Abstract
Preeclampsia is characterized by hypertension and proteinuria in pregnant women. Its exact cause is unknown. Preeclampsia increases the risk of maternal and fetal morbidity and mortality. Although delivery, often premature, is the only known cure, early targeted interventions may improve maternal and fetal outcomes. Successful intervention requires a better understanding of the molecular etiology of preeclampsia and the development of accurate methods to predict women at risk. To this end, we tested the role of miR-210, a miRNA up-regulated in preeclamptic placentas, in first-trimester extravillous trophoblasts. miR-210 overexpression reduced trophoblast invasion, a process necessary for uteroplacental perfusion, in an extracellular signal–regulated kinase/mitogen-activated protein kinase–dependent manner. Conversely, miR-210 inhibition promoted invasion. Furthermore, given that the placenta secretes miRNAs into the maternal circulation, we tested if serum expression of miR-210 was associated with the disease. We measured miR-210 expression in two clinical studies: a case-control study and a prospective cohort study. Serum miR-210 expression was significantly associated with a diagnosis of preeclampsia (P = 0.007, area under the receiver operator curves = 0.81) and was predictive of the disease, even months before clinical diagnosis (P < 0.0001, area under the receiver operator curve = 0.89). Hence, we conclude that aberrant expression of miR-210 may contribute to trophoblast function and that miR-210 is a novel predictive serum biomarker for preeclampsia that can help in identifying at-risk women for monitoring and treatment.
Hypertensive disorders of pregnancy (HDPs) have a large impact on maternal and fetal health. Preeclampsia, a multisystem disorder characterized by maternal hypertension and proteinuria, is one of the most common complications of pregnancy, with an occurrence of 5% to 10%.1–3 Although preeclampsia confers specific maternal risks, including end-organ injury, it also increases the risk of fetal and neonatal morbidity. Despite extensive research, the exact cause of preeclampsia is unknown. Current theories include defects in placental implantation,4 alterations in angiogenesis,5,6 and dysregulation of plasma volume.7 In addition, randomized controlled trials targeting placental vasoconstriction have been relatively unsuccessful.8,9 Delivery remains the only effective cure.
Recent research on the initiation of pathogenesis has focused on trophoblast/placental dysfunction. Implantation and successful placentation ensue from a distinct set of cellular events, including attachment of the embryo to the endometrial wall, proliferation and migration of embryonic trophoblast cells, and invasion of the trophoblast cells into the endometrium.7,10 Remodeling of the uterine spiral arteries by the invading extravillous trophoblast (EVT) cells increases vessel diameter and results in uteroplacental perfusion. Abnormalities in this process can cause placental hypoxia, resulting in trophoblast dysfunction and, presumably, preeclampsia.11 Therefore, identifying how this process is regulated is central to understanding the pathophysiological characteristics of preeclampsia.
Preeclampsia is often diagnosed in the third trimester of pregnancy. However, the underlying disorder is likely present much earlier. Identifying those at risk for developing preeclampsia before the onset of clinical symptoms may provide valuable insight into the etiology of the disease and improve health outcomes. Several biomarkers for preeclampsia have been suggested.12,13 Unfortunately, most of these biomarkers lack sufficient sensitivity and/or specificity for widespread adoption into clinical use. Furthermore, most studies have focused on proteins and metabolites in biofluids. However, few studies have evaluated circulating nucleic acids, such as miRNAs, which are becoming a powerful class of biomarkers for a wide variety of physiological states and diseases,14,15 as biomarkers for preeclampsia.
miRNAs are small (approximately 22 nucleotides), noncoding RNAs that play a critical role in post-transcriptional gene regulation.16 miRNAs regulate diverse cellular processes and target up to 60% of human genes.16,17 By binding to complementary sites in their target mRNA, miRNAs destabilize the transcript and repress translation.16 miR-210 is a common hypoxia-induced miRNA involved in a myriad of biological processes, including angiogenesis, cell differentiation, cell cycle regulation, proliferation and growth, inflammation, DNA damage repair, and mitochondrial metabolism.18–20 Because many of these processes have been shown to be abnormally regulated in preeclampsia, recent studies have evaluated miR-210 expression in preeclampsia.21–24 These studies found increased miR-210 expression in preeclamptic placentas. One study suggests that miR-210 levels are elevated in the plasma of women with preeclampsia at delivery.24 However, it is unknown whether miR-210 levels are elevated before the clinical presentation of symptoms. Thus, whether miR-210 levels can be a clinically useful predictor of the development of this disease and, hence, could be used to risk stratify women for interventions remains unstudied.
We hypothesized that dysregulated miR-210 expression may contribute to trophoblast dysfunction, and because the placenta secretes miRNAs during pregnancy,25 we also posited that miR-210 levels will be elevated at the time of clinical diagnosis of the disease and months before the onset of clinical symptoms. To test our hypothesis, we first determined the biological effect of miR-210 on trophoblast function. We tested the impact of inhibiting and overexpressing miR-210 on EVT invasiveness and investigated the underlying signaling pathway mediating this effect. To determine whether miR-210 levels are altered with the clinical onset of disease, we measured serum miR-210 expression in maternal blood from a case-control study of women with preeclampsia. To determine whether miR-210 levels could predict preeclampsia months before the clinical onset of symptoms, we assessed miR-210 levels in maternal blood collected in the early second trimester of pregnancy in asymptomatic, low-risk women.
Materials and Methods
Cell Culture
Primary EVTs were isolated from first-trimester villous tissue using a protocol that has been established and well documented by researchers.26–28 Briefly, finely minced chorionic villi collected from de-identified elective first-trimester pregnancy termination tissues (<12 weeks) were cultured at 37°C in RPMI 1640 medium containing 20% charcoal-stripped (steroid-free) fetal bovine serum (FBS). EVT cells, which outgrow from attached villous fragments, were separated from villous tissue during washing and passaging of the cells. The isolated EVT cells were cultured and propagated in RPMI 1640 medium containing 20% FBS. The EVT cells used in our experiments were characterized by immunostaining for trophoblast cell markers, cytokeratins 7, 8, and 18, and integrin α-1.26,29,30 These results are similar to those obtained by other investigators using the same EVT isolation methods and confirm the purity of the EVT cell preparations.27,28
EVT Transfection
EVT cells were plated at 1 × 105 cells per well in 6-well plates in antibiotic-free RPMI 1640 media containing 20% FBS. The cells were transfected with 40 nmol/L miRNA mimics or 60 μmol/L morpholino antisense oligos the next day. Hsa-miR-210 and miR-neg (nontargeting control) miRNA mimics were purchased from Life Technologies (Carlsbad, CA). The morpholino antisense oligo (MO)-210 (5′-AGATCAGCCGCTGTCACACGCACAG-3′) and MO-neg (nontargeting antisense oligo) were purchased from Gene Tools, LLC (Philomath, OR). Fluorescein-labeled negative control morpholino (Gene Tools, LLC) and TEX 615–labeled negative control siRNA (IDT, San Jose, CA) were used to assess transfection efficiency. Lipofectamine RNAiMAX (6 μmol/L; Life Technologies) and Endo-Porter (Gene Tools, LLC) were used for the transfection of the miRNA mimics and morpholino oligos, respectively, according to the manufacturer's protocols. Cells were transfected for 48 to 72 hours and maintained under normal growth conditions before using for invasion assays.
