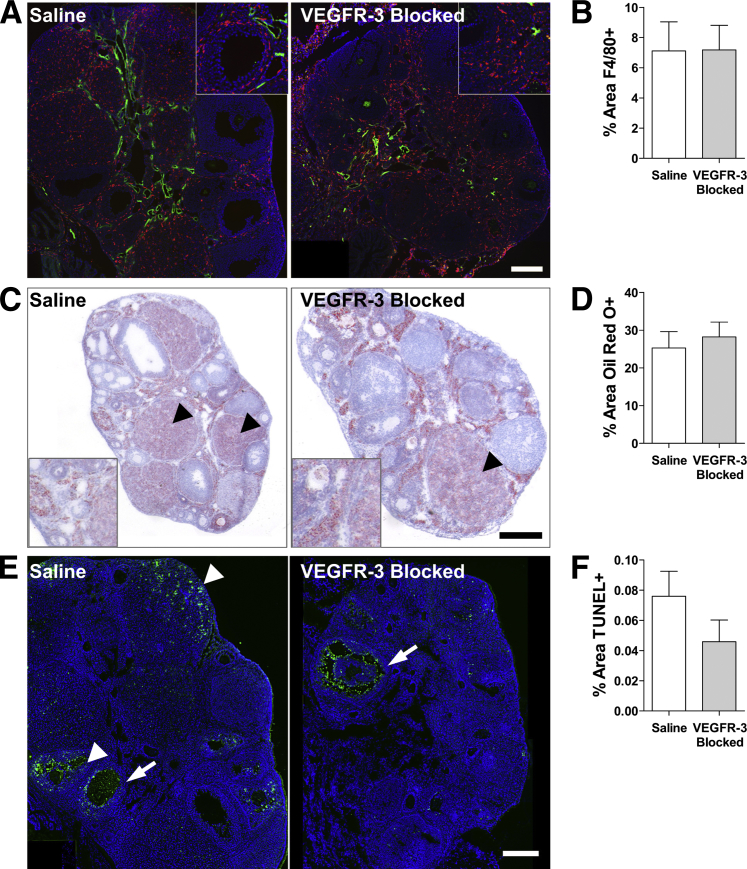
VEGFR-3 Blocking Does Not Alter Macrophage Recruitment, Lipid Accumulation, or Apoptosis within the Ovary
Our combined in vivo and in vitro results suggest that the failed pregnancies derived from alterations in the follicular environment due to inhibited lymphangiogenesis. Because immune function may be important in mediating the balance between hormone accumulation37 and follicle maturation and ovulation within the ovary38,39 and immune-cell traffic through lymphatic vessels,25,26 we labeled macrophages within the ovaries; however, we found no differences in density or localization (P = 0.980) (Figure 3, A and B).
Figure 3.
Macrophage recruitment, lipid accumulation, and cell apoptosis in the ovary appear to be unaffected by VEGFR-3 blockade. A: Macrophages (F4/80; red) present in the ovary are limited to follicular peripheries in both control and treated ovaries (LYVE-1; green). B: Total F4/80+ area within the ovary remains unchanged. C: The ovaries contain large amount of lipids (Oil Red O; red), particularly in the corpora lutea (arrowheads). D: Lipid accumulation (the Oil Red O+ area) in the ovary is not significantly changed between conditions. E: Apoptotic cells (TUNEL; green) are limited to interior granulosa cells (arrows) of regressing follicles and regular apoptosis of regressing corpora lutea and corpus hemorrhagicum (arrowheads) in both treatments. F: No difference was measured in the percent area of apoptotic cell nuclei within the ovary with mF4-31C1 treatment. Scale bars: 200 μm (A and E); 300 μm (B); insets, 2× zoom of main image to visualize localization.
Lymphatic insufficiencies have been linked to excessive tissue lipid accumulation in skin.40–42 Lipids are necessary for hormone synthesis by granulosa and luteal cells in the ovary43,44 and may thus be altered with VEGFR-3 blockade. We visualized lipid content in the ovaries via Oil Red O staining and observed no difference in total amount (P = 0.635) or localization of lipid (Figure 3, C and D).
Finally, we sought to determine whether blocking VEGFR-3 increases cell apoptosis throughout the folliculogenic cycle, which is a normal ovarian process.45 Consistent with findings of others,45,46 apoptotic cells were confined primarily to the interior layer granulosum of regressing antral follicles, regressing corpora lutea, and postovulatory cells at the ovarian wall in both groups (Figure 3E). A similar distribution and number of TUNEL+ cells was seen within the ovary (P = 0.226) of both groups (Figure 3F). Thus, neither the direct action of VEGFR-3 neutralization nor the reduced lymphatic vasculature decreased ovarian cell survival.
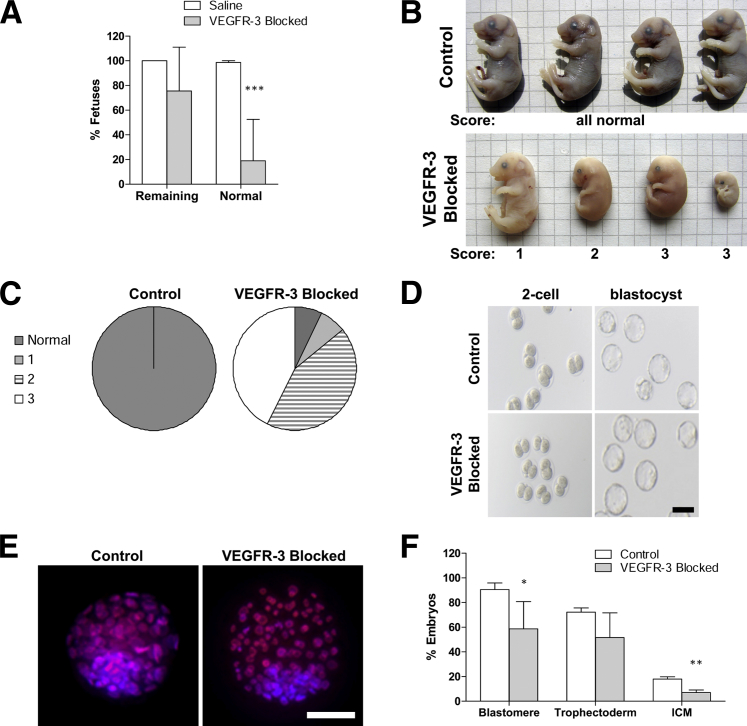
VEGFR-3 Neutralization Prior to Fertilization Retards Embryonic Development
Because pregnancies were unsuccessful in VEGFR-3 neutralized mice, we sought to determine at what stage after fertilization observable differences could be seen in embryonic and fetal development. Mice were treated for 2 weeks, and then mated; their uteri were examined at pregnancy days 9 and 17. We counted no differences in the number of implantation spots in the uteri of treated versus control mice, but fewer fetuses remained, per implantation spot, in mF4-31C1–treated mice at pregnancy day 17, although this trend was not significant (P = 0.12) (Figure 4A). More strikingly, the remaining fetuses were dramatically smaller and underdeveloped (P < 0.001), and not likely to be viable (Figure 4, A–C). A majority of fetuses exhibited severe and symmetric intrauterine growth restriction, with whole-body reduced growth; fetal size was independent of position within the uterus. Uteri examined at pregnancy day 9 did not, however, demonstrate these obvious visual differences (Supplemental Figure S3).
Figure 4.
Pregnancies occur after lymphangiogenesis is blocked, but embryonic development is severely impaired in the womb. A: In mice treated with mF4-31C1 before fertilization, few fetal bodies remained within the uterus at pregnancy day 17. The percentage is based on number of fetuses per implantation spot. B: Fetuses extracted at pregnancy day 17 from treated animals display a marked deficiency from control animals. They were scored as normal or as normal sized but discolored (1), deficient size and underdeveloped (2), and identifiable cell masses (3). C: The majority of fetal bodies are severely underdeveloped masses. D: Two-cell embryos were extracted from fertilized VEGFR-3–treated mice and cultured in vitro. Both two-cell embryos and blastomeres were identical in appearance to those taken from control animals. E: Effects of VEGFR-3 blockade prior to fertilization are manifest in blastocyst cell density. No differences were observed in the number of trophectodermal cells (propidium iodide and bisbenzimide; red/blue) that would later become the placenta, but fewer cells were identified in the inner cell mass (ICM) (bisbenzimide; blue) that would later develop into the fetus. F: Quantification indicated a significant reduction in cell numbers in each mass, compared with control. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bars: 100 μm (D); 50 μm (E). Grid = 4 mm.
To determine whether the numbers of ovulated oocytes and early blastocyst development from VEGFR-3–blocked mice were normal, mice were sacrificed 42 hours after mating and two-cell embryos were collected. The numbers of harvested two-cell embryos per mouse did not differ between saline-treated control mice and mF4-31C1–treated mice (8 ± 1 and 9 ± 2, respectively). All two-cell embryos were cultured in vitro to blastocysts with 100% success, regardless of treatment (Figure 4D); however, cell numbers within the blastocysts were reduced (P = 0.027). Blastocysts contain an inner cell mass that develops into the fetus and trophectoderm cells that develop into the placenta. The total number of cells in the inner cell mass was significantly reduced in blastocysts cultured from mF4-31C1–treated mice (P = 0.003), but not in the corresponding trophectoderm (P = 0.154) (Figure 4, E and F).
Maternal Environment Dictates Proper Fetal Development or Miscarriage of Transplanted Normal Embryos
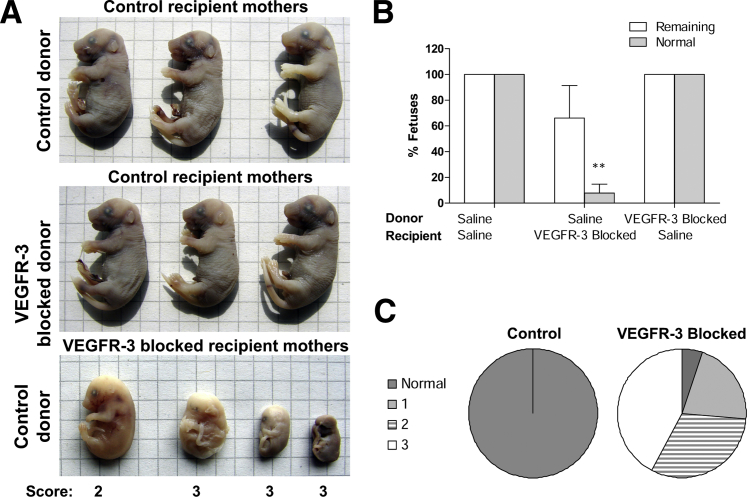
To verify that the maternal gestational environment was responsible for the observed developmental deficiencies, two-cell embryos were isolated from control or mF4-31C1–treated donor dams and implanted into control or mF4-31C1–treated pseudo-pregnant recipient dams. Regardless of the treatment of the donor dam, transplantation of embryos into normal dams resulted in normal implantation and fetal development, with only normal, viable fetuses found in the uterus at pregnancy day 17 (Figure 5A). However, fetal development of normal embryos in mF4-31C1–treated recipient dams was inhibited, and fewer than 10% developed normally (P = 0.002), despite normal implantation (Figure 5, B and C). These developmental deficiencies were nearly identical to those observed in previously examined in situ pregnancies (Figure 4, A and B). This finding, coupled with the earlier measured blastocyst retardation, further supports the notion that early ovarian lymphangiogenesis is independently necessary both for initial development after fertilization and for subsequent successful maintenance of pregnancy.
Figure 5.
VEGFR-3 blockade in the maternal gestational environment controls pregnancy success. A: Both normal and treated embryos implanted into normal, pseudo-pregnant recipient dams developed into normal, viable fetuses by pregnancy day 17. In pseudo-pregnant dams pretreated with mF4-31C1, no embryos developed into normal fetuses, despite normal implantations. B: Quantification of normal and remaining fetuses in implantation experiments. C: The distribution of fetal quality at pregnancy day 17 after normal embryo transfer to mF4-31C1–treated recipients closely replicates that of in situ fetal development in VEGFR-3–blocked pregnancies; the majority of fetuses were severely underdeveloped. ∗∗P < 0.01. Grid = 4 mm.
Reduction in Ovarian Lymphatic Vessels Alone Appears to Inhibit Fetal Development
It was previously reported that the rat monoclonal antibody mF4-31C1 does not sufficiently cross the placental barrier.18 Even though treatment was stopped before mating, we detected low levels of rat IgG in the fetus at day pregnancy day 8, although by day 13 it was no longer detected (Supplemental Figure S4A). We removed the Fc domain of mF4-31C1 by enzymatic digestion, to prevent transplacental transport (Supplemental Figure S4, B and C).47 Treatment with the F(ab′) fragment alone resulted in the same failed pregnancies and fetal development insufficiencies as with the full-form antibody (Supplemental Figure S4, D and E), demonstrating that fetal VEGFR-3 inhibition was not the primary cause of impaired development.