EVT Cell Treatments for Invasion Assays
EVTs were cultured as previously described and plated at 1 × 105 cells per well in 6-well plates. Experiments investigating the involvement of the mitogen-activated protein kinase (MAPK) pathway in trophoblast invasion were performed by transfecting EVTs with miR-210 for 48 hours, followed by treatment with 50 μmol/L MAPK/extracellular signal–regulated kinase (ERK) kinase (MEK) 1/2–specific inhibitor, U0126 (Calbiochem, Gibbstown, NJ), for 1 hour before the start of the invasion assay and for 72 hours during the invasion assay. MEK1/2 inhibitor vehicle control [dimethyl sulfoxide (DMSO)]-treated EVTs was included in these experiments. The cells were then plated into chambers for the invasion assay.
Matrigel Invasion Assay
The invasiveness of primary EVT cells through an extracellular matrix was measured using a commercially available cell invasion assay kit (Chemicon, Temecula, CA). Briefly, 300 μL of 1 × 106 EVT cells/mL suspensions were plated onto 8-μm pore size ECMatrix gel-coated cell culture inserts in 24-well plates. After 72 hours, the noninvading cells and the ECMatrix gel from the upper surface of the inserts were removed using a cotton-tipped swab. Invasive cells on the lower surface of the membrane were stained with 0.2% crystal violet for 20 minutes. The membranes were mounted onto microscope slides. Stained cells from five random microscope fields (at ×20 magnification) were imaged, counted, and analyzed. Data from experiments measuring EVT invasion are expressed as a percentage of control.
Case-Control Study
With Institutional Review Board approval, a case-control study (Preeclampsia: Mechanisms and Consequences) was performed between March 2005 and October 2009 at the Hospital of the University of Pennsylvania (Philadelphia, PA). Controls were defined as women without hypertension-related complications who presented for delivery at term (≥37 gestational weeks). Cases were identified based on prespecified maternal criteria, according to standard American College of Obstetricians and Gynecologists criteria. A diagnosis of preeclampsia is defined as elevated blood pressure (≥140/90 mm Hg on two measurements ≥6 hours apart), with ≥1+ proteinuria (0-trace protein for gestational hypertension (GHTN). Based on these prespecified criteria, case eligibility was determined at enrollment by the study investigators and not by the treating physician. Within 24 hours of enrollment into the study, before delivery, peripheral blood samples were collected from all pregnant patients. After sitting at room temperature for 30 to 60 minutes, samples were centrifuged at 3400 rpm (1000 × g) (Clay Adams Sero-fuge 2001, BD Biosciences, San Jose, CA) rpm for 20 minutes. Serum was extracted from whole blood and stored at −80°C until the analyses were performed. miR-210 levels were assessed by quantitative real time PCR (qPCR), as noted later. Clinical information, including race, body mass index (BMI) at first prenatal visit, maternal age, and other maternal and prenatal factors, were abstracted from the patient's medical record.
Prospective Cohort
To assess if miR-210 expression can predict the development of a hypertensive disorder of pregnancy months before the onset of clinical symptoms, a nested case-control study from a prospective cohort was performed. This cohort is an Institutional Review Board–approved prospective cohort of low-risk women (The Placenta Study) at the Hospital of the University of Pennsylvania. Pregnant women, with singleton gestations, presenting for fetal aneuploidy screening were eligible for enrollment. Maternal serum was obtained at the second-trimester blood draw (15 to 20 weeks) and stored at −80°C until use. After delivery, medical records were reviewed and women with a hypertensive disorder (preeclampsia or GHTN) or controls (women delivering at term with no evidence of any hypertensive disorder of pregnancy) were identified. A diagnosis of preeclampsia or GHTN was confirmed by the study investigator (N.S.). Controls were randomly selected from the same cohort. For this study, women with chronic hypertension were excluded. Clinical information, including race, BMI at first prenatal visit, maternal age, and other maternal and prenatal factors, was abstracted from the patient's medical record. miR-210 levels were assessed by qPCR, as noted later. Statistical analyses for clinical studies/univariate analyses of categorical data were performed using χ2 or Fisher's exact tests, as appropriate. Means were compared using Student's t-test and one-way analysis of variance, whereas medians were compared using Wilcoxon-rank-sum and Kruskal-Wallis tests. If statistical significance was reached, then a pairwise comparison by the Student-Newman-Keuls test was performed. Multivariable logistic regression was performed to model the association between miR-210 and disease state controlling for confounders. Finally, areas under the receiver-operator curve (AUC) to determine the test characteristics of miR-210 to predict disease state were calculated and compared. P < 0.05 was considered statistically significant for all analyses. All arithmetic means ± SEM are presented.
miRNA Extraction, cDNA Generation, and qPCR
Total RNA, including miRNAs and other small RNAs, was extracted from serum samples via phenol/chloroform extraction, followed by column-based purification. Briefly, 750 μL of TRIzol reagent (Life Technologies) was added to each 50 μL sample of serum. To this, 1 μg of carrier RNA (MS2 bacteriophage total RNA; Roche, Indianapolis, IN, catalog 10165948001) was added. Chloroform (160 μL) was then added to each sample and mixed vigorously for 15 seconds. The samples were then transferred to a phase lock gel tube (5′, 2302830), and spun at 12,000 × g for 15 minutes at 4°C. The resulting aqueous phase was carefully removed and purified using Qiagen's miRNeasy kit (Qiagen, Valencia, CA), following the manufacturer's protocol for total RNA isolation. The RNA was eluted in 30 μL of RNase-free water. cDNA was generated from 10 μL of the isolated miRNA using the miScript Reverse Transcription II kit (Qiagen), and qPCR was performed on the 7900HT Real-Time PCR System (Life Technologies) using the miScript SYBR Green PCR kit (Qiagen), according to the manufacturer's protocols. The ΔΔCT method was used for relative expression quantification using the RQ manager software version 2.4 (Life Technologies). The endogenous reference, RNU6B, was used for miRNA quantification. All primer sets were purchased from Qiagen: miR-210 (MS00003801) and RNU6B (MS0001400).