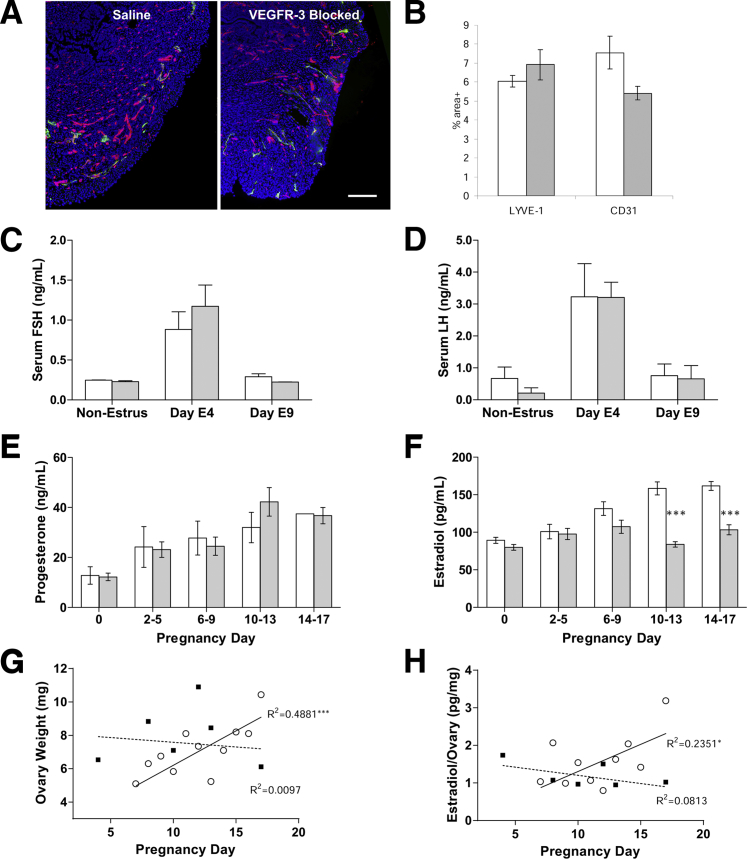
There was no significant change in the lymphatic vessel density of the uterus or the decidua in dams at pregnancy day 10 with or without blockade of VEGFR-3 (P = 0.283) (Figure 6, A and B). There was a small but nonsignificant reduction in the uterine blood vessel density at pregnancy day 10 (P = 0.083) (Figure 6, A and B), which corroborates a previous study using a high concentration bolus of mF4-31C1 after mating that also measured a minor reduction, but that also demonstrated no subsequent effects on pregnancy success with VEGFR-3 blockade.18
Figure 6.
Pregnancy failures are attributable only to the ovarian lymphatic vessels. A: Blood (CD31; red) and lymphatic (LYVE-1; green) capillaries in the uterine wall appear normal in both mF4-31C1–treated and untreated pregnant dams. B: Quantified vessel density exhibited a nonsignificant decrease in blood vessel density (in accord with past use of mF4-31C1 in pregnancy); lymphatic density was unchanged. C and D: Serum levels of the pituitary-derived reproductive hormones follicle-stimulating hormone (FSH) (C) and luteinizing hormone (LH) (D) were not significantly affected with mF4-31C1 treatment during early pregnancy. E: Progesterone levels during gestation were unaffected by VEGFR-3 blockade or by the failing fetuses. F: Estradiol was significantly reduced in circulation at pregnancy day 6 to 9 after VEGFR-3 blockade. G: There was no sustained weight increase in VEGFR-3–blocked ovaries during pregnancy. H: Estradiol levels in ovarian homogenates (pg estradiol/mg ovarian protein) were reduced, mirroring the decrease in circulation. ∗P < 0.05, ∗∗∗P < 0.001 between treatments or for regression line significantly different from zero. Scale bar = 200 μm. Saline, white bars; VEGFR3-blocked, shaded (B–F). Open circles, saline; black squares, VEGFR3-blocked (G and H).
In focusing the effects of VEGFR-3 neutralization on the ovary, we assessed hormone levels during pregnancy that are critical to the gestational environment and whose gonadal communication may be lymphatic transport dependent.48 Neither pituitary-secreted follicle-stimulating hormone levels (P = 0.567) nor luteinizing hormone levels (P = 0.676), important for ovulation and the development of the corpora lutea, were reduced in early pregnancy subsequent to VEGFR-3 neutralization (Figure 6, C and D). Circulating progesterone concentrations increased normally in mF4-31C1–treated dams during pregnancy (Figure 6E). Serum estradiol, however, was significantly reduced (P < 0.001, two-way analysis of variance) at pregnancy days 6 to 9 and then returned to baseline levels for the remainder of the pregnancy (P < 0.001, Bonferroni post hoc analysis) in dams with reduced ovarian lymphatic vessels (Figure 6F). Because there was no significant change in progesterone with VEGFR-3 blockade, even when pregnancies were failing late in term, and because progesterone therapy failed to rescue pregnancy maintenance (data not shown), this effect appeared to be estradiol-specific. With decreased lymphatic vessels, ovarian weight gain was significantly inhibited (P = 0.018) during pregnancy, and the concentration of estradiol in these ovaries mirrored the reduced circulating levels (P = 0.013) over time (Figure 6, G and H). In total, these data demonstrate that the ovarian lymphatic vasculature and lymphangiogenesis during follicle maturation and corpus luteum formation are important regulators of reproductive capacity.
Discussion
We have demonstrated here that VEGFR-3–dependent adult ovarian lymphangiogenesis occurring during follicle maturation is necessary for successful reproduction. Neutralization of VEGFR-3 effectively halted lymphangiogenesis of maturing follicles within the ovary, but did not visibly affect blood angiogenesis, macrophage recruitment, lipid accumulation, or cell apoptosis. In the absence of lymphangiogenesis, there were fewer patent secondary follicles, but folliculogenesis, ovulation, and fertilization were successful. Early cultured blastocysts from VEGFR-3–neutralized dams, however, exhibited reduced cell densities. These embryonic masses naturally implanted in the uterus, but exhibited growth restriction and all the pregnancies eventually miscarried; there were no successful births, despite a normal number of uterine implantation spots. Transplanting embryos to untreated dams permitted normal gestational development.
Although the role of, or even existence of, lymphatic vessels in the uterine wall, endometrium, and decidua is still unclear,49–52 we measured no major differences in LYVE-1+ cell densities in these tissues during pregnancy after VEGFR-3 blockade before mating. A previous report suggested that VEGFR-3 signaling occurs in endometrial lymphatic vessels,51 but high-dose mF4-31C1 (a dose five times higher than in the present study) administered at pregnancy day 3.75 had little effect on uterine vascularization and pregnancy success.18 Similarly, VEGF-C/VEGFR-3 signaling may occur in trophoblast cells, affecting placental growth and leading to intrauterine growth restriction.53 Indeed, this may explain the reduced blastocyst cell densities ex vivo. Again, however, delivery of mF4-31C1 at pregnancy day 3.75 did not limit pregnancy success, so this deficiency is corrected in gestation.18 These results, coupled with the lowered estradiol levels measured during pregnancy, suggest that it is specifically the absence of new follicular lymphatic vessels in the ovary that limits follicle health and ovarian support of pregnancy maintenance.
The ovarian microvasculature is critical in regulating hormonal transport during pregnancy. Normally, as follicles mature, the theca layers become vascularized by blood vessels54 and support follicles by synthesizing estrogen,55 and abnormalities in the theca cell layers can result in infertility.56 After implantation, proper blood vascularization is necessary for successful pregnancy,6 and blocking blood vessel formation in the corpus luteum leads to pregnancy failure.8 The developing blood vasculature of the corpus luteum permits this pseudo-organ to function properly, supplying increased progesterone and estrogen to the uterus to maintain pregnancy.8,35,57 We find lymphangiogenesis and ovarian lymphatic vessels to be of potentially equal importance. Indeed, VEGFR-3 blockade had the greatest effect on decreasing follicle-associated lymphatic capillaries on the normally dense lymphatic network around corpora lutea.58,59
Lymphatic vessels supply a route by which hormones produced within the ovary enter systemic circulation.60 Additionally, it has also been suggested that retrograde transfer of prostaglandin E2 (which is involved in many crucial processes of pregnancy, including maintenance of the corpora lutea) from the uterus to the ovary may occur via lymphatic transport.35,61 Indeed, various studies indicate that the uterus has a dense lymphatic network,5 that ovarian lymphatic vessels and lymphangiogenesis are essential for reproduction,2,3 and that the lymphangiogenic growth factors VEGF-C and VEGF-D cycle with estrous.58 The poorly connected granulosa of isolated follicles and lower levels of estradiol during pregnancy are likely related.62 The blocked growth of new lymphatic capillaries during folliculogenesis would disturb the local hormonal balance with VEGFR-3 neutralization. The lymphatic vasculature of these follicles is then insufficient to modulate the corpora lutea and their hormone secretions during pregnancy. Because VEGFR-3 signaling (and therefore lymphangiogenesis) was blocked only before mating, ovulation and fertilization and implantation all occurred normally in a sufficiently vascularized uterus.18 We have thus isolated a developmental period during which lymphangiogenesis appears to most critically occur.
The range in development across the same fetal cohort suggests that what is involved is not a systemic maternal response to factors such as reduced nutrition, glucose levels, or total circulating hormones. Although it is possible that the systemic reduction of hormones and ovarian estradiol production are linked to the poor development in late term resulting in feedback to the ovary from the uterus that reduces ovarian hormone production,63 this would also manifest as equivalent developmental retardation across the cohort. The differences in fetal size were not correlated with position within the uterus, but a reduction in uterine and placental blood flow may lead to intrauterine growth restriction, with reduced volumetric flow providing fewer nutrients to the fetus.64,65 Because in the present study fetuses appeared normal at pregnancy day 9, exhibited limb and eye formation, and some had normal skin vascularization, they were potentially size-restricted because of decreased placental or uterine blood flow. VEGFR-3 neutralization was stopped before mating, so it is unlikely to have directly regulated blood flow. Estrogens, however, are necessary to increase blood flow through the uterine and myometrial arteries.66,67 The measured reduction in estradiol levels resulting from mF4-31C1 delivery and inhibited follicular lymphangiogenesis therefore seems to be the likely local mediator controlling fetal nutrition.
Maintaining fluid balance and interstitial fluid pressure is another important role for the lymphatic system. In the ovary, follicles become increasingly vascularized as they grow and a fluid-filled antrum is formed. The interstitial fluid pressure in antra of developing follicles is approximately 15 mmHg, regardless of size, and drops rapidly to 5 mm Hg immediately preceding ovulation.68 After ovulation, the interstitial fluid pressure in the highly vascularized corpus luteum has been reported at a very high 50 mmHg.68 Ovarian lymphatic vessels must play a role in modulating these fluid pressures. Furthermore, concurrent lymphangiogenesis is likely necessary to drain extravasated fluid from the newly formed luteal blood capillaries26 and may help to regulate hormonal signaling on the luteal cells by controlling interstitial flow. VEGFR-3 neutralization may also dampen lymphatic pumping responses69 or alter capillary permeability,70 either of which could limit the ability of existing lymphatic vessels to balance interstitial fluid pressure.
Lack of follicular lymphatic vessels has been reported in ADAMTS-1 knockout mice,2,3 and Frizzled4 knockout mice exhibit low levels of ovarian VEGF-C, the primary ligand to VEGFR-3.71 Both of these strains are infertile, despite normal mating behavior; infertility in these mice is considered to be the result of failed hormone transport, intrafollicular pressure modulation, or maintenance of the corpus luteum. Mice possessing a mutation in the VEGFR-3 gene or with low levels of soluble VEGFR-3 exhibit poor lymphatic function,41 but are able to reproduce, albeit as heterozygotes and at a lower success rate than wild-type mice.72,73
In conclusion, our results demonstrate that VEGFR-3–mediated lymphangiogenesis in the ovary is necessary for pregnancy. Given that antilymphangiogenic therapies aimed at preventing tumor metastases are proposed as a cancer therapy33,34,74 and that prolymphangiogenic therapies are proposed for treating lymphedema,72,75,76 both targeting the VEGF-C/D–VEGFR-3 axis, it is critical to understand the role of lymphangiogenesis in the ovary and the role of reproductive tissue lymphatic vessels in fertility. Moreover, an increased knowledge of the physiological role of lymphangiogenesis in the ovaries may provide insight into causes of infertility (and into potential therapeutic strategies) and would permit a more careful examination of the pathological vasculogenesis and lymphangiogenesis inherent with ovarian cancers.
Acknowledgments
We thank Bronislaw Pytowski for helpful comments and discussion, ImClone Systems for the VEGFR-3 neutralizing antibody mF4-31C1, Carolyn Sacco for photography, and Veronique Borel and Sonia Verp for technical assistance with the animals during this study.
Footnotes
Supported in part by grants from the NIH (R01-HL075217 to M.A.S.), the Swiss National Science Foundation (107602 to M.A.S.), and the American Heart Association (12SDG12050287 to J.M.R.).