Statistical Analysis
Statistical analyses for all in vitro experiments were performed with Sigma Stat Software version 3.5 (Statistical Package for the Social Sciences Inc., Chicago, IL). For data that were normally distributed, one-way analysis of variance was used. If statistical significance was reached (P < 0.05), then pairwise comparison with a Student-Newman-Keuls test was performed.
Results
miR-210 Regulates Trophoblast Invasion
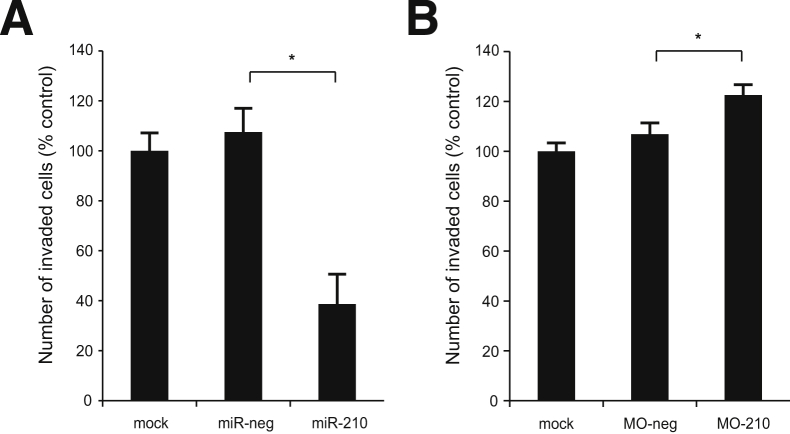
EVT invasion is crucial for proper implantation, and defects in this process have been associated with preeclampsia. Hence, we examined the role of miR-210 in EVT invasiveness. EVTs transfected with an miR-210 mimic had a significant decrease in invasion (approximately 60%) (P < 0.0001) relative to the mock and negative control transfected cells (Figure 1A). Transfection efficiency was confirmed via qPCR of miR-210 and fluorescent microscopy of cells transfected with a fluorescently labeled siRNA (Supplemental Figure S1, A and B). Next, we tested the effect of inhibiting endogenous miR-210 on trophoblast invasion using an antisense morpholino (MO). EVTs transfected with MO-210 showed a 20% increase in invasion when compared with both mock and negative control transfected EVTs (P = 0.03) (Figure 1B). Transfection efficiency was confirmed via fluorescent microscopy of cells transfected with a fluorescently labeled morpholino oligo (Supplemental Figure S1B). Representative images of stained membranes from the invasion assays are shown in Supplemental Figures S2 and S3. Together, these data strongly suggest that miR-210 inhibits trophoblast invasion.
Figure 1.
miR-210 regulates trophoblast invasion. Results from invasion assay of extravillous trophoblast (EVT) cells transfected with miR-210 mimic (A) or inhibitor (B). Values are means ± SEM. n = 6 (A); n = 3 (B). MO-neg, morpholino-negative control; MO-210, morpholino antisense oligo to miR-210. ∗P ≤ 0.05.
miR-210 Inhibition of Trophoblast Invasion Is Mediated by the ERK/MAPK Pathway
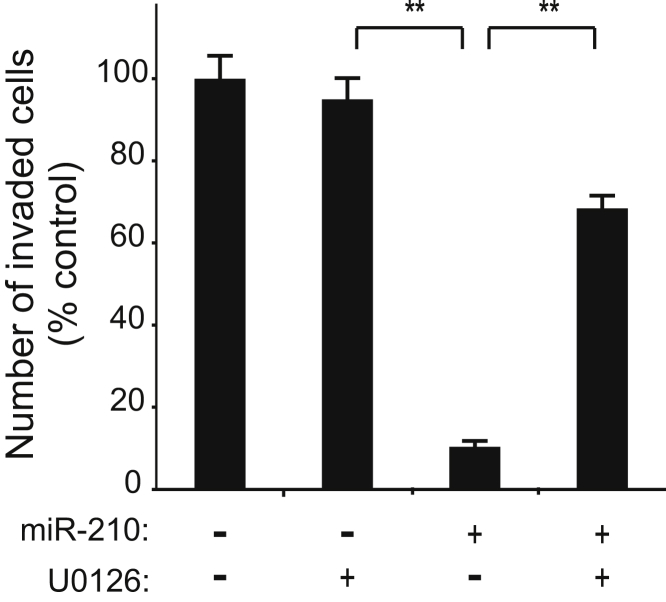
We previously demonstrated a role for ERK signaling in the regulation of trophoblast invasion.26 Hence, we tested if miR-210 inhibition of trophoblast invasion was mediated by the ERK/MAPK pathway. Treatment of EVTs with the MEK1/2 inhibitor, U0126, blocked the inhibitory effect of miR-210 on invasion by 40% compared with DMSO (vehicle control; P < 0.0001) (Figure 2). Representative images of stained membranes from the invasion assays are shown in Supplemental Figure S4. Hence, this suggested that miR-210 inhibited trophoblast invasion, in part, through the ERK signaling pathway.
Figure 2.
miR-210 regulation of extravillous trophoblast (EVT) invasion is MAPK/ERK dependent. Invasion assay of EVT cells transfected with miR-210 and treated with the MEK inhibitor, U0126. Negative U0126 columns represent DMSO vehicle-controls. Values are means ± SEM (n = 3). ∗∗P ≤ 0.01.
Case-Control Study
Serum miR-210 Expression Is Increased in Women with Hypertensive Disorders of Pregnancy
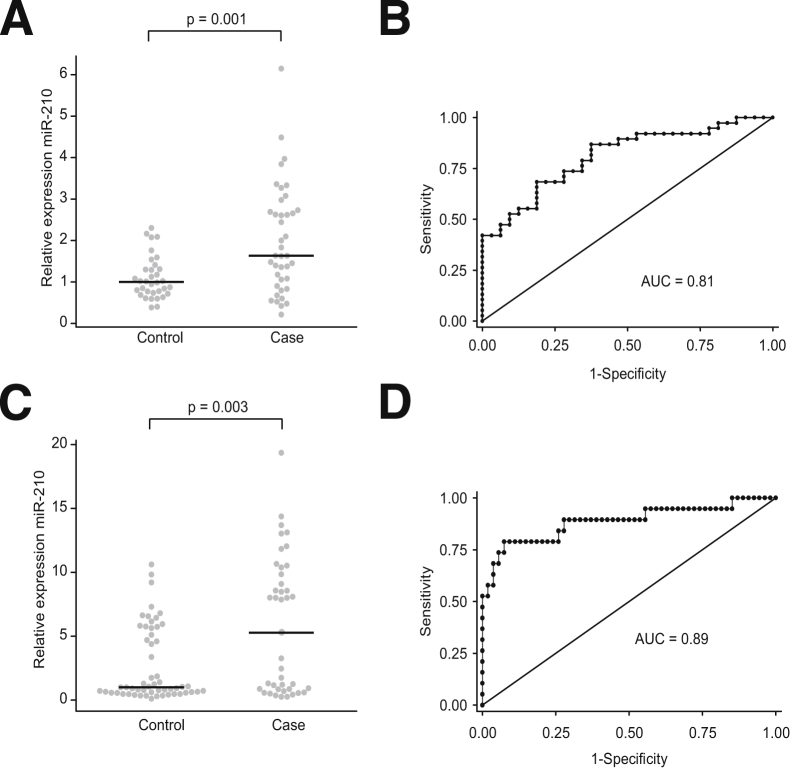
We measured serum miR-210 expression in women with (n = 40) and without (n = 33) HDP (including preeclampsia and GHTN). Demographic characteristics are detailed in Table 1. The median expression of miR-210 was 1.6-fold higher in cases than controls (P = 0.001) (Figure 3A). For each 5 U increase in miR-210, the odds of HDP increased 36-fold (95% CI 3.8 to 340.3; P = 0.002). Controlling for potential confounders, including maternal age, BMI, race, and parity, the odds of HDP increased 25-fold (95% CI 2.4 to 245.3; P = 0.007) for each 5 U increase in miR-210. Including these demographic variables increased the predictive ability of the model (AUC, 0.81 versus 0.72) (Figure 3B).
Table 1.
Term Case-Control Study Patient Demographics
| Demographic Variables | CNTL (N = 34) | GHTN (N = 7) | PEC (N = 33) | P value CNTL vs GHTN + PEC | P value CNTL vs PEC |
|---|---|---|---|---|---|
| African American | 29 (85) | 5 (71) | 28 (85) | 0.75 | 0.96 |
| Maternal age at delivery date | 26.2 (6.7) | 28.9 (8.3) | 25.5 (7.5) | 0.95 | 0.69 |
| Nulliparous: no prior pregnancy with GA >16 weeks | 9 (26) | 4 (57) | 20 (61) | 0.004 | 0.005 |
| BMI at first prenatal visit | 29.3 (7.7) | 33.5 (6.3) | 27.9 (7.1) | 0.79 | 0.47 |
Data are given as number (percentage) for discrete variables and mean (SD) for continuous demographic variables by pregnancy-induced hypertension diagnosis among the 74 women without chronic hypertension in the term case-control study. P values are based on the χ2 test for discrete variables or the t-test for continuous variables.
CNTL, control; GA, gestational age; PEC, preeclampsia.
Figure 3.
miR-210 expression is up-regulated in maternal serum in hypertensive disorders of pregnancy. A: Relative serum expression of miR-210 in case-control study (third trimester). Points represent individual samples. Black horizontal lines are the medians of the respective groups. The median of the control group is set to 1. B: Receiver-operator curve (ROC) showing specificity and sensitivity of miR-210 as a classifier for the disease state. C and D: Similar to A and B, but with second-trimester serum samples. ROC curves in B and D are corrected for maternal age, BMI, race, and parity.
Prospective Cohort
Serum miR-210 Expression Is Increased in Women Destined to Develop a Hypertensive Disorder of Pregnancy Months before the Clinical Onset of Symptoms
Next, we sought to determine whether serum miR-210 expression could predict HDP months before the onset of clinical symptoms. We measured serum miR-210 expression in women with (n = 41) and without (n = 56) HDP. Demographic characteristics are detailed in Table 2. The median expression of miR-210 was increased 5.3-fold in women who eventually developed HDP compared with those who did not (P = 0.003) (Figure 3C). For each 5-U increase in miR-210, the odds of HDP increased 1.8-fold (95% CI 1.3 to 2.6; P = 0.001). Controlling for race, tobacco use, BMI, and parity, the odds of HDP increased 2.7-fold (95% CI 1.6 to 4.6; P < 0.0001) for every 5-U increase of miR-210. When analyses were limited to only those women with the most severe phenotype (ie, only women with preeclampsia but not GHTN), each 5-U increase in miR-210 increased the odds of preeclampsia fourfold (95% CI 1.8 to 8.7; P < 0.0001; AUC = 0.89) (Figure 3D), controlling for the same clinical variables.
Table 2.
Second-Trimester Case-Control Study Patient Demographics
| Demographic Variables | CNTL (N = 56) | GHTN (N = 21) | PEC (N = 20) | P value CNTL vs GHTN + PEC | P value CNTL vs PEC |
|---|---|---|---|---|---|
| Race | 0.044 | 0.26 | |||
| African American | 25 (45) | 4 (19) | 12 (60) | ||
| Asian | 11 (20) | 1 (5) | 1 (5) | ||
| White/other | 20 (36) | 16 (76) | 7 (35) | ||
| Maternal age at delivery date | 29.7 (6.6) | 30.4 (7.2) | 31.2 (7.5) | 0.45 | 0.40 |
| Smoked tobacco in the first trimester | 6 (11) | 3 (14) | 2 (10) | 0.87 | 0.89 |
| Nulliparous: no prior pregnancy with GA >16 weeks | 0.18 | 0.36 | |||
| No, 1+ prior pregnancy | 20 (36) | 3 (14) | 5 (25) | ||
| Yes, first pregnancy | 18 (32) | 9 (43) | 5 (25) | ||
| Missing | 18 (32) | 9 (43) | 10 (50) | ||
| BMI at first prenatal visit | 24.9 (5.6) | 28.5 (6.8) | 31.0 (7.3) | 0.001 | 0.003 |
Data are given as number (percentage) for discrete variables and mean (SD) for continuous demographic variables by pregnancy-induced hypertension diagnosis among the 97 women without chronic hypertension in the second-trimester case-control study. P values are based on the χ2 test for discrete variables or the t-test for continuous variables.