Disclosures: The nonclinical neutralizing antibody mF4-31C1 was a gracious gift from ImClone Systems (Bronislaw Pytowski). Bronislaw Pytowski approved the manuscript.
Supplemental Data
Follicular vasculature was evaluated and quantitated at four stages of follicle maturation. A: Representation of follicles at various maturation states (as described under Materials and Methods) within the murine ovary. Vasculature increases with maturation. B: Each follicle was first identified and numbered on H&E-stained sections, where follicular structure was easier to recognize after fluorescence imaging of the entire ovarian slice. C: On the corresponding fluorescence image, each follicle was outlined using MetaMorph software (dotted lines indicate samples of the procedure), and the percent area of lymphatic and blood vessel coverage within each region of interest was quantified. D: There were more maturation category 2 follicles in VEGFR-3–blocked ovaries than in controls; the similar trend for maturation category 1 did not reach significance. Fewer Graafian follicles (category 3) and corpora lutea (category 4) were seen in VEGFR-3–blocked dams, but this reduction was not significant. Scale bar = 200 μm.
Follicle patency was not determined by basal lamina quality. No differences were noted in the follicular basement membrane proteins in vascular VEGFR-3–blocked ovaries. Antibodies against collagen IV (A), laminin (B), and perlecan (C) each labeled intact follicular membranes (green) in both control and VEGFR-3–blocked ovaries. Blood vascular structures also marked by these proteins (arrows) likewise appeared normal. Scale bar = 100 μm.
Uteri removed from control and VEGFR-3–blocked dams at pregnancy day 9 exhibit little visible difference in early pregnancy. Ovaries are included for anatomical reference. A rule is included for scale.
Failed pregnancies could not be accounted for by VEGFR-3 blockade directly in the fetus. The F(ab′)2 fragment of mF4-31C1 alone yielded the same failures in pregnancy and abnormal fetuses as documented with the full antibody. A: Residual mF4-31C1 was detected at low levels in the uterine wall (at high exposure) and within the developing fetus (anti-rat IgG; green) at pregnancy day 8 (arrow), but had cleared by day 13. B: The approximately 30-kDa Fc domain of mF4-31C1, which binds the Fc-receptor to allow IgGs to cross the placental barrier, was removed by enzymatic digestion. C: The mF4-31C1 antibody, both in its native form and as the F(ab′)2 fragment alone, was effective at blocking VEGF-C–induced VEGFR-3 phosphorylation in lymphatic endothelial cells, but a higher concentration of 50 μg/mL was necessary to achieve the same blockade as with mF4-31C1 in its native form at 10 μg/mL. D: Fetuses extracted at pregnancy day 17 from treated animals displayed a marked developmental deficiency, compared with control animals. E: A significant reduction in normal pregnancy day 17 fetuses was observed in mice treated with the F(ab′)2 fragment of mF4-31C1 alone. ∗P < 0.05. Scale bar = 100 μm. Grid = 4 mm.
References
- 1.Choi I., Lee S., Hong Y.K. The new era of the lymphatic system: no longer secondary to the blood vascular system. Cold Spring Harb Perspect Med. 2012;2:a006445. doi: 10.1101/cshperspect.a006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown H.M., Dunning K.R., Robker R.L., Pritchard M., Russell D.L. Requirement for ADAMTS-1 in extracellular matrix remodeling during ovarian folliculogenesis and lymphangiogenesis. Dev Biol. 2006;300:699–709. doi: 10.1016/j.ydbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Brown H.M., Robker R.L., Russell D.L. Development and hormonal regulation of the ovarian lymphatic vasculature. Endocrinology. 2010;151:5446–5455. doi: 10.1210/en.2010-0629. [DOI] [PubMed] [Google Scholar]
- 4.Gaytán F., Tarradas E., Bellido C., Morales C., Sánchez-Criado J.E. Prostaglandin E(1) inhibits abnormal follicle rupture and restores ovulation in indomethacin-treated rats. Biol Reprod. 2002;67:1140–1147. doi: 10.1095/biolreprod67.4.1140. [DOI] [PubMed] [Google Scholar]
- 5.Svingen T., François M., Wilhelm D., Koopman P. Three-dimensional imaging of Prox1-EGFP transgenic mouse gonads reveals divergent modes of lymphangiogenesis in the testis and ovary. PLoS One. 2012;7:e52620. doi: 10.1371/journal.pone.0052620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser H.M. Regulation of the ovarian follicular vasculature. Reprod Biol Endocrinol. 2006;4:18. doi: 10.1186/1477-7827-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaczmarek M.M., Schams D., Ziecik A.J. Role of vascular endothelial growth factor in ovarian physiology–an overview. Reprod Biol. 2005;5:111–136. [PubMed] [Google Scholar]
- 8.Pauli S.A., Tang H., Wang J., Bohlen P., Posser R., Hartman T., Sauer M.V., Kitajewski J., Zimmermann R.C. The vascular endothelial growth factor (VEGF)/VEGF receptor 2 pathway is critical for blood vessel survival in corpora lutea of pregnancy in the rodent. Endocrinology. 2005;146:1301–1311. doi: 10.1210/en.2004-0765. [DOI] [PubMed] [Google Scholar]
- 9.Skobe M., Hamberg L.M., Hawighorst T., Schirner M., Wolf G.L., Alitalo K., Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 11.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–478. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 12.Iijima K., Jiang J.Y., Shimizu T., Sasada H., Sato E. Acceleration of follicular development by administration of vascular endothelial growth factor in cycling female rats. J Reprod Dev. 2005;51:161–168. doi: 10.1262/jrd.51.161. [DOI] [PubMed] [Google Scholar]
- 13.Gómez R., Simón C., Remohí J., Pellicer A. Vascular endothelial growth factor receptor-2 activation induces vascular permeability in hyperstimulated rats, and this effect is prevented by receptor blockade. Endocrinology. 2002;143:4339–4348. doi: 10.1210/en.2002-220204. [DOI] [PubMed] [Google Scholar]