CNTL, control; GA, gestational age; PEC, preeclampsia.
Discussion
In this study, we provide evidence for a molecular mechanism by which miR-210 may alter trophoblast invasion, lead to the development of HDP, and demonstrate that miR-210 may be an accurate biomarker for hypertensive disorders of pregnancy. The increased expression of miR-210 in the placenta of women with preeclampsia suggests a possible functional role in the pathophysiological characteristics of this disease. In the placenta, miR-210 is primarily expressed in the villous and extravillous trophoblasts, with stronger in situ evidence in the latter.31 Furthermore, multiple studies have shown increased miR-210 expression in placental cells, such as BeWo, JEG-3, Swan 71, JAR, and cytotrophoblasts, exposed to hypoxia (1% to 2% O2).24,31,32 Interestingly, in a study done by Ishibashi et al,32 miR-210 expression was found to be highest in the trophoblast layer of the placenta (when compared with villous stroma and fetal endothelium). Hence, an increasing number of studies are attempting to determine the function of miR-210 in the placenta. In a study by Muralimanoharan et al,33 increased expression of miR-210 was found to decrease mitochondrial respiration and up-regulate reactive oxygen species in preeclampsia, suggesting a role for miR-210 in placental dysfunction. In our study, miR-210 repressed extravillous trophoblast invasion. Our results support a study done by Zhang et al,24 which found that miR-210 repressed cytotrophoblast invasion and migration. Extravillous trophoblasts are the cells that actually invade the uterine spiral arteries soon after embryo implantation. Therefore, our findings suggest a role for miR-210 in the regulation of trophoblast invasion during early pregnancy. Interestingly, a study by Lee et al31 suggested that miR-210 up-regulation may contribute to poor trophoblast invasion through the knockdown of iron-sulfur cluster scaffold homologue. Although our study demonstrates that miR-210 represses invasion, in certain disease states (eg, cancer), miR-210 has been demonstrated to promote tumor cell invasion.34,35 The differing molecular effects of miR-210 suggest that miR-210 likely acts in a cell- and disease-specific manner. Further research is needed to elucidate the role of miR-210 in trophoblast function and in the development of trophoblast-related adverse pregnancy outcomes.
The molecular mechanism by which miR-210 regulates trophoblast invasion is likely complex because miRNAs can target hundreds of genes. We determined that miR-210 inhibition of trophoblast invasion was dependent, at least in part, on MAPK signaling. In a previous study, we also found that lipopolysaccharide (LPS) inhibited trophoblast invasion in an MAPK-dependent manner.26 Interestingly, LPS activates miR-210 expression in murine macrophages.36 Hence, miR-210 may serve as a converging node for multiple stimuli (including hypoxia and inflammation) that regulate trophoblast invasion. Furthermore, both hypoxia and LPS activate ERK/MAPK signaling in trophoblast cells.37 This suggests that miR-210 may function upstream of MAPK, possibly promoting signaling and, consequently, repressing trophoblast invasion.
Because we determined a functional role for miR-210 in trophoblast dysfunction and the placenta secretes miRNAs into maternal circulation during pregnancy,25 we wanted to assess if serum levels of miR-210 could identify the onset of preeclampsia. By using our clinical specimens, we found that miR-210 was elevated in the serum of women with a diagnosis of preeclampsia compared with women without disease. Our finding that miR-210 levels are associated with the development of HDP corroborated a recent study by Zhang et al24 in a cohort of Chinese women. However, this study was limited in that it investigated plasma miR-210 levels in term pregnancies only with a total sample size of 45 patients. Our study, with a larger sample size, confirms these findings. Novel to our study, we have demonstrated that miR-210 levels can predict the future development of HDP months before the clinical onset of symptoms. To our knowledge, this is the first study to identify a biomarker with this degree of prediction for the future development of HDP. The most promising finding of our study is the ability of miR-210 to accurately predict the development of preeclampsia 8 to 12 weeks before the clinical onset of any symptoms. Although confirmation in larger cohorts is needed, these results suggest that miR-210 levels might provide a strong tool for identifying women at risk for HDP months before the clinical onset of disease. Thus, if validated, miR-210 might provide the opportunity to offer new therapeutic strategies to women at risk.
With the hopes of diagnosing preeclampsia before the development of clinical symptoms, investigators have focused on identifying early biomarkers for this disease. Several studies have investigated preeclampsia-associated pro- and anti-angiogenic factors, including soluble fms-like tyrosine kinase (sFlt-1), placental growth factor (PlGF), and soluble endoglin (sENG), as possible serum biomarkers for this disease.38–44 However, studies on the effectiveness of these factors as early biomarkers of preeclampsia are somewhat contradictory because some have found altered PlGF, sFlt-1, and sENG levels in the serum of women who developed preeclampsia and others found no association.38–41,45,46 Espinoza et al45 performed one of the largest prospective cohort studies (3348 patients) investigating the predictive ability of angiogenic factors (along with uterine artery Doppler velocimetry) to identify patients destined to develop preeclampsia. Maternal PlGF concentration predicted early-onset preeclampsia (AUC = 0.80) and severe preeclampsia (AUC = 0.789); however, maternal sFlt-1 levels were of limited use in the prediction of early-onset (AUC = 0.49) and severe (AUC = 0.54) preeclampsia.45 Another large (1622 patients) longitudinal cohort study, performed by Kusanovic et al,44 found lower predictive values for sFlt-1 (AUC = 0.587), PlGF (AUC = 0.647), and PlGF/sFlt-1 (AUC = 0.592) in early pregnancy (6 to 15 weeks). Similarly, AUCs were lower in mid-trimester (20 to 25 weeks) for sFlt-1 (0.524), PlGF (0.650), and PLF/sFlt-1 (0.602).44 In addition, we have demonstrated in a prospective case-control study that included 374 patients that sFlt-1, PlGF, and sENG do not perform well in discriminating cases from controls.46 Alterations in the ratios of angiogenic factors, including serum sFlt-1 to PlGF, may possibly be more accurate predictors of preeclampsia across different gestational time points.42–44 Although somewhat promising, overall, these biomarkers are not thought to be reliable enough for general application in predicting preeclampsia.47 Nevertheless, as previously suggested, a panel of biomarkers may be needed to effectively identify women early in pregnancy who are at the highest risk for developing preeclampsia. Although specific interactions between miR-210 and these angiogenic factors are unknown, future studies could explore if evaluating these factors together may more accurately predict preeclampsia. However, if the predictive ability of miR-210, as demonstrated in this study, is validated in larger prospective cohort studies, then miR-210 alone may prove to be a useful biomarker without additional analytes.