- 14.Hazzard T.M., Rohan R.M., Molskness T.A., Fanton J.W., D'Amato R.J., Stouffer R.L. Injection of antiangiogenic agents into the macaque preovulatory follicle: disruption of corpus luteum development and function. Endocrine. 2002;17:199–206. doi: 10.1385/ENDO:17:3:199. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann R.C., Hartman T., Bohlen P., Sauer M.V., Kitajewski J. Preovulatory treatment of mice with anti-VEGF receptor 2 antibody inhibits angiogenesis in corpora lutea. Microvasc Res. 2001;62:15–25. doi: 10.1006/mvre.2001.2312. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann R.C., Hartman T., Kavic S., Pauli S.A., Bohlen P., Sauer M.V., Kitajewski J. Vascular endothelial growth factor receptor 2-mediated angiogenesis is essential for gonadotropin-dependent follicle development. J Clin Invest. 2003;112:659–669. doi: 10.1172/JCI18740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stouffer R.L., Xu F., Duffy D.M. Molecular control of ovulation and luteinization in the primate follicle. Front Biosci. 2007;12:297–307. doi: 10.2741/2065. [DOI] [PubMed] [Google Scholar]
- 18.Douglas N.C., Tang H., Gomez R., Pytowski B., Hicklin D.J., Sauer C.M., Kitajewski J., Sauer M.V., Zimmermann R.C. Vascular endothelial growth factor receptor 2 (VEGFR-2) functions to promote uterine decidual angiogenesis during early pregnancy in the mouse. Endocrinology. 2009;150:3845–3854. doi: 10.1210/en.2008-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams R.H., Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 20.Norrmén C., Tammela T., Petrova T.V., Alitalo K. Biological basis of therapeutic lymphangiogenesis. Circulation. 2011;123:1335–1351. doi: 10.1161/CIRCULATIONAHA.107.704098. [Erratum appeared in Circulation 2011, 124:e901–e903] [DOI] [PubMed] [Google Scholar]
- 21.Goldman J., Rutkowski J.M., Shields J.D., Pasquier M.C., Cui Y., Schmokel H.G., Willey S., Hicklin D.J., Pytowski B., Swartz M.A. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 2007;21:1003–1012. doi: 10.1096/fj.06-6656com. [Erratum appeared in FASEB J 2007, 21:1942] [DOI] [PubMed] [Google Scholar]
- 22.Pytowski B., Goldman J., Persaud K., Wu Y., Witte L., Hicklin D.J., Skobe M., Boardman K.C., Swartz M.A. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- 23.Tammela T., Enholm B., Alitalo K., Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65:550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Ichikawa S., Uchino S., Hirata Y. Lymphatic and blood vasculature of the forming corpus luteum. Lymphology. 1987;20:73–83. [PubMed] [Google Scholar]
- 25.Otsuki Y., Magari S., Sugimoto O. Lymphatic capillaries in rabbit ovaries during ovulation: an ultrastructural study. Lymphology. 1986;19:55–64. [PubMed] [Google Scholar]
- 26.Otsuki Y., Magari S., Sugimoto O. Fine structure and morphometric analysis of lymphatic capillaries in the developing corpus luteum of the rabbit. Lymphology. 1987;20:64–72. [PubMed] [Google Scholar]
- 27.Demeestere I., Delbaere A., Gervy C., Van Den Bergh M., Devreker F., Englert Y. Effect of preantral follicle isolation technique on in-vitro follicular growth, oocyte maturation and embryo development in mice. Hum Reprod. 2002;17:2152–2159. doi: 10.1093/humrep/17.8.2152. [DOI] [PubMed] [Google Scholar]
- 28.Liu J., Van Der Elst J., Van Den Broecke R., Dumortier F., Dhont M. Maturation of mouse primordial follicles by combination of grafting and in vitro culture. Biol Reprod. 2000;62:1218–1223. doi: 10.1095/biolreprod62.5.1218. [DOI] [PubMed] [Google Scholar]
- 29.Thouas G.A., Korfiatis N.A., French A.J., Jones G.M., Trounson A.O. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online. 2001;3:25–29. doi: 10.1016/s1472-6483(10)61960-8. [DOI] [PubMed] [Google Scholar]
- 30.Inai T., Mancuso M., Hashizume H., Baffert F., Haskell A., Baluk P., Hu-Lowe D.D., Shalinsky D.R., Thurston G., Yancopoulos G.D., McDonald D.M. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol. 2004;165:35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Partanen T.A., Paavonen K. Lymphatic versus blood vascular endothelial growth factors and receptors in humans. Microsc Res Tech. 2001;55:108–121. doi: 10.1002/jemt.1162. [DOI] [PubMed] [Google Scholar]
- 32.Witmer A.N., van Blijswijk B.C., Dai J., Hofman P., Partanen T.A., Vrensen G.F., Schlingemann R.O. VEGFR-3 in adult angiogenesis. J Pathol. 2001;195:490–497. doi: 10.1002/path.969. [DOI] [PubMed] [Google Scholar]
- 33.Laakkonen P., Waltari M., Holopainen T., Takahashi T., Pytowski B., Steiner P., Hicklin D., Persaud K., Tonra J.R., Witte L., Alitalo K. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–599. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- 34.Roberts N., Kloos B., Cassella M., Podgrabinska S., Persaud K., Wu Y., Pytowski B., Skobe M. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–2657. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds L.P., Grazul-Bilska A.T., Redmer D.A. Angiogenesis in the corpus luteum. Endocrine. 2000;12:1–9. doi: 10.1385/ENDO:12:1:1. [DOI] [PubMed] [Google Scholar]
- 36.Tammela T., Zarkada G., Wallgard E., Murtomäki A., Suchting S., Wirzenius M., Waltari M., Hellström M., Schomber T., Peltonen R., Freitas C., Duarte A., Isoniemi H., Laakkonen P., Christofori G., Ylä-Herttuala S., Shibuya M., Pytowski B., Eichmann A., Betsholtz C., Alitalo K. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- 37.Pate J.L., Landis Keyes P. Immune cells in the corpus luteum: friends or foes? Reproduction. 2001;122:665–676. doi: 10.1530/rep.0.1220665. [DOI] [PubMed] [Google Scholar]
- 38.Hedger M.P., Qin J.X., Robertson D.M., de Kretser D.M. Intragonadal regulation of immune system functions. Reprod Fertil Dev. 1990;2:263–280. doi: 10.1071/rd9900263. [DOI] [PubMed] [Google Scholar]
- 39.Shakil T., Whitehead S.A. Inhibitory action of peritoneal macrophages on progesterone secretion from co-cultured rat granulosa cells. Biol Reprod. 1994;50:1183–1189. doi: 10.1095/biolreprod50.5.1183. [DOI] [PubMed] [Google Scholar]
- 40.Rockson S.G. Update on the biology and treatment of lymphedema. Curr Treat Options Cardiovasc Med. 2012;14:184–192. doi: 10.1007/s11936-012-0170-0. [DOI] [PubMed] [Google Scholar]