Circulating miRNAs have emerged as powerful biomarkers for human disease.48,49 They are resistant to degradation and are readily measured from small volumes of biofluid. miRNAs are released from many tissues/cell types into the circulation via exosomes and microvesicles.15 Therefore, expression changes in the circulation can proxy changes in the source tissue(s). For example, the chromosome 19 miRNA cluster is expressed predominantly in the placenta.50 During pregnancy, chromosome 19 miRNA cluster miRNAs are secreted via exosomes into the maternal circulation, where they remain highly expressed until delivery.25 Although the exact origin of miR-210 in the maternal circulation remains unclear, it is plausible that it is being released from the placenta. Prior work has demonstrated that miR-210 is expressed in placental tissues and that miR-210 levels are increased in the placentas from preeclamptic women.21–23 However, we cannot exclude other sources for miR-210 in maternal serum. Because miR-210 is expressed in endothelial cells and endothelial dysfunction is posited to contribute to preeclampsia pathophysiological characteristics,51 release of miR-210 from the vasculature of pregnant women may be a source of miR-210 in maternal serum.
In all, our results show that elevated levels of miR-210 lead to a decrease in extravillous trophoblast invasion via an MAPK-dependent mechanism. Also, we demonstrate that miR-210 has the potential to be a predictive and diagnostic biomarker for preeclampsia because serum miR-210 is elevated months before the onset of clinical symptoms. More studies are needed to understand the source and function of the elevated miR-210 in preeclampsia and how this relates to the etiological and pathophysiological characteristics of the disease. However, our findings may provide a new clinical strategy to identify women at risk for preeclampsia and apply and test intervention strategies to improve maternal and fetal health outcomes.
Acknowledgment
We thank Anita Weber for assistance with statistical analysis of the data.
Footnotes
Supported by The Penn Presbyterian George L. and Emily McMichael Harrison Fund for Research in Obstetrics and Gynecology and grant 1R03HD069742 (N.S.), NIH grants 2-R01-NS054794-06 and 5-R01-HL097800-04 (J.B.H.), DARPA grant 12-DARPA-1068 (J.B.H), and National Human Genome Research Institute training grant T32HG000046 (A.O.O.-G.).
L.A. and A.O.O.-G. contributed equally to this article.
Supplemental Data
Extravillous trophoblasts (EVTs) are efficiently transfected with siRNAs/miRNAs or morpholinos. A: EVTs were transfected with the indicated miRNA mimics, and miR-210 expression (relative to U6) was measured with qPCR. B: Fluorescent microscopy of EVTs transfected with fluorescently labeled negative control siRNA (TEX615-si-neg) or morpholino oligo (fluor-MO-neg) demonstrates high transfection efficiency.
Images of stained membranes from invasion assays of extravillous trophoblasts (EVTs) transfected with miRNA mimics. EVTs were transfected with the indicated miRNA mimics and applied to the chamber of the invasion assay (see Materials and Methods for details). Shown are two representative bright-field microscopy images of the membrane from two different experiments for each of the indicated mimics.
Images of stained membranes from invasion assays of extravillous trophoblasts (EVTs) transfected with morpholino oligos. EVTs were transfected with the indicated morpholino oligos and applied to the chamber of the invasion assay (see Materials and Methods for details). Shown are representative bright-field microscopy images of the membranes for each morpholino oligo.
Images of stained membranes from invasion assays of extravillous trophoblasts (EVTs) transfected with miRNA mimics and treated with the MEK inhibitor, U0126. EVTs were transfected with the indicated miRNA mimics and treated with the MEK inhibitor, U0126 or DMSO (vehicle control). EVTs were then added to the chamber of the invasion assay (see Materials and Methods for details). Shown are representative bright-field microcopy images of the membrane for each of the conditions. neg, Negative.
References
- 1.The Working Group on High Blood Pressure in Pregnancy . US Dept of Health and Human Services; Washington: 1991. National High Blood Pressure Education Program (NHPEP): Working Group Report on High Blood Pressure in Pregnancy: report 91-3029. [Google Scholar]
- 2.Chang J., Elam-Evans L.D., Berg C.J., Herndon J., Flowers L., Seed K.A., Syverson C.J. Pregnancy-related mortality surveillance–United States, 1991–1999. MMWR Surveill Summ. 2003;52:1–8. [PubMed] [Google Scholar]
- 3.Koonin L.M., Atrash H.K., Rochat R.W., Smith J.C. Maternal mortality surveillance: United States, 1980-1985. MMWR CDC Surveill Summ. 1988;37:19–29. [PubMed] [Google Scholar]
- 4.Meekins J.W., Pijnenborg R., Hanssens M., McFadyen I.R., van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- 5.Maynard S.E., Min J.-Y., Merchan J., Lim K.-H., Li J., Mondal S., Libermann T.A., Morgan J.P., Sellke F.W., Stillman I.E., Epstein F.H., Sukhatme V.P., Karumanchi S.A. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor R.N., Grimwood J., Taylor R.S., McMaster M.T., Fisher S.J., North R.A. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 7.Van Beek E., Ekhart T.H., Schiffers P.M., van Eyck J., Peeters L.L., de Leeuw P.W. Persistent abnormalities in plasma volume and renal hemodynamics in patients with a history of preeclampsia. Am J Obstet Gynecol. 1998;179:690–696. doi: 10.1016/s0002-9378(98)70066-3. [DOI] [PubMed] [Google Scholar]
- 8.Rossi A.C., Mullin P.M. Prevention of pre-eclampsia with low-dose aspirin or vitamins C and E in women at high or low risk: a systematic review with meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2011;158:9–16. doi: 10.1016/j.ejogrb.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Roberts J.M., Myatt L., Spong C.Y., Thom E.A., Hauth J.C., Leveno K.J., Pearson G.D., Wapner R.J., Varner M.W., Thorp J.M., Jr., Mercer B.M., Peaceman A.M., Ramin S.M., Carpenter M.W., Samuels P., Sciscione A., Harper M., Smith W.J., Saade G., Sorokin Y., Anderson G.B. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brosens I., Robertson W.B., Dixon H.G. The physiological response of the vessels of the placental bed to normal pregnancy. J Pathol Bacteriol. 1967;93:569–579. doi: 10.1002/path.1700930218. [DOI] [PubMed] [Google Scholar]