- 41.Rutkowski J.M., Markhus C.E., Gyenge C.C., Alitalo K., Wiig H., Swartz M.A. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol. 2010;176:1122–1129. doi: 10.2353/ajpath.2010.090733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rutkowski J.M., Moya M., Johannes J., Goldman J., Swartz M.A. Secondary lymphedema in the mouse tail: Lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvasc Res. 2006;72:161–171. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skarzyński D., Młynarczuk J., Kotwica J. Involvement of high-density lipoprotein in stimulatory effect of hormones supporting function of the bovine corpus luteum. Acta Vet Hung. 2003;51:111–120. doi: 10.1556/AVet.51.2003.1.11. [DOI] [PubMed] [Google Scholar]
- 44.Veldhuis J.D. Follicle-stimulating hormone regulates low density lipoprotein metabolism by swine granulosa cells. Endocrinology. 1988;123:1660–1667. doi: 10.1210/endo-123-3-1660. [DOI] [PubMed] [Google Scholar]
- 45.Rolaki A., Drakakis P., Millingos S., Loutradis D., Makrigiannakis A. Novel trends in follicular development, atresia and corpus luteum regression: a role for apoptosis. Reprod Biomed Online. 2005;11:93–103. doi: 10.1016/s1472-6483(10)61304-1. [DOI] [PubMed] [Google Scholar]
- 46.Nourani M.R., Owada Y., Kitanaka N., Sakagami H., Hoshi H., Iwasa H., Spener F., Kondo H. Occurrence of immunoreactivity for adipocyte-type fatty acid binding protein in degenerating granulosa cells in atretic antral follicles of mouse ovary. J Mol Histol. 2005;36:491–497. doi: 10.1007/s10735-006-9024-y. [DOI] [PubMed] [Google Scholar]
- 47.Chen P., Li C., Lang S., Zhu G., Reheman A., Spring C.M., Freedman J., Ni H. Animal model of fetal and neonatal immune thrombocytopenia: role of neonatal Fc receptor in the pathogenesis and therapy. Blood. 2010;116:3660–3668. doi: 10.1182/blood-2010-05-284919. [DOI] [PubMed] [Google Scholar]
- 48.Krzymowski T., Stefanczyk-Krzymowska S. Local retrograde and destination transfer of physiological regulators as an important regulatory system and its role. Facts and hypothesis. J Physiol Pharmacol. 2012;63:3–16. [PubMed] [Google Scholar]
- 49.Bellini C., Rutigliani M., Boccardo F., Campisi C., Bellini T., Bonioli E., Fulcheri E. Are there lymphatic vessels in the placenta? Lymphology. 2012;45:34–36. [PubMed] [Google Scholar]
- 50.Castro E., Tony Parks W., Galambos C. Neither normal nor diseased placentas contain lymphatic vessels. Placenta. 2011;32:310–316. doi: 10.1016/j.placenta.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 51.Donoghue J.F., McGavigan C.J., Lederman F.L., Cann L.M., Fu L., Dimitriadis E., Girling J.E., Rogers P.A. Dilated thin-walled blood and lymphatic vessels in human endometrium: a potential role for VEGF-D in progestin-induced break-through bleeding. PLoS One. 2012;7:e30916. doi: 10.1371/journal.pone.0030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volchek M., Girling J.E., Lash G.E., Cann L., Kumar B., Robson S.C., Bulmer J.N., Rogers P.A. Lymphatics in the human endometrium disappear during decidualization. Hum Reprod. 2010;25:2455–2464. doi: 10.1093/humrep/deq224. [DOI] [PubMed] [Google Scholar]
- 53.Dunk C., Ahmed A. Expression of VEGF-C and activation of its receptors VEGFR-2 and VEGFR-3 in trophoblast. Histol Histopathol. 2001;16:359–375. doi: 10.14670/HH-16.359. [DOI] [PubMed] [Google Scholar]
- 54.Vollmar B., Laschke M.W., Rohan R., Koenig J., Menger M.D. In vivo imaging of physiological angiogenesis from immature to preovulatory ovarian follicles. Am J Pathol. 2001;159:1661–1670. doi: 10.1016/S0002-9440(10)63013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Acosta T.J., Miyamoto A. Vascular control of ovarian function: ovulation, corpus luteum formation and regression. Anim Reprod Sci. 2004;82–83:127–140. doi: 10.1016/j.anireprosci.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 56.Magoffin D.A. Ovarian theca cell. Int J Biochem Cell Biol. 2005;37:1344–1349. doi: 10.1016/j.biocel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 57.Jabbour H.N., Kelly R.W., Fraser H.M., Critchley H.O. Endocrine regulation of menstruation. Endocr Rev. 2006;27:17–46. doi: 10.1210/er.2004-0021. [DOI] [PubMed] [Google Scholar]
- 58.Nitta A., Shirasuna K., Haneda S., Matsui M., Shimizu T., Matsuyama S., Kimura K., Bollwein H., Miyamoto A. Possible involvement of IFNT in lymphangiogenesis in the corpus luteum during the maternal recognition period in the cow. Reproduction. 2011;142:879–892. doi: 10.1530/REP-11-0157. [DOI] [PubMed] [Google Scholar]
- 59.Xu F., Stouffer R.L. Existence of the lymphatic system in the primate corpus luteum. Lymphat Res Biol. 2009;7:159–168. doi: 10.1089/lrb.2009.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stefańczyk-Krzymowska S., Krzymowski T. Local adjustment of blood and lymph circulation in the hormonal regulation of reproduction in female pigs–facts, conclusions and suggestions for future research. Reprod Biol. 2002;2:115–132. [PubMed] [Google Scholar]
- 61.Stefanczyk-Krzymowska S., Chlopek J., Grzegorzewski W., Radomski M. Local transfer of prostaglandin E2 into the ovary and its retrograde transfer into the uterus in early pregnant sows. Exp Physiol. 2005;90:807–814. doi: 10.1113/expphysiol.2005.031112. [DOI] [PubMed] [Google Scholar]
- 62.Drummond A.E., Findlay J.K. The role of estrogen in folliculogenesis. Mol Cell Endocrinol. 1999;151:57–64. doi: 10.1016/s0303-7207(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 63.Krzymowski T., Stefańczyk-Krzymowska S. The role of the endometrium in endocrine regulation of the animal oestrous cycle. Reprod Domest Anim. 2008;43:80–91. doi: 10.1111/j.1439-0531.2007.00859.x. [DOI] [PubMed] [Google Scholar]
- 64.Battaglia F.C. Clinical studies linking fetal velocimetry, blood flow and placental transport in pregnancies complicated by intrauterine growth retardation (IUGR) Trans Am Clin Climatol Assoc. 2003;114:305–313. [PMC free article] [PubMed] [Google Scholar]
- 65.Gilbert M., Leturque A. Fetal weight and its relationship to placental blood flow and placental weight in experimental intrauterine growth retardation in the rat. J Dev Physiol. 1982;4:237–246. [PubMed] [Google Scholar]
- 66.Khan L.H., Rosenfeld C.R., Liu X.T., Magness R.R. Regulation of the cGMP-cPKG pathway and large-conductance Ca2+-activated K+ channels in uterine arteries during the ovine ovarian cycle. Am J Physiol Endocrinol Metab. 2010;298:E222–E228. doi: 10.1152/ajpendo.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagar D., Liu X.T., Rosenfeld C.R. Estrogen regulates {beta}1-subunit expression in Ca(2+)-activated K(+) channels in arteries from reproductive tissues. Am J Physiol Heart Circ Physiol. 2005;289:H1417–H1427. doi: 10.1152/ajpheart.01174.2004. [DOI] [PubMed] [Google Scholar]
- 68.Espey L.L., Lipner H. Measurements of intrafollicular pressures in the rabbit ovary. Am J Physiol. 1963;205:1067–1072. doi: 10.1152/ajplegacy.1963.205.6.1067. [DOI] [PubMed] [Google Scholar]
- 69.Breslin J.W., Gaudreault N., Watson K.D., Reynoso R., Yuan S.Y., Wu M.H. Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am J Physiol Heart Circ Physiol. 2007;293:H709–H718. doi: 10.1152/ajpheart.00102.2007. [DOI] [PubMed] [Google Scholar]
- 70.Pietrowski D., Szabo L., Sator M., Just A., Egarter C. Ovarian hyperstimulation syndrome is correlated with a reduction of soluble VEGF receptor protein level and a higher amount of VEGF-A. Hum Reprod. 2012;27:196–199. doi: 10.1093/humrep/der349. [DOI] [PubMed] [Google Scholar]
- 71.Hsieh M., Boerboom D., Shimada M., Lo Y., Parlow A.F., Luhmann U.F., Berger W., Richards J.S. Mice null for Frizzled4 (Fzd4-/-) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod. 2005;73:1135–1146. doi: 10.1095/biolreprod.105.042739. [DOI] [PubMed] [Google Scholar]
- 72.Karkkainen M.J., Saaristo A., Jussila L., Karila K.A., Lawrence E.C., Pajusola K., Bueler H., Eichmann A., Kauppinen R., Kettunen M.I., Yla-Herttuala S., Finegold D.N., Ferrell R.E., Alitalo K. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA. 2001;98:12677–12682. doi: 10.1073/pnas.221449198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mäkinen T., Jussila L., Veikkola T., Karpanen T., Kettunen M.I., Pulkkanen K.J., Kauppinen R., Jackson D.G., Kubo H., Nishikawa S., Ylä-Herttuala S., Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 74.Achen M.G., Mann G.B., Stacker S.A. Targeting lymphangiogenesis to prevent tumour metastasis. Br J Cancer. 2006;94:1355–1360. doi: 10.1038/sj.bjc.6603120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szuba A., Skobe M., Karkkainen M.J., Shin W.S., Beynet D.P., Rockson N.B., Dakhil N., Spilman S., Goris M.L., Strauss H.W., Quertermous T., Alitalo K., Rockson S.G. Therapeutic lymphangiogenesis with human recombinant VEGF-C. FASEB J. 2002;16:1985–1987. doi: 10.1096/fj.02-0401fje. [DOI] [PubMed] [Google Scholar]
- 76.Yoon Y.S., Murayama T., Gravereaux E., Tkebuchava T., Silver M., Curry C., Wecker A., Kirchmair R., Hu C.S., Kearney M., Ashare A., Jackson D.G., Kubo H., Isner J.M., Losordo D.W. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest. 2003;111:717–725. doi: 10.1172/JCI15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Follicular vasculature was evaluated and quantitated at four stages of follicle maturation. A: Representation of follicles at various maturation states (as described under Materials and Methods) within the murine ovary. Vasculature increases with maturation. B: Each follicle was first identified and numbered on H&E-stained sections, where follicular structure was easier to recognize after fluorescence imaging of the entire ovarian slice. C: On the corresponding fluorescence image, each follicle was outlined using MetaMorph software (dotted lines indicate samples of the procedure), and the percent area of lymphatic and blood vessel coverage within each region of interest was quantified. D: There were more maturation category 2 follicles in VEGFR-3–blocked ovaries than in controls; the similar trend for maturation category 1 did not reach significance. Fewer Graafian follicles (category 3) and corpora lutea (category 4) were seen in VEGFR-3–blocked dams, but this reduction was not significant. Scale bar = 200 μm.
Follicle patency was not determined by basal lamina quality. No differences were noted in the follicular basement membrane proteins in vascular VEGFR-3–blocked ovaries. Antibodies against collagen IV (A), laminin (B), and perlecan (C) each labeled intact follicular membranes (green) in both control and VEGFR-3–blocked ovaries. Blood vascular structures also marked by these proteins (arrows) likewise appeared normal. Scale bar = 100 μm.
Uteri removed from control and VEGFR-3–blocked dams at pregnancy day 9 exhibit little visible difference in early pregnancy. Ovaries are included for anatomical reference. A rule is included for scale.
Failed pregnancies could not be accounted for by VEGFR-3 blockade directly in the fetus. The F(ab′)2 fragment of mF4-31C1 alone yielded the same failures in pregnancy and abnormal fetuses as documented with the full antibody. A: Residual mF4-31C1 was detected at low levels in the uterine wall (at high exposure) and within the developing fetus (anti-rat IgG; green) at pregnancy day 8 (arrow), but had cleared by day 13. B: The approximately 30-kDa Fc domain of mF4-31C1, which binds the Fc-receptor to allow IgGs to cross the placental barrier, was removed by enzymatic digestion. C: The mF4-31C1 antibody, both in its native form and as the F(ab′)2 fragment alone, was effective at blocking VEGF-C–induced VEGFR-3 phosphorylation in lymphatic endothelial cells, but a higher concentration of 50 μg/mL was necessary to achieve the same blockade as with mF4-31C1 in its native form at 10 μg/mL. D: Fetuses extracted at pregnancy day 17 from treated animals displayed a marked developmental deficiency, compared with control animals. E: A significant reduction in normal pregnancy day 17 fetuses was observed in mice treated with the F(ab′)2 fragment of mF4-31C1 alone. ∗P < 0.05. Scale bar = 100 μm. Grid = 4 mm.