- 11.Norwitz E.R., Schust D.J., Fisher S.J. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 12.Scazzocchio E., Figueras F. Contemporary prediction of preeclampsia. Curr Opin Obstet Gynecol. 2011;23:65–71. doi: 10.1097/GCO.0b013e328344579c. [DOI] [PubMed] [Google Scholar]
- 13.Carty D.M., Delles C., Dominiczak A.F. Novel biomarkers for predicting preeclampsia. Trends Cardiovasc Med. 2008;18:186–194. doi: 10.1016/j.tcm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Driscoll L. Extracellular nucleic acids and their potential as diagnostic, prognostic and predictive biomarkers. Anticancer Res. 2007;27:1257–1265. [PubMed] [Google Scholar]
- 15.Kosaka N., Iguchi H., Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101:2087–2092. doi: 10.1111/j.1349-7006.2010.01650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 17.Friedman R.C., Farh K.K.-H., Burge C.B., Bartel D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulshreshtha R., Davuluri R.V., Calin G.A., Ivan M. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15:667–671. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- 19.Huang X., Le Q.-T., Giaccia A.J. MiR-210: micromanager of the hypoxia pathway. Trends Mol Med. 2010;16:230–237. doi: 10.1016/j.molmed.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hua Z., Lv Q., Ye W., Wong C.K., Cai G., Gu D., Ji Y., Zhao C., Wang J., Yang B.B., Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enquobahrie D.A., Abetew D.F., Sorensen T.K., Willoughby D., Chidambaram K., Williams M.A. Placental microRNA expression in pregnancies complicated by preeclampsia. Am J Obstet Gynecol. 2011;204 doi: 10.1016/j.ajog.2010.09.004. 178.e12–e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pineles B.L., Romero R., Montenegro D., Tarca A.L., Han Y.M., Kim Y.M., Draghici S., Espinoza J., Kusanovic J.P., Mittal P., Hassan S.S., Kim C.J. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol. 2007;196 doi: 10.1016/j.ajog.2007.01.008. 261.e1–e6. [DOI] [PubMed] [Google Scholar]
- 23.Zhu X., Han T., Sargent I.L., Yin G., Yao Y. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol. 2009;200 doi: 10.1016/j.ajog.2008.12.045. 661.e1–e7. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Fei M., Xue G., Zhou Q., Jia Y., Li L., Xin H., Sun S. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. J Cell Mol Med. 2012;16:249–259. doi: 10.1111/j.1582-4934.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo S.-S., Ishibashi O., Ishikawa G., Ishikawa T., Katayama A., Mishima T., Takizawa T., Shigihara T., Goto T., Izumi A., Ohkuchi A., Matsubara S., Takeshita T., Takizawa T. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod. 2009;81:717–729. doi: 10.1095/biolreprod.108.075481. [DOI] [PubMed] [Google Scholar]
- 26.Anton L., Brown A.G., Parry S., Elovitz M.A. Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: possible mechanisms of first trimester placental dysfunction. Hum Reprod. 2012;27:61–72. doi: 10.1093/humrep/der362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham C.H., Lysiak J.J., McCrae K.R., Lala P.K. Localization of transforming growth factor-beta at the human fetal-maternal interface: role in trophoblast growth and differentiation. Biol Reprod. 1992;46:561–572. doi: 10.1095/biolreprod46.4.561. [DOI] [PubMed] [Google Scholar]
- 28.Getsios S., Chen G.T., Huang D.T., MacCalman C.D. Regulated expression of cadherin-11 in human extravillous cytotrophoblasts undergoing aggregation and fusion in response to transforming growth factor beta 1. J Reprod Fertil. 1998;114:357–363. doi: 10.1530/jrf.0.1140357. [DOI] [PubMed] [Google Scholar]
- 29.Neudeck H., Oei S.L., Stiemer B., Hopp H., Graf R. Binding of antibodies against high and low molecular weight cytokeratin proteins in the human placenta with special reference to infarcts, proliferation and differentiation processes. Histochem J. 1997;29:419–430. doi: 10.1023/a:1026499203743. [DOI] [PubMed] [Google Scholar]
- 30.Koi H., Zhang J., Makrigiannakis A., Getsios S., MacCalman C.D., Strauss J.F., 3rd, Parry S. Syncytiotrophoblast is a barrier to maternal-fetal transmission of herpes simplex virus. Biol Reprod. 2002;67:1572–1579. doi: 10.1095/biolreprod.102.004325. [DOI] [PubMed] [Google Scholar]
- 31.Lee D.-C., Romero R., Kim J.-S., Tarca A.L., Montenegro D., Pineles B.L., Kim E., Lee J., Kim S.Y., Draghici S., Mittal P., Kusanovic J.P., Chaiworapongsa T., Hassan S.S., Kim C.J. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am J Pathol. 2011;179:590–602. doi: 10.1016/j.ajpath.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishibashi O., Ohkuchi A., Ali M.M., Kurashina R., Luo S.-S., Ishikawa T., Takizawa T., Hirashima C., Takahashi K., Migita M., Ishikawa G., Yoneyama K., Asakura H., Izumi A., Matsubara S., Takeshita T., Takizawa T. Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: a novel marker for predicting preeclampsia. Hypertension. 2012;59:265–273. doi: 10.1161/HYPERTENSIONAHA.111.180232. [DOI] [PubMed] [Google Scholar]
- 33.Muralimanoharan S., Maloyan A., Mele J., Guo C., Myatt L.G., Myatt L. MIR-210 modulates mitochondrial respiration in placenta with preeclampsia. Placenta. 2012;33:816–823. doi: 10.1016/j.placenta.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ying Q., Liang L., Guo W., Zha R., Tian Q., Huang S., Yao J., Ding J., Bao M., Ge C., Yao M., Li J., He X. Hypoxia-inducible microRNA-210 augments the metastatic potential of tumor cells by targeting vacuole membrane protein 1 in hepatocellular carcinoma. Hepatology. 2011;54:2064–2075. doi: 10.1002/hep.24614. [DOI] [PubMed] [Google Scholar]
- 35.Rothé F., Ignatiadis M., Chaboteaux C., Haibe-Kains B., Kheddoumi N., Majjaj S., Badran B., Fayyad-Kazan H., Desmedt C., Harris A.L., Piccart M., Sotiriou C. Global microRNA expression profiling identifies miR-210 associated with tumor proliferation, invasion and poor clinical outcome in breast cancer. PLoS One. 2011;6:e20980. doi: 10.1371/journal.pone.0020980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qi J., Qiao Y., Wang P., Li S., Zhao W., Gao C. microRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-κB1 in murine macrophages. FEBS Lett. 2012;586:1201–1207. doi: 10.1016/j.febslet.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 37.Park M.-H., Galan H.L., Arroyo J.A. Effect of hypoxia on endothelial nitric oxide synthase, NO production, intracellular survival signaling (p-ERK1/2 and p-AKT) and apoptosis in human term trophoblast. Am J Reprod Immunol. 2011;65:407–414. doi: 10.1111/j.1600-0897.2010.00886.x. [DOI] [PubMed] [Google Scholar]
- 38.Thadhani R., Mutter W.P., Wolf M., Levine R.J., Taylor R.N., Sukhatme V.P., Ecker J., Karumanchi S.A. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 39.Baumann M.U., Bersinger N.A., Mohaupt M.G., Raio L., Gerber S., Surbek D.V. First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. Am J Obstet Gynecol. 2008;199 doi: 10.1016/j.ajog.2008.06.069. 266.e1–e6. [DOI] [PubMed] [Google Scholar]
- 40.Akolekar R., de Cruz J., Foidart J.-M., Munaut C., Nicolaides K.H. Maternal plasma soluble fms-like tyrosine kinase-1 and free vascular endothelial growth factor at 11 to 13 weeks of gestation in preeclampsia. Prenat Diagn. 2010;30:191–197. doi: 10.1002/pd.2433. [DOI] [PubMed] [Google Scholar]
- 41.Foidart J.M., Schaaps J.P., Chantraine F., Munaut C., Lorquet S. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin) in preeclampsia: a step forward but not the definitive answer. J Reprod Immunol. 2009;82:106–111. doi: 10.1016/j.jri.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Levine R.J., Maynard S.E., Qian C., Lim K.-H., England L.J., Yu K.F., Schisterman E.F., Thadhani R., Sachs B.P., Epstein F.H., Sibai B.M., Sukhatme V.P., Karumanchi S.A. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 43.Erez O., Romero R., Espinoza J., Fu W., Todem D., Kusanovic J.P., Gotsch F., Edwin S., Nien J.K., Chaiworapongsa T., Mittal P., Mazaki-Tovi S., Than N.G., Gomez R., Hassan S.S. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kusanovic J.P., Romero R., Chaiworapongsa T., Erez O., Mittal P., Vaisbuch E., Mazaki-Tovi S., Gotsch F., Edwin S.S., Gomez R., Yeo L., Conde-Agudelo A., Hassan S.S. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espinoza J., Romero R., Nien J.K., Gomez R., Kusanovic J.P., Gonçalves L.F., Medina L., Edwin S., Hassan S., Carstens M., Gonzalez R. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196 doi: 10.1016/j.ajog.2006.11.002. 326.e1–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivas S.K., Larkin J., Sammel M.D., Appleby D., Bastek J., Andrela C.M., Ofori E., Elovitz M.A. The use of angiogenic factors in discriminating preeclampsia: are they ready for prime time? J Matern Fetal Neonatal Med. 2010;23:1294–1300. doi: 10.3109/14767051003677988. [DOI] [PubMed] [Google Scholar]
- 47.Verlohren S., Stepan H., Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin Sci. 2012;122:43–52. doi: 10.1042/CS20110097. [DOI] [PubMed] [Google Scholar]
- 48.Chen X., Ba Y., Ma L., Cai X., Yin Y., Wang K., Guo J., Zhang Y., Chen J., Guo X., Li Q., Li X., Wang W., Zhang Y., Wang J., Jiang X., Xiang Y., Xu C., Zheng P., Zhang J., Li R., Zhang H., Shang X., Gong T., Ning G., Wang J., Zen K., Zhang J., Zhang C.-Y. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 49.Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L., Peterson A., Noteboom J., O'Briant K.C., Allen A., Lin D.W., Urban N., Drescher C.W., Knudsen B.S., Stirewalt D.L., Gentleman R., Vessella R.L., Nelson P.S., Martin D.B., Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bentwich I., Avniel A., Karov Y., Aharonov R., Gilad S., Barad O., Barzilai A., Einat P., Einav U., Meiri E., Sharon E., Spector Y., Bentwich Z. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- 51.Granger J.P., Alexander B.T., Llinas M.T., Bennett W.A., Khalil R.A. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension. 2001;38:718–722. doi: 10.1161/01.hyp.38.3.718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extravillous trophoblasts (EVTs) are efficiently transfected with siRNAs/miRNAs or morpholinos. A: EVTs were transfected with the indicated miRNA mimics, and miR-210 expression (relative to U6) was measured with qPCR. B: Fluorescent microscopy of EVTs transfected with fluorescently labeled negative control siRNA (TEX615-si-neg) or morpholino oligo (fluor-MO-neg) demonstrates high transfection efficiency.
Images of stained membranes from invasion assays of extravillous trophoblasts (EVTs) transfected with miRNA mimics. EVTs were transfected with the indicated miRNA mimics and applied to the chamber of the invasion assay (see Materials and Methods for details). Shown are two representative bright-field microscopy images of the membrane from two different experiments for each of the indicated mimics.
Images of stained membranes from invasion assays of extravillous trophoblasts (EVTs) transfected with morpholino oligos. EVTs were transfected with the indicated morpholino oligos and applied to the chamber of the invasion assay (see Materials and Methods for details). Shown are representative bright-field microscopy images of the membranes for each morpholino oligo.
Images of stained membranes from invasion assays of extravillous trophoblasts (EVTs) transfected with miRNA mimics and treated with the MEK inhibitor, U0126. EVTs were transfected with the indicated miRNA mimics and treated with the MEK inhibitor, U0126 or DMSO (vehicle control). EVTs were then added to the chamber of the invasion assay (see Materials and Methods for details). Shown are representative bright-field microcopy images of the membrane for each of the conditions. neg, Negative.